Abstract
Top-down regulation in the human brain and anatomical connections between the prefrontal cortex (PFC) and specific catecholamine-related regions have been well-studied. However, the way in which the PFC modulates downstream neuro-networks in terms of serotonin and the autonomic nervous system (ANS) by variation in the level of brain-derived neurotrophic factor (BDNF) is still unclear. We recruited sixty-seven healthy subjects. Serotonin transporter (SERT) availability was examined by SPECT with [123I]ADAM analysis; heart rate variability (HRV) testing was performed, and the BDNF level was measured. The Wisconsin card-sorting test (WCST), which assesses PFC activation, was also conducted. The interactions of BDNF level and SERT availability were significant in relation to the HRV indexes of low frequency, high frequency, total power, and mean heart rate range. Moderate to significant positive correlations between SERT availability and the above-mentioned HRV indexes existed only in subjects with a low BDNF level. Furthermore, in the low BDNF level group, only those with high WCST perseveration errors or low category completions exhibited significant positive correlations between SERT availability and HRV indexes. A lower BDNF level and poorer PFC function might modulate the synergistic effects of serotonergic and ANS systems in order to maintain brain physiological and psychological homeostasis.
Introduction
The human prefrontal cortex (PFC) manages several aspects of cognitive- emotional-behavioral regulation and plays crucial roles in problem-solving, strategy-shifting and error-monitoring1. In addition, top-down regulation from the PFC is connected to different brain regions and exerts neuromodulatory effects under stressful environments, which involve evocation of several neurochemical factors to orchestrate brain activities2.
For example, a high level of brain-derived neurotrophic factor (BDNF) in the PFC has been found to prevent or reverse stress-related mental illness by enhancing stress resilience3. Furthermore, BDNF can cross the blood-brain barrier, and its levels in serum and plasma are highly correlated with its level in the cerebrospinal fluid4. Serotonin is an important monoamine that manages several important domains of human cognition, emotional regulation, and behavior responses, and even exerts neuromodulatory effects under stress5. The serotonin transporter (SERT), which transports excessive serotonin from the synapse cleft to pre-synapse neurons, is important in terms of its antidepressant effects6 and several serotonin-related activities7, and altered SERT availability has been observed in major depressive disorder (MDD) and other anxiety disorders. More importantly, a connection between the PFC and the dorsal raphe nucleus (DRN), which is abundant in serotonin, has been demonstrated during stress management8. In addition, a previous study showed that SERT function is modulated by BDNF9, added to which the interaction effects of BDNF and serotonin have been demonstrated to be involved in the management of emotional regulation10.
The above-mentioned neurobiological systems that manage stress under challenging environments may be clinically measurable by the autonomic nervous system (ANS) performance, which alters human psycho-physiological conditions directly in order to cope with stress (fight or flight)11. For example, high frequency (HF) heart rate variability (HRV) predicts depressive symptoms in adolescents12, and reduction in HRV may lead to a greater risk of cardiovascular disease13. In addition, mentally perceived stress was found to be negatively correlated with the HF component of HRV14. Correlation between serotonin and the ANS, both of which have similar regulatory functions, has been reported previously15–19. Structurally, serotonergic neurons originating from the raphe nucleus in the midbrain project onto the nucleus tractus solitarius, which is responsible for cardiovascular reflexes and ANS function15,16. Clinically, the tryptophan depletion test, which decreases the serotonin level, may lead to lower HRV and more severe depressive symptoms17. Our previous study also showed that the effect of anxiety on HRV is related to serotonin vulnerability20. The antidepressant effect also alters HRV and may increase the risk of cardiovascular disease18. Genetically, serotonergic gene polymorphism modulates parasympathetic activity under conditions of greater stress19. Although there exists no direct evidence of a correlation between BDNF and the ANS, genetic analysis has shown that BDNF genes influence sympathovagal tone21. Moreover, the PFC modulates ANS function when facing physiological or psychological stress22.
To conclude, besides the ascertained structural connection, a frontal-subcortical functional connection and connections between subcortical activities might exist. However, the effects of BDNF and serotonin, as mentioned in previous studies, on the ANS remain unclear, and how regulatory effects of the PFC modulate downstream effects needs to be clarified. Therefore, we used [123I]-labeled 2-((2-((dimethylamino)methyl) phenyl)thio)-5- iodophenylamine ([123I]ADAM), a radioligand with a high selectivity and a strong affinity to SERT, combined with the techniques of single-photon emission computed tomography (SPECT), to examine SERT availability in the midbrain, and employed HRV frequent domain measurement to represent ANS function. We also used the Wisconsin card-sorting test (WCST), which is a psychological test that assesses prefrontal cognitive flexibility and executive function, and is correlated with stress management23. Hence, our study aimed to (i) explore the possible effects of BDNF and serotonin availability on the ANS; (ii) identify correlations among serotonin, each HRV component (subcortical-subcortical correlations), and BDNF level; and (iii) investigate the modulatory effect of the PFC in terms of WCST performance on the above associations (frontal-subcortical associations).
Results
Demographic data and interactions of BDNF level and SERT availability with HRV indexes
Table 1 presents the basic demographic data for the whole group of normal healthy participants. In line with the aims of the present study, we first examined the interactions of BDNF level and SERT availability with ANS function, and significant interactions were found with LF (F = 4.00, p = 0.0497), HF (F = 4.20, p = 0.045), total power (F = 4.53, p = 0.037), and MHRR (F = 6.42, p = 0.014) (Table 2). Full results of the interactions of BDNF level and SERT availability with ANS functions are presented in Supplemental Table 1. Subjects were divided into 2 groups according to the median BDNF level for further analysis. Differences in the demographic and other variables between the 2 groups are shown in Table 1. Besides the significant BDNF level difference due to stratification, only LF/HF was significantly higher in the low BDNF level group than in the high BDNF level group (p = 0.01).
Table 1.
Demographic data and comparison of low and high BDNF level groups.
| Total (n = 67) | Low (n = 33) | High (n = 34) | Statistic | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | Mean | ± SD | t/χ2 | p | |
| Age | 36.10 | ±13.30 | 34.09 | ±12.85 | 38.06 | ±13.62 | −1.23 | 0.22 |
| Sex (M/F) | 26/ | 41 | 14/ | 19 | 12/ | 22 | 0.36 | 0.55 |
| Smoking (Y/N) | 6/ | 61 | 3/ | 30 | 3/ | 31 | 0.00 | 0.97 |
| Alcohol (Y/N) | 5/ | 62 | 2/ | 31 | 3/ | 31 | 0.19 | 0.67 |
| Educational years | 13.31 | ±3.83 | 12.91 | ±4.64 | 13.71 | ±2.86 | −0.85 | 0.40 |
| Body mass index | 22.92 | ±3.79 | 23.52 | ±4.41 | 22.33 | ±3.02 | 1.29 | 0.20 |
| Systolic blood pressure (mmHg) | 115.63 | ±16.02 | 116.10 | ±16.26 | 115.21 | ±16.05 | 0.22 | 0.83 |
| Diastolic blood pressure (mmHg) | 73.94 | ±10.47 | 75.27 | ±10.88 | 72.73 | ±10.09 | 0.96 | 0.34 |
| ln (LF) | 5.86 | ±0.82 | 5.89 | ±0.91 | 5.83 | ±0.75 | 0.31 | 0.76 |
| ln (HF) | 5.36 | ±1.06 | 5.18 | ±1.10 | 5.55 | ±1.00 | −1.45 | 0.15 |
| ln (LF/HF) | 0.50 | ±0.65 | 0.72 | ±0.56 | 0.28 | ±0.67 | 2.85 | 0.01 |
| ln (total power) | 7.07 | ±0.78 | 7.04 | ±0.86 | 7.10 | ±0.70 | −0.32 | 0.75 |
| MHRR | 13.70 | ±5.63 | 13.35 | ±5.47 | 14.05 | ±5.84 | −0.51 | 0.61 |
| SERT availability | 2.18 | ±0.54 | 2.06 | ±0.48 | 2.30 | ±0.58 | −1.80 | 0.08 |
| BDNF (pg/ml) | 9178 | ±5652 | 4613 | ±2294 | 13610 | ±4207 | −10.82 | <0.001 |
Note: subjects were split into low and high BDNF level groups according to the median (8,500 pg/ml).
BDNF: brain-derived neurotrophic factor.
PSQI: Pittsburgh sleep quality index.
RLCQ: recent life change questionnaire.
LF: low frequency.
HF: high frequency.
MHRR: mean heart rate range.
SERT: serotonin transporter.
Table 2.
Interactions of BDNF level and SERT availability with HRV indexes and Spearman’s rho correlations between SERT availability and HRV indexes in subject groups with low and high BDNF levels.
| BDNFхSERTa | SERT | |||||
|---|---|---|---|---|---|---|
| Low BDNF (n = 33) | High BDNF (n = 34) | |||||
| F | p | ρ b | p | ρ b | p | |
| ln (LF) | 4.00 | 0.0497 | 0.49 | 0.004 | 0.07 | 0.68 |
| ln (HF) | 4.20 | 0.045 | 0.43 | 0.012 | −0.04 | 0.80 |
| ln (LF/HF) | 0.65 | 0.42 | −0.07 | 0.69 | 0.09 | 0.62 |
| ln (total power) | 4.53 | 0.037 | 0.47 | 0.006 | 0.04 | 0.82 |
| MHRR | 6.42 | 0.014 | 0.68 | <0.001 | 0.01 | 0.94 |
aThe results were similar if the BDNF group was used instead of BDNF level.
bThe results were still significant if partial correlation was used after controlling for age and sex, except ln (HF) (r = 0.30, p = 0.10).
Note: subjects were split into low and high BDNF level groups according to the median (8,500 pg/ml).
BDNF: brain-derived neurotrophic factor.
SERT: serotonin transporter.
HRV: heart rate variability.
LF: low frequency.
HF: high frequency.
MHRR: mean heart rate range.
Correlations between SERT availability and HRV indexes in subjects with different BDNF levels
We examined the correlations between SERT availability and HRV indexes in the different groups. The results showed that SERT was significantly correlated with LF (ρ = 0.49, p = 0.004), HF (ρ = 0.43, p = 0.012), total power (ρ = 0.47, p = 0.006), and MHRR (ρ = 0.68, p < 0.001) in the low BDNF group, but not in the high BDNF group. These significant correlations still existed after controlling for age and sex, with the exception of the marginal significant correlation with HF (ρ = 0.30, p = 0.10) (Table 2).
WCST modulated the correlations between SERT availability and HRV indexes in different BDNF level groups
We examined whether these findings were similar in groups with different levels of prefrontal cognitive flexibility function, as assessed by the two performance indexes of the WCST. The results showed that only subjects with a low BDNF level and a poorer WCST performance (higher perseveration errors or lower categorical completion) exhibited significant positive correlations between SERT availability and HRV indexes (see Tables 3 and 4, and Fig. 1).
Table 3.
Spearman’s correlations between SERT availability and HRV indexes in low and high BDNF level groups modulated by the perseveration error of WCST.
| SERT availability | ||||||||
|---|---|---|---|---|---|---|---|---|
| Low perseveration error | High perseveration error | |||||||
| Low BDNF (n = 18) | High BDNF (n = 16) | Low BDNF (n = 15) | High BDNF (n = 13) | |||||
| ρ | p | ρ | p | ρ | p | ρ | p | |
| ln (LF) | 0.24 | 0.33 | −0.24 | 0.37 | 0.88 | <0.001a | 0.34 | 0.25 |
| ln (HF) | 0.12 | 0.63 | −0.19 | 0.48 | 0.91 | <0.001a | 0.07 | 0.83 |
| ln (LF/HF) | 0.38 | 0.12 | 0.00 | 0.99 | −0.56 | 0.031 | 0.07 | 0.83 |
| ln (total power) | 0.18 | 0.47 | −0.16 | 0.54 | 0.88 | <0.001a | 0.25 | 0.42 |
| MHRR | 0.54 | 0.021 | −0.10 | 0.70 | 0.86 | <0.001a | 0.20 | 0.51 |
aA significant correlation still existed after controlling for age and sex.
Note: subjects were split into low and high BDNF level, perseveration error, and categories completion groups at the median (8,500 pg/ml, 8.5, and 3.5, respectively).
SERT: serotonin transporter
HRV: heart rate variability
BDNF: brain-derived neurotrophic factor
WCST: Wisconsin card-sorting test
LF: low frequency
HF: high frequency
MHRR: mean heart rate range.
Table 4.
Spearman’s correlations between SERT availability and HRV indexes in low and high BDNF level groups modulated by the categories completion of WCST.
| SERT availability | ||||||||
|---|---|---|---|---|---|---|---|---|
| Low categories completion | High categories completion | |||||||
| Low BDNF (n = 22) | High BDNF (n = 14) | Low BDNF (n = 11) | High BDNF (n = 15) | |||||
| ρ | p | ρ | p | ρ | p | ρ | p | |
| ln (LF) | 0.63 | 0.002a | 0.18 | 0.53 | 0.34 | 0.31 | −0.20 | 0.47 |
| ln (HF) | 0.77 | <0.001a | −0.17 | 0.56 | 0.01 | 0.98 | 0.02 | 0.94 |
| ln (LF/HF) | −0.48 | 0.023 | 0.41 | 0.14 | 0.46 | 0.15 | −0.44 | 0.10 |
| ln (total power) | 0.67 | 0.001a | 0.08 | 0.78 | 0.31 | 0.36 | −0.13 | 0.64 |
| MHRR | 0.71 | <0.001 | −0.06 | 0.84 | 0.78 | 0.005 | −0.28 | 0.31 |
aA significant correlation still existed after controlling for age and sex.
Note: subjects were split into low and high BDNF level, perseveration error, and categories completion groups at the median (8,500 pg/ml, 8.5, and 3.5, respectively).
SERT: serotonin transporter
HRV: heart rate variability
BDNF: brain-derived neurotrophic factor
WCST: Wisconsin card-sorting test
LF: low frequency
HF: high frequency
MHRR: mean heart rate range.
Figure 1.
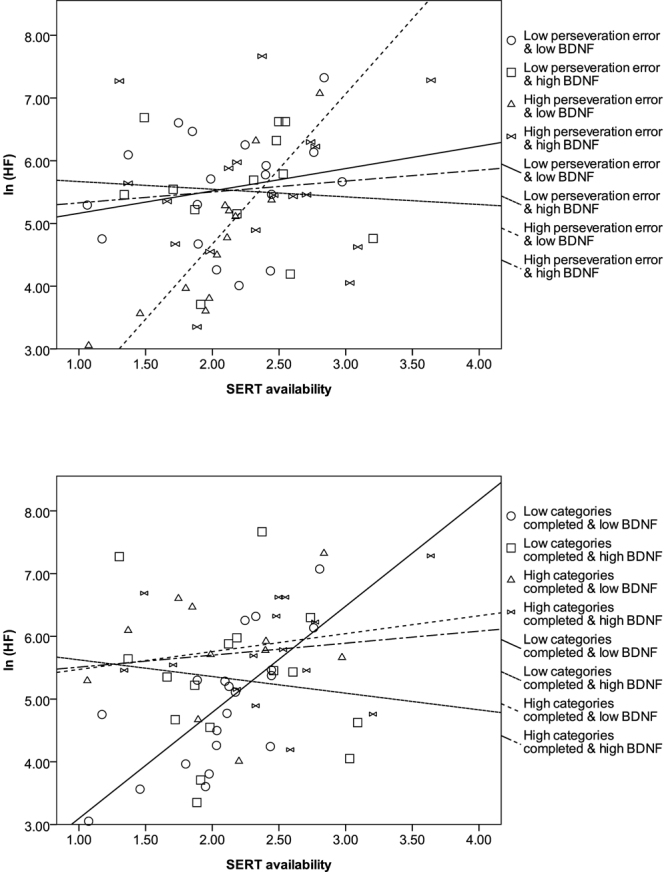
Correlation between SERT and ANS function in subjects with different BDNF levels, and modulation effects of PFC function in terms of WCST examination. Parasympathetic predominant domains may be positively correlated with SERT in subjects with a low BDNF level and poorer PFC function.
Discussion
Previous studies have demonstrated a correlation between BDNF and serotonin24. Our study further showed that a positive correlation existed between serotonin and the ANS in the low BDNF level group. Moreover, the functions of the PFC, represented by the top-down regulatory center during stress and in several neuropsychiatric illnesses8, may be modulated by compensatory effects of serotonin and ANS regulation when the PFC function is poor, especially in those with a lower BDNF level.
Substantial evidence has suggested that interaction of BDNF and serotonin may influence neuron structural plasticity25, exert genetic interaction effects that alter depression susceptibility26, modulate stress-buffering effects27, and could even be correlated with risk of depression when facing stressful life events28. Also, BDNF and serotonergic neurons have bidirectional effects that modulate their functions29,30. Neuro-imaging study has demonstrated a correlation between SERT availability and BDNF level31. Our results were in line with previous findings, and showed the existence of a significant interaction between BDNF and SERT.
Furthermore, a positive correlation between SERT and the ANS only existed in subjects with a low BDNF level. Previous studies have demonstrated that effective antidepressant treatment may synergistically enhance the extracellular serotonin level and the BDNF level, therefore improving symptoms of depression and anxiety disorders32. Meanwhile, sympathetic-related symptoms can be relieved with enhancement of the extracellular serotonin level33. Therefore, in the low BDNF level group, the reduced extracellular serotonin level could be associated with lower ANS function, and vice versa. This result was similar to previous findings in animal models, which showed that 5HT-2A and 5HT-3 receptor activation in the nucleus tractus solitaries regulates ANS function in terms of bradycardia and other vagal activities34,35. Interestingly, identical positive correlations between SERT and LF, HF, and total power existed in our study. Although previous studies have suggested that LF might be an indicator of sympathetic and partially parasympathetic regulation, this interpretation is controversial, and recent studies have demonstrated that LF, like HF, also has a predominant parasympathetic function36. This indicates that parasympathetic function might play a crucial role in ANS function to modulate symptoms related to an altered serotonin level37. The role of parasympathetic activities in our study could be strengthened by MHRR, which is also an index of parasympathetic functioning after deep breathing38, and has a positive correlation with SERT. Furthermore, stress-related downregulation of the BDNF level might be partly mediated by the serotonin level27 and ANS function39 to relieve symptoms. Our study produced similar results, showing that in order to maintain physiological homeostasis, modulations of serotonin and the ANS occur to prevent the detrimental effects of an inadequate BDNF level. Our results may explain why biofeedback management, including muscle relaxation and deep breathing, re-regulates the ANS function and relieves symptoms related to a lower serotonin level (anxiety, palpitations, etc.).
As mentioned, top-down regulation of the PFC to associated neuro-networks could prevent potential damage caused by challenging environments. Therefore, preserved PFC function learned from previous stressful experiences blocks serotonergic activation in the DRN when under stress40. On the contrary, one animal study showed that PFC inactivation increased the downstream serotonergic activation in the DRN41. Moreover, 5-HT2A receptor activation enhances the working memory function regulated by the dorsolateral PFC42. Our findings are consistent with previous findings showing that subjects with poorer PFC function exhibit a significant positive correlation between SERT and ANS function, especially subjects with a low BDNF level. In addition, when subjects have a better PFC function in terms of low perseveration errors or high categories completion on the WCST, the downstream modulating effects of the ANS and serotonin vanish, regardless of BDNF level. Our results implied that preservation of PFC function in order to cope with external stimulation might be sufficient to maintain neuro-physiological homeostasis, but poorer PFC function might switch the control of behavior and emotion to downstream neuro-networks for compensation. When the compensatory effects are disrupted, several psychiatric illnesses could occur, such as major depressive disorder43 and schizophrenia44, etc. However, interpretation should be performed cautiously, as the compensatory effects may not necessarily be in the form of bottom-up regulation. We do not have any evidence of bottom-up regulation owing to the study design, but the downstream effects for compensation of prefrontal malfunction in our study may be worthy of further exploration in order to prove the bidirectional mechanism in the human brain45. Another important result of our study was that in the group of subjects with poorer PFC function, a higher BDNF level seemed to offset the PFC inadequacy. Although the exact mechanisms are unclear, a buffering effect of BDNF in dysregulated cortical function has been reported previously46. Therefore, the associations between SERT and the ANS in order to maintain physiological homeostasis in subjects with poorer PFC function may be medicated by a high BDNF level. The exact mechanism merits further investigation.
Several limitations existed in our study, and the results should be interpreted with caution. First, the sample sizes were small, and several factors related to the ANS could not be tested; for example, effects following stratification by gender. Second, although we attempted to avoid several major issues that may influence ANS and SERT functioning, for example, medications use or even BMI47, several factors such as lifestyle (including exercise behavior) could not be controlled. Third, the cross-sectional correlation design of our study could not confirm a causal relationship between the ANS and serotonin. Therefore, further challenge tests are required to determine this causal relationship. Fourth, the effects of estrogen might influence the level of serotonin in different menstrual stages48. Although we did not control estrogenic effects in our study, previous studies have indicated that the estrogenic effect might have an insignificant influence on SERT availability49. Also, individual differences in the equilibrium time and clearance rate of the radiotracer may alter the imaging results. Examination of the metabolism/protein binding ratio of each subject in our study may solve this problem, but limitations with regards to the techniques possible in our laboratory hinder this potential solution. Finally, the model of PFC-serotonin-ANS associations may be over-simplified. A number of studies have shown that other monoamines are also responsible for stress modulation (for example, dopamine and norepinephrine)1. Future studies should integrate other monoamines for more complete neuro-network analysis.
Our study demonstrated a possible model of cognitive (PFC)-emotional (serotonin)-behavioral (ANS) association in the human brain (see Fig. 2). Malfunction in top-down regulation may be compensated by downstream neuro-network collaborations. Therefore, clinicians should emphasize cognitive rehabilitation in certain neuropsychiatric illnesses for better emotional regulation and improvement of a subject’s ability to cope with stress.
Figure 2.
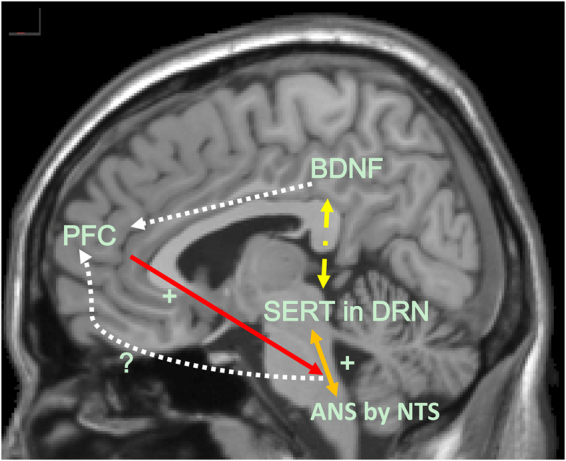
Proposed model of interaction between the PFC, SERT, ANS and BDNF level. Possible top-down regulation from the PFC may modulate downstream associations. PFC: prefrontal cortex. SERT: serotonin transporter. ANS: autonomic nervous system. BDNF: brain-derived neurotrophic factor. DRN: dorsal raphe nucleus. NTS: nucleus tractus solitaries
Methods
Subjects
Sixty-seven healthy controls, ranging from the age of 18 to 65, evaluated to have no other mental, medical, or neurological problems, were recruited from the community using advertisements for various studies50,51. The health of the participants was assessed by a physician, and an experienced psychiatrist used the Chinese version of the Mini International Neuropsychiatric Interview (MINI)52 to exclude individuals with any possible psychiatric disorders. Excluded subjects included (i) individuals with medical or mental illnesses identified when examined/interviewed; (ii) pregnant women, or participants suspected to be pregnant, and nursing women; and (iii) patients with drug or alcohol dependence or abuse in the past six months.
No subjects were in receipt of any medication that would affect the study results, including benzodiazepines, antihistamines, beta-adrenergic antagonists, or antidepressants. In addition, participants were not allowed to use alcohol, nicotine, or caffeine on the day of measurement. Before any procedure was performed, written informed consent was obtained from each of the participants after a complete explanation of the study. The Ethical Committee for Human Research at the National Cheng Kung University Hospital approved the study protocol. The methods were carried out in accordance with the relevant guidelines, including any relevant details.
BDNF measurement
Plasma BDNF level was measured in blood samples taken within a 2-h interval in the morning, between 9 AM and 11 AM, after patients had fasted for at least 8 h. Ten milliliters of whole blood were withdrawn from the antecubital vein of each patient into a vacuum tube containing ethylenediamine tetraacetic acid (EDTA) (Greiner Bio-One Vacuette; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and the blood sample was kept on ice for up to 30 min. To isolate plasma, whole blood was then centrifuged at 3000 × g for 15 min at 4 °C and immediately stored at −80 °C. A BDNF kit (Quantikine Human BDNF kit; R&D Systems, Minneapolis, MN, USA) and an enzyme-linked immunosorbent assay (ELISA) reader (Spectra-Max-M2; Molecular Devices, Sunnyvale, CA, USA) were used to analyze the level of plasma BDNF, the minimum detectable dose of which is typically < 80 pg/ml for humans. All samples were assayed in duplicate by laboratory staff blind to patient status.
SERT availability in the midbrain
For the SPECT examination with [123I]ADAM, the protocol, intravenous administration time of the radioligand, MRI co-registration, and reasons for selecting the midbrain as the region of interest (ROI) have been described in our previous studies50,53.
HRV measurements
ANS activity
A full 20-minute period of recumbent acclimatization in a quiet room that was maintained at a comfortable temperature (25–27 °C) preceded the cardiovascular measurements, which started at 10 AM. Subjects were asked to relax and breathe normally to avoid hyperventilation. Cardiac autonomic function was calculated by the geometric method, which is based on short-term measurements of the interbeat interval (IBI)54. Briefly, the sequence of IBI (IBI1, IBI2, …, IBIn) was transformed into a figure on a two-dimensional plane by plotting IBIk+1 against IBIk. The length of the transverse axis (T) is affected by both the sympathetic and parasympathetic blockades, whereas the length of the longitudinal axis (L) is affected only by the parasympathetic blockade. Thus, log10 (L × T) is a cardiac vagal index, whereas the L/T ratio is a cardiac sympathetic index. These two indices have been demonstrated to be more reliable than conventional measures including spectral analysis54. This geometric method has been found to be a sound measurement55, and its advantages include: (i) controlled respiration or other maneuvers are not required, and (ii) as few as 100 IBIs are sufficient for the assessment54.
Power spectral density analysis of HRV was performed by fast Fourier transformation56. Several spectral components were defined as follows: low frequency (LF) (0.04 to 0.15 Hz), HF (0.15 to 0.40 Hz), and total power (≤0.4 Hz). The very low frequency (VLF) data were excluded, because the available VLF data in short-term recording is dubious and should be avoided57. The total power represents the total autonomic activity. The HF power of HRV represents an index of cardiac parasympathetic (vagal) activity, whereas the LF power represents an index of vasomotor sympathetic activity, or both sympathetic and vagal activities. The LF/HF ratio has been proposed as an index of the relative balance of sympatho-vagal influences on the heart, with higher LH/HF ratios reflecting increased sympathetic activity or decreased parasympathetic modulation58. These indexes were ln-transformed to correct the skewness.
Autonomic function during resting
The beat-to-beat blood pressure (BP) of the left radial artery and the heart rate were monitored for 5 minutes while subjects remained in the supine position. BP and heart rate were continuously monitored using a Tonometry BP Monitor (Colin BP-508, Colin Co., Komaki-City, Aichi, Japan) and input into a computer console. The referential BP was recorded using a sphygmomanometer cuff over the right brachial artery and measured at intervals of 2.5 minutes. Whenever the tonometry BP measurement was questionable or failed, cuff measurement for calibration was automatically started.
Autonomic function during the respiratory challenge test
The subjects were asked to take a deep breath and the heart rate was continuously recorded. Each deep breath cycle contained a five-second inspiration and a five-second expiration; 5 successive breath cycles were measured in one assessment. We subtracted the minimum HR during expiration from the maximum HR during inspiration for each cycle of breathing, the time interval between two cycles being one minute, and then determined the mean of the differences. The differences were recorded as the mean heart rate range (MHRR), which is one of the most widely-used methods by which to assess HRV under respiratory challenge testing59.
WCST measurements
During the WCST, patients were required to match response cards to four stimulus cards, along with one of three dimensions (color, form, or number) on the basis of verbal feedback (correct or wrong) without being given any information about the dimensions. Once the subject had sorted a series of 10 cards by one category, they were then asked to sort the cards again by a different category. There were 64 cards in the present test. All definitions of indices are described in the WCST manual60. There are many measures by which to determine WCST performance. In this study, we selected the number of WCST categories that patients completed and the perseveration errors as indexes of WCST performance.
Statistical analysis
First, multiple regression was used to test the interactions of BDNF level and SERT availability with each individual HRV index, within which the HRV indexes were the dependent variables, and SERT, BDNF, and interaction were independent variables. Subjects were further split into high and low BDNF groups by the median for further stratified correlation analysis. Spearman’s rho correlation and partial correlation, controlling for age and sex, were both used to examine the correlations between SERT availability and the HRV indexes in different groups. Independent t tests and Chi-square tests were used to assess differences between groups.
Because the results of the WCST (categories completed and perseveration errors) might moderate the aforementioned results, median-splitting of categories completed or perseveration errors was conducted. Correlations between SERT availability and HRV indexes in subjects with differing WCST performances and BDNF levels were probed. The data were analyzed using Statistical Package for Social Science software version 17 (SPSS Inc., Chicago, IL, USA). The threshold for statistical significance was set at p < 0.05.
Electronic supplementary material
Supplementary Table S1. Full results of interactions of BDNF level and SERT availability with ANS function.
Acknowledgements
This study was supported by grants from the National Science Council of Taiwan (NSC 93-2314-B-006-107, NSC 97-2314-B-006-006-MY3, and NSC 101-2314-B-006-065), Ministry of Science and Technology, R.O.C. (MOST 106-2314-B-006-036), and the Atomic Energy Council of Taiwan (NSC 99-NU-E-006-003). The authors wish to thank Ms. Tsai Hua Chang, Mr. Chien Ting Lin and Professor Yuan-Hwa Chou from Taipei Veterans General Hospital for their administrative support.
Author Contributions
Y.K.Y. designed the study and wrote the protocol. W.J.Y. and N.T.C. helped to design the study. S.-H.L. and P.S.C. contributed to the statistical analyses. W.H.C. wrote the first draft of the manuscript. I.H.L., M.H.C., K.C.C., and Y.K.Y. managed the data collection. All authors interpreted the analysis of the results and helped to revise the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20923-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/S0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 5.Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Taylor MJ, Sen S, Bhagwagar Z. Antidepressant response and the serotonin transporter gene-linked polymorphic region. Biol. Psychiatry. 2010;68:536–543. doi: 10.1016/j.biopsych.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesse S, Barthel H, Schwarz J, Sabri O, Muller U. Advances in in vivo imaging of serotonergic neurons in neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2004;28:547–563. doi: 10.1016/j.neubiorev.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Challis C, Berton O. Top-down control of serotonin systems by the prefrontal cortex: a path toward restored socioemotional function in depression. ACS Chem. Neurosci. 2015;6:1040–1054. doi: 10.1021/acschemneuro.5b00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mossner R, et al. Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF) Neurochem. Int. 2000;36:197–202. doi: 10.1016/S0197-0186(99)00122-9. [DOI] [PubMed] [Google Scholar]
- 10.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez L, et al. High frequency heart-rate variability predicts adolescent depressive symptoms, particularly anhedonia, across one year. J. Affect. Disord. 2016;196:243–247. doi: 10.1016/j.jad.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleiger RE, Miller JP, Bigger JT, Jr., Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 14.Dishman RK, et al. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int. J. Psychophysiol. 2000;37:121–133. doi: 10.1016/S0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 15.Schaffar N, Kessler JP, Bosler O, Jean A. Central serotonergic projections to the nucleus tractus solitarii: evidence from a double labeling study in the rat. Neuroscience. 1988;26:951–958. doi: 10.1016/0306-4522(88)90111-X. [DOI] [PubMed] [Google Scholar]
- 16.Sevoz-Couche C, Brouillard C. Key role of 5-HT3 receptors in the nucleus tractus solitarii in cardiovagal stress reactivity. Neurosci. Biobehav. Rev. 2017;74:423–432. doi: 10.1016/j.neubiorev.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Booij L, et al. Tryptophan depletion affects heart rate variability and impulsivity in remitted depressed patients with a history of suicidal ideation. Biol. Psychiatry. 2006;60:507–514. doi: 10.1016/j.biopsych.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Kemp AH, et al. Effects of depression, anxiety, comorbidity, and antidepressants on resting-state heart rate and its variability: an ELSA-Brasil cohort baseline study. Am. J. Psychiatry. 2014;171:1328–1334. doi: 10.1176/appi.ajp.2014.13121605. [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, et al. Serotonin 2A receptor (5-HT2A) gene promoter variant interacts with chronic perceived stress to modulate resting parasympathetic activity in humans. Psychoneuroendocrinology. 2017;76:119–126. doi: 10.1016/j.psyneuen.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao CY, et al. The association between baseline subjective anxiety rating and changes in cardiac autonomic nervous activity in response to tryptophan depletion in healthy volunteers. Medicine (Baltimore). 2016;95:e3498. doi: 10.1097/MD.0000000000003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang AC, et al. BDNF Val66Met polymorphism alters sympathovagal balance in healthy subjects. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;153B:1024–1030. doi: 10.1002/ajmg.b.31069. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan TW, et al. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35:56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shields GS, Sazma MA, Yonelinas AP. The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neurosci. Biobehav. Rev. 2016;68:651–668. doi: 10.1016/j.neubiorev.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 25.Trajkovska V, et al. BDNF downregulates 5-HT(2A) receptor protein levels in hippocampal cultures. Neurochem. Int. 2009;55:697–702. doi: 10.1016/j.neuint.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Grabe HJ, et al. Genetic epistasis between the brain-derived neurotrophic factor Val66Met polymorphism and the 5-HTT promoter polymorphism moderates the susceptibility to depressive disorders after childhood abuse. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36:264–270. doi: 10.1016/j.pnpbp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya VA, Terwilliger RM, Duman RS. Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci. Lett. 1999;262:1–4. doi: 10.1016/S0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 28.Kim JM, et al. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol. Psychiatry. 2007;62:423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Siuciak JA, Boylan C, Fritsche M, Altar CA, Lindsay RM. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996;710:11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- 30.Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J. Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou YH, et al. Association of brain serotonin transporter availability and brain-derived neurotrophic factor in models of serotonin transporter genotypes in healthy subjects. J. Clin. Psychopharmacol. 2013;33:432–435. doi: 10.1097/JCP.0b013e3182904a80. [DOI] [PubMed] [Google Scholar]
- 32.Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:261–265. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Olbrich S, et al. CNS- and ANS-arousal predict response to antidepressant medication: Findings from the randomized iSPOT-D study. J. Psychiatr. Res. 2016;73:108–115. doi: 10.1016/j.jpsychires.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Comet MA, Bernard JF, Hamon M, Laguzzi R, Sevoz-Couche C. Activation of nucleus tractus solitarius 5-HT2A but not other 5-HT2 receptor subtypes inhibits the sympathetic activity in rats. Eur. J. Neurosci. 2007;26:345–354. doi: 10.1111/j.1460-9568.2007.05673.x. [DOI] [PubMed] [Google Scholar]
- 35.Doucet E, et al. Immunolabeling of the rat central nervous system with antibodies partially selective of the short form of the 5-HT3 receptor. Neuroscience. 2000;95:881–892. doi: 10.1016/S0306-4522(99)00494-7. [DOI] [PubMed] [Google Scholar]
- 36.Reyes del Paso GA, Langewitz W, Mulder LJ, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- 37.Jordan D, Mifflin SW, Spyer KM. Hypothalamic inhibition of neurones in the nucleus tractus solitarius of the cat is GABA mediated. J. Physiol. 1988;399:389–404. doi: 10.1113/jphysiol.1988.sp017087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fouad FM, Tarazi RC, Ferrario CM, Fighaly S, Alicandri C. Assessment of parasympathetic control of heart rate by a noninvasive method. Am. J. Physiol. 1984;246:H838–842. doi: 10.1152/ajpheart.1984.246.6.H838. [DOI] [PubMed] [Google Scholar]
- 39.Pal R, Singh SN, Chatterjee A, Saha M. Age-related changes in cardiovascular system, autonomic functions, and levels of BDNF of healthy active males: role of yogic practice. Age (Dordr) 2014;36:9683. doi: 10.1007/s11357-014-9683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J. Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amat J, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 42.Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J. Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bremner JD, et al. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch. Gen. Psychiatry. 1997;54:364–374. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- 44.Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res. Brain Res. Rev. 2000;31:302–312. doi: 10.1016/S0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AG, Goehler LE, Galper DI, Innes KE, Bourguignon C. Top-down and bottom-up mechanisms in mind-body medicine: development of an integrative framework for psychophysiological research. Explore (NY) 2010;6:29–41. doi: 10.1016/j.explore.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Winkel M, et al. Impact of variation in the BDNF gene on social stress sensitivity and the buffering impact of positive emotions: replication and extension of a gene-environment interaction. Eur. Neuropsychopharmacol. 2014;24:930–938. doi: 10.1016/j.euroneuro.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Hinderberger P, et al. The effect of serum BDNF levels on central serotonin transporter availability in obese versus non-obese adults: A [(11)C]DASB positron emission tomography study. Neuropharmacology. 2016;110:530–536. doi: 10.1016/j.neuropharm.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 48.Biegon A, McEwen BS. Modulation by estradiol of serotonin receptors in brain. J. Neurosci. 1982;2:199–205. doi: 10.1523/JNEUROSCI.02-02-00199.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hesse S, et al. Is correction for age necessary in neuroimaging studies of the central serotonin transporter? Eur. J. Nucl. Med. Mol. Imaging. 2003;30:427–430. doi: 10.1007/s00259-002-1044-6. [DOI] [PubMed] [Google Scholar]
- 50.Yang YK, et al. Association between serotonin transporter availability and hostility scores in healthy volunteers - a single photon emission computed tomography study with [(123)I] ADAM. Psychiatry Res. 2007;154:281–284. doi: 10.1016/j.pscychresns.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Yeh TL, et al. Availability of dopamine and serotonin transporters in opioid-dependent users-a two-isotope SPECT study. Psychopharmacology (Berl). 2012;220:55–64. doi: 10.1007/s00213-011-2454-6. [DOI] [PubMed] [Google Scholar]
- 52.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 53.Chang WH, et al. Which demographic variables are necessary to correct in neuroimaging studies of serotonin transporter availability? A SPECT study with [(1)(2)(3)I]ADAM. Psychiatry Res. 2015;231:320–324. doi: 10.1016/j.pscychresns.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Toichi M, Sugiura T, Murai T, Sengoku A. A new method of assessing cardiac autonomic function and its comparison with spectral analysis and coefficient of variation of R-R interval. J. Auton. Nerv. Syst. 1997;62:79–84. doi: 10.1016/S0165-1838(96)00112-9. [DOI] [PubMed] [Google Scholar]
- 55.Kamen PW, Krum H, Tonkin AM. Poincare plot of heart rate variability allows quantitative display of parasympathetic nervous activity in humans. Clin. Sci. 1996;91:201–208. doi: 10.1042/cs0910201. [DOI] [PubMed] [Google Scholar]
- 56.Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 17, 354–381 (1996). [PubMed]
- 57.Task Force of the European Society of Cardiology the North American Society of Pacing. Heart rate variability. Standards of measurement, physiologic interpretation, and clinical use. Circulation93, 1043–1065 (1996). [PubMed]
- 58.Malliani A, Lombardi F, Pagani M, Cerutti S. Clinical exploration of the autonomic nervous system by means of electrocardiography. Ann. N. Y. Acad. Sci. 1990;601:234–246. doi: 10.1111/j.1749-6632.1990.tb37304.x. [DOI] [PubMed] [Google Scholar]
- 59.Shields RW., Jr. Heart rate variability with deep breathing as a clinical test of cardiovagal function. Cleve. Clin. J. Med. 2009;76(Suppl 2):S37–40. doi: 10.3949/ccjm.76.s2.08. [DOI] [PubMed] [Google Scholar]
- 60.Heaton, R. K., Grant, I. & Mathews, C. G. Comprehensive norms for an expanded halstead-reitan battery: demographic corrections, research findings, and clinical applications. (Psychological Assessment Resources, 1991).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Full results of interactions of BDNF level and SERT availability with ANS function.


