Abstract
Intracerebral hemorrhage (ICH) has the highest mortality rate in all strokes. However, controversy still exists concerning the association between plasma homocysteine (Hcy) and ICH. A systematic review and meta-analysis was conducted using Pubmed, Embase, and Web of Science up to April 18, 2017. Standard mean difference (SMD) for mean differences of plasma Hcy levels with 95% confidence intervals (CI) was calculated. Seven studies including 667 ICH patients and 1821 ischemic stroke patients were identified for meta-analysis. Our results showed that Hcy levels in ICH patients were significantly higher than those in healthy controls (SMD = 0.59, 95% CI = 0.51–0.68, P < 0.001); no statistic differences were found in the comparisons of Hcy levels between ICH and ischemic stroke (SMD = −0.03, 95% CI = −0.13–0.06, P > 0.05); further subgroup analysis of ethnicity (Asians: SMD = 0.57, 95% CI = 0.48–0.66, P < 0.001; Caucasians: SMD = 0.77, 95% CI = 0.51–1.02, P < 0.001) and sample size (small samples: SMD = 0.55, 95% CI = 0.30–0.80, P < 0.001; large samples size: SMD = 0.60, 95% CI = 0.51–0.69, P < 0.001) in relation to Hcy levels between ICH and healthy controls did not change these results. In conclusion, Hcy level may be an aggravating factor in atherosclerosis, which is positively associated with high risk of ICH. Race-specific differences between Asians and Caucasians have no impact on the risk of ICH.
Introduction
Intracerebral hemorrhage (ICH), which accounts for 10–15% of all strokes, is the second most detrimental subtype of stroke1,2. ICH is more severe and disabling than ischemic stroke; it has the highest mortality rate of all the strokes3,4. Though our understanding of ICH has advanced in recent decades, so far this knowledge has not been translated into significant improvement in efficient therapies and prognosis5–7. Therefore, emphasis has been placed on preventive medicine aiming at reducing traditional risk factors of ICH, mainly hypertension, hyperlipidemia, diabetes mellitus and smoking8–11. A high level of homocysteine (Hcy) has been regarded as a qualifiable risk factor for ischemic stroke, and Hcy-lowering therapy is widespread used for those patients12,13. According to guidelines for healthcare professionals from the American Heart Association/American Stroke Association, though no randomized, placebo-controlled study has shown that Hcy-lowering therapy reduces ischemic stroke recurrence, it is recommended that supplements of folic acid, vitamin B6 and vitamin B12 reduce levels of homocysteine and may be considered for patients with ischemic stroke and hyperhomocysteinemia14. However, the relationship between Hcy and ICH remains controversial. Preview study showed no obvious association between Hcy level and risk of hemorrhagic stroke15. Recently, some studies revealed that patients with ICH exhibited higher concentrations of Hcy than that of healthy controls, which implied an underlying mechanism linking plasma Hcy levels with ICH16–18.
Homocysteine, an intermediate sulfhydryl-containing amino acid, is synthesized by the liver in a reaction involving the removal of the methyl group from methionine19,20. High level of Hcy is mainly found in patients with a nutritional deficiency of B vitamins as well as being present in genetic diseases where there is an insufficient level of cystathionine beta-synthase enzyme (CβS) or a mutation inn the enzyme 5,10-methylenetetrahydrofolate reductase (MTHFR). Other factors can elevate the level of Hcy i.e. a diet with high methionine content, smoking and a sedentary lifestyle21–24. According to epidemiological evidence, Hcy is considered to be a potential predictor of pathophysiology of neuronal system disorders25,26. High level of Hcy induces a neuro-inflammatory process due to its abilities to react with cytokines in the brain, leading to increased permeability of blood-brain barrier (BBB) and impaired integrity of cerebral vessels27,28. There is convincing evidence that elevated plasma Hcy level is associated with higher mortality rates from ischemic stroke, cardiovascular diseases and peripheral artery disease29–32. However, it is still controversial whether elevated plasma Hcy level is a risk factor for ICH. Therefore, we performed a systematic overview of retrospective studies to ascertain the association between Hcy and the risk of ICH.
Materials and Methods
Inclusion and Exclusion Criteria
To identify eligible studies investigating the association between plasma Hcy levels and ICH, published studies meeting the following criteria were considered eligible for inclusion: (1) case-control studies written in English; (2) studies which assessed the correlation between Hcy and patients with ICH; (3) the cases were confirmed ICH patients and the controls were healthy people; (4) providing information of plasma Hcy levels in the control group and case group at the onset of ICH. The exclusion criteria were as follows: (1) conflicts with the inclusion criteria; (2) publications with duplication or studies with overlapping data from the same study; (3) abstracts, case report, proceedings, letters, reviews, or meta-analysis.
Literature Search
Databases of Pubmed, Embase, and Web of Science were comprehensively searched for articles up to April 18, 2017. The terms searched were as follows: (“plasma homocysteine” OR homocysteine OR Hcy OR “plasma Hcy”) AND (“intracerebral hemorrhage” OR “cerebral hemorrhage” OR “brain hemorrhage” OR “hemorrhagic stroke”). We also screened the reference lists of relevant reviews and included relevant articles in these lists.
Data Extraction and Quality Assessment
Two reviewers independently extracted the data and information from the finally selected trials according to a standard protocol, and then discussed or consulted the original report to reach a consensus when discrepancies occurred. In the case of eligible studies, the following information was collected: first author, publication year, country, detection method for plasma Hcy, proportion of men, number of participants as well as plasma Hcy levels in the groups of ICH, control and ischemic stroke. Guidelines of preferred reporting items for systematic reviews and meta-analyses (PRISMA) were followed by the study. Quality assessment was conducted according to the Newcastle-Ottawa Scale (NOS) criteria for retrospective studies33. A score of up to 9 points was assigned to each study: 4 points for selection, 2 points for comparability, and 3 points for assessment of outcomes. Studies with 6 or more points were considered as high quality, while those with less than 6 were considered to be of suboptimal quality.
Statistical Analysis
Statistical analysis in this meta-analysis was performed with the software Review Manager 5.2 and STATA version 14.0. Standard mean difference (SMD) with 95% confidence intervals (CI) was calculated by random effects model or fixed effects model to measure the differences in plasma Hcy levels between the case group and control group, the significance of which was assessed by utilizing the Z test. I2 test (25, 50, and 75% represented low, moderate, and high degrees of heterogeneity) was applied to assess the heterogeneity among studies34–36. Random effects models were applied for the evidence of significant heterogeneity (I2 test exhibited > 50%); otherwise, the fixed effects model was utilized34,35,37. Hcy levels from plasma and serum were used equally. Subgroup analyses by ethnicity and sample size of participants (≥50 or less) were also conducted. Sensitivity analysis was carried out by sequentially omitting one study to examine its influence on the pooled risk estimates. A publication bias was detected by visual inspection of a funnel plot i.e. an asymmetric funnel plot indicated a possible risk of a publication bias.
Results
Study selection and characteristics
A total of 241 abstracts were retrieved initially. After exclusion of 219 irrelevant or duplicated reports, 22 potentially eligible studies were assessed by reviewing the full text15–18,38–55. In all, 15 studies were further excluded, including 4 studies with irrelevant outcomes, 3 studies containing ischemic stroke patients, 6 studies in which there was a lack of usable data and 2 prospective studies. Thus, 7 studies were finally included into the current meta-analysis15,50–55 (Fig. 1). According to the NOS criteria (Table 1), these seven studies were categorized as high quality. The main characteristics of the studies included in the meta-analysis are detailed in Table 2. Those 7 studies involved a total of 667 patients with ICH, 1821 patients with ischemic stroke and 2500 healthy controls15,50–55. In six of the seven trials, the study subjects were Asians, the seventh trial was performed in Caucasians. When examining the sample sizes, 2 studies with large samples (n ≥ 50) whereas the other five studies examined small samples (n < 50). Figure 1 shows the flowchart of the study selection process and the screening steps.
Figure 1.
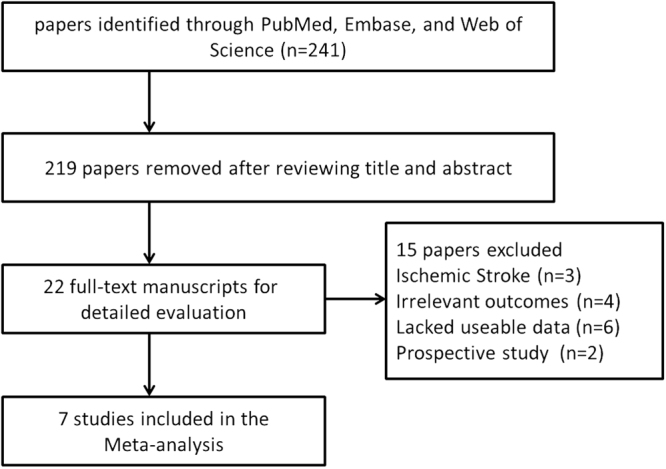
Flow chart of study selection in the meta-analysis.
Table 1.
The Newcastle-Ottawa Quality Assessment Scale for case-control studies.
| Study | Selection | Comparability | Outcome | Summary |
|---|---|---|---|---|
| Bokhari FA (2012) | 4 | 2 | 2 | 8 |
| Hiroyasu I (2004) | 3 | 2 | 2 | 7 |
| Fang X (2005) | 4 | 2 | 2 | 8 |
| Li ZH (2003) | 4 | 2 | 2 | 8 |
| Araki A (1989) | 4 | 2 | 2 | 8 |
| Perini F (2005) | 4 | 2 | 2 | 8 |
| Peng H (2000) | 4 | 2 | 2 | 8 |
Table 2.
General characteristics of the included studies.
| First author | Year | Country | Detecting methods | Hemorrhagic | Controls | Ischemic | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of male patients | tHcy, μmol/L Mean ± SD | Age, Years | No. of male patients | tHcy, μmol/L Mean±SD | Age, Years | No. of male patients | tHcy, μmol/L Mean±SD | Age, Years | ||||
| Bokhari FA | 2012 | Pakistan | ELISA | 4/9 | 23.73 ± 13.61 | NR | NR/33 | 20.73 ± 8.59 | NR | 10/22 | 20.45 ± 9.47 | NR |
| Hiroyasu I | 2004 | Japan | HPLC | 17/38 | 10.4 ± 2.86 | 64.7 | 51/114 | 9.2 ± 3.1 | 64.4 | 60/98 | 9.8 ± 3.99 | 65.9 |
| Fang X(+/−) | 2005 | Mongolia | HPLC | NR/12 | 15.6 ± 4.6 | NR | NR/6 | 12.9 ± 5.3 | NR | NR/18 | 18.4 ± 8.9 | NR |
| Fang X(+/+) | 2005 | Mongolia | HPLC | NR/5 | 20 ± 6.4 | NR | NR/2 | 15.7 ± 6.3 | NR | NR/11 | 21.3 ± 1.8 | NR |
| Li ZH | 2003 | China | HPLC | 318/503 | 14.6 ± 1.3 | 58.2 ± 9.6 | 1052/1832 | 12.8 ± 3.47 | 59.6 ± 8.8 | 513/807 | 14.7 ± 8.6 | 61.3 ± 9.7 |
| Araki A | 1989 | Japan | HPLC | 13/20 | 9.6 ± 3.9 | 65.4 ± 9.7 | 30/45 | 7.3 ± 2.9 | 62.9 ± 10.8 | 30/45 | 13.1 ± 5.6 | 63.3 ± 10.9 |
| Perini F | 2005 | Italy | HPLC | 39/70 | 18.18 ± 14.08 | 69.5 ± 13.8 | 201/421 | 11.97 ± 6.61 | 56.6 ± 14.8 | 399/775 | 18.94 ± 13.65 | 71.6 ± 12.1 |
| Fang X(−/−) | 2005 | Mongolia | HPLC | NR/3 | 20.5 ± 8.6 | NR | NR/16 | 15.4 ± 4.2 | NR | NR/13 | 18.9 ± 8.0 | NR |
| Peng H | 2000 | China | HPLC | NR/7 | 18.77 ± 5.58 | NR | 26/31 | 11.46 ± 5.96 | 51.8 | NR/32 | 18.72 ± 10.55 | NR |
M male, n number, tHcy total homocysteine, NR not reported, ELISA enzyme-linked immunosorbent assay, HPLC high-performance liquid chromatography, +/+ homozygous methylenetetrahydrofolate reductase gene mutation, +/− heterozygous methylenetetrahydrofolate reductase gene mutation, −/− wild-type.
Meta-analysis
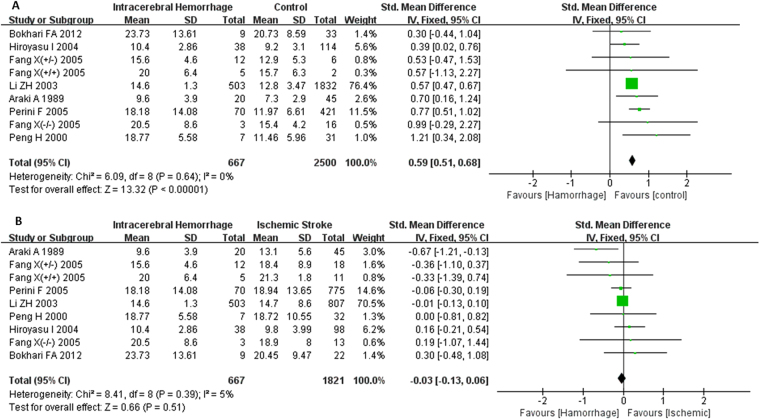
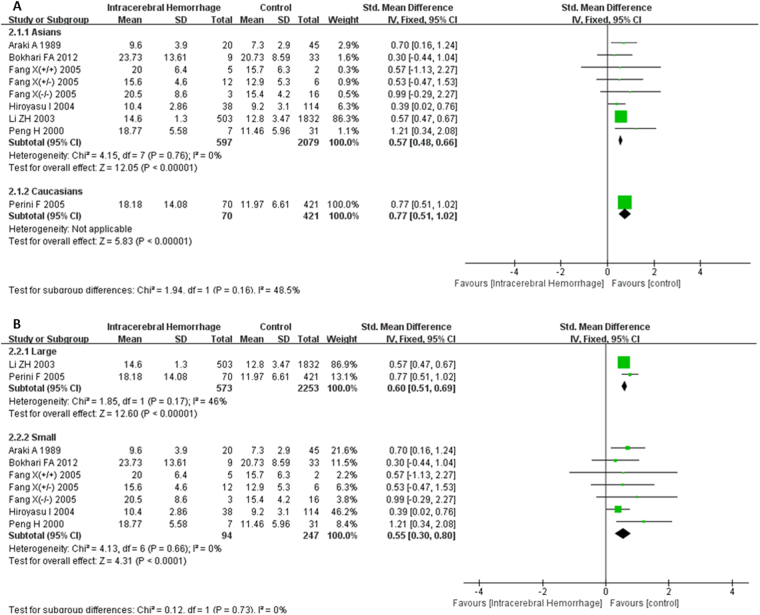
There was no heterogeneity among those 7 studies reporting differences of plasma Hcy levels between groups of ICH and healthy controls (I2 = 0%), as well as groups of ICH and ischemic stroke (I2 = 5%); thus, the fixed-effects model was used to pool data. Patients with ICH had a higher serum Hcy level compared with control group (SMD = 0.59, 95% CI = 0.51–0.68, P < 0.001) (Fig. 2A). There were no significant differences in the comparisons of plasma Hcy levels between ICH group and ischemic stroke group (SMD = −0.03, 95% CI = −0.13–0.06, P > 0.05) (Fig. 2B). The results of subgroup analysis based on ethnicity indicated that the plasma Hcy levels of ICH patients were significantly higher than those of healthy controls in both Asians and Caucasians (Asians: SMD = 0.57, 95% CI = 0.48–0.66, P < 0.001; Caucasians: SMD = 0.77, 95% CI = 0.51–1.02, P < 0.001), and there were no statistical differences between those two groups (P > 0.05) (Fig. 3A). A further subgroup analysis on sample size revealed that ICH patients displayed higher plasma Hcy levels than healthy controls in both small samples (n < 50) and large samples (n ≥ 50) (small samples: SMD = 0.55, 95% CI = 0.30–0.80, P < 0.001; large samples size: SMD = 0.60, 95% CI = 0.51–0.69, P < 0.001), and there were no statistical differences between those two groups (P > 0.05) (Fig. 3B).
Figure 2.
Forest plots for the comparisons of plasma Hcy levels between intracerebral hemorrhage (ICH) patients and healthy controls (A); Forest plots for the comparisons of plasma Hcy levels between intracerebral hemorrhage (ICH) patients and ischemic stroke patients (B). CI: confidence interval.
Figure 3.
Forest plots of the subgroup analyses on ethnicity (A) and sample size (B) in relation to plasma Hcy levels between intracerebral hemorrhage (ICH) patients and healthy controls. CI: confidence interval.
Sensitivity Analysis and Publication bias
Sensitivity analysis performed by omitting one individual study sequentially showed that no single study had any obvious influence on the pooled mean difference (Fig. 4). Since the funnel plot for overall analysis was symmetrical (Fig. 5), there was no obvious risk of publication bias in the meta-analysis.
Figure 4.
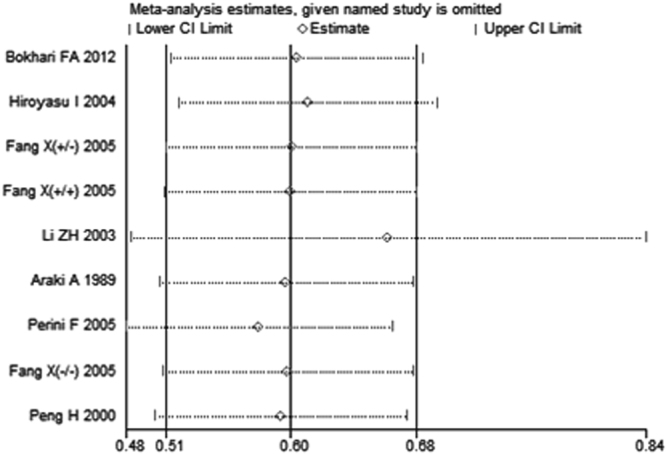
Forest plot in the sensitivity analysis of the present meta-analysis (individual names for each study have been omitted). CI: confidence interval.
Figure 5.
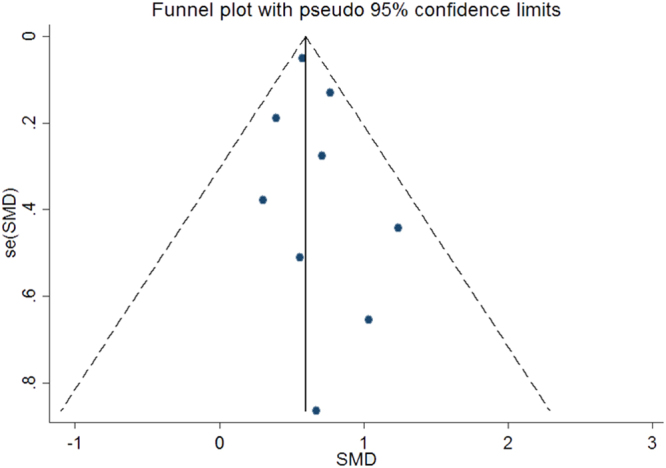
Funnel plot to detect risk of publication bias in the meta-analysis. SMD: standard mean difference.
Discussion
As far as we are aware, there has been no relevant systematic review of case-control studies carried out to explore whether plasma Hcy are associated with the risk of ICH. Seven eligible studies with a total of 667 ICH patients were included into our meta-analysis15,50–55. In comparison with a single study, the meta-analysis makes it possible to have a more accurate estimate and evaluation of the impact of Hcy levels on ICH. The findings emerging from comprehensive meta-analysis confirmed that patients with ICH exhibited prominently higher plasma Hcy levels than healthy controls, and this association could not be affected by study quality, participant characteristics and publication bias.
The precise mechanism of Hcy on the susceptibility of ICH remains unresolved. However, our meta-analysis supported that the pathogenesis of ICH likely involves some factor elevating Hcy levels. Experimental studies have verified multiple biological responses triggered by Hcy during the process of atherosclerosis, for example, Hcy inhibits glutathione peroxidase to stimulate the proliferation of smooth muscle cells, by which endothelial nitric oxide synthase (eNOS) can be suppressed via the asymmetric dimethyl-L-arginine (ADMA) pathway, leading to endothelial dysfunction and vascular spasms26,56–58. In addition, Hcy enhances the release of arachidonic acid from platelets to generate reactive oxygen species (ROS), leading to the deposition of calcium and lipid in the endothelial wall; these changes degrade arterial elasticity and accelerate the process of atherosclerosis21,59–61. In the present results, the comparisons of plasma Hcy levels between ICH and ischemic stroke showed no significant difference. This implied that the plasma Hcy level was likely identified as an aggravating factor in atherosclerosis, owing to which the pathophysiology of endothelial degeneration and necrosis of vessel walls could elevate the risk of ischemic stroke and cerebral hemorrhage62.
However, we are unable to rule out the possibility that the high levels of Hcy may be attributable to the acute stress reaction evoked by stroke, as all blood samples of plasma Hcy were obtained at the onset of ICH rather than before its occurrence. There is one prospective study in which participants were invited to donate a fasting blood sample for future research; this showed that plasma Hcy levels were significantly higher in patients who would develop ICH compared to their healthy controls49. Another study reported that Hcy levels initially decreased within 24 hours and then gradually raised within 48 hours in patients with hemorrhagic stroke, whereas differences in mean Hcy levels at 0, 24 and 48 hours post stroke were insignificant compared with mean Hcy levels in controls54. Accordingly, it is speculated that Hcy levels have been already elevated before the onset of ICH and that their levels could not be affected by acute stress reaction after a stroke. Further studies will be needed to assess the influence of the acute stress reaction during follow-up on the association between Hcy and ICH.
Consistent with the overall results, subgroup analysis on either ethnicity or sample size confirmed that plasma Hcy levels of ICH patients were significantly higher than those of healthy controls. Preview study revealed that Africans had apparently higher risk of ICH compared with Caucasians, suggesting that race played an important role in the ICH risk pattern63. Interestingly, in the present study, the subgroup analysis based on ethnicity in relation to plasma Hcy levels between Asians and Caucasians had no statistic differences, which implied that race-specific differences exerted no effect on the results of our meta-analysis. The reason for this inconsistency might be that race-specific differences between Africans and Caucasians had a profound impact on the risk of ICH rather than race-specific differences between Asians and Caucasians in our study. Although our meta-analysis revealed no statistical heterogeneity, there can also be clinical heterogeneity or methodological heterogeneity between studies. Some possible sources of clinical heterogeneity such as inclusion and exclusion criteria, intervention measures, defining indicators may be the differences. Other sources of methodology heterogeneity such as experimental design, bias risk and outcome integrity may also affect the results.
Limitations
Several potential limitations of this meta-analysis must be acknowledged. All included studies followed a retrospective design, which may be more subjected to bias and artifact than prospective studies. The different detection methods employed for plasma Hcy levels may have sensitivity and reliability issues. We were unable to analyze the effect of the acute stress reaction of ICH on plasma Hcy levels since there were insufficient data about Hcy levels before the onset of ICH. Although the result of the funnel plot did not suggest any publication bias, there is the possibility of journals publishing only positive findings as only published data were utilized in the meta-analysis. Additionally, the relatively limited number of studies and the fact that most of patients were Asians, may also influence the sensitivity of statistical analysis. Further randomized controlled trials and larger numbers of participants will be necessary to confirm these findings.
Conclusions
Our results of meta-analysis provide support for the hypothesis that elevated level of Hcy in blood is positively associated with the incidence of ICH. Hcy level may be an aggravating factor in atherosclerosis which contributes to ICH. There is no impact of race-specific differences between Asians and Caucasians on the risk of ICH. More clinical trials with more comprehensive data, using larger sample sizes and better design, will be needed to validate our results.
Acknowledgements
We would like to appreciate all the authors of studies included in this paper. This work was supported by National Natural Science Foundation of China (No. 81372104, Chuansheng Zhao); The Shenyang Population and Health Technical Critical Special Project (No. F16–206–9–01, Chuansheng Zhao); The Program of the Distinguished Professor of Liaoning Province, Neurology (Chuansheng Zhao).
Author Contributions
Zhou, Z.K., Teng, W.Y. and Zhao, C.S. conceived the study. Zhou, Z.K. and Liang, Y.F. selected reports and extracted the data. Zhou, Z.K., Qu, H.L., Zhao, M. and Guo, F. analysed and interpreted the data. Zhou, Z.K., Teng, W.Y. and Zhao C.S. wrote the first draft of the manuscript. All authors reviewed the manuscript and approved the final version.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chuansheng Zhao, Email: cszhao@cmu.edu.cn.
Weiyu Teng, Email: wyteng@cmu.edu.cn.
References
- 1.Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012;14:300–6. doi: 10.1007/s11883-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thabet AM, Kottapally M, Hemphill JC. Management of intracerebral hemorrhage. Handb Clin Neurol. 2017;140:177–94. doi: 10.1016/B978-0-444-63600-3.00011-8. [DOI] [PubMed] [Google Scholar]
- 3.Cai X, Rosand J. The Evaluation and Management of Adult Intracerebral Hemorrhage. Semin Neurol. 2015;35:638–45. doi: 10.1055/s-0035-1564687. [DOI] [PubMed] [Google Scholar]
- 4.Zheng H, Chen C, Zhang J, Hu Z. Mechanism and Therapy of Brain Edema after Intracerebral Hemorrhage. Cerebrovasc Dis. 2016;42:155–69. doi: 10.1159/000445170. [DOI] [PubMed] [Google Scholar]
- 5.Provencio JJ, Da Silva IR, Manno EM. Intracerebral hemorrhage: new challenges and steps forward. Neurosurg Clin N Am. 2013;24:349–59. doi: 10.1016/j.nec.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Kreitzer N, Adeoye O. An update on surgical and medical management strategies for intracerebral hemorrhage. Semin Neurol. 2013;33:462–7. doi: 10.1055/s-0033-1364210. [DOI] [PubMed] [Google Scholar]
- 7.Jiang B, et al. Role of Glibenclamide in Brain Injury After Intracerebral Hemorrhage. Transl Stroke Res. 2017;8:183–93. doi: 10.1007/s12975-016-0506-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, et al. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke. 2013;44:1833–9. doi: 10.1161/STROKEAHA.113.001326. [DOI] [PubMed] [Google Scholar]
- 9.Tsai CF, Anderson N, Thomas B, Sudlow CL. Comparing Risk Factor Profiles between Intracerebral Hemorrhage and Ischemic Stroke in Chinese and White Populations: Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0151743. doi: 10.1371/journal.pone.0151743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulouis G, Morotti A, Goldstein JN, Charidimou A. Intensive blood pressure lowering in patients with acute intracerebral haemorrhage: clinical outcomes and haemorrhage expansion. Systematic review and meta-analysis of randomised trials. J Neurol Neurosurg Psychiatry. 2017;88:339–45. doi: 10.1136/jnnp-2016-315346. [DOI] [PubMed] [Google Scholar]
- 11.Woo D, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190–5. doi: 10.1161/01.STR.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]
- 12.Lehotský J, et al. Role of Homocysteine in the Ischemic Stroke and Development of Ischemic Tolerance. Front Neurosci. 2016;10:538. doi: 10.3389/fnins.2016.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng, L. S., Tu, W. J., Shen, Y., Zhang, L. J. & Ji, K. Combination of High-Sensitivity C-Reactive Protein and Homocysteine Predicts the Post-Stroke Depression in Patients with Ischemic Stroke. Mol Neurobiol., 10.1007/s12035-017-0549-8 (2017). [DOI] [PubMed]
- 14.Kernan WN, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 15.Hiroyasu I, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109:2766–72. doi: 10.1161/01.CIR.0000131942.77635.2D. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, et al. Elevated homocysteine levels contribute to larger hematoma volume in patients with intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:784–8. doi: 10.1016/j.jstrokecerebrovasdis.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Yao ES, et al. Elevated Homocysteine Level Related to Poor Outcome After Thrombolysis in Acute Ischemic Stroke. Med Sci Monit. 2016;22:3268–73. doi: 10.12659/MSM.900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan S, Zhang S, Li L, Ren C, Xie J. Carotid artery intima-media thickness associated with prognosis of intracranial branch atheromatous disease. Int J Neurosci. 2017;127:361–7. doi: 10.3109/00207454.2016.1174120. [DOI] [PubMed] [Google Scholar]
- 19.Zhong C, et al. High homocysteine and blood pressure related to poor outcome of acute ischemia stroke in Chinese population. PLoS One. 2014;9:e107498. doi: 10.1371/journal.pone.0107498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira IO, et al. Interactions between lifestyle and MTHFR polymorphisms on homocysteine concentrations in young adults belonging to the 1982 Pelotas Birth Cohort. Eur J Clin Nutr. 2017;71:259–66. doi: 10.1038/ejcn.2016.193. [DOI] [PubMed] [Google Scholar]
- 21.Chen SF, Cui CL, Wu P, Xie NZ. Relationship of serum homocysteine level with nutritional status and HbA1c level in elderly inpatients. Int J Clin Exp Med. 2013;6:779–84. [PMC free article] [PubMed] [Google Scholar]
- 22.Mandaviya PR, Stolk L, Heil SG. Homocysteine and DNA methylation: a review of animal and human literature. Mol Genet Metab. 2014;113:243–14. doi: 10.1016/j.ymgme.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia P, Singh N. Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundam Clin Pharmacol. 2015;29:142–8. doi: 10.1111/fcp.12145. [DOI] [PubMed] [Google Scholar]
- 24.Dinç N, Yücel SB, Taneli F, Sayın MV. The effect of the MTHFR C677T mutation on athletic performance and the homocysteine level of soccer players and sedentary individuals. J Hum Kinet. 2016;51:61–9. doi: 10.1515/hukin-2015-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann W, Obeid R. Homocysteine: a biomarker in neurodegenerative diseases. Clin Chem Lab Med. 2011;49:435–41. doi: 10.1515/CCLM.2011.084. [DOI] [PubMed] [Google Scholar]
- 26.Petras M, et al. Hyperhomocysteinemia as a risk factor for the neuronal system disorders. J Physiol Pharmacol. 2014;65:15–23. [PubMed] [Google Scholar]
- 27.Kamat PK, Vacek JC, Kalani A, Tyaqi N. Homocysteine Induced Cerebrovascular Dysfunction: A Link to Alzheimer’s Disease Etiology. Open Neurol J. 2015;9:9–14. doi: 10.2174/1874205X01509010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamat PK, Kyles P, Kalani A, Tyaqi N. Hydrogen Sulfide Ameliorates Homocysteine-Induced Alzheimer’s Disease-Like Pathology, Blood-Brain Barrier Disruption, and Synaptic Disorder. Mol Neurobiol. 2016;53:2451–67. doi: 10.1007/s12035-015-9212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams SR, et al. Genome-wide meta-analysis of homocysteine and methionine metabolism identifies five one carbon metabolism loci and a novel association of ALDH1L1 with ischemic stroke. PLoS Genet. 2014;10:e1004214. doi: 10.1371/journal.pgen.1004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S, Yin L, Xu Y, Zou C, Chen L. The homocysteine associated variant rs548987 of SLC17A3 confers susceptibility to ischemic stroke in Chinese population. J Neurol Sci. 2016;370:78–81. doi: 10.1016/j.jns.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 31.Zulli A. Endoplasmic Stress Inhibitors for Homocysteine Induced Cardiovascular Disease. Curr Pharm Des. 2016;22:2704–8. doi: 10.2174/1381612822666160406121342. [DOI] [PubMed] [Google Scholar]
- 32.Katsiki N, Perez-Martinez P, Mikhailidis DP. Homocysteine and Non-Cardiac Vascular Disease. Curr Pharm Des. 2017;23:3224–32. doi: 10.2174/1381612823666170317124913. [DOI] [PubMed] [Google Scholar]
- 33.Wells G, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Applied Engineering in Agriculture. 2014;18:727–34. [Google Scholar]
- 34.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672–3. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 35.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–37. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 38.Kopyta I, Zimny M, Sarecka-Hujar B. The role of biochemical risk factors in the etiology of AIS in children and adults. Int J Neurosci. 2015;125:875–84. doi: 10.3109/00207454.2014.991925. [DOI] [PubMed] [Google Scholar]
- 39.Cnossen MH, van Ommen CH, Appel IM. Etiology and treatment of perinatal stroke; a role for prothrombotic coagulation factors? Semin Fetal Neonatal Med. 2009;14:311–7. doi: 10.1016/j.siny.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Ovbiagele B, Liebeskind DS, Pineda S, Saver JL. Strong independent correlation of proteinuria with cerebral microbleeds in patients with stroke and transient ischemic attack. Arch Neurol. 2010;67:45–50. doi: 10.1001/archneurol.2009.310. [DOI] [PubMed] [Google Scholar]
- 41.Van Guelpen B, et al. Folate, vitamin B12, and risk of ischemic and hemorrhagic stroke: a prospective, nested case-referent study of plasma concentrations and dietary intake. Stroke. 2005;36:1426–31. doi: 10.1161/01.STR.0000169934.96354.3a. [DOI] [PubMed] [Google Scholar]
- 42.Hogeveen M, et al. Hyperhomocysteinemia as risk factor for ischemic and hemorrhagic stroke in newborn infants. J Pediatr. 2002;141:429–31. doi: 10.1067/mpd.2002.126598. [DOI] [PubMed] [Google Scholar]
- 43.Owolabi MO, Agunloye AM. Which risk factors are more associated with ischemic rather than hemorrhagic stroke in black Africans? Clin Neurol Neurosurg. 2013;115:2069–74. doi: 10.1016/j.clineuro.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Somarajan BI, Kalita J, Mittal B. Evaluation of MTHFR C677T polymorphism in ischemic and hemorrhagic stroke patients. A case-control study in a Northern Indian population. J Neurol Sci. 2011;304:67–70. doi: 10.1016/j.jns.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Boysen G, Brander T, Christensen H, Gideon R, Truelsen T. Homocysteine and risk of recurrent stroke. Stroke. 2003;34:1258–61. doi: 10.1161/01.STR.0000069017.78624.37. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Hu J, Ma Q, Ma J, Liu B. Correlation between biochemical indicators of blood lipid with cerebral vascular diseases. Int J Clin Exp Pathol. 2015;8:3022–6. [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell DJ, et al. Low-density lipoprotein particles and risk of intracerebral haemorrhage in subjects with cerebrovascular disease. Eur J Cardiovasc Prev Rehabil. 2007;14:413–8. doi: 10.1097/HJR.0b013e328010f275. [DOI] [PubMed] [Google Scholar]
- 48.Cheng X, Zhang L, Xie NC, Ma YQ, Lian YJ. High Plasma Levels of D-Dimer Are Independently Associated with a Heightened Risk of Deep Vein Thrombosis in Patients with Intracerebral Hemorrhage. Mol Neurobiol. 2016;53:5671–8. doi: 10.1007/s12035-015-9487-5. [DOI] [PubMed] [Google Scholar]
- 49.Hultdin J, et al. Prospective study of first stroke in relation to plasma homocysteine and MTHFR 677C > T and 1298A > C genotypes and haplotypes - evidence for an association with hemorrhagic stroke. Clin Chem Lab Med. 2011;49:1555–62. doi: 10.1515/CCLM.2011.234. [DOI] [PubMed] [Google Scholar]
- 50.Araki A, et al. Plasma sulfhydryl-containing amino acids in patients with cerebral infarction and in hypertensive subjects. Atherosclerosis. 1989;79:139–46. doi: 10.1016/0021-9150(89)90118-4. [DOI] [PubMed] [Google Scholar]
- 51.Peng H, et al. Study on the relationship between plasma homocysteine and acute cerebral vascular disease. J Tongji Med Univ. 2000;20:330–1. doi: 10.1007/BF02888195. [DOI] [PubMed] [Google Scholar]
- 52.Li Z, et al. Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: a Multicenter Case-Control Study in China. Stroke. 2003;34:2085–90. doi: 10.1161/01.STR.0000086753.00555.0D. [DOI] [PubMed] [Google Scholar]
- 53.Perini F, et al. Elevated plasma homocysteine in acute stroke was not associated with severity and outcome: stronger association with small artery disease. Neurol Sci. 2005;26:310–8. doi: 10.1007/s10072-005-0505-7. [DOI] [PubMed] [Google Scholar]
- 54.Bokhari FA, Ambreen B, Syed A, Farkhanda G. Serial changes in plasma homocysteine in acute clinical stroke. Translational Neuroscience. 2012;3:41–5. doi: 10.2478/s13380-012-0005-1. [DOI] [Google Scholar]
- 55.Fang X, Namba H, Akamine S, Sugiyama K. Methylenetetrahydrofolate reductase gene polymorphisms in patients with cerebral hemorrhage. Neurol Res. 2005;27:73–6. doi: 10.1179/016164105X18313. [DOI] [PubMed] [Google Scholar]
- 56.Tyagi N, et al. Hydrogen sulfide mitigates matrix metalloproteinase-9 activity and neurovascular permeability in hyperhomocysteinemic mice. Neurochem Int. 2010;56:301–7. doi: 10.1016/j.neuint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pushpakumar S, Kundu S, Sen U. Endothelial dysfunction: the link between homocysteine and hydrogen sulfide. Curr Med Chem. 2014;21:3662–72. doi: 10.2174/0929867321666140706142335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinna A, et al. Plasma Homocysteine and Asymmetrical Dimethyl-l-Arginine (ADMA) and Whole Blood DNA Methylation in Early and Neovascular Age-Related Macular Degeneration: A Pilot Study. Curr Eye Res. 2016;41:88–96. doi: 10.3109/02713683.2014.1002044. [DOI] [PubMed] [Google Scholar]
- 59.Dionisio N, Jardín I, Salido GM, Rosado JA. Homocysteine, intracellular signaling and thrombotic disorders. Curr Med Chem. 2010;17:3109–19. doi: 10.2174/092986710791959783. [DOI] [PubMed] [Google Scholar]
- 60.Tranchina L, et al. Levels of plasma homocysteine in pseudoexfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2011;249:443–8. doi: 10.1007/s00417-010-1487-6. [DOI] [PubMed] [Google Scholar]
- 61.Bao XM, Zheng H. Atorvastatin attenuates homocysteine-induced migration of smooth muscle cells through mevalonate pathway involving reactive oxygen species and p38 MAPK. Clin Exp Pharmacol Physiol. 2015;42:865–73. doi: 10.1111/1440-1681.12435. [DOI] [PubMed] [Google Scholar]
- 62.Sato S, et al. Intra- and extracranial atherosclerotic disease in acute spontaneous intracerebral hemorrhage. J Neurol Sci. 2013;332:116–20. doi: 10.1016/j.jns.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 63.Howard G, et al. Risk factors for intracerebral hemorrhage: the REasons for geographic and racial differences in stroke (REGARDS) study. Stroke. 2013;44:1282–7. doi: 10.1161/STROKEAHA.111.000529. [DOI] [PMC free article] [PubMed] [Google Scholar]