To the Editor
Gene-environment interactions are thought to play a critical role in determining asthma incidence and severity. A human interleukin 4 receptor alpha chain gene (IL4R) variant that results in a glutamine to arginine substitution at amino acid residue 576 (IL4Rα-Q576R polymorphism) is associated with asthma diagnosis and severity 1. We have recently shown that the IL4RαR576 variant promotes severe airway inflammation by uniquely linking the IL4R to a growth-factor-receptor-bound protein 2 (GRB2)-dependent intracellular signaling pathway that destabilizes allergen-specific regulatory T cells to drive mixed TH2/TH17 cell inflammation 2. School endotoxin exposure is associated with asthma morbidity 3. High endotoxin exposure is associated with increased TH17 cell skewing 4, leading us to test the hypothesis that there is an interaction between the IL4Rα-Q576R polymorphism, school endotoxin exposure, and asthma symptoms.
The School Inner-city Asthma Study (SICAS) is a single-center prospective cohort study conducted between 2008 and 2013 of children with persistent asthma attending inner-city elementary schools in a northeastern United States city (Online Repository). Repeated assessment of asthma symptom days over a 14-day period was assessed by parental or caretaker survey at three month intervals. One-week classroom air samples were collected twice during the school year using charged particle samplers (Quadra; Sharper Image/Camelot Venture Group); endotoxin levels were assessed using the Limulus Amebocyte Lysate assay 3. Genotyping of the IL4RQ576 and IL4RR576 alleles was carried out using the amplification resistance mutation screen PCR method 2 on DNA was extracted from either whole blood (Gentra Puregene Blood Kit; Qiagen) or saliva (prepIT L2P; DNA Genotek).
The IL4Rα-Q576R genotypes were modeled as a three-group categorical variable (Q/Q, Q/R, or R/R genotypes, with the Q/Q genotype as the reference group). To test the hypothesis that the genotypes differed by self-reported race, Fisher’s exact tests were performed. To test the hypothesis that a gene-environment interaction was present, a multiplicative term between classroom endotoxin levels (continuous) and genotype (3-level categorical variable) was included in binomial family generalized estimating equations with a logit link and an overdispersion parameter. These models included only observations during the school year, and adjusted for age, gender, self-reported race, allergic sensitization, baseline asthma severity, use of asthma controller medications, annual income, and season (using linear and quadratic terms for days since school start). All statistical analyses were performed in Stata version 13.1 (StataCorp). Two-sided p-values of <0.05 were considered statistically significant for main effects; p-values of <0.10 were considered statistically significant for interactions.
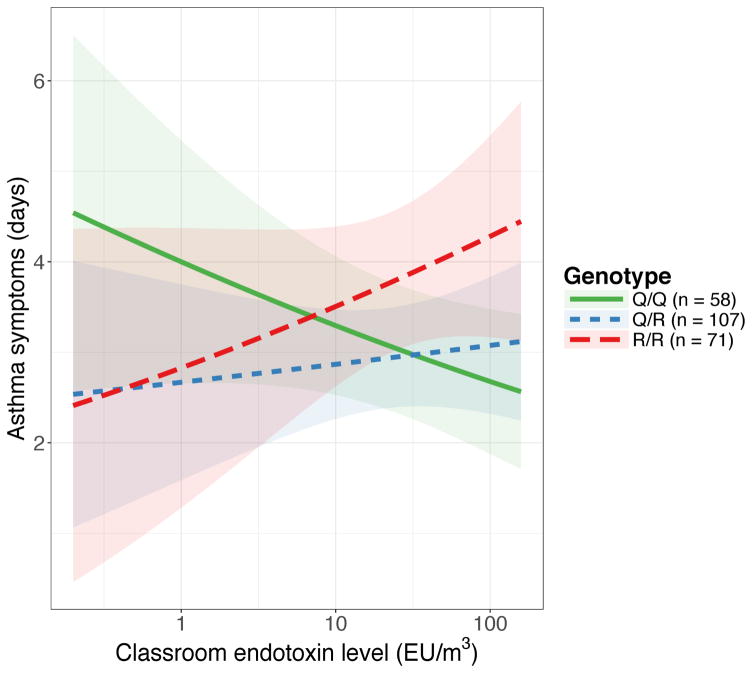
Baseline characteristics of the 236 children included in the analysis are depicted in Table 1; 51.7% were male, the average age was 8.0 ± 1.9 years, and 5.1% self-identified as being Caucasian. The distribution of genotypes differed by race; 75.0%, 13.1%, and 31.7% of Caucasian, African-American, and Hispanic children had the Q/Q genotype, whereas 0%, 47.6%, and 14.6% of Caucasian, African-American, and Hispanic children had the R/R genotype (p < 0.001). The frequency of the Q allele was highest in children of Caucasian race, whereas the frequency of the R allele was the highest in children of African-American or mixed race (Supplementary Table 1). Median measured classroom endotoxin levels were 27.5 EU/m3 [interquartile range 7.4 – 71.8 EU/m3]. In univariate analyses, the main effects of the genotype and of classroom endotoxin levels on asthma symptoms did not reach statistical significance. In a multivariate model, the effect of classroom endotoxin levels on asthma symptom days varied by genotype (overall interaction p-value = 0.09; pairwise interaction p-values for Q/R vs. Q/Q = 0.115, R/R vs. Q/Q = 0.049). Higher classroom endotoxin levels were associated with fewer asthma symptoms for children with the Q/Q genotype (OR 0.76 [0.55 – 1.04]), an equivocal effect for the Q/R genotype (OR 1.10 [0.78 – 1.55]), and increased asthma symptoms for the R/R genotype (OR 1.34 [0.85 – 2.13], Figure 1, Supplementary Table 2).
Table 1.
Baseline characteristics of study participants obtained during initial study visit, overall and stratified by IL4Rα-Q576R genotype. P-values represent univariate testing between genotype. Distribution of genotype differs by self-reported race, p < 0.001.
| Characteristic | Overall | Q/Q genotype | Q/R genotype | R/R genotype | p-value |
|---|---|---|---|---|---|
| n | 236 | 58 | 107 | 71 | |
| Age in years (mean ± sd) | 7.96 ± 1.91 | 8.00 ± 1.84 | 7.84 ± 2.01 | 8.11 ± 1.82 | 0.641 |
| Male gender, n (%) | 122 (51.7%) | 27 (46.6%) | 62 (57.9%) | 33 (46.5%) | 0.216 |
| Self-reported race, n (%) | <0.001 | ||||
| Caucasian | 12 (5.1%) | 9 (15.5%) | 3 (2.8%) | 0 (0.0%) | |
| African-American | 84 (35.6%) | 11 (19.0%) | 33 (30.8%) | 40 (56.3%) | |
| Hispanic | 82 (34.7%) | 26 (44.8%) | 44 (41.1%) | 12 (16.9%) | |
| Other | 42 (17.8%) | 11 (19.0%) | 19 (17.8%) | 12 (16.9%) | |
| Mixed | 16 (6.8%) | 1 (1.7%) | 8 (7.5%) | 7 (9.9%) | |
| Annual income >$25,000, n (%) | 104 (44.1%) | 30 (51.7%) | 46 (43.0%) | 28 (39.4%) | 0.359 |
| Family history of asthma, n (%) | 187 (79.2%) | 43 (74.1%) | 88 (82.2%) | 56 (78.9%) | 0.470 |
| Physician diagnosis of hayfever/allergic rhinitis, n (%) | 35 (14.9%) | 7 (12.3%) | 16 (15.0%) | 12 (16.9%) | 0.766 |
| Physician diagnosis of eczema, n (%) | 108 (45.8%) | 19 (32.8%) | 52 (48.6%) | 37 (52.1%) | 0.065 |
| Allergic sensitization a (%) | 159 (67.4%) | 40 (69.0%) | 77 (72.0%) | 42 (59.2%) | 0.195 |
| Asthma severity b (%) | 0.742 | ||||
| mild | 20 (8.5%) | 2 (3.4%) | 11 (10.3%) | 7 (9.9%) | |
| intermittent | 47 (19.9%) | 10 (17.2%) | 21 (19.6%) | 16 (22.5%) | |
| moderate | 69 (29.2%) | 18 (31.0%) | 31 (29.0%) | 20 (28.2%) | |
| severe | 100 (42.4%) | 28 (48.3%) | 44 (41.1%) | 28 (39.4%) | |
| Controller medication c use, n (%) | 129 (54.7%) | 30 (51.7%) | 59 (55.1%) | 40 (56.3%) | 0.864 |
| Use of rescue inhaler > 1x/week (%) | 51 (21.6%) | 11 (19.0%) | 21 (19.6%) | 19 (26.8%) | 0.449 |
| Asthma symptom days (mean ± sd) d | 2.86 ± 4.09 | 2.60 ± 4.05 | 2.79 ± 4.05 | 3.18 ± 4.20 | 0.703 |
Allergic sensitization was defined as any positive skin prick test to 14 common aeroallergens or specific IgE level ≥ 0.35kU/L on serologic testing
Asthma severity is graded based on recommendations from the National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma.
Controller medications are defined as use of an inhaled corticosteroid or leukotriene modifier, as ascertained by review of active prescriptions during baseline study visit.
Number of days with asthma symptoms noted by parent or caretaker in 14 days prior to initial clinic visit
Figure 1.
Predicted asthma symptom days per two-week period associated with classroom endotoxin levels (log-transformed) stratified by IL4Rα-Q576R genotype demonstrates a gene by environment (G×E) interaction. The overall interaction p-value = 0.09; pairwise interaction p-values for Q/R vs. Q/Q = 0.115, R/R vs. Q/Q = 0.049.
In this study, we identify a gene-environment interaction associated with pediatric asthma symptoms. Few gene-environment interaction studies involving the IL4Rα-Q576R polymorphism have been performed. In a humanized mouse model of the IL4Rα-R576 variant (Il4raR576 mice), exposure of the mice to fine and ultrafine particles from vehicular exhaust exacerbated allergic inflammation in association with augmented mixed Th2/Th17 airway cell responses 5, demonstrating the plausibility that interactions between this allele and environmental exposures contribute to asthma morbidity. Similarly, an exaggerated mixed Th2-Th17 cell inflammation may underlie the interaction between the IL4Rα-R576 variant and endotoxin exposure.
Of note, high endotoxin exposure in the presence of the Q/Q genotype was associated with a protective effect against asthma symptoms. A pro-con effect of endotoxin exposure on asthma has been well described in epidemiologic studies. For example, Tischer et al 6, in an analysis of three large European birth cohorts, reported a protective effect of early life endotoxin exposure on later asthma development in the Spanish cohort, an equivocal effect in the German cohort, and a harmful effect in the Dutch cohort. Our findings support the possibility that the IL4R Q versus R variants influence the outcome of endotoxin exposure on asthma.
Our study has a few limitations. Our sample size was modest, thus we had limited power to detect associations, and we were not able to account for possible confounding due to population stratification by performing stratified analyses using self-reported race. We did not validate our findings in an independent cohort, and there is linkage disequilibrium between the polymorphism studied and other IL4R polymorphisms; thus, our findings are preliminary and would need to be verified in larger studies. However, it has been suggested that gene-environment interactions contribute to racial disparities in asthma outcomes 7. United States poverty rates are highest in ethnic minorities 8; a prior nationwide study found that poverty is associated with higher home endotoxin exposure 9. It is possible that the gene-environment interaction we observed contributes to racial disparities in pediatric asthma. While it may be difficult to address individual home environmental conditions, a public health initiative addressing the environment of inner-city schools, where there is a high proportion of ethnic minorities, may improve observed disparities in asthma.
Supplementary Material
Acknowledgments
Support: This work was supported in part by NIH grants R01 AI 073964, U01 AI 110397, K24 AI 106822, U01 AI 126614 (to W.P), R01 AI065617 (to T.A.C.), K23 ES023700 (to P.S.L), and P30 ES000002 (to D.D.) and 5T32AI007512 (to M.X.). This work was also supported by the American College of Allergy, Asthma, and Immunology Young Faculty Award. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
We thank the following companies for their generous donations. Lincoln Diagnostics, Inc., Decatur, IL, for Multi-Test II devices; Greer, Inc, Lenoir, NC allergenic extracts for skin testing; Thermo Fisher Inc. for ImmunoCAP® testing; Monaghan Medical, Inc. for aerochambers; Aeorcrine, Inc. for NiOx Machines; The Allergy and Asthma Awareness Initiative, Inc. for school and community supportive materials.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- 1.Rosa-Rosa L, Zimmermann N, Bernstein JA, Rothenberg ME, Khurana Hershey GK. The R576 IL-4 receptor alpha allele correlates with asthma severity. J Allergy Clin Immunol. 1999;104(5):1008–14. doi: 10.1016/s0091-6749(99)70082-5. [DOI] [PubMed] [Google Scholar]
- 2.Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med. 2016;22(9):1013–22. doi: 10.1038/nm.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai PS, Sheehan WJ, Gaffin JM, Petty CR, Coull BA, Gold DR, Phipatanakul W. School Endotoxin Exposure and Asthma Morbidity in Inner-city Children. Chest. 2015;148(5):1251–8. doi: 10.1378/chest.15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glader P, Smith ME, Malmhall C, Balder B, Sjostrand M, Qvarfordt I, Linden A. Interleukin-17-producing T-helper cells and related cytokines in human airways exposed to endotoxin. Eur Respir J. 2010;36(5):1155–64. doi: 10.1183/09031936.00170609. [DOI] [PubMed] [Google Scholar]
- 5.Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, Chatila ZK, Daher N, Sioutas C, Chatila TA. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 2015;136(2):441–53. doi: 10.1016/j.jaci.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tischer C, Casas L, Wouters IM, Doekes G, Garcia-Esteban R, Gehring U, Hyvarinen A, Oldenwening M, Kerkhof M, Sunyer J, Standl M, Thiering E, Torrent M, Heinrich J group Hs. Early exposure to bio-contaminants and asthma up to 10 years of age: results of the HITEA study. Eur Respir J. 2015;45(2):328–37. doi: 10.1183/09031936.00060214. [DOI] [PubMed] [Google Scholar]
- 7.Forno E, Celedon JC. Asthma and ethnic minorities: socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. 2009;9(2):154–60. doi: 10.1097/aci.0b013e3283292207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macartney S, Bishaw A, Fontenot K. Poverty Rates for Selected Detailed Race and Hispanic Groups by State and Place: 2007–2011. U.S. Census Bureau; 2013. Contract No.: Document Number|. [Google Scholar]
- 9.Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, Rose KM, Zeldin DC. Endotoxin Exposure: Predictors and Prevalence of Associated Asthma Outcomes in the United States. Am J Respir Crit Care Med. 2015;192(11):1287–97. doi: 10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.