Abstract
Objective
To determine the prevalence of isolated and multiple neurodevelopmental impairments at age 10 years among children born extremely preterm (<28 weeks gestational age) and to offer a framework for categorizing neurological limitations.
Design
A multicenter, prospective cohort follow-up study (Extremely Low Gestational Age Newborn Study) recruited 889 10 year-old children (92% of eligible children) born from 2002–2004. We assessed prevalence of cognitive impairment, measured by intelligent quotient (IQ) and tests of executive function, cerebral palsy (CP), autistic spectrum disorder (ASD), and epilepsy singly and in combination. The three-levels of impairment severity were: Category I: no major neurodevelopmental impairment. Category II: normal cognitive ability with CP, ASD, and/or epilepsy. Category III: children with cognitive impairment.
Results
214 of 873 children (25%) had cognitive impairment, 93 of 849 children (11%) had CP, 61 of 857 children (7%) had ASD, and 66 of 888 children (7%) had epilepsy. 19% of all children had 1 diagnosis, 10% had 2 diagnoses, 3% had 3 diagnoses, and none had 4 diagnoses. Decreasing gestational age was associated with increasing number of impairments (p<0.001). Half the children with cognitive impairment and a third of children with CP, ASD, or epilepsy had a single impairment. 601 (68% [95% CI, 64.5%–70.7%]) of children were in Category I, 74 (8% [95% CI, 6.6%–10.3%]) were in Category II, and 214 (24% [95% CI 21.7%–27.4%]) were in Category III.
Conclusions
Three quarters of children had normal intellect at age 10 years; nearly 70% were free of neurodevelopmental impairment. 40% of those with impairments had multiple diagnoses.
Keywords: Extremely preterm, neurological, follow-up, multiple disabilities
Introduction
Over the past two decades, studies of children born extremely preterm (EP) have found that the prevalence of major adverse neurodevelopmental disorders ranges from 15 to 40% for deficient IQ,1–7 5 to 18% for cerebral palsy (CP),8–13 7 to 8% for autism spectrum disorder (ASD),14,15 and 2 to 10% for epilepsy.16,17 Prevalence data such as these usually do not account for multiple disorders occurring in the same child. Yet, disorders of development occur together more often than expected by chance. For example, a third to half of children with CP have deficient IQ18 but only 1 to 3% of children in the general population without CP have deficient IQ.19
Children born EP are at particularly increased risk of having multiple neurodevelopmental disorders, including deficient IQ, impaired executive functioning, CP, ASD, and epilepsy. In the Extremely Low Gestational Age Newborn (ELGAN) cohort, the prevalence of CP at age 2 years was 11%.20 In the same cohort, at age 10 years, the prevalence of IQ less than 70 was 15%,1 cognitive impairment as assessed by a summary categorization of IQ and executive function (EF) ability was 25%,21 ASD was 7%,22 and epilepsy was 7%.22 Here we report the frequency with which these disorders occur in isolation and in combination.
Beyond whether children born EP have single or multiple impairments is the question of how to understand the severity of the neurological burden carried by the child. Most often, overall impairment is determined either by specific criteria for each neurological disorder or, less commonly, by assigning a composite descriptive designation based on a combination of findings.3,6,7,23,24 Studies of EP cohorts born in the past 20 years largely using such combinations estimate rates for moderate to severe overall impairment ranging from 19 to 45%.2,3,5–7,23,24 We propose a conceptual framework for categorizing neurodevelopmental impairment based on four of the most common neurological impairments in children born EP that we reason will impact the ability to live independent adult lives - cognitive impairment, cerebral palsy, autism, and epilepsy.25
Methods
Participants
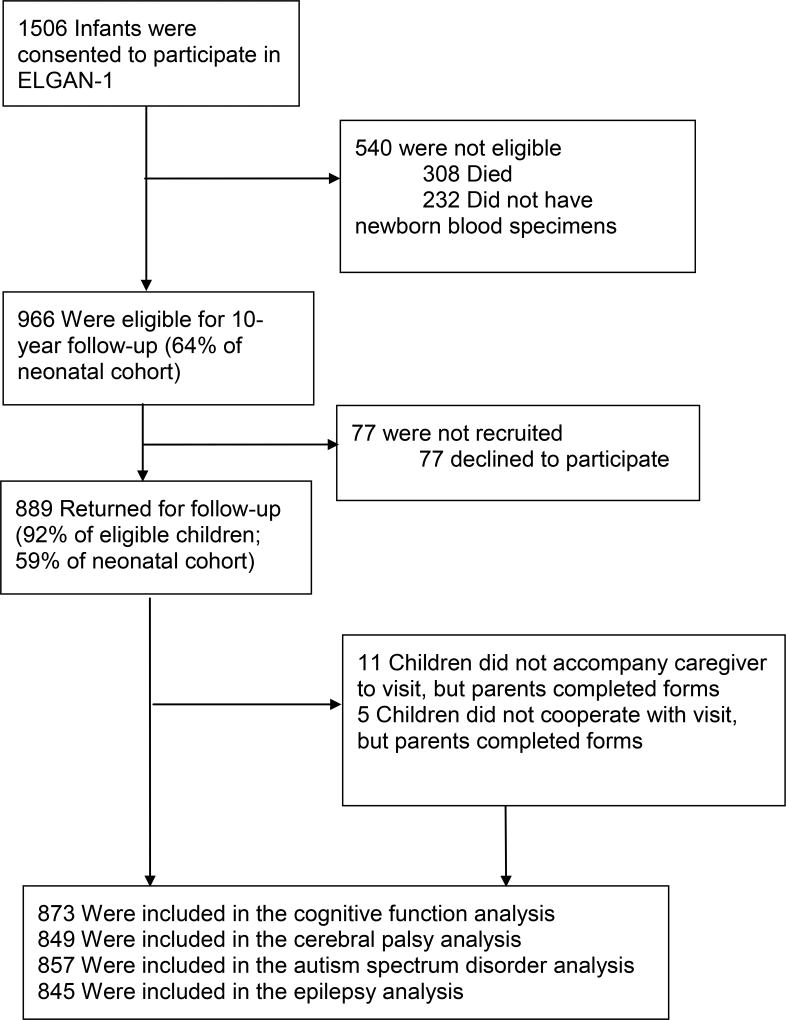
The ELGAN Study is a multicenter observational study of the risk of structural and functional neurologic disorders in EP infants. One thousand two hundred forty-nine mothers delivering 1506 live-born infants before 28 weeks gestation were enrolled between 2002–2004 (Figure 1). From the 1198 ELGAN Study children who survived to 10 years of age, we actively recruited the 966 surviving members of the ELGAN cohort from whom we had collected blood spots during the first postnatal month for the measurement of inflammation-related proteins. The institutional review boards of all participating institutions approved the study. Because of a combination of severe motor, visual and cognitive disability, 40 children were assigned the lowest score on some or all tests. Eleven children did not accompany the caregiver during the follow-up visit, and 5 children could not complete the assessment,1 leaving 873 children available for analyses.
Figure 1.
Enrollment
Procedures
Cognitive evaluations were administered by certified child psychologists and all examiners underwent in-person training and verification of competency for administration of the test battery. Further, all autism evaluators participated in research-level training in administration and scoring of the Autism Diagnostic Interview - Revised (ADI-R)26 and Autism Diagnostic Observation Schedule-2 (ADOS).27 In this paper we use the term deficient IQ when talking about an IQ less than 70, and restrict the use of the terms cognitive ability/cognitive impairment to children who have deficiencies in the latent profile analysis construct of IQ and executive function (see below).
Intellectual quotient (IQ)
IQ was assessed with the School-Age Differential Ability Scales–II (DAS-II)28 Verbal and Nonverbal Reasoning scales.29 The mean of these two measures was used as an estimate of overall IQ because the DAS-II Verbal and Nonverbal IQ scores were strongly correlated within the sample. An IQ score more than two standard deviations below the normative mean (i.e., < 70) is considered in the intellectually disabled or deficient range.
Executive function (EF)
Attention and EF were assessed with the DAS-II and the NEPSY-II30 for measures of verbal working memory, auditory attention, set switching and inhibition, concept generation, and mental flexibility.
Latent profile analysis (LPA)
Using LPA, we classified children in our sample into subgroups based on similarities in their profiles of IQ and EF scores. We have found that this profile is a more sensitive predictor of academic achievement than IQ alone,21 and is likely to have long-term implications for individual and societal burden.31 LPA identified four subgroups corresponding to the following levels of “cognitive functional class” (CFC): normal (CFC 1) in 34% of the cohort with mean IQ and EF scores within normal range on all measures, low-normal (CFC 2) in 41% of the cohort with mean IQ and EF scores ranging from 0.5 to 1 standard deviation (SD) below the norm, moderately impaired (CFC 3) in 17% of the cohort with mean IQ and EF measures between 1.5 and 2.5 SD below the norm, and severely impaired (CFC 4) in 8% of the cohort with mean IQ and EF measures 3 to 4 SD below the norm.21
Cerebral palsy (CP)
For the diagnosis of CP, neurologic examiners utilized a standardized manual and data collection form, and viewed an instructional CD designed to minimize examiner variability.32
Autism spectrum disorder (ASD)
All children determined to be at risk on the Social Communication Questionnaire (SCQ)33 were assessed with the Autism Diagnostic Interview – Revised (ADI-R),26 an in-depth parent interview. Children meeting ADI-R criteria for ASD were administered the Autism Diagnostic Observation Schedule-2 (ADOS-2).27 Children meeting standardized research criteria for ASD on the ADOS-2 were classified as having ASD.
Seizure and epilepsy determination
Identification of seizures involved a two-stage process.34,35 Parents of children were asked to complete part one of a validated seizure screen.34 Parents of children with a positive part one screen completed a structured interview with a study coordinator followed by an open-ended interview with a pediatric epileptologist. Then, a second epileptologist independently reviewed interview responses and similarly rated event types as seizures or not. A third epileptologist served as a tie-breaker in the 3% for which the evaluators were discordant. For these analyses, we defined epilepsy as having 2 or more unprovoked seizures.35
Impairment severity
We devised a three-level categorization of impairment for neurodevelopmental burden among EP infants. The first level (Category I) constitutes children free of major neurodevelopmental impairment. The second level (Category II) includes children who have normal IQ (greater than or equal to 70) or normal range cognitive ability (CFC 1 or 2) but have one or more of the other three neurological impairments, CP, ASD, and epilepsy. The third level (Category III) includes children with cognitive impairment whether or not co-morbidities coexist. We do not include visual or hearing impairment in our categorization because only seven children were blind or hearing impaired.1
Statistical analyses
When determining the prevalence of isolated conditions and severity, we restrict analysis to the children for whom we had data on the condition: status of cognition for 873 children, CP for 849 children, ASD for 857 children, and epilepsy for 845 children (Figure 1). When analyzing comorbid conditions, we included all children and treated missing data as unimpaired.
Forty-three of 273 children screened at-risk for seizures could not be contacted for interview by the epileptologist and so were missing data on seizures. We used inverse probability weighting to account for this missing data when analyzing seizures, with the probability of missingness based on gestational age (GA) and the result of the initial seizure screen.36 Weighted counts and prevalence are given for analyses involving seizures.
We conducted two sets of analyses. In the first set, we defined cognitive impairment as levels of cognitive functional class (CFC 3 or 4), which, as noted above, includes measures of IQ and EF. In the second set, we considered only IQ less than 70, and these results are described in the Supplement.
Prevalence is described through percentages and 95% confidence intervals. The association between number of impairments and GA category was tested through the Mantel-Haenszel chi-square test for trend. The increased risk for another impairment, for children with one particular impairment, was described through relative risks and 95% confidence intervals. Statistical analyses were conducted using the Stata Release 1437 and SAS Version 9.338 software packages.
Results
Sample description (eTable 1 in the Supplement)
Of children with any neurodevelopmental impairment, 52% (n=97) were born at 23–24 weeks GA, 32% (n=128) were born at 25–26 weeks GA, and 21% (n=63) were born at 27 weeks GA. Demographic characteristics associated with any neurodevelopmental impairment were mother’s identification as black, mother’s age less than 21 years, single marital status of mother, and mother’s enrollment in public insurance. Newborn characteristics associated with neurodevelopmental impairment were lower GA, lower BW Z-score, and male sex.
Percentage of children with impairments
Twenty-five percent (n=214) of children had cognitive impairment (CFC 3 or 4), 11% (n=93) of children had CP, 7% (n=61) of children had ASD, and 7% (n=66) of children had epilepsy (Table 1). Sixty-eight percent of children did not meet criteria for any diagnosis at age 10 years when cognitive impairment included measures of IQ and EF (CFC 3 or 4) (Table 2) and 77% did not meet criteria for any diagnosis when considering only IQ less than 70 (eTable 2 in the Supplement).
Table 1.
Distribution of each outcome among those with isolated impairments or with multiple impairments (row percents).
| Adverse impairment (n) |
% with isolated impairment (n=288) |
% with multiple impairments (n=117) |
Among those with multiple impairments, % of each adverse impairment associated with other impairments (maximum n=117) |
|||
|---|---|---|---|---|---|---|
| CFC 3 or 4 | CP | ASD | Epilepsy | |||
| CFC 3 or 4 (214) | 49% (104/214) | 51% (110/214) | 55% (58/106) | 48% (40/84) | 38% (41/109) | |
| CP at age 2 (93) | 32% (30/93) | 68% (63/93) | 94% (58/62) | 20% (8/40) | 34% (21/62) | |
| ASD (61) | 30% (18/61) | 70% (43/61) | 93% (40/43) | 20% (8/41) | 21% (9/43) | |
| Epilepsy (66) | 29% (19/66) | 71% (47/66) | 89% (41/46) | 47% (21/45) | 26% (9/35) | |
Table 2.
Number of impairments (CFC 3 or 4, CP, ASD, and/or epilepsy) according to gestational age (column percents).
| Number of impairments | n (% of all children) | n (%) according to GA (weeks) | ||
|---|---|---|---|---|
| (n=889) | 23–24 W (n=187) | 25–26 W (n=400) | 27 W (n=302) | |
| 0 | 601 (68%) | 90 (48%) | 271 (68%) | 240 (79%) |
| 1 | 171 (19%) | 49 (26%) | 82 (21%) | 40 (13%) |
| 2 | 90 (10%) | 39 (21%) | 38 (10%) | 13 (4%) |
| 3 | 25 (3%) | 9 (5%) | 8 (2%) | 8 (3%) |
| 4 | 2 (0%) | 0 (0%) | 1 (0.25%) | 1 (0.3%) |
p<0.001 from Mantel-Haenszel test for trend
When cognitive impairment included measures of IQ and EF (CFC 3 or 4), 19% of all children had 1 diagnosis, 10% of children had 2 diagnoses, 3% of children had 3 diagnoses, and no child had all 4 diagnoses; i.e., 60% of children with impairment had a single diagnosis (Table 2). There was a significant trend for decreasing number of impairments with increasing GA (p<0.001). These percentages did not change substantially if we assumed that missing values represented presence of a diagnosis.
Description of co-morbidities (Table 3)
Table 3.
Relative risks of having other impairments, given presence of one impairment
| Relative risk (95% CI): |
Given Condition | |||
|---|---|---|---|---|
| CFC 3 or 4 | CP at age 2 | ASD | Epilepsy | |
| CFC 3 or 4 | 3.29 (2.66, 4.08) | 3.58 (2.84, 4.52) | 2.96 (2.36, 3.72) | |
| CP at age 2 | 5.51 (3.70, 8.19) | 1.69 (0.85, 3.35) | 3.62 (2.39, 5.49) | |
| ASD | 7.09 (4.25, 11.82) | 1.71 (0.84, 3.46) | 2.57 (1.34, 4.94) | |
| Epilepsy | 5.29 (3.28, 8.55) | 4.01 (2.50, 6.45) | 2.61 (1.24, 5.08) | |
Conditions are not mutually exclusive
Children with cognitive impairment measured by IQ and EF (CFC 3 or 4) had more than 5 times the risk for having CP and/or epilepsy and 7 times the risk for having ASD, compared to children without cognitive impairment. At age 10 years, those who had been diagnosed with CP at age 2 years had 3.3 times the risk for having cognitive impairment (CFC 3 or 4) and 4.0 times the risk for having epilepsy, compared with children without CP at age 10. Children with ASD had 3.6 times the risk of having cognitive impairment (CFC 3 or 4) and 2.6 times the risk for having epilepsy compared to children without ASD. Children with epilepsy had between 2.5 and 3.6 times the risk of having ASD, cognitive impairment (CFC 3 or 4), and/or CP compared to children without epilepsy.
Percent of children with single impairments or with co-morbidities
One-third of the cohort had at least one neurodevelopmental impairment, and of these children, 40% had more than one other impairment (Table 2). Of the children with only one finding, 61% had cognitive impairment measured by IQ and EF (CFC 3 or 4), 17.5% had CP, 10.5% had ASD, and 11% had epilepsy. Among the 117 children with multiple impairments, 94% were classified as CFC 3 or 4, 54% had CP, 37% had ASD, and 40% had epilepsy, leaving only 6% (n=7) of the children with multiple deficits and preserved cognitive abilities.
Cognition based only on IQ
When deficient IQ was analyzed without consideration of EF, 14% of children had 1 diagnosis, 6% of children had 2 diagnoses, 2% of children had 3 diagnoses, and no child had all 4 diagnoses; 64% of children with impairment had a single diagnosis (eTable 2 in Supplement). Of those children with only one diagnosis, 14% had IQ less than 70, 37% had CP, 25% had ASD, and 24% had epilepsy. Among the 75 children with multiple impairments, 84% involved deficient IQ, 61% had CP, 39% had ASD, and 48% had epilepsy, leaving 15% children with multiple deficits and preserved IQ (eTable 3 in the Supplement). Children with IQ less than 70 had 6.9, 9.8, and 6.6 times the risk for having CP, ASD, and/or epilepsy, compared to children with IQ greater than or equal to 70 (eTable 4 in the Supplement). Children with CP had 8.0 times the risk for having IQ less than 70 and 4.0 times the risk for having epilepsy, compared to children without CP. Children with ASD had 10.5 times the risk for having IQ less than 70 and 2.6 times the risk for having epilepsy, compared to children without ASD. Finally, children with epilepsy had 6.0, 3.6, and 2.6 times the risk for having IQ less than 70, CP, and/or ASD compared to children without epilepsy.
Impairment severity
When cognitive impairment was defined by both IQ and EF using latent profile analysis (CFC 3 or 4), 601 (68% [95% CI, 64.5%–70.7%]) of children were free of major impairment (Category I). Seventy-four (8% [95% CI, 6.6%–10.3%]) of the 873 children had normal or low-normal cognitive abilities (CFC 1 or 2), but had one or more other impairments (Category II): CP (5%), ASD (3%), and/or epilepsy (4%). Two hundred fourteen (24% [95% CI 21.7%–27.4%]) of the 873 children had moderate to severe cognitive impairment (CFC 3 or 4) (Category III).
When we considered deficient IQ (less than 70) without regard to EF, 687 (77% [95% CI, 74.5%–80.0%]) of children were free of major impairment (Category I). One hundred twenty-one children of the 873 children (14% [95% CI, 11.6%–16.2%]) did not have deficient IQ, but had one or more other impairments (Category II): CP (7%), ASD (5%), and/or epilepsy (5%). Eighty-one of the 873 children (9% [95% CI, 7.4%–11.2%]) had deficient IQ (Category III).
Discussion
In this sample of 889 children born from 2002 to 2004 before 28 weeks gestation, 68% percent were free of major impairment. Among the 32% with cognitive impairment as assessed by IQ and EF (CFC 3 or 4), CP, ASD, and/or epilepsy, 19% had 1 diagnosis, 10% had 2 diagnoses, 3% had 3 diagnoses, and no child had all 4 diagnoses considered here. Half the children with cognitive impairment (CFC 3 or 4) and one-third of children with CP, ASD, or epilepsy had a single impairment. The remainder had multiple impairments.
IQ and EF as a measure of cognitive function
According to the American Association on Intellectual and Developmental Disabilities (AAIDD), intellectual disability is defined and characterized by significant limitations both in intellectual functioning and in adaptive behavior as expressed in conceptual, social, and practical adaptive skill.25 Adaptive function impairment appears to be closely aligned with disturbances in EF.39–42
Category III is based on the premise that impaired cognition involves both IQ and EF, approximating the AAIDD definition of intellectual disability, and predicts a child’s ability to function later as an independent adult.25 We posit that children in Category II will have less impact on family43,44 and a greater capacity to live independent lives45,46 than children in Category III.
Supplementing IQ measures with EF assessments appears to add to the precision in predicting academic and other outcomes21,47 and clarifies the individual and societal burden of extreme prematurity when compared to IQ alone,21 a point that Chung et al highlight: “When executive function deficits and intellectual deficits are considered together, as they are in the LPA groupings, the life-long individual and societal burden of extreme prematurity becomes clear. Outside of complex chronic disease, the single most individually and societally costly childhood condition might be school failure. School failure is a threshold event, creating sudden and marked discontinuities in long-term economic and civic potential and productivity, thus predisposing individuals, and even subsequent generations, to early morbidity and mortality.31” In our sample, the prevalence of cognitive impairment as assessed by IQ and EF (24%) and the prevalence of major neurodevelopmental disorders (32% in Categories II + III) is substantially higher than rates of deficient IQ (9%) or major neurodevelopmental disorders (23% in Categories II + III) when using IQ alone (IQ less than 70).
Comparison with prior studies
The majority of follow-up studies of children born EP over the past 20 years, which are listed in Table 4, use IQ scores without consideration of EF as a key measure of cognition when categorizing impairment severity. Applying similar IQ-only criteria for cognitive outcome to our cohort, 23% of children are categorized as having moderate to severe impairment (Categories II + III), which falls on the low end of the range (18 – 45%) of impairment reported in other studies.2,3,5–7,23,24
Table 4.
Comparison with prior studies
| Study | Mod/Sev | Mild | None |
|---|---|---|---|
|
| |||
| Johnson et al,1 2009a | 45% | 39% | 16% |
| IQ≤70, CP with GMFCS>2, mod/sev impaired vision and/or hearing loss | IQ 71–85, CP with GMFCS 1–2, mild visual impairment and/or hearing loss | ||
|
| |||
| Roberts et al,2 2010b | 19% | 40% | 41% |
| IQ<70, CP with mod/sev limitations, blindness, deafness | IQ 70–85, CP with mild limitations | ||
|
| |||
| Herber-Jonat et al,3 2014c | 24% | 35% | 41% |
| IQ<70, abnormal neurodevelopmental exam with mod/sev impaired mobility (GMFCS≥2), severe visual and/or hearing impairment | IQ 70–84, abnormal neurodevelopmental examination with normal/mildly impaired mobility (GMFCS≤1) | ||
|
| |||
| Holsti et al,4 2014d | 34% | 31% | 35% |
| IQ≤72, mod/sev CP, severe visual impairment, hearing impairment with bilateral hearing aids | IQ 73–88, mild CP, unilateral blindness | ||
|
| |||
| Serenius et al,5 2016e | 33% | 30% | 36% |
| IQ<77, CP with GMFCS≥2, mod/sev visual impairment, hearing impairment | IQ 77–89 or mild cognitive disability by a clinical examination or record review, CP with GMFCS=1, mild visual impairment | ||
|
| |||
| Stahlmann et al,6 2009f | 31% | 39% | 30% |
| IQ<70, CP with GMFCS≥1, abnormal neurodevelopmental signs with severe difficulties of muscle tone regulation, coordination and balance, blindness, deafness | IQ 70–85, weak muscle tone, difficulties in coordination, balance, or clumsiness | ||
|
| |||
| Neubauer et al,7 2008g | 28% | 42% | 28% |
| IQ<70, CP, blindness, deafness, intractable epilepsy | IQ 70–84, gross and fine motor deficits, language disorders, visual and audit defects, ADHD, abnormal socio-emotional development | ||
|
| |||
| ELGANh (Proposed) | Category III: 24% | Category II: 8% | Category I: 68% |
| CFC 3 or 4 (with or without CP, ASD, epilepsy) | CFC 1 or 2 with CP, ASD, and/or epilepsy* | CFC 1 or 2 without CP, ASD, or epilepsy | |
Abbreviations: ASD, autism spectrum disorder; CFC, cognitive functional class; CP, cerebral palsy; GMFCS, Gross Motor Function Classification System; IQ, General cognitive ability; Mod/sev, moderate to severe
Inclusion criteria (weeks GA):
≤25
22–27
<25
23–25
22–27
<27 weeks
<26 (n=50)
<28
63–67% of all children with CP, ASD, and/or epilepsy had comorbid cognitive impairment (CFC 3 or 4)
The relatively low prevalence of subnormal IQ may be due to several factors. First, it may reflect advances in care and interventions.48–53 Second, some studies listed in Table 4 use lower GA criteria, often associated with increased severity of neurodevelopmental impairment, compared to our cutoff of 28 weeks.2,3,7,24 Third, our study used published IQ test norms as measures of comparison rather than data from a control population. Published norms may underestimate contemporary measures of IQ (Flynn effect),54 leading to an underestimate of the prevalence of deficient IQ.2,3,6,7,55–57
Given these caveats, about one-third of EP children have moderate to severe impairment when EF is taken into account (CFC 3 or 4). A recent Swedish study showed that 15% of children born EP with no neurosensory impairment and a normal IQ had an EF score more than 2 standard deviations below the mean compared to 3% among control children.58 That study further showed that these executive dysfunctions were strongly associated with academic, behavioral, and learning skill deficits.
To our knowledge, no other studies have evaluated the co-occurrence of the 4 neurodevelopmental disabilities included here - cognitive and motor disorders, ASD, and epilepsy. The EPICURE Study investigated cognitive, motor, vision, and hearing impairments and found 75% of children with major impairment in 1 domain, 17% in 2 domains, 8% in 3 domains, and none in all domains.2 Other studies that defined disabilities more broadly to include behavioral, attentional and/or mild impairments found multiple impairments in 30%59 and 44% of children.5,60 The biological basis of multiple impairments in EP children remains to be defined and is likely multifactorial, involving both pre- and post-natal influences on brain development.1
Strengths/Limitations
Strengths of our study include a large number of infants with minimal attrition. We selected infants based on GA, not birth weight, in order to minimize confounding related to fetal growth restriction.61 Well-validated tools are used for assessment of neurodevelopmental functions, and examiners were unaware of the children’s medical histories. We also characterized severity at school age, when assessment of cognitive and neurodevelopmental deficits is more reliable than at earlier ages.6,7,55,62
Limitations include possible underestimation of the prevalence of impairment in our cohort because some children who were missing data were considered to not have an impairment. However, if we assumed presence of impairment, our findings did not change substantially. Another limitation is that we used standardized population-based normative means and standard deviations rather than control term peers. We did not conduct analyses of antecedent risk factors using the disability construct proposed in this paper, although previously we have reported antecedent risks for each of the individual outcomes considered.1,14,22,35 While psychiatric and behavioral outcomes might contribute to school failure and independence, we did not consider them at age 10 because they will manifest most clearly in adolescence. We also did not consider bilateral visual or hearing impairments because there were very few children with these disorders in our cohort.
Conclusions and Implications
Approximately one-third of children who were born extremely preterm had major impairment at 10 years of age, and cognitive deficits were the most prevalent. Nearly 70% of children had no major neurodevelopmental impairment. Our findings have implications for predicting prognosis of neurodevelopmental outcomes among school-age children born extremely preterm.
Supplementary Material
Acknowledgments
Dr. Frazier has received research support from Janssen Research and Development, SyneuRex International Corp, Neuren Pharmaceuticals, and Roche Pharmaceuticals over the past two years. In addition, she has served on a data safety monitoring board for Forrest Pharmaceuticals (but none of these are relevant to this article).
Funding/Support: This study was supported by cooperative agreements with the National Institute of Neurological Disorders and Stroke, Bethesda, Maryland (5U01NS040069-05; 2R01NS040069 - 06A2; U01NS040069), a center grant award from the National Institute of Child Health and Human Development, Bethesda, Maryland (5P30HD018655-28), and the National Institutes of Health, Bethesda, Maryland (1UG3OD023348).
Group Information: The ELGAN Group members are as follows:
Boston Children’s Hospital, Boston, MA:
Janice Ware, PhD
Taryn Coster, BA
Brandi Hanson, PsyD
Rachel Wilson, PhD
Kirsten McGhee, PhD
Patricia Lee, PhD
Aimee Asgarian, PhD
Anjali Sadhwani, PhD
Tufts Medical Center, Boston, MA:
Ellen Perrin, MD
Emily Neger, MA
Kathryn Mattern, BA
Jenifer Walkowiak, PhD
Susan Barron, PhD
Baystate Medical Center
Bhavesh Shah, MD
Rachana Singh, MD, MS
Anne Smith, PhD
Deborah Klein, BSN, RN
Susan McQuiston, PhD
University of Massachusetts Medical School, Worcester, MA:
Lauren Venuti, BA
Beth Powers, RN
Ann Foley, Ed M
Brian Dessureau, PhD
Molly Wood, PhD
Jill Damon-Minow, PsyD
Yale University School of Medicine, New Haven, CT:
Richard Ehrenkranz, MD
Jennifer Benjamin, MD
Elaine Romano, APRN
Kathy Tsatsanis, PhD
Katarzyna Chawarska, PhD
Sophy Kim, PhD
Susan Dieterich, PhD
Karen Bearrs, PhD
Wake Forest University Baptist Medical Center, Winston-Salem, NC:
Nancy Peters, RN
Patricia Brown, BSN
Emily Ansusinha, BA
Ellen Waldrep, PhD
Jackie Friedman, PhD
Gail Hounshell. PhD
Debbie Allred, PhD
University Health Systems of Eastern Carolina, Greenville, NC:
Stephen C. Engelke, MD
Nancy Darden-Saad, BS, RN, CCRC
Gary Stainback, PhD
North Carolina Children’s Hospital, Chapel Hill, NC:
Diane Warner, MD, MPH
Janice Wereszczak, MSN, PNP
Janice Bernhardt, MS, RN
Joni McKeeman, PhD
Echo Meyer, PhD
Helen DeVos Children’s Hospital, Grand Rapids, MI:
Steve Pastyrnak, PHD
Julie Rathbun, BSW, BSN, RN
Sarah Nota, BS
Teri Crumb, BSN, RN, CCRC
Sparrow Hospital, Lansing, MI:
Madeleine Lenski, MPH
Deborah Weiland, MSN
Megan Lloyd, MA, EdS
University of Chicago Medical Center, Chicago, IL:
Scott Hunter, PhD
Michael Msall, MD
Rugile Ramoskaite, BA
Suzanne Wiggins, MA
Krissy Washington, MA
Ryan Martin, MA
Barbara Prendergast, BSN, RN
Megan Scott, PhD
William Beaumont Hospital, Royal Oak, MI:
Judith Klarr, MD
Beth Kring, RN
Jennifer DeRidder, RN
Kelly Vogt, PhD.
Additional Contributions: We acknowledge the inspiration, guidance and collaboration of Alan Leviton and Elizabeth Allred in conducting the ELGAN Study. We are also grateful to ELGAN Study participants and their families for their willingness to be engaged in the study for these many years and for the commitment and extra efforts that have made this work possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None of the other authors have conflicts of interest relevant to this article to disclose.
References
- 1.Joseph RM, O'Shea TM, Allred EN, et al. Neurocognitive and academic outcomes at age 10 years of extremely preterm newborns. Pediatrics. 2016;137(4) doi: 10.1542/peds.2015-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson S, Fawke J, Hennessy E, et al. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics. 2009;124(2):e249–257. doi: 10.1542/peds.2008-3743. [DOI] [PubMed] [Google Scholar]
- 3.Holsti A, Adamsson M, Serenius F, Hagglof B, Farooqi A. Two-thirds of adolescents who received active perinatal care after extremely preterm birth had mild or no disabilities. Acta Paediatr. 2016;105(11):1288–1297. doi: 10.1111/apa.13499. [DOI] [PubMed] [Google Scholar]
- 4.Stahlmann N, Eisemann N, Thyen U, Herting E, Rapp M. Long-term health outcomes and health-related quality of life in adolescents from a cohort of extremely premature infants born at less than 27 weeks of gestation in northern Germany. Neuropediatrics. 2016;47(6):388–398. doi: 10.1055/s-0036-1593373. [DOI] [PubMed] [Google Scholar]
- 5.Stahlmann N, Rapp M, Herting E, Thyen U. Outcome of extremely premature infants at early school age: health-related quality of life and neurosensory, cognitive, and behavioral outcomes in a population-based sample in northern Germany. Neuropediatrics. 2009;40(3):112–119. doi: 10.1055/s-0029-1243166. [DOI] [PubMed] [Google Scholar]
- 6.Roberts G, Anderson PJ, De Luca C, Doyle LW. Changes in neurodevelopmental outcome at age eight in geographic cohorts of children born at 22–27 weeks' gestational age during the 1990s. Arch Dis Child Fetal Neonatal Ed. 2010;95(2):F90–94. doi: 10.1136/adc.2009.165480. [DOI] [PubMed] [Google Scholar]
- 7.Serenius F, Ewald U, Farooqi A, et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in sweden. JAMA Pediatr. 2016;170(10):954–963. doi: 10.1001/jamapediatrics.2016.1210. [DOI] [PubMed] [Google Scholar]
- 8.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343(6):378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 9.Doyle LW. Neonatal intensive care at borderline viability--is it worth it? Early Hum Dev. 2004;80(2):103–113. doi: 10.1016/j.earlhumdev.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Piecuch RE, Leonard CH, Cooper BA, Kilpatrick SJ, Schlueter MA, Sola A. Outcome of infants born at 24–26 weeks' gestation: II. Neurodevelopmental outcome. Obstet Gynecol. 1997;90(5):809–814. doi: 10.1016/S0029-7844(97)00429-8. [DOI] [PubMed] [Google Scholar]
- 11.Emsley HC, Wardle SP, Sims DG, Chiswick ML, D'Souza SW. Increased survival and deteriorating developmental outcome in 23 to 25 week old gestation infants, 1990–4 compared with 1984–9. Arch Dis Child Fetal Neonatal Ed. 1998;78(2):F99–104. doi: 10.1136/fn.78.2.f99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Msall ME, Buck GM, Rogers BT, et al. Multivariate risks among extremely premature infants. J Perinatol. 1994;14(1):41–47. [PubMed] [Google Scholar]
- 13.Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Arch Pediatr Adolesc Med. 1998;152(5):425–435. doi: 10.1001/archpedi.152.5.425. [DOI] [PubMed] [Google Scholar]
- 14.Joseph RM, Korzeniewski SJ, Allred EN, et al. Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a large cohort study of 10-year-old children born at 23–27 weeks' gestation. Am J Obstet Gynecol. 2017;216(3):304.e301–304.e316. doi: 10.1016/j.ajog.2016.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. J Pediatr. 2010;156(4):525–531. e522. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Moore GP, Lemyre B, Barrowman N, Daboval T. Neurodevelopmental outcomes at 4 to 8 years of children born at 22 to 25 weeks' gestational age: a meta-analysis. JAMA Pediatr. 2013;167(10):967–974. doi: 10.1001/jamapediatrics.2013.2395. [DOI] [PubMed] [Google Scholar]
- 17.Marret S, Marchand-Martin L, Picaud JC, et al. Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One. 2013;8(5):e62683. doi: 10.1371/journal.pone.0062683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuban KCK, Leviton A. Cerebral palsy. N Engl J Med. 1994;330(3):188–195. doi: 10.1056/NEJM199401203300308. [DOI] [PubMed] [Google Scholar]
- 19.Schieve LA, Gonzalez V, Boulet SL, et al. Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006–2010. Res Dev Disabil. 2012;33(2):467–476. doi: 10.1016/j.ridd.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Kuban KCK, Allred E, O’Shea TM, Paneth N, Pagano M, Leviton A. An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr. 2008;153(4):466–472. doi: 10.1016/j.jpeds.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heeren T, Joseph RM, Allred E, O’Shea TM, Leviton A, Kuban KCK. Cognitive Functioning at Age 10 Years Among Children Born Extremely Preterm: A Latent Profile Approach. Pediatr Res. 2017 doi: 10.1038/pr.2017.82. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuban KCK, Joseph RM, O'Shea TM, et al. Girls and boys born before 28 weeks gestation: risks of cognitive, behavioral, and neurologic outcomes at age 10 years. J Pediatr. 2016;173:69–75. e61. doi: 10.1016/j.jpeds.2016.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herber-Jonat S, Streiftau S, Knauss E, et al. Long-term outcome at age 7–10 years after extreme prematurity - a prospective, two centre cohort study of children born before 25 completed weeks of gestation (1999–2003) J Matern Fetal Neonatal Med. 2014;27(16):1620–1626. doi: 10.3109/14767058.2013.871699. [DOI] [PubMed] [Google Scholar]
- 24.Neubauer AP, Voss W, Kattner E. Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr. 2008;167(1):87–95. doi: 10.1007/s00431-007-0435-x. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JR, Bradley VJ, Buntinx WH, et al. Conceptualizing supports and the support needs of people with intellectual disability. Intellect Dev Disabil. 2009;47(2):135–146. doi: 10.1352/1934-9556-47.2.135. [DOI] [PubMed] [Google Scholar]
- 26.LeCouteur A, Lord C, Rutter M. Autism Diagnostic Interview – Revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 27.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I): Modules 1–4. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- 28.Elliott CD. Differential Ability Scales-II (DAS-II) San Antonio, TX: Pearson Education; 2007. [Google Scholar]
- 29.Joseph RM, Fein D. The significance of IQ and differential cognitive abilities for understanding ASD. The Neuropsychology of Autism. 2011:281–294. [Google Scholar]
- 30.Korkman M, Kirk U, Kemp S. NEPSY-II: Clinical and Interpretive Manual. San Antonio, TX: The Psychological Corporation; 2007. [Google Scholar]
- 31.Chung PJ, Opipari VP, Koolwijk I. Executive function and extremely preterm children. Pediatr Res. 2017;82(4):565–566. doi: 10.1038/pr.2017.184. [DOI] [PubMed] [Google Scholar]
- 32.Kuban KCK, O'Shea M, Allred E, et al. Video and CD-ROM as a training tool for performing neurologic examinations of 1-year-old children in a multicenter epidemiologic study. J Child Neurol. 2005;20(10):829–831. doi: 10.1177/08830738050200101001. [DOI] [PubMed] [Google Scholar]
- 33.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 34.Douglass LM, Kuban KCK, Tarquinio D, et al. A novel parent questionnaire for the detection of seizures in children. Pediatr Neurol. 2016;54:64–69. e61. doi: 10.1016/j.pediatrneurol.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Douglass L, Heeren T, Stafstrom CE, et al. The cumulative incidence of seizures and epilepsy in 10-year-old children born before 28 weeks gestation. Epilepsia. 2017 doi: 10.1016/j.pediatrneurol.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 37.Stata Statistical Software: Release 14. [computer program] College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 38.Base SAS® 9.4 Procedures Guide. [computer program] Cary, NC: SAS Institute Inc.; 2011. [Google Scholar]
- 39.Xu XJ, Wang LL, Zhou N. Ecological executive function characteristics and effects of executive function on social adaptive function in school-aged children with epilepsy. Zhonghua yi xue za zhi. 2016;96(7):517–521. doi: 10.3760/cma.j.issn.0376-2491.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 40.King TZ, Smith KM, Ivanisevic M. The mediating role of visuospatial planning skills on adaptive function among young-adult survivors of childhood brain tumor. Arch Clin Neuropsychol. 2015;30(5):394–403. doi: 10.1093/arclin/acv033. [DOI] [PubMed] [Google Scholar]
- 41.Loe IM, Feldman HM, Huffman LC. Executive function mediates effects of gestational age on functional outcomes and behavior in preschoolers. J Dev Behav Pediatr. 2014;35(5):323–333. doi: 10.1097/DBP.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ware AL, Crocker N, O'Brien JW, et al. Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res. 2012;36(8):1431–1441. doi: 10.1111/j.1530-0277.2011.01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephens BE, Bann CM, Poole WK, Vohr BR. Neurodevelopmental impairment: predictors of its impact on the families of extremely low birth weight infants at 18 months. Infant Ment Health J. 2008;29(6):570–587. doi: 10.1002/imhj.20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein RE, Jessop DJ. The impact on family scale revisited: further psychometric data. J Dev Behav Pediatr. 2003;24(1):9–16. [PubMed] [Google Scholar]
- 45.Fortuna RJ, Robinson L, Smith TH, et al. Health conditions and functional status in adults with autism: a cross-sectional evaluation. J Gen Intern Med. 2016;31(1):77–84. doi: 10.1007/s11606-015-3509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong V, Chung B, Hui S, et al. Cerebral palsy: correlation of risk factors and functional performance using the Functional Independence Measure for Children (WeeFIM) J Child Neurol. 2004;19(11):887–893. doi: 10.1177/08830738040190110701. [DOI] [PubMed] [Google Scholar]
- 47.Aylward GP. Cognitive and neuropsychological outcomes: more than IQ scores. Ment Retard Dev Disabil Res Rev. 2002;8(4):234–240. doi: 10.1002/mrdd.10043. [DOI] [PubMed] [Google Scholar]
- 48.Hintz SR, Kendrick DE, Wilson-Costello DE, et al. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks' gestational age. Pediatrics. 2011;127(1):62–70. doi: 10.1542/peds.2010-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hack M, Wilson-Costello D, Friedman H, Minich N, B S. Early childhood outcomes of infants born at the limits of viability have not improved in 2000–2004. E-PAS. 2008:536521. [Google Scholar]
- 50.Vaucher YE, Peralta-Carcelen M, Finer NN, et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. 2012;367(26):2495–2504. doi: 10.1056/NEJMoa1208506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt B, Whyte RK, Asztalos EV, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309(20):2111–2120. doi: 10.1001/jama.2013.5555. [DOI] [PubMed] [Google Scholar]
- 52.Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368(22):2094–2104. doi: 10.1056/NEJMoa1302298. [DOI] [PubMed] [Google Scholar]
- 53.Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120(6):1337–1351. doi: 10.1213/ANE.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flynn J. Searching for justice: The discovery of IQ gains over time. American Psychologist. 1999;54(1):5–20. [Google Scholar]
- 55.Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Paper presented at: Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): 2007. [Google Scholar]
- 56.Wolke D, Ratschinski G, Ohrt B, Riegel K. The cognitive outcome of very preterm infants may be poorer than often reported: an empirical investigation of how methodological issues make a big difference. Eur J Pediatr. 1994;153(12):906–915. doi: 10.1007/BF01954744. [DOI] [PubMed] [Google Scholar]
- 57.Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131(4):e1053–1061. doi: 10.1542/peds.2012-2311. [DOI] [PubMed] [Google Scholar]
- 58.Farooqi A, Adamsson M, Serenius F, Hagglof B. Executive functioning and learning skills of adolescent children born at fewer than 26 weeks of gestation. PLoS One. 2016;11(3):e0151819. doi: 10.1371/journal.pone.0151819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodward LJ, Moor S, Hood KM, et al. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed. 2009;94(5):F339–344. doi: 10.1136/adc.2008.146282. [DOI] [PubMed] [Google Scholar]
- 60.van Baar AL, van Wassenaer AG, Briet JM, Dekker FW, Kok JH. Very preterm birth is associated with disabilities in multiple developmental domains. J Pediatr Psychol. 2005;30(3):247–255. doi: 10.1093/jpepsy/jsi035. [DOI] [PubMed] [Google Scholar]
- 61.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134(6):604–613. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- 62.Voss W, Neubauer AP, Wachtendorf M, Verhey JF, Kattner E. Neurodevelopmental outcome in extremely low birth weight infants: what is the minimum age for reliable developmental prognosis? Acta Paediatr. 2007;96(3):342–347. doi: 10.1111/j.1651-2227.2006.00130.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.