Abstract
Glyphosate-containing herbicides are among the most widely-used in the world. Although glyphosate itself is relatively non-toxic, growing evidence suggests that commercial herbicide formulations may lead to increased oxidative stress and mitochondrial inhibition. In order to assess these mechanisms in vivo, we chronically (24 h) exposed Caenorhabditis elegans to various concentrations of the glyphosate-containing herbicide TouchDown (TD). Following TD exposure, we evaluated the function of specific mitochondrial electron transport chain complexes. Initial oxygen consumption studies demonstrated inhibition in mid- and high-TD concentration treatment groups compared to controls. Results from tetramethylrhodamine ethyl ester and ATP assays indicated reductions in the proton gradient and ATP levels, respectively. Additional studies were designed to determine whether TD exposure resulted in increased reactive oxygen species (ROS) production. Data from hydrogen peroxide, but not superoxide or hydroxyl radical, assays showed statistically significant increases in this specific ROS. Taken together, these data indicate that exposure of Caenorhabditis elegans to TD leads to mitochondrial inhibition and hydrogen peroxide production.
Keywords: Glyphosate, C. elegans, mitochondrial inhibition, reactive oxygen species, hydrogen peroxide, herbicide
1.0 Introduction
The increasing adoption and planting of genetically modified, herbicide-resistant agricultural crops (e.g., corn, soy, and wheat) has been mirrored by an increase in the overall amount of glyphosate-containing herbicides applied in these occupational settings (Benbrook, 2012). While pesticides are used by many populations (landscape professionals, home owners, small-scale family gardeners), they are primarily applied by workers in the world’s agricultural industry (Grube et al., 2011). Furthermore, the leading class of pesticides consists of herbicide formulations that contain glyphosate (Grube et al., 2011).
Previous studies focused predominantly on the toxicity of the active ingredient, glyphosate, which is relatively non-toxic in both rats (oral LD50 = 2 g/kg) and mice (oral LD50 = 10 g/kg) (Tomlin, 2006). In light of this low toxicity, research has shifted from assessing glyphosate toxicity alone to determining the toxicity of formulations to which agricultural and industrial workers are actually exposed (de Liz Oliveira Cavalli et al., 2013; Mesnage et al., 2014; Peixoto, 2005). Studies with the agricultural formulation have demonstrated that it is much more toxic than either glyphosate alone or the putative relevant surfactants (Kim et al., 2013). This research, however, was typically performed in vitro or in isolated organelles rather than in vivo.
It is well-documented that occupational pesticide exposure is associated with an increased risk for some chronic neurodegenerative diseases (i.e., Parkinson’s disease (Allen and Levy, 2013); Alzheimer’s disease (Baltazar et al., 2014)). In light of reports of greater potency resulting from the readily available products, we sought to investigate whether mitochondrial inhibition or oxidative stress resulted from TD exposure, and could potentially explain the neurodegeneration we previously reported in C. elegans treated with TD (Negga et al., 2011; Negga et al., 2012). In those studies, worms treated chronically with concentrations of TD also used in this research showed neurodegeneration in both dopaminergic (DAergic) and GABAergic neurons. Degeneration of these neuron populations are involved in numerous chronic diseases (Coune et al., 2013; Kalia et al., 2013; Rousseaux et al., 2012; Schwab et al., 2013; Wang et al., 2010). Since the neurodegeneration is often attributable to mitochondrial inhibition and/or oxidative stress (Bhat et al., 2015; Kamat et al., 2016; Mostafalou and Abdollahi, 2013; Okazawa et al., 2014; Piccoli et al., 2008), we wanted to determine if TD exposure would result in modulation of these endpoints.
2.0 Materials and Methods
2.1 Worm and Escherichia coli strains
Wild-type (N2) and CL2166 worms, as well as NA22 Escherichia coli (E. coli) and OP50-1 E. coli were obtained from the Caenorhabditis Genetics Center (CGC). In CL2166 worms (dvIs19 [(pAF15)gst-4p::gfp::NLS] III), an oxidative stress-inducible green fluorescent protein gene (gfp) is fused with the glutathione-S-transferase 4 promoter (gst-4p) region (http://www.wormbase.org/db/get?name=cl2166;class=strain).
2.2 Synchronization
Protocols for synchronization, treatment, and endpoint assays used in our lab were previously published in detail (Bailey et al., 2016; Todt et al., 2016). Briefly, C. elegans were grown at 20°C on 8P plates (51.3 mM NaCl, 25.0 g bactoagar/L, 20.0 g bactopeptone/L, 1 mM CaCl2, 500 µM KH2PO4 (pH 6), 13 µM cholesterol (95% ethanol), and 1 mM MgSO4) with a lawn of NA22 E. coli (grown in 16 g tryptone/L, 10 g yeast extract/L, 85.5 mM NaCl) until gravid. Synchronization was accomplished by isolating eggs via hypochlorite treatment. Once eggs were removed, they were washed and incubated at 20°C in an M9 buffer (20 mM KH2PO4, 40 mM Na2HPO4, 68 mM NaCl) for 18 h.
2.3 Treatments
Worms were exposed to TD following established protocols (Negga et al., 2011) that have been extensively described (Bailey et al., 2016). Briefly, 5,000 worms/treatment group (n = 3 treatment groups/TD concentration/synchronization were exposed to 2.7%, 5.5%, or 9.8% glyphosate as TD (TouchDown® Hitech, formulation of 52.3% glyphosate, Syngenta AG, Wilmington, DE). These concentrations were used previously (Negga et al., 2011; Negga et al., 2012), and are within application limits recommended by herbicide manufacturers. In order to enable comparison of these results with similar herbicide studies, herbicide concentrations were normalized to percent active ingredient in the sample (glyphosate) rather than total percent of the parent pesticide formulation.
Following exposure to TD for 30 min, washed worms were poured onto nematode growth medium plates (NGM plates; 51.3 mM NaCl, 17.0 g bactoagar/L, 2.5 g bactopeptone/L, 1 mM CaCl2, 1 mM MgSO4, 500 µM KH2PO4 (pH 6.0), 12.9 mM cholesterol (95% ethanol), 1.25 mL nystatin/L, 200 mg streptomycin/L) with a lawn of OP50-1 E. coli for an additional 24 h at 20°C. Since concentrated TD is diluted with H2O in agricultural settings, H2O was used as the control treatment for each study reported here. Following the treatments, worms were assessed for either mitochondrial function or reactive oxygen species (ROS) production.
2.4 Mitochondrial Assays
2.4.1 Polarographic measurements
All endpoint assays have been previously described in detail (Todt et al., 2016). Briefly, all treatment groups were standardized to yield 10,000 live worms/mL/treatment concentration. Using an oxygen probe (YSI 5304), oxygen measurements were recorded every ten seconds for five minutes at 22°C (water bath chambers YSI 5301B Standard Bath).
2.4.2 Proton gradient integrity
For these studies, worm solutions were standardized to 1,000 worms/mL/treatment group. Worms from each treatment group were incubated with a final concentration of 50 µM tetramethylrhodamine ethyl ester (TMRE; Biotium, Hayward, CA) in dimethyl sulfoxide (DMSO; final concentration of 0.5% DMSO) for 1 h. Following extensive washing, images were taken with a digital camera attached to a fluorescence microscope.
2.4.3 Relative ATP amount
For these studies, worms were counted such that 250 live worms/treatment group were added to each well of a 96-well plate. ATP concentration was determined with the Promega Mitochondrial ToxGlo™ Assay (Promega Corporation, Madison, WI). Sodium azide was used as a negative control. Fluorogenic peptide substrate (obtained from the assay kit) was added to each well, and incubated at 20°C for 60 min to determine viability. Fluorescence was measured per the guidelines provided in the assay kit. Afterwards, worms were incubated for 15 min with the luciferin-based ATP probe. Luminescence was measured as previously reported (Todt et al., 2016).
2.5 Reactive oxygen species detection
2.5.1 Superoxide detection
Normalized worm solutions were prepared to yield 5,000 live worms/mL/treatment group. Worm were then incubated for three hours in 250 µM dihydroethidium (DHE; Merck KGaA, Darmstadt, Germany). During the incubation period, pictures were taken by placing three 50 µL samples per treatment group on a UV light box (UVP Benchtop 2UV Transilluminator, Upland, CA) at a wavelength of 302 nm.
2.5.2 Hydrogen peroxide detection
For these studies, a total of 200 worms/treatment group/well (in a 96-well plate) were treated with 50 µL of the Amplex® Red (Life Technologies, Grand Island, NY) as previously described (Todt et al., 2016). Negative and positive controls were generated per the protocol accompanying the reagent kit. Aluminum foil-covered plates were incubated for 1 h, at which time fluorescence was assessed as indicated in the kit instructions.
2.5.3 Hydroxyl radical detection
Treated worms were normalized to 1,000 live worms/mL/treatment group. The hydroxyphenyl fluorescein (HPF) probe (Life Sciences, Grand Island, NY) was added, and the solutions incubated for 1.5 h at 20°C per published protocols (Todt et al., 2016). Fluorescence associated with 250 live worms was then determined using a Promega® GloMax-Multi+ Detection System.
2.5.4 Glutathione-S-transferase (GST) up-regulation
Glutathione-S-transferase (GST) facilitates the conjugation of reduced glutathione to electrophilic xenobiotics. In C. elegans, up-regulation of GST has been used to assess oxidative stress. In order to determine if GST transcription and translation occurred post-TD treatment, CL2166 worms (dvIs19 [(pAF15)gst-4p::gfp::NLS] III) were photographed using a digital camera attached to a fluorescence microscope (see Fluorescence microscopy section).
2.6 Fluorescence microscopy
Photomicrographs were taken per published protocols (Negga et al., 2012; Todt et al., 2016). A Leitz & Wetzlar (Halco Instruments, Inc) microscope equipped with a 50-W AC mercury source lamp (E. Leitz, Rockleigh, NJ) and 40× objective (Leitz & Wetzlar, Halco Instruments, Inc.) was coupled to a digital camera (Micrometrics, MilesCo Scientific, Princeton, MN) operated by Micrometrics software (SE Premium, v2.7). Photomicrographs were analyzed to determine red (TMRE) or green (GST::GFP) pixel intensity.
2.7 Statistical analysis
All data were analyzed using GraphPad Prism (version 6.0 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com). Polarographic data are presented as mean ± standard error of the mean (SEM) and represent N ≥ 4 separate synchronizations, with at least 15,000 worms per intra-experimental replication (n ≥ 3). Differences in slope were determined using linear regression followed by one-way analysis of variance (ANOVA) of the regression lines. If the ANOVA resulted in an overall significant p value (p < 0.05), a post-hoc Tukey’s test was performed.
Data the ATP assays are shownnas mean ± SEM and represent N ≥ 3 separate synchronizations with at least 500 worms per intra-experimental replication (n ≥ 3). A one-way ANOVA, followed by a post-hoc Dunnett’s test, was used to determine statistically significant differences among groups. Red or green pixel intensity of individual worms (n > 5 for each independent replication) was measured TMRE and GST regulation assays. Data are represented as mean ± SEM and represent N ≥ 3 separate synchronizations, of at least three independent replicates (n ≥ 3). One-way ANOVA, followed by a post-hoc Dunnett’s test, was used to assess whether statistically significant differences among groups could be attributed to treatment.
3.0 Results
3.1 Overall mitochondrial inhibition
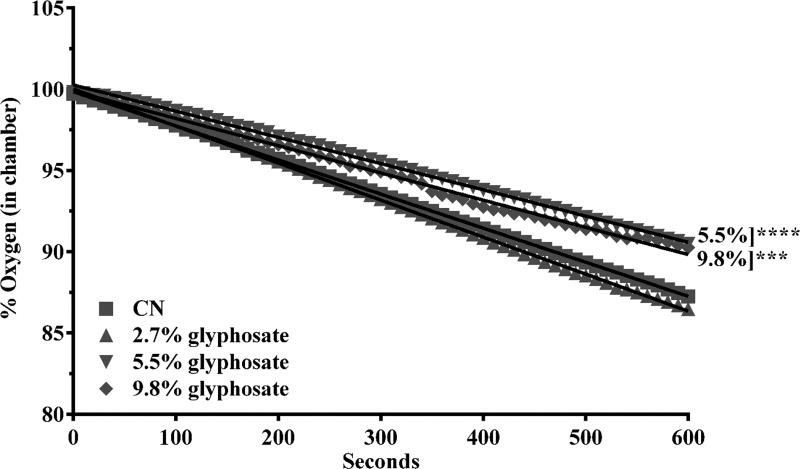
To determine whether glyphosate inhibited mitochondrial function following exposure to TD, oxygen consumption from whole worms was measured over the course of a five-minute assessment period. Oxygen consumption during this period indicated that chronic treatment with 5.5% or 9.8% glyphosate (as TD) resulted in statistically significant decreases in oxygen consumed per time (slope) compared to control (****p < 0.001 and ***p < 0.001, respectively) Figure 1.
Figure 1.
Decreased mitochondrial respiration following treatment with TD. Following chronic treatment with TD, worms in the mid- and high-concentration groups consistently showed a statistically significant decrease in oxygen consumption. Data are presented as mean percent oxygen consumed and represent N ≥ 4 separate synchronizations. ***p < 0.001, or ****p < 0.001 compared to controls.
3.2 Decreased proton gradient integrity
To further ascertain whether decreased oxygen consumption observed in Figure 1 impaired the ability of the mitochondria to establish a proton gradient, worms were incubated with TMRE after TD treatment. TMRE accumulates within active mitochondria if there is an intact proton gradient. Analysis of red pixels (TMRE fluorescence) was completed, the data indicated 29% fewer red pixels associated with worms in the 9.8% TD group. This difference was statistically significant when compared to control worms (***p <0.0001) Figure 2.
Figure 2.
Proton gradient integrity in TD-treated worms. Following treatment with TD, ANOVA indicated a statistically significant decrease in number of red pixels associated with worms in the highest treatment group. Although not statistically significant, a trend (p = 0.0625) towards a decrease in fluorescence was observed in the 2.7% group. Data are presented as mean pixel number ± SEM and represent N ≥ 3 separate synchronizations. ***p < 0.001 compared to control.
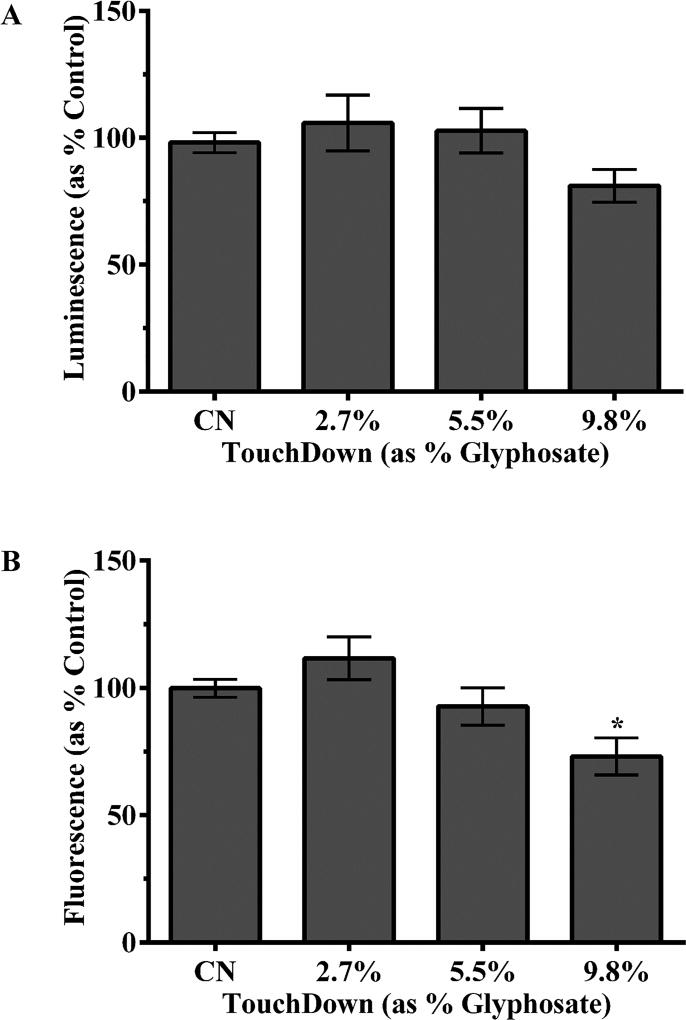
3.3 Decreased relative ATP levels
Relative ATP concentrations were also measured to see if mitochondrial inhibition resulted in less ATP. Following incubation with the luminescent probe (ATP detection), analysis indicated that TD-exposed worms had around 17% less ATP than control worms (Figure 3A), This, however, did not reach the level of statistical significance. Since lower relative concentrations of ATP could either result from an actual decrease in ATP levels, or a decrease in the number of live worms, we followed up the ATP assessment by determining activity of a necrotic protease. Results from this assay (Figure 3B) revealed a statistically significant decrease of 27% in relative fluorescence for worms in the 9.8% TD group compared controls (*p < 0.05). This further analysis suggested that decreased ATP in the highest treatment group was likely due to decreased worm viability.
Figure 3.
Relative ATP amount following TD treatment of worms. One-way ANOVA indicated no statistically significant decrease in luminescence (A), a measure of ATP, even though viability in the same group was statistically significantly lower (B). Data are presented as mean intensity ± SEM and represent N ≥ 3 separate synchronizations. *p < 0.05 compared to control worms.
3.4 Superoxide and hydrogen peroxide detection following treatment
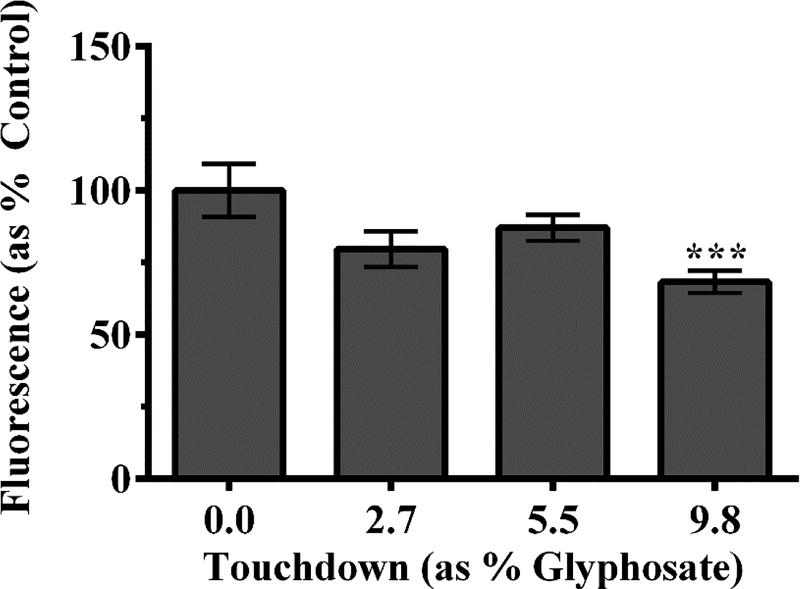
Since we observed mitochondrial inhibition, we next sought to identify the particular ROS produced in vivo. DHE is oxidized in the presence of , resulting in the formation of a red fluorescent product. Analysis of red pixel number indicated that there was no statistically significant difference between treatment groups compared to control (Figure 4A).
Figure 4.
Use of fluorescence to assess ROS production following TD treatment of worms. In order to assess whether TD exposure could lead to increased oxidative stress, worms were exposed to DHE, to assess superoxide production (A). No statistically significant changes in DHE fluorescence was observed in groups compared to control. Chronic treatment with 5.5% or 9.8% TD resulted in a statistically significant decrease in fluorescence of AmplexRed, a probe used to detect H2O2 (B). Data are presented as mean intensity ± SEM and represent N ≥ 3 separate synchronizations. ****p < 0.001 compared to control.
Since no significant production was detected, we next assessed the presence of hydrogen peroxide (H2O2). We chose to look at this ROS next because a one-electron transfer to could yield H2O2, perhaps explaining the lack of in the previous assay. Following exposure of worms to TD, Amplex®Red was added to assay tubes as described. Analysis indicated (Figure 4B) that chronic treatment with 5.5% or 9.8% TD resulted in a statistically significant decrease in H2O2 production compared to control worms (***p < 0.001).
3.5 Hydroxyl radical detection following treatment
In light of the high H2O2 amounts compared to controls, we wanted to determine if hydroxyl radicals (•OH) were also present in detectable levels. This was important because •OH can be produced from a one-electron transfer to H2O2, To this end, C. elegans were incubated with HPF, which oxidizes and fluoresces in the presence of •OH. Analysis indicated were no statistically significant differences among any of the groups (data not shown).
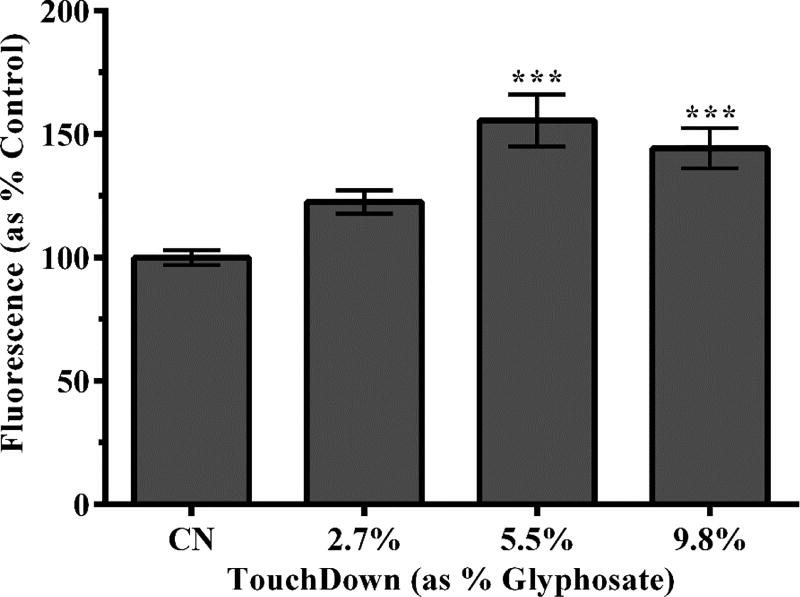
3.6 GST up-regulation
In light of decreased oxidative stress, as inferred by H2O2 assessment (Figure 5), we used a transgenic worm strain, CL2166 (gst-4p::gfp), which has a gfp construct attached to the promoter region of glutathione-S-transferase. When these worms experience increased oxidative stress, gst-4 transcription and translation is increased, and the worms demonstrate increased fluorescence. Following treatment with TD, green pixel analysis indicated that worms exposed to the two highest concentrations of the herbicide resulted in a statistically significant increase in the number of green pixels compared to controls (***p <0.001) (Figure 5).
Figure 5.
Increased GST-4::GFP fluorescence following treatment with TD. Transgenic worms treated with the mid- and high-level concentrations of TD showed statistically significant increases in green fluorescence compared to control worms. Data are presented as mean intensity ± SEM and represent N ≥ 4 separate synchronizations. ***p <0.001 compared to controls.
4.0 Discussion
Given that both mitochondrial dysfunction and reactive oxygen species (ROS) production are associated with numerous diseases (Chakraborty et al., 2013; Wang et al., 2013), we wanted to determine whether exposure to TD would also inhibit mitochondrial respiration. We also wanted to investigate the possibility that TD may initiate ROS production. Although mitochondrial respiration studies are often conducted in isolated mitochondrial fractions, we completed these in vivo using C. elegans to observe the effect TD had on intact mitochondria in a live organism. As C. elegans are transparent, we used fluorometric markers to facilitate visualization of mitochondrial inhibition and ROS levels in whole organisms. While it is the case that this worm is a much simpler model organism than even zebrafish (Danio rerio), these worms have many similarities to mammals (Avila et al., 2012; Calahorro and Ruiz-Rubio, 2011). For instance, worms have numerous gene homologs and orthologs associated with various human diseases (Mukherjee et al., 2017; Sutphin et al., 2017; Wang et al., 2017). Critically important to our studies, there is also a high degree of similarity of neurotransmitters, pre-synaptic neurotransmitter transporters, post-synaptic receptors, and catabolic and anabolic enzymes associated with the neurotransmitter neuronal populations in humans (Bargmann and Kaplan, 1998; Chase and Koelle, 2007; Jin, 2005). As such, worms are now widely-used in various toxicology and neurodegenerative studies (Wolozin et al., 2011). More importantly for our current work, they are also sensitive to TD neurotoxicity (Negga et al., 2011; Negga et al., 2012) at concentrations used in occupational, agricultural, and industrial settings (Syngenta, 2010).
Previous research regarding glyphosate alone or a commercially-available herbicide formulation showed that the formulation was significantly more toxic than glyphosate in various cell lines (Benachour and Seralini, 2009; Kim et al., 2013; Mesnage et al., 2014). Furthermore, the increased toxicity was still observed when isolated mitochondria were treated with either glyphosate or the herbicide (Olorunsogo et al., 1979; Peixoto, 2005). Based on these reports and our previous research showing neurodegeneration in C. elegans following treatment with a glyphosate formulation (Negga et al., 2011; Negga et al., 2012), we sought to test the hypothesis that mitochondrial inhibition and/or oxidative stress could be possible mechanisms of cell death following exposure to TouchDown (TD; Syngenta). The concentrations used in these studies were consistent with those used in our previous work (Negga et al., 2011), and are also within the range of those which occupational agricultural and pesticide workers would be routinely using. For example, glyphosate formulations sold in ready-to-use formulations are often around 2.7% glyphosate (Monsanto, 2014), which is the lowest concentration we used on C. elegans. On the other hand, concentrated formulations are manufactured with 37.0–53.2% glyphosate, with recommended application concentrations ranging from 0.4–2.2% for general field spraying, or from 20–30% for spot spraying (Syngenta, 2010). These latter concentrations are well-above those that induced various levels of neurodegeneration in our previous studies (Negga et al., 2011). Since the focus of our current work was to determine potential mechanisms responsible for the neurodegeneration, we used doses that were (1) within the levels used in agricultural settings, and (2) provided various degrees of neuronal damage in C. elegans.
The oxygen consumption studies (Figure 1) provided initial data that indicated worms treated with the two highest concentrations of TD did not respire at the same rate as control worms. While this did not provide specific information for proteins in the electron transport chain, it did suggest that TD could potentially inhibit mitochondria. Thus, our next step was to try to determine whether the observed inhibition also resulted in a compromised proton gradient or ATP production. Results from those studies indicated that decreased oxygen consumption was associated with both. TMRE data (Figure 2) confirmed a decrease in TMRE accumulation at the highest TD concentration, which could have caused the trend for decreased ATP production (Figure 3). On the other hand, we cannot completely rule out the possibility that the decreased ATP levels may be due to increased worm death (Figure 3B).
It is often the case that mitochondrial inhibition is accompanied by increased ROS production. For this reason, we also wanted to determine if we could detect changes in various ROS molecules. Initially we assayed for production, since it is one of the major ROS associated with mitochondrial inhibition (Turrens, 1997). Instead, we saw changes in H2O2 production (Figure 4B) that strongly correlated with up-regulation of GST-4 (Figure 5). Although gst-4 encodes putative glutathione-requiring prostaglandin D synthase (Sternberg et al., 2017), its regulation is tightly controlled by SKN-1 and its transcription and translation has been used as an indicator of oxidative stress in C. elegans (Detienne et al., 2016; Leiers et al., 2003; Zhang et al., 2014)
Several pesticides, as well as classic toxicants (e.g., 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)(Schober, 2004) used to model Parkinson’s disease, are known to inhibit mitochondrial function (Keeney et al., 2006; Testa et al., 2005) and/or increase oxidative stress (McCormack et al., 2005). Additionally, both of these endpoints are often observed in patients with Parkinson’s disease (Celardo et al., 2014; Hauser and Hastings, 2013) and other pathologies (Barnham et al., 2004; Lin and Beal, 2006). While genetic mutations are also linked to neurodegeneration (Bagyinszky et al., 2014; Zuo and Motherwell, 2013), there is considerable evidence that exposure to environmental toxicants also correlates with pathogenesis (Bouchard et al., 2010; Dick et al., 2007; Reitz et al., 2011). Due to the wide-spread agricultural and occupational use of glyphosate-containing herbicides, chronic exposure to a mitochondrial inhibitor could render a person more susceptible to neurodegeneration, although parkinsonism following a single exposure to a glyphosate-based herbicide has been reported (Barbosa and Leite, 2001).
Although worms are evolutionarily distant from humans, they provide valuable information that can inform future studies in higher organisms (Teschendorf and Link, 2009). Our current data suggest that chronic exposure to TD likely leads mitochondiral inhibition and increased ROS production. Furthermore, these results occur following exposure to the environmentally relevant formulation actually used in agricultural and occupational settings using concentrations well within those suggested by the manufacturer. Thus, we suggest that additional work is needed in higher model organisms using the pesticide formulations to better characterize potential risks of neurodegeneration.
Acknowledgments
Funding
This work was supported by the National Institute of Environmental Health Sciences [R15 ES015628-01A1 and ES015628-01A2 to V.A.F].
Worm strains and bacteria were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health Office of Research Infrastructure Programs [P40 OD010440].
Abbreviations
- ROS
Reactive oxygen species
- C. elegans
Caenorhabditis elegans
- E. coli
Escherichia coli
- CGC
Caenorhabditis Genetics Center
- GFP
Green fluorescent protein
- GST-4p
Glutathione-S-transferase 4 promoter
- NGM
Worm growth media
- TMRE
Tetramethylrhodamine ethyl ester
- DMSO
Dimethyl sulfoxide
- DHE
Dihydroethidium
- HPF
Hydroxyphenyl fluorescein
- ANOVA
Analysis of variance
- TD
TouchDown
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MT, Levy LS. Parkinson's Disease and Pesticide Exposure: A New Assessment. Crit Rev Toxicol. 2013;43:515–534. doi: 10.3109/10408444.2013.798719. [DOI] [PubMed] [Google Scholar]
- Avila D, Helmcke K, Aschner M. The Caenorhabiditis elegans Model as a Reliable Tool in Neurotoxicology. Hum Exp Toxicol. 2012;31:236–243. doi: 10.1177/0960327110392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagyinszky E, Youn YC, An SS, Kim S. The Genetics of Alzheimer's Disease. Clinical Intervent Aging. 2014;9:535–551. doi: 10.2147/CIA.S51571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DC, Todt CE, Orfield SE, Denney RD, Snapp IB, Negga R, Montgomery KM, Bailey AC, Pressley AS, Traynor WL, Fitsanakis VA. Caenorhabditis elegans Chronically Exposed to a Mn/Zn Ethylene-bis-Dithiocarbamate Fungicide Show Mitochondrial Complex I Inhibition and Increased Reactive Oxygen Species. Neurotoxicology. 2016;56:170–179. doi: 10.1016/j.neuro.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, Tsatsakis AM, Duarte JA, Carvalho F. Pesticides Exposure as Etiological Factors of Parkinson's Disease and Other Neurodegenerative Diseases: A Mechanistic Approach. Toxicol Lett. 2014;230:85–103. doi: 10.1016/j.toxlet.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Barbosa ER, Leite CC. Parkinsonism after Glycine-Derivate Exposure. Movement Disord. 2001;16:565–568. doi: 10.1002/mds.1105. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Kaplan JM. Signal Transduction in the Caenorhabditis elegans Nervous System. Annu Rev Neurosci. 1998;21:279–308. doi: 10.1146/annurev.neuro.21.1.279. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative Diseases and Oxidative Stress. Nature Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- B Benachour N, Seralini GE. Glyphosate Formulations Induce Apoptosis and Necrosis in Human Umbilical, Embryonic, and Placental Cells. Chem Res Toxicol. 2009;22:97–105. doi: 10.1021/tx800218n. [DOI] [PubMed] [Google Scholar]
- Benbrook CM. Impacts of Genetically Engineered Crops on Pesticide Use in the U.S.: The First Sixteen Years. Environ Sci Eur. 2012;24:1–13. [Google Scholar]
- Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA. Oxidative Stress, Mitochondrial Dysfunction and Neurodegenerative Diseases: A Mechanistic Insight. Biomed Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-Deficit/Hyperactivity Disorder and Urinary Metabolites of Organophosphate Pesticides. Pediatrics. 2010;125:e1270–1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calahorro F, Ruiz-Rubio M. Caenorhabditis elegans as an Experimental Tool for the Study of Complex Neurological Diseases: Parkinson's Disease, Alzheimer's Disease and Autism Spectrum Disorder. Invert Neurosci. 2011;11:73–83. doi: 10.1007/s10158-011-0126-1. [DOI] [PubMed] [Google Scholar]
- Celardo I, Martins LM, Gandhi S. Unravelling Mitochondrial Pathways to Parkinson's Disease. Brit J Pharmacol. 2014;171:1943–1957. doi: 10.1111/bph.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Bornhorst J, Nguyen TT, Aschner M. Oxidative Stress Mechanisms Underlying Parkinson's Disease-Associated Neurodegeneration in C. elegans. Internat J Molec Sci. 2013;14:23103–23128. doi: 10.3390/ijms141123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Koelle MR. Biogenic Amine Neurotransmitters in C. elegans. WormBook. 2007:1–15. doi: 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coune PG, Craveiro M, Gaugler MN, Mlynarik V, Schneider BL, Aebischer P, Gruetter R. An in vivo Ultrahigh Field 14.1 T (1)H-MRS Study on 6-OHDA and Alpha-Synuclein-Based Rat Models of Parkinson's Disease: GABA as an Early Disease Marker. NMR Biomed. 2013;26:43–50. doi: 10.1002/nbm.2817. [DOI] [PubMed] [Google Scholar]
- de Liz Oliveira Cavalli VL, Cattani D, Heinz Rieg CE, Pierozan P, Zanatta L, Benedetti Parisotto E, Wilhelm Filho D, Mena Barreto Silva FR, Pessoa-Pureur R, Zamoner A. Roundup Disrupts Male Reproductive Functions by Triggering Calcium-Mediated Cell Death in Rat Testis and Sertoli Cells. Free Radic Biol Med. 2013;65:335–346. doi: 10.1016/j.freeradbiomed.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Detienne G, Van de Walle P, De Haes W, Schoofs L, Temmerman L. Skn-1-Independent Transcriptional Activation of Glutathione-S-Transferase 4 (Gst-4) by Egf Signaling. Worm. 2016;5:e1230585. doi: 10.1080/21624054.2016.1230585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Counsell C, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Soderkvist P, Felice A. Environmental Risk Factors for Parkinson's Disease and Parkinsonism: The Geoparkinson Study. Occup Environ Med. 2007;64:666–672. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube A, Donaldson D, Kiely T, Wu L. Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates. In: United States Environmental Protection Agency, editor. Biological and Economic Analysis Division. Office of Pesticide Programs; 2011. [Google Scholar]
- Hauser DN, Hastings TG. Mitochondrial Dysfunction and Oxidative Stress in Parkinson's Disease and Monogenic Parkinsonism. Neurobiol Dis. 2013;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. Synaptogenesis. In: Jorgensen E, Kaplan J, editors. Wormbook. The C. elegans Research Community; 2005. [Google Scholar]
- Kalia LV, Brotchie JM, Fox SH. Novel Nondopaminergic Targets for Motor Features of Parkinson's Disease: Review of Recent Trials. Mov Disord. 2013;28:131–144. doi: 10.1002/mds.25273. [DOI] [PubMed] [Google Scholar]
- Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer's Disease: Understanding the Therapeutics Strategies. Molec Neurobiol. 2014;53:648–661. doi: 10.1007/s12035-014-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson's Disease Brain Mitochondrial Complex I Has Oxidatively Damaged Subunits and Is Functionally Impaired and Misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Hong JR, Gil HW, Song HY, Hong SY. Mixtures of Glyphosate and Surfactant TN20 Accelerate Cell Death Via Mitochondrial Damage-Induced Apoptosis and Necrosis. Toxicol In Vitro. 2013;27:191–197. doi: 10.1016/j.tiv.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Leiers B, Kampkotter A, Grevelding CG, Link CD, Johnson TE, Henkle-Duhrsen K. A Stress-Responsive Glutathione-S-Transferase Confers Resistance to Oxidative Stress in Caenorhabditis elegans. Free Radic Biol Med. 2003;34:1405–1415. doi: 10.1016/s0891-5849(03)00102-3. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of Oxidative Stress in Paraquat-Induced Dopaminergic Cell Degeneration. J Neurochem. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- Mesnage R, Defarge N, Spiroux de Vendomois J, Seralini GE. Major Pesticides Are More Toxic to Human Cells Than Their Declared Active Principles. BioMed Res Internat. 2014;2014:179691. doi: 10.1155/2014/179691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsanto. Roundup® Ready-to-Use Weed & Grass Killer III with One-Touch Wand 2014 [Google Scholar]
- Mostafalou S, Abdollahi M. Pesticides and Human Chronic Diseases: Evidences, Mechanisms, and Perspectives. Toxicol Appl Pharmacol. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Russell JC, Carr DT, Burgess JD, Allen M, Serie DJ, Boehme KL, Kauwe JS, Naj AC, Fardo DW, Dickson DW, Montine TJ, Ertekin-Taner N, Kaeberlein MR, Crane PK. Systems Biology Approach to Late-Onset Alzheimer's Disease Genome-Wide Association Study Identifies Novel Candidate Genes Validated Using Brain Expression Data and Caenorhabditis elegans Experiments. Alzheimers Dement. 2017;S1552–5260:30042–30050. doi: 10.1016/j.jalz.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negga R, Rudd DA, Davis NS, Justice AN, Hatfield HE, Valente AL, Fields AS, Fitsanakis VA. Exposure to Mn/Zn Ethylene-Bis-Dithiocarbamate and Glyphosate Pesticides Leads to Neurodegeneration in Caenorhabditis elegans. Neurotoxicology. 2011;32:331–341. doi: 10.1016/j.neuro.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negga R, Stuart JA, Machen ML, Salva J, Lizek AJ, Richardson SJ, Osborne AS, Mirallas O, McVey KA, Fitsanakis VA. Exposure to Glyphosate- and/or Mn/Zn-Ethylene-Bis-Dithiocarbamate-Containing Pesticides Leads to Degeneration of Gamma-Aminobutyric Acid and Dopamine Neurons in Caenorhabditis elegans. Neurotox Res. 2012;21:281–290. doi: 10.1007/s12640-011-9274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa H, Ikawa M, Tsujikawa T, Kiyono Y, Yoneda M. Brain Imaging for Oxidative Stress and Mitochondrial Dysfunction in Neurodegenerative Diseases. Q J Nucl Med Mol Imaging. 2014;58:387–397. [PubMed] [Google Scholar]
- Olorunsogo OO, Bababunmi EA, Bassir O. Effect of Glyphosate on Rat Liver Mitochondria in vivo. Bull Environ Contam Toxicol. 1979;22:357–364. doi: 10.1007/BF02026955. [DOI] [PubMed] [Google Scholar]
- Peixoto F. Comparative Effects of the Roundup and Glyphosate on Mitochondrial Oxidative Phosphorylation. Chemosphere. 2005;61:1115. doi: 10.1016/j.chemosphere.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Piccoli C, Sardanelli A, Scrima R, Ripoli M, Quarato G, D'Aprile A, Bellomo F, Scacco S, De Michele G, Filla A, Iuso A, Boffoli D, Capitanio N, Papa S. Mitochondrial Respiratory Dysfunction in Familiar Parkinsonism Associated with Pink1 Mutation. Neurochem Res. 2008;33:2565–2574. doi: 10.1007/s11064-008-9729-2. [DOI] [PubMed] [Google Scholar]
- Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer Disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux MW, Marcogliese PC, Qu D, Hewitt SJ, Seang S, Kim RH, Slack RS, Schlossmacher MG, Lagace DC, Mak TW, Park DS. Progressive Dopaminergic Cell Loss with Unilateral-to-Bilateral Progression in a Genetic Model of Parkinson Disease. Proc Natl Acad Sci U S A. 2012;109:15918–15923. doi: 10.1073/pnas.1205102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A. Classic Toxin-Induced Animal Models of Parkinson's Disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Schwab C, Yu S, Wong W, McGeer EG, McGeer PL. Gad65, Gad67, and GABAT Immunostaining in Human Brain and Apparent Gad65 Loss in Alzheimer's Disease. J Alzheimers Dis. 2013;33:1073–1088. doi: 10.3233/JAD-2012-121330. [DOI] [PubMed] [Google Scholar]
- Sternberg P, Kersey P, Berriman M, Stein L. Wormbase: Gst-4 2017 [Google Scholar]
- Sutphin GL, Backer G, Sheehan S, Bean S, Corban C, Liu T, Peters MJ, van Meurs JB, Murabito JM, Johnson AD, Korstanje R Cohorts for Heath and Aging Research in Genomic Epidemiology (CHARGE) Consortium Gene Expression Working, G. Caenorhabditis elegans Orthologs of Human Genes Differentially Expressed with Age Are Enriched for Determinants of Longevity. Aging Cell. 2017;16:672–682. doi: 10.1111/acel.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syngenta. Touchdown Hi Tech. In: Syngenta, editor. Product Label. Syngenta Crop Protection; Greensboro, NC: 2010. [Google Scholar]
- Teschendorf D, Link CD. What Have Worm Models Told Us About the Mechanisms of Neuronal Dysfunction in Human Neurodegenerative Diseases? Mol Neurodegener. 2009;4:38. doi: 10.1186/1750-1326-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Sherer TB, Greenamyre JT. Rotenone Induces Oxidative Stress and Dopaminergic Neuron Damage in Organotypic Substantia Nigra Cultures. Molec Brain Res. 2005;134:109. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Todt CE, Bailey DC, Pressley AS, Orfield SE, Denney RD, Snapp IB, Negga R, Bailey AC, Montgomery KM, Traynor WL, Fitsanakis VA. Acute Exposure to a Mn/Zn Ethylene-bis-Dithiocarbamate Fungicide Leads to Mitochondrial Dysfunction and Increased Reactive Oxygen Species Production in Caenorhabditis elegans. Neurotoxicology. 2016;57:112–120. doi: 10.1016/j.neuro.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin, C.D.S. The Pesticide Manual: A World Compendium. 14. British Crop Protection Council; Hampshire, UK: 2006. pp. 545–548. [Google Scholar]
- Turrens JF. Superoxide Production by the Mitochondrial Respiratory Chain. Bioscience Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer's Disease. Biochim Biophys Acta. 2013;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang QJ, Liu J, Ali U, Gui ZH, Hui YP, Chen L, Wang T. Changes in Firing Rate and Pattern of GABAergic Neurons in Subregions of the Substantia Nigra Pars Reticulata in Rat Models of Parkinson's Disease. Brain Res. 2010;1324:54–63. doi: 10.1016/j.brainres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Wang YA, Kammenga JE, Harvey SC. Genetic Variation in Neurodegenerative Diseases and Its Accessibility in the Model Organism Caenorhabditis elegans. Hum Genomics. 2017;11:12. doi: 10.1186/s40246-017-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Gabel C, Ferree A, Guillily M, Ebata A. Watching Worms Whither: Modeling Neurodegeneration in C. elegans. Prog Mol Biol Transl Sci. 2011;100:499–514. doi: 10.1016/B978-0-12-384878-9.00015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Li Y, He Q, Qin J, Yu Y, Li X, Zhang L, Yao M, Liu J, Chen Z. Microfluidic Platform Integrated with Worm-Counting Setup for Assessing Manganese Toxicity. Biomicrofluidics. 2014;8:054110. doi: 10.1063/1.4896663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Motherwell MS. The Impact of Reactive Oxygen Species and Genetic Mitochondrial Mutations in Parkinson's Disease. Gene. 2013;532:18–23. doi: 10.1016/j.gene.2013.07.085. [DOI] [PubMed] [Google Scholar]