1. Introduction
Knowledge of the pathogenesis of pre-clinical Alzheimer’s disease (AD) has grown enormously. Several National Institute on Aging and the Alzheimer’s Association (NIA-AA) joint working groups have developed guidelines for the stages of preclinical Alzheimer’s disease and revised criteria for diagnoses [1–3]. The pre-clinical period begins years before onset of clinical disease [4, 5]. The diagnosis of persons with preclinical disease is potentially important because persons may be more likely to benefit from disease modifying treatments if interventions occur before the occurrence of significant brain damage [6]. We use the terms primary prevention to refer to interventions designed to be implemented before the occurrence of brain pathology, and secondary preventions to refer to interventions designed to slow progression to clinical disease (e.g., mild cognitive impairment or Alzheimer disease) among persons who already have some brain pathology [7]. A recent consensus report that reviewed the current state of evidence on interventions to prevent cognitive decline and onset of dementia concluded that while at present no specific prevention interventions are strongly supported by the available scientific evidence, cognitive training, blood pressure management and increased physical activity may provide some prevention benefit [8]. Recently a number of promising drugs failed to show clinical benefit in double blind placebo controlled trials in persons with mild to moderate dementia due to AD, and one hypothesis for those disappointing findings is that the drugs were administered too late in the disease course [9]. The development of prevention interventions is a rapidly evolving field especially with increased understanding of biomarkers and the preclinical course of Alzheimer’s disease.
Forecasts of pre-clinical and clinical disease stages are important from a number of perspectives. First, the resources needed to care for patients vary considerably by clinical stage. Second, prevalence estimates by disease stage are important for planning as they provide information about the numbers of persons who could benefit from potential primary and secondary preventions.
Two approaches have been described for estimating national AD prevalence [10]. The first approach is based on probability based nationally representative prevalence surveys such as the Aging, Demographics and Memory Study (ADAMS) [11]. The second approach, called forward calculation, uses AD incidence rates from epidemiological cohort (longitudinal) studies, mortality rates, and population projections in a multistate model to forecast AD prevalence and incidence numbers [12–14]. An advantage of the forward calculation method is that it can be used to evaluate the potential impact of preventive and therapeutic advances that delay progression of disease. Here, we generalize the forward calculation method to incorporate preclinical disease and mild cognitive impairment states into a multistate model. We use the model to forecast U.S. prevalence of preclinical and clinical disease and to evaluate the potential impact of primary and secondary preventions on those forecasts.
2. Methods
2.1 Multistate Model
A National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroup proposed a framework for the preclinical stages of AD [1]. The framework posits that the Alzheimer’s disease process typically begins with asymptomatic amyloidosis which refers to Amyloid β (Aβ) deposition which can be detected by specific biomarkers for Aβ accumulation such as positron emission tomography (PET) amyloid imaging or low Aβ 42 in the cerebrospinal fluid (CSF). The framework postulates that sometime after the onset of amyloidosis, the disease process advances to neurodegeneration which can be detected by biomarkers including elevated CSF tau, neuronal dysfunction based on fluorodeoxyglucose (FDG) PET, or hippocampal atrophy/cortical thinning on volumetric magnetic resonance imaging (MRI). Neurodegeneration is followed by subtle cognitive decline, onset of mild cognitive impairment due to AD [2], and ultimately clinical AD [3].
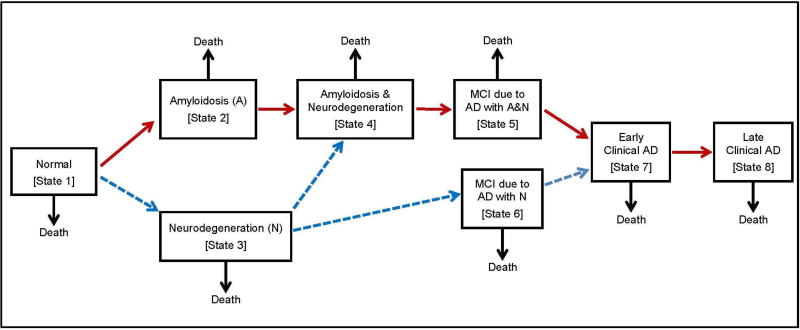
We use a multi-state model largely based on the NIA-AA framework for the preclinical stages of AD [1]. Our model includes 9 states: 8 preclinical or clinical disease states plus the death state (Figure 1). Persons can die in any state. The model allows several pathways to AD. One pathway (red pathway in Figure 1) assumes persons progress sequentially through the following: normal (state 1), preclinical amyloidosis (state 2), amyloidosis with neurodegeneration (state 4), mild cognitive impairment due to AD with both amyloidosis and neurodegeneration (state 5), early clinical AD (state 7) and late (or advanced) clinical AD (state 8). Persons in states 7 and 8 have reached the threshold for a clinical diagnosis of AD, that is, dementia due to AD. While the red pathway is the primary pathway posited by the NIA-AA working group and most consistent with the amyloid hypothesis of AD [15], evidence supporting the occurrence of Alzheimer’s disease in the absence of amyloidosis have also been described [16]. While such pathways have been termed suspected non-Alzheimer’s disease pathophysiology (SNAP), there is controversy as to whether such pathways should or should not be considered part of the AD pathological processes [17, 18]. Here we allow for such alternative pathways including occurrence of neurodegeneration before amyloidosis, and occurrence of MCI due to AD in the presence of neurodegeneration but not amyloidosis (blue pathways in Figure 1).
Figure 1.
Multistate model of the progression of Alzheimer’s disease through preclinical and clinical disease states.
The model in Figure 1 differs from the NIA framework in that we do not include a stage of amyloidosis and neurodegeneration with subtle cognitive decline (called stage 3 in [1]) because we do not believe there is adequate data at this time to provide reliable estimates of transition rates to and from that stage. Instead, this stage is subsumed into state 4 in Figure 1. The model in Figure 1 differs from the model of Jack and colleagues [19] in that we include mild cognitive impairment states due to AD; specifically, an MCI state with both amyloidosis and neurodegeneration (state 5) and an MCI state with only neurodegeneration (state 6). We refer to the states before the onset of MCI (states 1–4) as preclinical although we recognize that subtle cognitive impairment may be present in some of these states.
We use a Markov model that allows the transition rates from one state to another state to depend on person’s current age, and calendar year. The dependence on calendar year is to allow for the introduction of prevention interventions. The Markov model does not allow the transition rate to depend on the duration of time the person has already spent in a state, but rates are allowed to depend on chronological age. The model is implemented as a discrete –time model in which transitions occur at the end of calendar years. Discrete time models rather than continuous time models are adequate approximations to Alzheimer’s pathogenesis because of difficulties in establishing exact ages of state transitions and AD diagnoses.
Here we provide an overview of the model inputs and methods. The Supplementary Material gives the technical details including the transition rates used in the model and their sources, definitions of biomarker defined states, sensitivity analysis methods, and the underlying forecasting equations.
2.2 Transition Rates
Preclinical and Clinical Transition Rates
The Mayo Clinic Study of Aging is a longitudinal population-based cohort study of cognitive aging in Olmstead County, Minnesota [20]. Using data from 1541 participants from the Mayo Clinic Study of Aging, Jack and colleagues provided a thorough analysis of the preclinical transition rates into states 2, 3 and 4 in Figure 1 [19]. We used the study by Vos and colleagues [21] to estimate transition rates from MCI to AD (i.e., transitions from states 5 to 7, and from states 6 to 7). The Vos study recruited subjects from 13 cohorts in Europe and the United States and is the largest published study of progression (i.e., transition) rates from MCI to clinical AD that measured neurodegeneration and amyloidosis at baseline with at least 3 years of follow-up that performed time to event (survival) analyses. The study included follow-up of 353 persons in the MCI state 5 and 222 persons in the MCI state 6.
The Mayo Clinic Study of Aging did not separate out MCI in their analyses of transition rates in [19] but did report transition rates from the combined states 4 and 5 to AD (state 7), and transition rates from the combined states 3 and 6 to AD (state 7). We used those estimates in combination with the progression rates from MCI to AD from the Vos study [21] to determine transition rates into the MCI states using statistical analyses described in Supplementary Material.
The clinical course of Alzheimer’s disease is generally progressive. Over the course of illness, the level of care required for a patient can range from adult day care in the early stage of the clinical illness to intensive nursing home care in late stage of illness. We divided the clinical period of disease into two states: the early clinical and late clinical stages of AD (states 7 and 8 in Figure 1). We used an annual transition rate of 1/6= 0.167 years of progressing from early to late clinical AD based on studies that suggested AD patients require an intensive level of care similar to that of a nursing home after an average of approximately 6 years from clinical diagnosis [22, 23]. While the clinical progression rates from early clinical AD (state 7) to late clinical AD (state 8) may depend on age and gender, we do not believe at this point in time there is adequate clinical data to more precisely characterize rates of progression for our modeling, however we do report a sensitivity analysis to this parameter.
Death rates
Mortality rates among persons with Alzheimer’s disease are higher than that among the general population. Previous analyses indicated that excess mortality associated with Alzheimer’s disease can be described by an additive model whereby death rates for patients with late stage clinical AD (state 8) are the background age-gender specific mortality rates plus an additional excess mortality of 7.8% per year but there is no excess in mortality rates during early stage clinical disease (state 7) [24]. The mortality rates predicted by this model are in good agreement with empirical studies [25–26]. We used that model in conjunction with United States background death rates by age, gender, and calendar year [26]. We also report a sensitivity analysis to evaluate the impact of excess mortality over background rates in the MCI states and early clinical AD state (i.e., states 5, 6 and 7) as well as the late clinical AD state (state 8).
2.3 Modeling Prevention Interventions
We considered potential impacts of disease modifying prevention interventions on prevalence of preclinical and clinical AD. Transition rates prior to the introduction of the intervention which are called the baseline rates rij (a), refer to the annual rate of transitioning from state i at age a to state j (see Supplementary Material). After introduction of the intervention, the model assumes that the baseline rates are multiplied by proportionality constants (i.e., the relative risks): specifically, transition rates become θijrij (a). The proportionality constants (or the relative risks) θij characterize the effectiveness of the interventions and specify which transition rates are altered by the interventions. We considered the three scenarios described below.
First, we considered a primary prevention (I) that lowered risks of amyloidosis. We modeled the effects of such an intervention by choosing values for θ12 (transition from state 1 to state 2) and θ34 (transition from state 3 to state 4) that are less than 1 (namely, θ12 = θ34 = 0.25,0.50,0.75,0.90) and setting other values for θij equal to 1. For example, θ12 = θ34 =0.25 corresponds to a primary prevention that reduces the annual risk of transitioning to amyloidosis from either state 1 or state 3 by 75% (i.e., risk with intervention for transitioning to amyloidosis from state 1 or state 3, relative to no intervention is 0.25).
Second, we considered a secondary prevention (II) that delays progression to MCI from a preclinical state of neurodegeneration without or with amyloidosis (state 3 or state 4). We modeled the effects of such an intervention by choosing values for the parameters θ45 and θ36 that are less than 1 and setting other values for θij equal to 1 (namely, θ45 = θ36 = 0.25,0.50,0.75,0.90).
Third, we considered a secondary prevention (III) that decreases the progression from MCI to clinical AD. We modeled effects of such an intervention by choosing values for parameters θ57 and θ67 that are less than 1 and setting other values for θij equal to 1 (namely, θ57 = θ67 = 0.50,0.75,0.90,0.95). For this scenario, we considered a modest relative risk (0.95) because the intervention is acting late in the disease course.
2.4 Forecasting Prevalence of Preclinical and Clinical Disease
We used the multistate model to forecast the age and gender specific prevalence rates by disease state through calendar year 2060. Persons are assumed in the healthy state before age 30. We calculated prevalence rates by matrix multiplication of the one step transition matrices (see Supplementary Material). We forecast numbers of individuals living in each disease state by multiplying age and gender specific prevalence rates by U.S Census population projections [28]. Our calculations also stratified on gender because U.S. death rates and census population projections depend on gender.
2.5 Sensitivity Analyses and Corroboration
To address uncertainties in transition rates, we performed sensitivity analyses. We calculated ranges for prevalences by using a high and low series of transition rates. The high and low series of transition rates were based on 95% confidence intervals for each transition rate..
To provide some independent corroboration for our model, we compared age specific AD incidence rates derived from the multistate model to age specific AD incidence rates from a worldwide systematic review of cohort studies [14]. The systematic review of AD incidence rates was based only on direct empirical observations of ages of AD diagnoses among members of 27 cohorts from around the world and were not based on any biomarker assessments or multistate modeling.
3. Results
Table 1 shows prevalence estimates of pre-clinical and clinical AD in the United States. In 2017 there were 3.65 million cases of clinical AD in the United States (range, 1.70–7.62 million). We estimate that approximately 1.54 million (42%) of the 3.65 million cases living today have late stage clinical Alzheimer’s disease who need level of care equivalent to nursing homes. We predict by 2060, U.S prevalence of clinical AD will grow to 9.30 million (range, 4.60–17.82 million). We estimate in 2017 there were 2.43 million Americans afflicted with mild cognitive impairment due to AD (range, 1.41–4.02 million), which will grow to 5.70 million by 2060 (range, 3.61–8.34 million). Approximately 73% of those MCI cases in 2017 have both neurodegeneration and amyloidosis while the remaining 27% have only neurodegeneration. In 2017, 6.08 million Americans were in one of the clinical disease states (MCI due to AD, early clinical AD or late clinical AD) (range, 3.11–11.64 million) and that will grow to 15.0 million by 2060. In 2017, 46.7 million Americans were in one of the preclinical Alzheimer’s disease states (range, 36.23–57.79 million): 22.14 million persons had amyloidosis, 8.33 million persons had only neurodegeneration, and 16.23 million persons had both amyloidosis and neurodegeneration. The number of persons with preclinical AD will increase to 75.68 million by 2060. We estimate the annual incidence of new cases of clinical AD in 2017 was 540,000 (range, 280,000–973,000) of whom approximately 89% arose from a state of MCI with both amyloidosis and neurodegeneration (range, 82%–93%) while 11% arose from an MCI state with only neurodegeneration.
Table 1.
Prevalence (in millions) of pre-clinical and clinical disease states of Alzheimer’s disease in the United States in 2017 and 2060 based on multistate model [ranges generated by high and low series of transition rates]
| CALENDAR YEAR | ||
|---|---|---|
| DISEASE STATE | 2017 | 2060 |
| Amyloidosis only [state 2] | 22.14 [18.70–26.70] | 31.90 [28.04–36.75] |
| Neurodegeneration only [state 3] | 8.33 [5.68–9.16]- | 13.60 [8.47–16.11]- |
| Amyloidosis & Neurodegeneration [state 4] | 16.23[11.85–21.93] | 30.18 [23.49–37.78] |
| MCI due to Alzheimer’s disease [states 5+6] | 2.43 [1.41–4.02] | 5.70 [3.61–8.34] |
| With neurodegeneration | 0.66 [0.28–1.51] | 1.23 [0.56–2.49] |
| With Amyloidosis and neurodegeneration | 1.77[1.13–2.51] | 4.47 [3.05–5.85] |
| Clinical Alzheimer’s Disease [states 7+8] | 3.65[1.70–7.62] | 9.30 [4.58–17.82] |
| Early Stage | 2.11 [1.03–4.12] | 5.29 [2.73–9.40] |
| Late Stage | 1.54 [0.67–3.50] | 4.01 [1.85–8.42] |
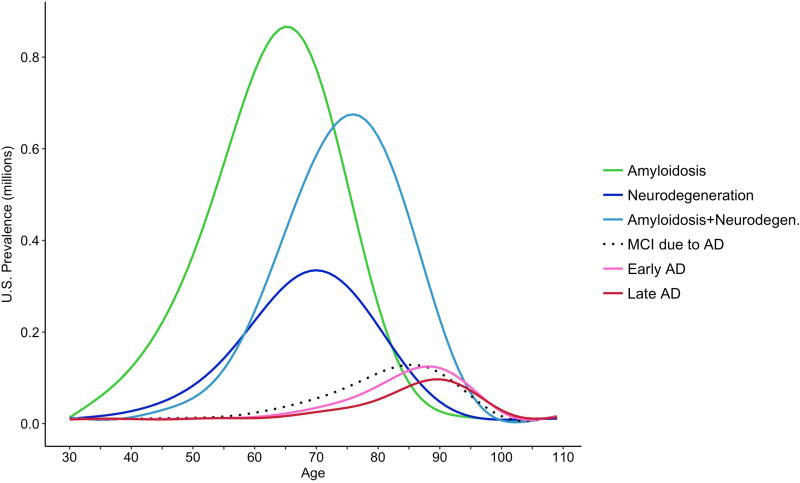
Figure 2 shows the prevalence (in millions) in 2017 by single year of chronological age for each preclinical and clinical disease state. More than half of those persons currently living with only amyloidosis are under age 70. The modes of the prevalence curves in Figure 2 increase in the following order: amyloidosis only, neurodegeneration only, amyloidosis and neurodegeneration, mild cognitive impairment due to AD (states 5 and 6 combined), early stage clinical AD, and late stage clinical AD. Figure 2 illustrates that the vast majority of persons who are living with some AD pathophysiology (amyloidosis or neurodegeneration or both) are in one of the preclinical states (states 2–4 in Figure 1), while only a small percentage (6.9 %) are in one of the clinical state (states 5–8).
Figure 2.
Prevalence (in millions) of Alzheimer’s disease preclinical and clinical disease states in the United States in 2017 by single year of age. Note: The prevalence of MCI (due to AD) includes persons with MCI who have both amyloidosis and neurodegeneration, and also persons with MCI who have only neurodegeneration.
Table 2 shows impacts of potential primary and secondary prevention interventions on the prevalence of MCI and clinical AD by 2060 (ranges based on the upper and lower series of transition rates are presented in the Supplementary Material). A primary prevention that reduces annual risks of onset of amyloidosis by 50% would in 2060 decrease the prevalence of MCI by 0.69 million (from 5.70 to 5.01) and the prevalence of AD by 2.35 million persons (from 9.3 to 6.95). A secondary prevention aimed at reducing annual risk of progression to MCI by 50% would in 2060 decrease the prevalence of MCI by 2.14 million (from 5.70 to 3.56) and the prevalence of AD by 3.84 million persons (from 9.3 to 5.46). A secondary prevention aimed at reducing the annual risk of progression from MCI to AD by 50% would in 2060 actually increase the prevalence of MCI by 2.85 million persons (from 5.70 to 8.55) but would decrease the prevalence of AD by 2.54 million (from 9.30 to 6.76).
Table 2.
Prevalence (in millions) of Alzheimer’s clinical disease states in the United States in 2060 with various primary and secondary prevention interventions. Interventions are assumed to begin in 2017.
| MCI due to AD | Clinical AD | |||
|---|---|---|---|---|
| Relative Risks (θ) | [States 5+6] | Early stage [state 7] |
Late stage [state 8] |
Total [states 7+8] |
| No Intervention | 5.70 | 5.29 | 4.01 | 9.30 |
| Primary Prevention: Delay Amyloidosis onset | ||||
| θ12 = θ34 | ||||
| 0.25 | 4.34 | 2.92 | 2.22 | 5.14 |
| 0.50 | 5.01 | 3.98 | 2.97 | 6.95 |
| 0.75 | 5.43 | 4.74 | 3.56 | 8.30 |
| 0.90 | 5.60 | 5.09 | 3.84 | 8.93 |
| Secondary Prevention: Delay progression to MCI | ||||
| θ45 = θ36 | ||||
| 0.25 | 2.04 | 1.78 | 1.21 | 3.00 |
| 0.50 | 3.56 | 3.20 | 2.26 | 5.46 |
| 0.75 | 4.74 | 4.34 | 3.19 | 7.53 |
| 0.90 | 5.34 | 4.93 | 3.70 | 8.63 |
| Secondary Prevention Delay progression from MCI to AD | ||||
| θ57 = θ67 | ||||
| 0.50 | 8.55 | 3.91 | 2.85 | 6.76 |
| 0.75 | 6.85 | 4.73 | 3.54 | 8.27 |
| 0.90 | 6.11 | 5.09 | 3.84 | 8.93 |
| 0.95 | 5.91 | 5.19 | 3.93 | 9.12 |
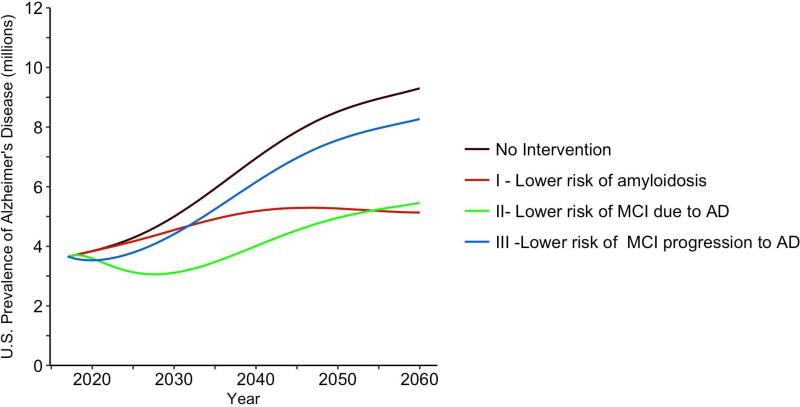
Figure 3 illustrates the impact of three prevention interventions in Table 2 on Alzheimer’s disease prevalence for the years 2017–2060: (I) highly effective primary prevention (red curve) that lowers annual risks of onset of amyloidosis by 75% (i.e., θ12=θ34=0.25); (II) moderately effective secondary prevention (green curve) that lowers annual risks of progression to MCI by 50% (i.e., θ45=θ36=0.50) ; (III) modestly effective secondary prevention (blue curve) that lowers annual risks of progression from MCI to Alzheimer’s disease by 25% (i.e., θ57=θ67=0.75). Figure 3 also illustrates no intervention (black curve). We find that the highly effective primary prevention strategy (I) resulted in the lowest AD prevalence by the year 2060. However, that primary prevention (I) was associated with the largest AD prevalence in the 15 years immediately following its introduction compared to interventions II or III. The explanation for this finding is that the full benefits of delaying amyloidosis in terms of reduced AD prevalence is not realized for many years because of the long lag time between amyloidosis and clinical AD. A take home message of Figure 3 is that the full impact on disease burden of primary prevention that targets the early stages of the pathogenesis of AD on clinical disease burden may not be realized for decades.
Figure 3.
Forecasts of the numbers of persons (in millions) living with Alzheimer’s disease (early or late stage clinical disease) in the United States from 2017–2060 for three prevention intervention scenarios. Note: Primary prevention scenario I reduces the transition rates to amyloidosis by 75% (θ12=θ34=0.25); Secondary prevention scenario II reduces the transition rates to MCI by 50% (θ45=θ36=0.50); Secondary prevention scenario III reduces the transition rates from MCI to Alzheimer’s disease by 25% (θ57=θ67=0.75).
The moderately effective secondary prevention in Figure 3 (green curve, II) resulted in the greatest reduction in AD prevalence for most of the years illustrated in the figure but was ultimately surpassed by the primary prevention (red curve, I) beginning in 2054. The modestly effective secondary prevention (blue curve, III) resulted in the greatest AD prevalence by year 2060 compared to interventions I and II. Intervention III yielded a slightly lower AD prevalence for 3 years immediately following its introduction compared to interventions I and II. The explanation for that finding is that MCI is proximate to clinical AD diagnosis, and thus the impact of delaying progression of MCI will be seen relatively quickly on AD prevalence compared to interventions that delay onset of amyloidosis or MCI.
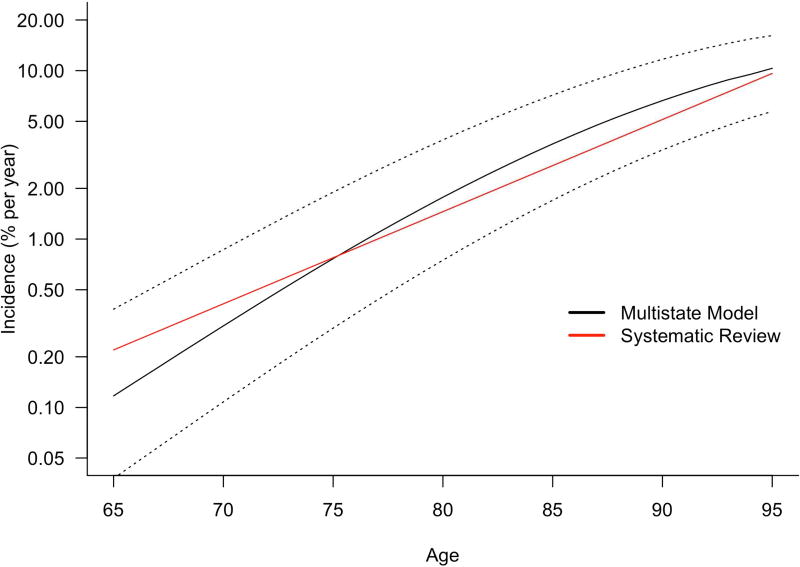
We sought to determine if AD incidence rates derived from the multistate model are consistent with worldwide literature. A systematic review of the worldwide literature of AD incidence found that annual age specific incidence rates grow exponentially and is given by 0.117e.127(a−60) % per year at age a (for ages ≥ 60) [12]. The systematic review was based only on direct observations of ages of diagnoses of AD in cohorts and not on any biomarker assessments or multistate modeling. Figure 4 compares age specific incidence rates of AD from the systematic review (red curve) with that from the multistate model (black curve). Annual age specific incidence rates (in % per year) from the multistate model at ages 70, 75, 80, 85, 90 and 95 were 0.31, 0.77, 1.77, 3.54, 6.65 and 10.33 respectively. Annual incidence rates (in % per year) from the systematic review at the same ages were 0.42, 0.79, 1.48, 2.80, 5.28, and 9.96 respectively. The incidence rates produced by the multistate model are in good agreement with that from the systematic review. Figure 4 also shows AD incidence rates produced by the multistate model based on low and high series of transition rates used in the sensitivity analyses (black dotted lines). Estimates from the systematic review are well within the bounds produced by the high and low series of transition rates. We recognize that these comparisons should not be construed to validate each individual transition rate used in the model; nevertheless they provide some corroborating evidence that transition rated used in the multistate model produce AD incidence rates consistent with worldwide literature.
Figure 4.
Comparison of age-specific Alzheimer’s disease incidence rates expressed as percent per year from a worldwide systematic review [12] (red curve) and from the multistate model (black curve) on a log scale. Also shown are the AD incidence rates from the multistate model based on the series of high and low transition rates that were used in the sensitivity analysis (black dotted curves).
We also performed a sensitivity analysis to the progression rate from early clinical AD (state 7) to late clinical AD (state 8). We find that if the mean durations of early clinical AD were 4, 6 and 8 years, then the percentage of prevalent clinical AD cases in 2017 (states 7 or 8) with late clinical AD (state 8) are 53%, 42% and 35%, respectively; however, the total prevalence of clinical AD (states 7 plus 8 combined) changes by no more than 5%. The prevalence estimates of the preclinical and MCI states (i.e., states 1 through 6) are unaffected by changing the progression rate from state 7 to state 8. In addition, we performed a sensitivity analysis to evaluate the impact of excess mortality over the background rates in the MCI states (states 5 and 6) and the early clinical AD state (state 7). To obtain mortality rates in states 5, 6 and 7, we added half of the excess over background mortality that was used in state 8 (that is, we added 7.8%/2= 3.9% per year to the background mortality). We found with that adjustment that the estimated 2017 prevalence of the clinical states (MCI due to AD, early clinical AD and late clinical AD; i.e., states 5–8 combined) is decreased by approximately 11%.
4. Discussion
We find that the majority of persons currently living with AD brain pathology (i.e., amyloidosis or neurodegeneration or both) are preclinical. Many of these persons may never progress to either MCI or AD during their lifetimes because of the increasing risks of death with age and long pre-clinical periods.
The prevention intervention scenarios we considered in Table 2 were disease modifying interventions and by that we mean they delayed the onset of clinical AD. For example, the primary prevention we considered reduced the transition rates to amyloidosis but did not alter any of the other transition rates. However, suppose an intervention simply masked or removed amyloid without changing the underlying disease process. In that case, θ12 and θ34 would be less than 1 but conceivably θ13, θ36 and θ67 would be greater than 1. We could find that there would be a decrease in numbers of persons following the red pathway to AD in Figure 1, a corresponding increase in numbers of persons following the lower blue pathway to AD in Figure 1 (SNAP), and no net delay in ages of onset of clinical AD. Table 2 considered the potential public health impact under hypothetical intervention scenarios. As the field of AD prevention develops and new candidate prevention interventions become available, the multistate modeling framework can be utilized to evaluate their potential public health impact.
The main sources of uncertainty in our results are transition rates. We based transition rates on two of the largest studies of their kind published to date. The ranges we cited on prevalence numbers were based on sensitivity analyses using confidence limits of transition rates. Nevertheless, there are other systematic sources of potential bias in our results. We recognize that the participants in the studies may not be representative of the underlying populations. However, the Mayo Clinic Study of Aging did not report any significant difference between participants who were imaged and those who were not or with regard to demographic characteristics or dementia rates [19]. The transition rates based on the Mayo Clinic Study of Aging [19] did not distinguish Alzheimer’s dementia from other dementia types, although the investigators of that study noted most of their cases of incident dementia were AD. If the transition rates in [19] were based solely on incident AD, presumably the transition rates would decrease which would decrease our AD prevalence estimates.
An additional complication is the impact of vascular pathology on our results. For example, consider the possibility that vascular pathology in the presence of AD pathology accelerates progression to clinical AD. If the study populations in references [19] and [21] adequately represented the prevalence of such mixed pathologies, then the transition rates we used would account for that possible complication. However, for populations with higher prevalence of vascular pathology than those in the study populations in references [19] and [21], it is possible the transition rates could be higher than those used here. Furthermore, vascular pathology could affect our results on the impact of interventions. For example, interventions that are designed to target both AD and vascular pathology could have a synergistic effect on decreasing transition rates to clinical AD, and as such, the efficacy of such interventions would depend on the prevalence of mixed pathologies in populations.
We also recognize that preclinical transition rates depend on the specific biomarkers and cut-points used to define preclinical states, although one study suggested that different definitions of the states of amyloidosis or neurodegeneration (cut points and biomarkers) yield similar findings [29]. In spite of these concerns about systematic biases in the transition rates used in the multistate model, we find that AD incidence rates produced by the multistate model were consistent with a worldwide systematic review of clinical AD incidence. Our prevalence estimates also rely on the accuracy of U.S Census population projections of the aging of the U.S. population.
Ongoing studies will provide improved and more detailed estimates of the transition rates allowing future refinements to the multistate model. For example, multistate models that subdivide states by quantitative levels of biomarkers (e.g. high, medium, low, and negative) rather than dichotomize (e.g., biomarker positive vs. negative) may prove useful. Future studies of transition rates from ethnically diverse populations are important.
Primary preventions offer the greatest potential for reducing AD prevalence. However, the benefits of primary prevention for reducing disease burden would not be fully realized for decades because of the long preclinical period. Because large numbers of persons are currently living with preclinical disease, our results highlight the public health importance of the development of secondary interventions targeted at persons most likely to progress to clinical disease during their lifetimes, the need for improved diagnostics for identifying such persons, as well as development of primary interventions for persons who do not yet have any AD brain pathology.
Supplementary Material
HIGHLIGHTS.
Forecasted preclinical and clinical AD in the U.S. using multistate model
Most persons living with AD brain pathology do not have clinical disease
15 million persons in U.S. will be living with MCI due to AD or clinical AD by 2060
Primary and secondary preventions have differential impacts on future disease burden
RESEARCH IN CONTEXT.
SYSTEMATIC REVIEW
The authors searched PubMed for articles on forecasting prevalence of preclinical and clinical Alzheimer’s disease. No previous study has provided population forecasts of preclinical disease or evaluated potential impacts of primary and secondary prevention on disease burden.
INTERPRETATION
While primary prevention offer the greatest potential in the long run for reducing AD prevalence, its full impact on reducing disease burden would not be realized for decades because of the long preclinical period of disease and large numbers of people currently living with AD brain pathology.
FUTURE DIRECTIONS
Because large numbers of persons are currently living with preclinical disease, our results highlight the public health importance of development of secondary interventions targeted at those persons with preclinical disease most likely to progress to clinical disease during their lifetimes together with improved diagnostics for identifying them as well as primary interventions for persons who have not yet developed preclinical disease.
Acknowledgments
Role of Funding Source:
This research was funded by a grant from the National Institutes of Health (1R21AG055361). The study sponsor had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
RB reports fees from Takeda Inc. for serving as a member of a data safety monitoring board. NA has nothing to disclose. CK has nothing to disclose. MMC has nothing to disclose.
Contributor Information
Ron Brookmeyer, Department of Biostatistics, University of California, Los Angeles, CA 90095, rbrookmeyer@ucla.edu
Nada Abdalla, Department of Biostatistics, University of California, Los Angeles, CA 90095, nada.a.abdallah@gmail.com
Claudia H. Kawas, Departments of Neurology, Neurobiology and Behavior, Epidemiology and Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, CA 92697, ckawas@uci.edu
María M. Corrada, Departments of Neurology, Epidemiology and Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, CA 92697, mcorrada@uci.edu
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Park DC. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. The Lancet Neurology. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Masters CL. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. The Lancet Neurology. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 6.Cummings JL. Defining and labeling disease modifying treatments for Alzheimer’s disease. Alzheimer’s & Dementia. 2009;5:406–418. doi: 10.1016/j.jalz.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC. Barriers for Prevention and Prodromal AD Trials. Journal of Prevention of Alzheimer’s disease. 2016;3(2):66–67. doi: 10.14283/jpad.2016.96. [DOI] [PubMed] [Google Scholar]
- 8.National Academies of Science, Engineering and Medicine. Preventing Cognitive Decline and Dementia: A Way Forward. Washington, DC: The National Academies Press; 2017. Doi: https://doi.org/10.17226/24782. [PubMed] [Google Scholar]
- 9.Amanatkar HR, Papagiannopoulos B, Grossberg GT. Analysis of recent failures of disease modifying therapies in Alzheimer’s disease suggesting a new methodology for future studies. Expert Review of Neurotherapeutics. 2017;17(1):7–16. doi: 10.1080/14737175.2016.1194203. [DOI] [PubMed] [Google Scholar]
- 10.Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL, Kukull WA. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimer's & Dementia. 2011;7(1):61–73. doi: 10.1016/j.jalz.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007 Oct 29;29(1–2):125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health. 1998;88(9):1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Archives of Neurology. 2003;60(8):1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 14.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer's & Dementia. 2007;3(3):186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 15.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 16.Knopman DS, Jack CR, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, Roberts RO. Brain injury biomarkers are not dependent on β-amyloid in normal elderly. Annals of Neurology. 2013;73(4):472–480. doi: 10.1002/ana.23816. (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR., Jr PART and SNAP. Acta neuropathologica. 2014 Dec 1;128(6):773–6. doi: 10.1007/s00401-014-1362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR, Jr, Knopman DS, Chételat G, Dickson D, Fagan AM, Frisoni GB, Jagust W, Mormino EC, Petersen RC, Sperling RA, Van Der Flier WM. Suspected non-Alzheimer disease pathophysiology - concept and controversy. Nature Reviews Neurology. 2016;12:117–124. doi: 10.1038/nrneurol.2015.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack CR, Therneau TM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Mielke MM, Vemuri P, Roberts RO, Machulda MM, Senjem ML. Transition rates between amyloid and neurodegeneration biomarker states and to dementia: a population-based, longitudinal cohort study. The Lancet Neurology. 2016;15(1):56–64. doi: 10.1016/S1474-4422(15)00323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vos SJ, Verhey F, Frölich L, Kornhuber J, Wiltfang J, Maier W, Peters O, Rüther E, Nobili F, Morbelli S, Frisoni GB. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138:1327–1338. doi: 10.1093/brain/awv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann PJ, Araki SS, Arcelus A, Longo A, Papadopoulos G, Kosik KA, Kuntz KM, Bhattacharjya A. Measuring Alzheimer’s disease progression with transition probabilities Estimates from CERAD. Neurology. 2001;57(6):957–64. doi: 10.1212/wnl.57.6.957. [DOI] [PubMed] [Google Scholar]
- 23.Stern Y, Tang MX, Albert MS, Brandt J, Jacobs DM, Bell K, Marder K, Sano M, Devanand D, Albert SM, Bylsma F. Predicting time to nursing home care and death in individuals with Alzheimer disease. Journal of the American Medical Association. 1997;277(10):806–12. 12. [PubMed] [Google Scholar]
- 24.Johnson E, Brookmeyer R, Ziegler-Graham K. Modeling the effect of Alzheimer's disease on mortality. The International Journal of Biostatistics. 2007;3(1) doi: 10.2202/1557-4679.1083. Article 13. [DOI] [PubMed] [Google Scholar]
- 25.Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD, Teri L, Kukull WA. Survival after initial diagnosis of Alzheimer disease. Annals of Internal Medicine. 2004;140:501–9. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 26.Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Archives of Neurology. 2002;59(11):1764–1767. doi: 10.1001/archneur.59.11.1764. [DOI] [PubMed] [Google Scholar]
- 27.Human Mortality Database. University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany); Available at www.mortality.org (download 12/09/2015) [Google Scholar]
- 28.U.S. Census Bureau, Population Projections. 2014 National Population Projection Datasets. [accessed 9/25/2017]; Available at https://www.census.gov/programs-surveys/popproj.html.
- 29.Jack CR, Wiste HJ, Weigand SD, Knopman DS, Mielke MM, Vemuri P, Machulda MM. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain. 2015;138(12):3747–3759. doi: 10.1093/brain/awv283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.