Abstract
Prostaglandins are formed by enzymatic and non-enzymatic mechanisms. They have been detected in human ovarian follicular fluid (HFF), a medium rich in growth factors and nutrients important for oocyte growth and fertility. However, the comprehensive identification of HFF prostaglandins has not been addressed. Here we use hybrid triple quadrupole time-of-flight and triple quadrupole mass spectrometers to comprehensively analyze prostaglandins in HFF. We identified PGE1, PGE2, PGF2α, and other prostaglandins synthesized via prostaglandin-endoperoxide synthase (i.e. Cox) cascades. We also identified specific PGF2α isomers (F2-isoprostanes) and PGF3α analogs whose structures are inconsistent with Cox-dependent formation. A prospective cohort pilot study of infertility patient subtypes revealed two potential associations. F2-isoprostanes are decreased in the diminished ovarian reserve subtype and elevated PGF2α may be associated with decreased live birth. Other than PGF2α, only body mass index > 25 kg/m2 correlated with poor in vitro fertilization outcome. Our studies suggest that HFF contains prostaglandins formed from at least two mechanisms, which may correlate with distinct clinical parameters.
Keywords: prostaglandin, mass spectrometry, ovary, lipid, polyunsaturated fatty acid, fertilization, cyclooxygenase
1. Introduction
Prostaglandins (PGs) are signaling molecules derived from dietary fats with clinically relevant roles in reproductive biology [1–4]. PGE2, for instance, promotes ovulation downstream of the luteinizing hormone surge [5, 6]. Excess consumption of nonsteroidal anti-inflammatory drugs, which inhibit prostaglandin-endoperoxide synthase (a.k.a. cyclooxygenase or Cox), is associated with reversible female infertility, likely due to failed ovulation [7, 8]. On the other hand, proinflammatory cytokines increase PGF2α associated with corpus luteum development and immune cell recruitment [9]. The mature human follicle contains mural and cumulus granulosa cells surrounding a single oocyte. During follicle development, an antrum forms that is filled with fluid containing PGs, steroids, peptide growth factors, and metabolites [10]. Human ovarian follicular fluid (HFF) is collected along with cumulus-oocyte complexes from mature follicles in patients undergoing in vitro fertilization (IVF), providing a window into the physiological signaling processes occurring in fertile and infertile women. Although PGs have been analyzed in HFF, most studies used radio- and enzyme-immunoassays, which lack the specificity to distinguish among the complexity of PG types and isomers known to exist today [11–13].
PGs are synthesized from the 20-carbon polyunsaturated fatty acids (PUFAs) dihommo-gamma-linolenic acid (DGLA), arachidonic acid (AA), and eicosapentaenoic acid (EPA) [1, 14, 15]. A key structural feature is the cyclopentane ring, which contains side groups that define classes. For instance, the F-series class member PGF2α is synthesized from AA and contains hydroxyl groups at the carbon-9 (C9) and C11 positions in the cyclopentane ring [16]. Classical PG synthesis is initiated by Cox enzymes, which convert AA into the bicyclic endoperoxide PGG2 and then PGH2 [17, 18]. PGD, PGE, and PGF synthases convert PGH2 into bioactive forms [16, 19, 20]. PGs are also formed non-enzymatically by free radical-induced peroxidation [21, 22]. In this mechanism, reactive oxygen species (ROS) produced during oxidative stress act on C20 PUFAs in phospholipids. PGs generated by Cox or ROS can be distinguished through structural information [23–25]. Cox pathways generate free PGs with specific stereochemistry [17]. In contrast, ROS produce a broad spectrum of PG classes and isomers that are esterified to phospholipids (Fig. 1). As auto-oxidation reactions lack specificity, free radical-induced peroxidation generates 64 esterified PGF2α isomers alone (termed F2-isoprostanes), comprising four regioisomeric families each with 16 isomeric members. These families are called 5-F2-isoprostanes, 8-F2-isoprostanes, 12-F2-isoprostanes, and 15-F2-isoprostanes, based on position of the cyclopentane ring in the carbon chain [21, 25]. Sensitive and specific analytical methods are necessary to resolve these PG species.
Figure 1. PGF2α isomers in HFF.
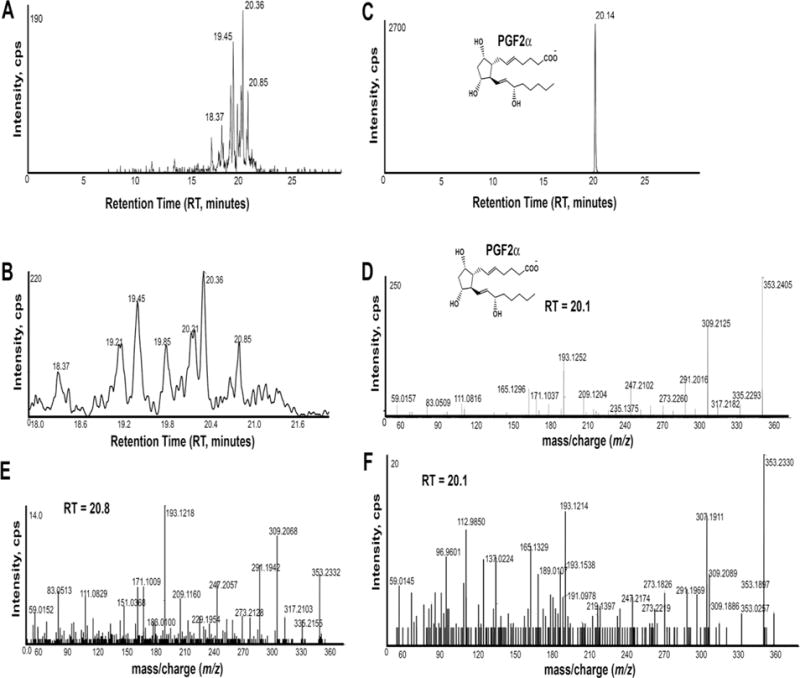
An extracted ion chromatogram (XIC) generated from the product ion m/z 193.122 that is found in 15-F2-isoprostanes (A). A magnified chromatogram focusing on RT 18 to 22 minutes is shown below (B). An XIC for product ion m/z 193.122 shows a single peak at RT 20.14 minutes from the PGF2α standard (10ng/ml) (C). MS/MS product ion spectrum of the PGF2α standard (D). MS/MS product ion spectrum of m/z 353.2323 at RT 20.8 minutes (E). MS/MS product ion spectrum of m/z 353.232 at RT 20.2 minutes (F). This spectrum contains overlapping isobaric precursor ions, possibly a steroidal sulfate(s), as evidenced by m/z 96.960 (HSO4−). Panels A, B, E, and F are from a single control patient. An XIC for a second control patient is shown in Supplemental Fig. 1B. Cps, counts per second.
There is recent evidence for a third PG metabolism pathway. The nematode C. elegans produces specific F1-isoprostanes, F2-isoprostanes, and F3-isoprostanes independent of Cox enzymes [26–28]. These F-series PGs are formed from DGLA, AA, and EPA precursors, respectively [27], and have an important function to attract migrating sperm to oocytes within the oviduct [27–29]. While the metabolism pathway is not well understood, insulin and TGF-β signaling pathways regulate ovarian F-series PG metabolism [26, 28, 30]. Genetic ablation of the two Cox genes in mice eliminates PGI2 and other classical PGs. However, specific F2-isoprostanes are still observed in wild-type and Cox null mice that are similar to those in C. elegans [26]. A distinguishing feature thus far is specificity for F-series versus D-series, E-series, and I-series PGs [27]. The extent to which the metabolic process and products generated overlaps with free radical-induced peroxidation is not clear. An important distinction is that C. elegans PG metabolism is strictly regulated and has a function unrelated to oxidative stress.
In this study, we used a system comprising nanoscale liquid chromatography coupled to a triple-TOF 5600 instrument, a hybrid Q-time-of-flight tandem mass spectrometer (qTOF), to comprehensively analyze PGs in HFF. To correlate selected PG concentrations across patient HFF samples, we used a conventional liquid chromatography tandem mass spectrometry (LC-MS/MS) system on a triple quadruplole mass spectrometer operated in multiple reaction monitoring (MRM) mode. The most abundant PGs across all samples are PGE2 and PGF2α. In addition, PGE1, PGF1α, and specific F2-isoprostanes and PGF3α analogs were detected. Compared to control HFF from oocyte donor and male infertility patients, HFF from patients with diminished ovarian reserve (DOR) contained significantly reduced concentration of F2-isoprostanes co-eluting with the 8-iso-PGF2α standard (also known as iPF2α-III or 15-F2t-IsoP). These results document diverse PG types in HFF and suggest that multiple F-series PGs are important for female fertility.
2. Materials and methods
2.1. Chemicals
PGF2α-d9, PGD2-d9, PGE2-d4, PGF1α-d9, PGF2α, 8-iso-PGF2α, ent-PGF2α, ent-8-iso-PGF2α ent-8-iso-15(S)-PGF2α, 15(R)-PGF2α, 8-iso-15(R)-PGF2α, 11β-PGF2α, 8-iso-9β-PGF2α, PGF1α, PGF3α, PGD2, PGE2, PGE1, PGD1, 6-keto-PGF1α, PGA1, and PGA2 standards were obtained from Cayman Chemical (Ann Arbor, MI). All HPLC solvents and reagents were purchased from Fisher Scientific Co. (Norcross, GA) and were of HPLC grade.
2.2 Patients and HFF collection
All patients gave consent for HFF donation for research purposes as part of a University of Alabama at Birmingham Institutional Review Board approved protocol. This work has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Subjects were premenopausal women presenting with subfertility or as oocyte donors. No patients undergoing oocyte retrieval were excluded from this study. Pertinent patient characteristics, including laboratory values, IVF cycle laboratory values, and outcome measures were recorded. Specific variables analyzed included subject age, history of live birth, body mass index, anti-mullerian hormone level (AMH), blastocyst conversion, and IVF outcomes (no pregnancy, biochemical pregnancy, spontaneous abortion, or ongoing pregnancy). Subjects were categorized by subfertility diagnosis that led to IVF. A control group was comprised of oocyte donors or patient couples presenting with male factor only infertility as a diagnosis.
Transvaginal cyst puncture was performed after controlled ovarian hyperstimulation with gonadotropins (recombinant follicle stimulating hormone, human menopausal gonadotropins). Protocols included long leuprolide, antagonist, and micro-dose leuprolide flares. HFF from the first entered follicle bilaterally was included in this study to limit potential specimen blood contamination, which may contain different PG types. The first follicles entered bilaterally were separately collected from each patient. Oocyte retrieval needles were flushed prior to follicle entry. HFF was obtained from mature sized follicles (≥16 mm diameter), with HFF from a total of 93 patients being collected. 42 patient samples were analyzed. Fresh HFF was stored in a −20°C freezer within 30 minutes after oocyte retrieval, and transferred to a −80°C freezer for further storage. PGF2α–d9 (1.00 ng/ml) was added to every sample as an internal standard. HFF samples were thawed in a warm bath prior to PG extraction.
2.3 Prostaglandin extraction
PGs were extracted using a liquid-liquid extraction method with minor modifications [31, 32]. Briefly, 2 ml of HFF (with the PGF2α–d9 internal standard) were mixed with 4 ml of acetone. The mixture was transferred to conical glass tubes (10 ml), mixed with hexane, and vortexed. The upper hexane soluble layer was removed and the bottom layer was mixed with 2 ml chloroform. After separation, the chloroform layer was evaporated to dryness under Nitrogen and capped immediately. The dried extract was stored in Nitrogen gas at −80°C for a brief period (typically less than 3 days) before analysis. Butylated hydroxytoluene was used in solutions to prevent oxidation. This method produced consistent results when the same HFF sample was extracted multiple times and analyzed using LC-MS/MS operated in MRM mode (N=9). Similar results were obtained using PGD2-d9, PGE2-d4, PGF1α-d9, and PGA2 internal standards. Dried samples were resuspended in 200 μl 80% MeOH for analysis, providing 10-fold increased concentration relative to the original HFF sample.
2.4 Prostaglandin identification and quantification
For comprehensive PG detection, 2 μl extracted HFF samples were loaded onto a Nano cHiPLC 200μm × 0.5mm ChromXP C18-CL 3μm 120Å reverse-phase trap cartridge (Eksigent, Dublin, CA) at 2 ml/min using an Eksigent autosampler. After washing the cartridge for 5 min with 0.1% formic acid in double distilled H20, the bound lipids were flushed with a 15 minute linear (5–95%) acetonitrile gradient in 0.1% formic acid at 1000 nl/min using an Eksigent 415 NanoLC system. The column was washed with 95% acetonitrile-0.1% formic acid for 5 min and then re-equilibrated with 5% acetonitrile-0.1% formic acid for 5 min. The Sciex 5600 Triple-TOF mass spectrometer (Sciex, Toronto, Canada) was used to analyze PGs. The IonSpray voltage for positive and negative modes were ±2300 V and the declustering potential was ±80 V. Ionspray and curtain gases were set at 10 psi and 25 psi, respectively. The interface heater temperature was 120°C. Eluted compounds were subjected to a time-of-flight survey scan from m/z 50–1000 to determine the most intense ions for MS/MS analysis. Product ion time-of-flight scans at 50 msec using a collision energy spread of 15 eV with a set collision point of 35 eV were carried out to obtain the tandem mass spectra of selected parent ions. Spectra were centroided and de-isotoped by Analyst software, version 1.6 TF (Sciex, Toronto, Canada).
Relative quantification of selected PGs was performed with liquid chromatography tandem mass spectrometry (LC-MS/MS) operated in MRM mode, following our previously published method [26–28]. Briefly, LC-MS/MS analyses were performed using a system consisting of a Shimadzu Prominence high performance liquid chromatography column with a refrigerated auto sampler (Shimadzu Scientific Instruments, Inc., Columbia, MD) and an API 4000 (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada) triple quadrupole mass spectrometer. The chromatographic separation was performed on a Synergy hydro RP-C18 column pre-equilibrated with 0.1% formic acid. The mobile phase consists of 0.1% formic acid [A] and acetonitrile containing 0.1% formic acid [B] and was pumped at a flow rate of 0.2 ml/min. The gradient started with 10% B and went up to 80% B from 0–11 min, 80–100% B from 11–14 min and returned back to 10% B at 16 min. The column effluent was introduced into the mass spectrometer using an ESI interface operating in negative ion mode. Nitrogen was used as a nebulizer and curtain gas (CUR = 10). The MRM analysis was conducted by monitoring the precursor ion to product ion transitions from m/z 353/193 and 353/309 for PGF2α and 8-iso-PGF2α, m/z 355/311 for PGF1α, m/z 351/191 and 351/193 for PGF3α, m/z 351/189 for PGE2 and PGD2, m/z 369/245 for 6-keto-PGF1α, m/z 369/169 for thromboxane B2, m/z 353/273 and 353/191 for PGE1 and PGD1, m/z 333/189 for PGA2, and m/z 335/273 for PGA1. The collision gas, collision energy and temperature were set at 10, −35 eV and 600°C, respectively. The LC-MS/MS system was controlled by BioAnalyst 1.4.2 software.
To estimate PG concentration, a stock solution of PG standard (1 mg/ml in 80% MeOH) was serially diluted with the same solvent to obtain 0.1, 0.5, 1.0, 10.0, and 50.0 ng/ml concentrations. The samples were analyzed by the MRM method. The standard curves exhibited excellent linearity in the range of concentration 0.1–1000 ng/ml with correlation coefficients > 0.99. Patient HFF samples were normalized to the internal control. The method exhibits excellent reproducibility and average concentration for each PG is comparable across HFF samples. Absolute PG concentrations are estimates due to potential matrix effects that can be different at each retention time [33]. Matrix effects are difficult to accurately quantify in HFF, which contains endogenous PGs. Based on analysis with PGF2α–d9, PGD2-d9, PGE2-d4, PGF1α-d9, and PGA2 internal standards, we estimated a 50% extraction and detection efficiency for all PGs.
2.5 Statistics
Mean PG levels were compared to clinical variables of interest using a student’s t-test with Levene’s Test for Equality of Variances. For Figure 4C, PGE2 and PGF2α levels were categorized as high and low by dichotomizing levels above and below the median of the 42 samples analyzed (n=21 per cohort). High and low cohorts were compared to clinical variables of interest using chi-square testing, along with calculations for relative risk with 95% confidence interval. Analysis was performed using SPSS version 21.0, and a P value of <0.05 was considered to be statistically significant. Clinical pregnancy was considered positive if the patient had a normal fetus on ultrasound and a fetal heartbeat at 8 weeks or more of gestation.
Figure 4. Clinical associations with 15-F2-isoprotanes.
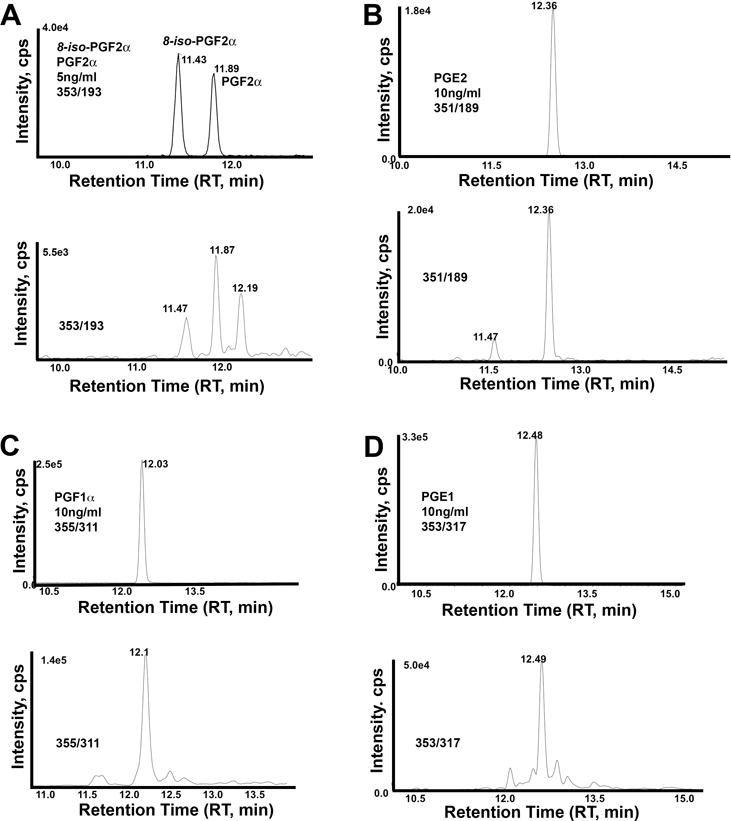
8-iso-PGF2α concentration in HFF is plotted according to patient age (A). The dotted line represents the best fit from a linear regression model. Average 8-iso-PGF2α concentration in control patients (egg donor or male infertility) and diminished ovarian reserve (DOR) patients (B). Dots represent individual data points. Bars are standard deviation. Average 8-iso-PGF2α concentration is reduced in DOR patients compared to controls using a student’s t-test (P < 0.05). 8-iso-PGF2α concentration was calculated from the peak at RT 11.4 to 11.5 in Fig. 3A. Similar results were obtained from the unknown 15-F2-isoprostane peak at RT 12.2 in Fig. 3A. Number of live births and negative pregnancies in patients with low PGF2α concentration (below the median) and high PGF2α concentration (above the median) (C). Negative pregnancy was assessed by HCG serum testing and included biochemical pregnancy and spontaneous abortion.
3. Results
3.1. Comprehensive PG analysis in control patients
PGF2α and PGE2 have been detected in HFF previously, but the analytical methods were not specific enough to distinguish among PG isomers or comprehensively identify PG types [11–13]. To address this issue, we developed an analytical method consisting of a nano-LC separation column coupled to the triple-TOF instrument. This hybrid Q-TOF platform has accurate mass (<5 ppm), high resolution (30,000), and fast acquisition. Nano-flow chromatography (1000 nl/min) resulted in increased sensitivity and was capable of resolving isobaric and isomeric compounds, including the stereoisomers PGF2α, ent-PGF2α, 8-iso-PGF2α, and ent-8-iso-PGF2α (Supplemental Fig. 1A).
We extracted HFF PGs from two fertile female IVF patients. As our extraction procedure removes neutral lipids, including phospholipids containing esterified PGs, we analyzed PGs in HFF as free carboxylic acids. Based on monoisotopic exact mass, retention time (RT) of commercial standards, and MS/MS spectra, we identified PGF2α, PGF1α, and PGE2 (Fig. 1, Supplemental Fig. 2, and Supplemental Fig. 3). PGD2, PGF3α, PGA1, PGA2, and 6-keto-PGF1α, the stable metabolite of PGI2, were low or undetectable (Supplemental Fig. 3 and data not shown). Other than occasional low PGD2, we did not detect multiple PGE2 or PGF1α isomers. A series of specific F2-isoprostanes and PGF3α analogs were detected, as described below.
F2-isoprostanes were detected by searching for the deprotonated molecular ion m/z 353.2328 [M-H]−. In both patients, extracted ion chromatograms (XIC) for m/z 353.2328 showed multiple peaks ranging in RT from 18–22 minutes. 15-F2-isoprostanes, such as PGF2α generate the characteristic product ion C12H17O2− with mass m/z 193.122 [23]. An XIC generated from this product ion indicated several peaks from RT 18–22 minutes (Fig. 1A, B). The ion m/z 353.233 at RT 20.1 was identified as PGF2α (Fig. 1). The extracted ion at RT 20.8 minutes showed MS/MS product ions almost identical to PGF2α, but with different RT (Fig. 1). The second patient’s HFF contained a peak at RT 19.4 minutes that co-eluted with 8-iso-PGF2α, in addition to peaks at RT 20.0, 20.1, and 20.3 minutes (Supplemental Fig. 1). Thus, HFF contains PGF2α, 8-iso-PGF2α, and at least one other 15-F2-isoprostane.
The MS/MS spectrum of m/z 353.233 at RT 20.3 minutes showed the product ion m/z 115.0402 (Supplemental Fig. 4A), which is characteristic of 5-F2-isoprostanes [21, 34]. An XIC for this product ion indicated a single prominent 5-F2-isoprostane peak in one patient (Supplemental Fig. 5) and two less abundant peaks in the other patient (not shown). In both patients, MS/MS showed product ions typical of 5-F2-isoprostanes (Supplemental Fig. 4B, C) [25, 34]. We did not detect evidence for 8-F2-isoprostanes or 12-F2-isoprostanes [21, 34] (Supplemental Fig. 5 and data not shown).
A search for the deprotonated ion m/z 351.2177 revealed several ions with MS/MS spectra similar to PGF3α, but different RTs. MS/MS showed the product ion m/z 191.1416 and an XIC for this product ion produced several peaks (Fig. 2). The most prominent peak at RT 19.1 minutes had an MS/MS spectrum typical of PGF3α, including product ions m/z 333, 307, 289, 245, 193, and 191 [23, 27] (Fig. 2B). However, the presence of major ions m/z 167.1067 and m/z 153.0912 suggests that this lipid is similar to a PGF3α analog synthesized by C. elegans oocytes [27]. MS/MS spectra for peaks at RT 20.32 minutes and 20.66 minutes are also consistent with these lipids being PGF3α analogs (data not shown). We did not detect 5-F3-isoprostanes or 8-F3-isoprostanes containing the product ions m/z 115.0402 or m/z 127.0759, respectively [35]. Therefore, HFF contains a number of specific PGF3α analogs.
Figure 2. PGF3α analogs in HFF.
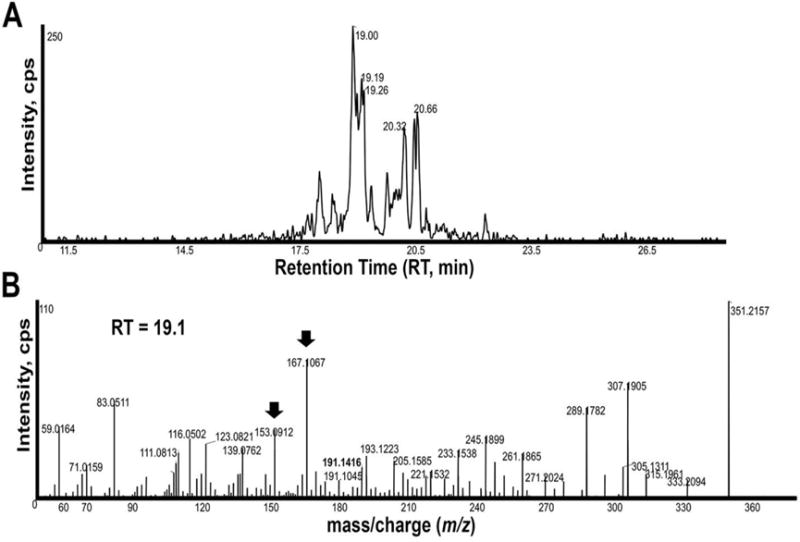
An XIC generated from the product ion m/z 191.145 that is found in PGF3α isomers (A). MS/MS product ion spectrum of m/z 351.215 at RT 19.1 minutes in the extracted HFF sample (B). The product ions m/z 333, 315, 307, 289, 245, 193, and 191 are observed in authentic PGF3α [23, 27]. Standards also available online at http://www.lipidmaps.org/data/standards/index.html. Arrows indicate major product ions that are not observed in PGF3α, but are observed in a PGF3α analog from C. elegans [27]. Peaks at RT 20.35 minutes and 20.65 minutes have several product ions observed in PGF3α spectra, including m/z 333, 315, 289, 245, 193, and 191. Cps, counts per second.
3.2 Targeted analysis of selected PGs across patients
MRM on a triple quadrupole instrument is well suited for comparing relative PG concentrations across samples. In MRM mode, the parent ion to product ion mass transition is specified, providing excellent specificity and sensitivity [23, 27, 36]. We previously developed a reversed-phase LC-MS/MS method operated in MRM mode to detect and measure a wide array of PGs [27, 28, 32]. Our method is able to separate most PG isomers and stereoisomers [27].
To complement our comprehensive studies above, we selected MRM mass transitions for detecting and quantifying major PG classes (Table 1) in HFF from 42 patients, including fertile and infertile females. The most consistently detected PGs across the samples co-elute in MRM mode with PGF2α, PGF1α, PGE2, and PGE1 standards (Fig. 3A–D). The MRM results showed good agreement with the nano-LC qTOF method. In particular, MRM mass transition m/z 353/193, which detects 15-F2-isoprostanes such as PGF2α, showed three major peaks in most patients (Fig. 3A). The peak at RT 11.4 to 11.5 minutes eluted near the 8-iso-PGF2α standard, similar to analysis using nano-LC qTOF (Fig. 1 and Supplemental Fig. 1). The peak at RT 11.9 minutes eluted near the PGF2α standard (Supplemental Fig. 6A). The peak at RT 12.2 minutes is also observed in C. elegans and Cox-1; Cox-2 knockout mice [26, 27] (Supplemental Fig. 6) and may represent the unknown PGF2α isomer at RT 20.8 in nano-LC qTOF (Fig. 1A and 1F). We did not focus on uncharacterized PGF3α analogs because co-eluting standards are not available. The estimated concentrations of selected PGs in HFF across all samples are shown in Table 1. PGE2 and PGF2α concentration varied widely in a small subset of patients, perhaps due to temporal regulation during oocyte maturation [6, 37] or an underlying inflammatory condition (see Discussion). In MRM mode, we did not consistently detect 6-keto-PGF1α, PGA1, PGA2, PGF3α, or PGD2 in most patient’s HFF (see Discussion).
Table 1.
PG MRM mass transitions and average concentration across all patients.
| Standard | RT (min) | MRM mass transition m/z | Average estimated PG concentration |
|---|---|---|---|
| PGA1 | 13.5 | 335/273 | ND |
| PGA2 | 13.3 | 333/189 | ND |
| PGD2 | 12.6 | 351/189 | ND |
| PGE1 | 12.4 | 353/317 | 70 ± 21 pg/ml |
| PGE2 | 12.3 | 351/189 | 638 ± 235 pg/ml |
| 6-keto-PGF1α | 10.7 | 369/245 | ND |
| PGF1α | 11.9 | 355/311 | 38 ± 8 pg/ml |
| PGF2α | 11.9 | 353/193 | 654 ± 269 pg/ml* |
| 8-iso-PGF2α | 11.5 | 353/193 | 50 ± 6 pg/ml* |
| PGF3α | 11.3 | 351/191 | ND |
ND, not determined because most HFF samples had low or undetectable PG concentration. Mean ± standard error of the mean (SEM) is shown. Average estimated concentration is in picograms (pg) per milliliter (ml) of HFF, assuming a 50% extraction/detection efficiency (see Methods). HFF was concentrated 10-fold in 80% MeOH prior to analysis. RT, Retention Time in minutes.
this peak may contain multiple 15-F2-isoprostanes (see Discussion).
Figure 3. Representative MRM chromatograms of PG standards and HFF.
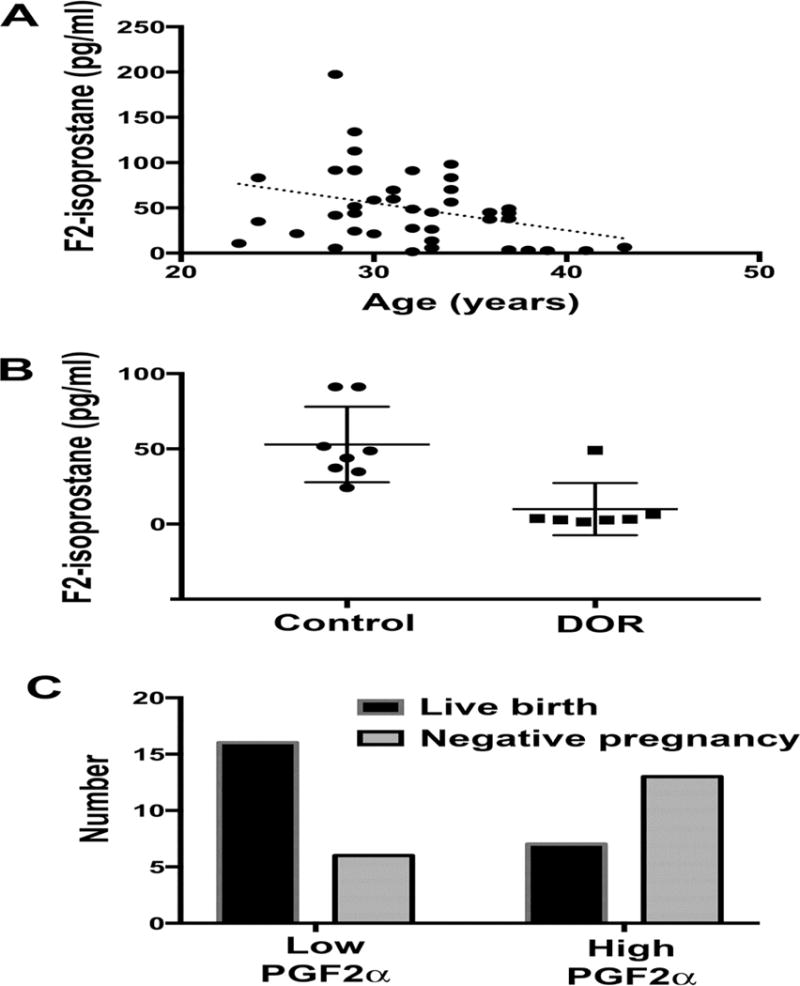
MRM using mass transition m/z 353/193 detects 8-iso-PGF2α and PGF2α standards (A, top). Three major peaks are observed in most HFF samples (A, bottom). Based on nano-LC qTOF analysis, these peaks may contain co-eluting PGF2α isomers (see Discussion). MRM using mass transition m/z 351/189 detects PGE2 (B, top) and PGD2 standards (not shown at RT = 12.6 minutes). A single major peak is observed in most HFF samples corresponding to PGE2 (B, bottom). MRM using mass transition m/z 355/311 detects the PGF1α (C, top) standard. A peak at RT 12.1 is observed in most HFF samples corresponding to PGF1α (C, bottom). MRM using mass transition m/z 353/317 detects the PGE1 (D, top) standard. A peak at RT 12.5 is observed in most HFF samples corresponding to PGE1 (C, bottom). Cps, counts per second.
3.3 Clinical characteristics
Subjects were categorized by subfertility diagnosis that led to IVF. 42 patient samples were analyzed, including 7 with endometriosis, 7 with polycystic ovary syndrome (PCOS), 7 with diminished ovarian reserve (DOR), 10 patients with unexplained infertility, and 8 fertile women undergoing IVF served as the controls. Three patients did not fall into the diagnostic categories. Endometriosis was defined by presence of endometrial implants noted during previous laparoscopy with pathologic confirmation of implants biopsied. Polycystic ovary syndrome (PCOS) was defined when the subject met at least 2 of 3 NIH Criteria for PCOS. DOR or low egg number relative to age was diagnosed with AMH <1.0 ng/mL, or FSH >10 IU/L. Unexplained infertility was diagnosed with normal ovarian reserve testing, normal male partner semen analysis, and normal uterus and fallopian tubes on hysterosalpingogram. The control group was comprised of fertile women who were oocyte donors or presented with male factor infertility only. Patient demographics are listed in Table 2. As expected, PCOS patients had a significantly higher BMI, and patients with DOR were older and had less oocytes retrieved. We found that endometriosis and PCOS patients had a trend toward elevated PGE2 and PGF2α levels, but this increase did not reach significance when compared to the control group. Age, body mass index, IVF stimulation protocol, and anti-mullerian hormone did not demonstrate a significant relationship with PG levels.
Table 2.
Patient clinical characteristics.
| Control (n=8) |
Unex (n=10) |
Endo (n=7) |
PCOS (n=7) |
DOR (n=7) |
|
|---|---|---|---|---|---|
| Age | 30.00 | 32.20 | 31.57 | 29.29 | 38.14* |
| BMI | 21.99 | 21.66 | 25.23 | 31.29* | 24.34 |
| History of Live Birth | 5/8 | 1/10* | 3/7 | 0/7* | 1/7 |
| Number Oocytes Retrieved | 18.13 | 17.20 | 17.71 | 19.43 | 6.71* |
| Total Blastocyst Number | 7.25 | 5.60 | 3.00 | 4.86 | 2.43* |
| IVF Cycle Negative HCG | 2/8 | 2/10 | 2/7 | 2/7 | 5/7 |
| IVF Cycle Biochemical or SAB | 0/8 | 1/10 | 1/7 | 4/7 | 0/7 |
| IVF Cycle Ongoing Pregnancy | 7/8 | 7/10 | 4/7 | 1/7 | 3/7 |
Number of patients is in parenthesis. BMI, Body Mass Index; Unex, Unexplained Infertility; Endo, Endometriosis; PCOS, Polycystic Ovary Syndrome; DOR, Diminished Ovarian Reserve; IVF, in vitro Fertilization; HCG, Human Chorionic Gonadotropin; SAB, Spontaneous Abortion.
P<0.05 comparing diagnostic groups versus control group using a student’s t-test and chi-square testing for parametric and non-parametric groups, respectively.
3.4 Clinical associations with F2-isoprostanes
F2-isoprostanes such as 8-iso-PGF2α are often linked to oxidative stress [21], but they might also have physiologic functions important for follicle development, ovulation, or fertilization [27]. To investigate free F2-isoprostanes in HFF, we measured the peak area in each HFF sample that corresponded to the 8-iso-PGF2α standard RT in MRM mode with mass transition m/z 353/193 (Fig. 3A). Concentration was calculated using an 8-iso-PGF2α standard curve and the internal standard. We then compared F2-isoprostane concentration in control patients (52 ± 26 pg/ml) to PCOS (82 ± 28 pg/ml) and endometriosis (76 ± 64 pg/ml) patients. Both disorders are linked to altered inflammatory state and ROS levels [38–42]. Average F2-isoprostane concentration is not statistically different in the three groups (P > 0.2 using a T-test with Levene’s Test for Equality of Variances). PGE2 and PGF2α are 5-fold to 15-fold increased in PCOS or endometriosis samples relative to the control. These elevated values did not reach significance, however, possibly due to low sample number. Additionally, ROS damage to lipids is thought to accumulate with age [43]. F2-isoprostane concentration did not increase significantly when patients were plotted according to age (Fig. 4A). A similar trend was observed for the unidentified peak at RT 12.2 min (Fig. 3A). Therefore, increased free F2-isoprostane concentration in HFF is a poor marker for PCOS, endometriosis, and other conditions associated with oxidative stress.
When we investigated F2-isoprostane concentration in differing infertility diagnoses, the only association that reached significance was DOR patients (Fig. 4B). In these patients, the F2-isoprostane peak was significantly decreased compared to controls. As DOR patients tend to be older, this trend was opposite to that expected if this F2-isoprostane(s) is a byproduct of oxidative stress. These data raise the possibility that specific free F2-isoprostanes have a physiologic role.
3.5 Elevated PGF2α may be associated with decreased live birth
We noticed that PGF2α and PGE2 concentrations varied extensively in a subset of patients. To test whether high or low PG levels are associated with a clinical outcome, we first calculated medium PGF2α and PGE2 concentrations. Patients were split into groups that fell above and below the median (referred to as high and low throughout). We then compared these groups to clinical variables of interest. PGE2 was not significantly associated with a clinical outcome. High PGF2α was significantly associated with a poor pregnancy outcome for the IVF cycle (negative pregnancy by HCG serum testing, biochemical pregnancy, or spontaneous abortion) when compared to low PGF2α levels (Fig. 4C). Similarly, the low PGF2α cohort had significantly higher live birth rate when compared to the high PGF2α cohort (Fig. 4C). These results suggest that elevated PGF2α concentration is detrimental to reproduction or implantation.
4. Discussion
Here we use multiple liquid chromatography mass spectrometry approaches to identify and compare relative PG concentrations in HFF. Two fertile patients were analyzed using a nano-LC Q-TOF method for comprehensive PG analysis. A targeted approach using MRM was used to detect and compare relative concentrations of selected PGs in a broader population of 42 patients. We found known PGs, such as PGF2α and PGE2, as well as specific F2-isoprostanes and PGF3α analogs with unknown functions.
A comprehensive analysis of two control patients identified multiple 15-F2-isoprostanes and one to two 5-F2-isoprostanes. 8-F2-isoprostanes and 12-F2-isoprostanes were not detected in the two samples. MRM using mass transition m/z 353/193 for 15-F2-isoprostanes detected three prominent peaks in most patients. One peak eluted with the 8-iso-PGF2α standard and a second eluted with the PGF2α standard. These peaks could contain multiple PGF2α isomers, as a small number of F2-isoprostanes co-elute in nano-LC and LC methods. The third peak did not co-elute with available standards. Less than half of the 64 total F2-isoprostanes are commercially available, making it difficult to develop LC methods to separate all possible isomers. MS/MS spectra among members of each regioisomer class is nearly identical. Thus, it is not currently possible to identify each F2-isoprostane and PGF3α analog present in HFF. Nevertheless, it is safe to assume that HFF has specific PG composition, as opposed to a nonselective mixture. Besides PGF2α, the other F2-isoprostanes and PGF3α analogs that were detected are likely formed independent of Cox enzymes. Consistent with this idea, 15-F2-isoprostane chromatograms from HFF closely resemble those from Cox-deficient mice and C. elegans [26, 27] (Supplemental Fig. 6).
Multiple PGF3α analogs were detected in nano-LC Q-TOF that did not co-elute with Cox-derived PGF3α. These PGs have MS/MS spectra similar to PGF3α-like lipids from C. elegans [27]. Their spectra do not contain abundant product ions, such as m/z 115.0402 or m/z 127.0759 from PGF3α isomers associated with oxidative stress [34, 35]. Cox pathways specifically generate PGE2 and PGD2. Chromatograms focusing on PGE2 isomers are dominated by abundant PGE2 and occasional minor PGD2. Other isomers were not detected. Taken together, these data suggest that HFF contains PGs made by Cox pathways, as well as an alternative pathway(s).
The concentrations of free PGs in HFF are estimated (see Methods) to be in a range consistent with physiological function. While it is difficult to quantify unknown PGF2α and PGF3α analogs, we were able to measure peaks eluting with PGF2α and 8-iso-PGF2α standards in MRM mode. In control patients, the average PGF2α peak concentration is about 144 pg/ml or 406 pM. The average estimated F2-isoprostane peak (RT 11.5 min) concentration is about 52 pg/ml or 150 pM. In general, PGF2α binding to its receptors occurs between 100 pM and 10 nM with reported dissociation constants in the high pM to low nM range, depending on the study [44–48]. An important mechanistic consideration comes from C. elegans ovaries, where numerous F-series PGs act collectively [27]. If this signaling mechanism is valid in human ovaries, the collective concentrations of PGF1α, F2-isoprostanes, and PGF3α analogs could occupy many receptor sites.
It is important to note PGs that were not consistently detected in our samples. PGD1, PGD2, PGF3α, PGA1, PGA2, and PGI2 were either close to or below the detection limit for most samples, suggesting that these PGs do not play an important role in the mature follicle. PGI2, in particular, was readily detectable in HFF containing blood, which could be a contaminating source. Previous studies using radio-immunoassays detected 6-keto-PGF1α, the stable metabolite of PGI2, in HFF [12, 13]. The discrepancy between these studies and ours could be due to blood contamination, poor specificity of the radioimmunoassays, or the patient population. PGE1, PGF1α, and PGA1 have been shown to bind the sperm cation channel CatSper, suggesting that these PGs might have a role in fertilization [49, 50]. We detected PGE1 and PGF1α in HFF, but not PGA1. However, the PG composition in the oviduct after ovulation might not reflect the composition in HFF. PGD2 has been implicated in mouse folliculogenesis [51]. Although PGD2 levels were low or undetectable in HFF from most sampled patients, it may be important early in follicle development.
Stratifying patients based on clinical diagnosis, age, and IVF outcome revealed several trends worth further investigation. Free F2-isoprostane concentration was not significantly increased with age or in conditions like PCOS and endometriosis. These findings are at odds with the conventional assumption that 8-iso-PGF2α is a marker for oxidative stress. Instead, they suggest that F2-isoprostanes and PGF3α analogs in HFF may have a function(s) unrelated to stress or inflammation. Another important consideration is that we did not measure esterified PGs, which might better reflect oxidative damage.
PGF2α levels above the median were associated with decreased live birth rates in our cohort undergoing IVF. PGF2α concentration varied widely among a small subset of patients. Some of this variation might reflect increased synthesis in a temporally restricted fashion following gonadotropin stimulation [5, 6]. Several patients, particularly those diagnosed with endometriosis and PCOS, exhibited very high PGF2α and PGE2 levels. One possibility is that these patients have an underlying inflammatory condition causing increased PGF2α synthesis throughout the ovary. Indeed, elevated PGF2α and PGE2 levels have been observed in endometriosis patients [39]. Excess inflammation could influence embryo implantation. Alternatively, high PGF2α in HFF could indicate compromised oocyte development, leading to failed embryogenesis. Future work is necessary to determine the function of PGF2α in HFF and the effects, if any, of elevated PGF2α on IVF outcome.
In conclusion, HFF contains a specific composition of structurally related PGs that require sensitive analytical methods to detect. While PGE2 is important for ovulation, the roles of other PGs are not well understood. In particular, the presence of multiple F-series PGs in HFF suggests that there is more to learn about these important lipid mediators in reproduction than previously appreciated.
Supplementary Material
Highlights.
Comprehensive profiling of prostaglandins in 42 human follicular fluid samples identified PGE1, PGE2, PGF1α, and PGF2α
Specific free F2-isoprostanes and PGF3α analogs were detected whose structures are inconsistent with synthesis by cyclooxygenase pathways
F2-isoprostane concentration was decreased in diminished ovarian reserve patients
High PGF2α concentration was associated with decreased live birth
Acknowledgments
Supported by NIH grants R01GM085105 (to MAM) and R01GM118361 (to MAM and JKP). The UAB Targeted Metabolomics and Proteomics Laboratory has been supported by the UAB-UCSD O’Brien Acute Kidney Injury Center (P30 DK079337), UAB Lung Health Center (HL114439, HL110950), and UAB Center for Free Radical Biology. Purchase of the mass spectrometers in the Targeted Metabolomics and Proteomics Laboratory came from funds provided by the NCRR for the SCIEX 5600 TripleTOF (S10 RR027822-01) and the UAB Health Services Foundation General Endowment Fund for the SCIEX 4000.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest.
Author’s Contributions
BP, JKP, JWE, GWB, and MAM conceived and designed the experiments. BP, JKP, and MAM wrote the manuscript. BP, LW, AA, and RM executed the experiments. JKP and MAM assisted with data interpretation. BP, JWE, and GWB helped secure clinical samples and consent.
References
- 1.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.Gross GA, Imamura T, Luedke C, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci U S A. 1998;95:11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergstrom S, Diczfalusy E, Borell U, Karim S, Samuelsson B, Uvnas B, Wiqvist N, Bygdeman M. Prostaglandins in Fertility Control. Science. 1972;175:1280–1287. doi: 10.1126/science.175.4027.1280. [DOI] [PubMed] [Google Scholar]
- 4.Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Dore M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update. 2004;10:373–385. doi: 10.1093/humupd/dmh032. [DOI] [PubMed] [Google Scholar]
- 5.Sirois J. Induction of prostaglandin endoperoxide synthase-2 by human chorionic gonadotropin in bovine preovulatory follicles in vivo. Endocrinology. 1994;135:841–848. doi: 10.1210/endo.135.3.8070377. [DOI] [PubMed] [Google Scholar]
- 6.Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol Hum Reprod. 2001;7:731–739. doi: 10.1093/molehr/7.8.731. [DOI] [PubMed] [Google Scholar]
- 7.Calmels C, Dubost JJ, Jasmin-Lebrun C, Sauvezie B. A new case of NSAID-induced infertility. Rev Rhum Engl Ed. 1999;66:167–168. [PubMed] [Google Scholar]
- 8.Mendonca LL, Khamashta MA, Nelson-Piercy C, Hunt BJ, Hughes GR. Non-steroidal anti-inflammatory drugs as a possible cause for reversible infertility. Rheumatology (Oxford) 2000;39:880–882. doi: 10.1093/rheumatology/39.8.880. [DOI] [PubMed] [Google Scholar]
- 9.Luo W, Salih SM, Bormann CL, Wiltbank MC. Induction of chemokines and prostaglandin synthesis pathways in luteinized human granulosa cells: potential role of luteotropin withdrawal and prostaglandin F2alpha in regression of the human corpus luteum. Reprod Biol. 2015;15:247–256. doi: 10.1016/j.repbio.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Hennet ML, Combelles CM. The antral follicle: a microenvironment for oocyte differentiation. Int J Dev Biol. 2012;56:819–831. doi: 10.1387/ijdb.120133cc. [DOI] [PubMed] [Google Scholar]
- 11.Bergqvist A, Brunkwall J, Ploman F. Concentrations of prostaglandin F2alpha in follicular fluid from women with endometriosis. Hum Reprod. 1997;12:1789–1793. doi: 10.1093/humrep/12.8.1789. [DOI] [PubMed] [Google Scholar]
- 12.Ylikorkala O, Tenhunen A. Follicular fluid prostaglandins in endometriosis and ovarian hyperstimulation. Fertil Steril. 1984;41:66–69. doi: 10.1016/s0015-0282(16)47543-6. [DOI] [PubMed] [Google Scholar]
- 13.Jeremy JY, Okonofua FE, Thomas M, Wojdyla J, Smith W, Craft IL, Dandona P. Oocyte maturity and human follicular fluid prostanoids, gonadotropins, and prolactin after administration of clomiphene and pergonal. J Clin Endocrinol Metab. 1987;65:402–406. doi: 10.1210/jcem-65-3-402. [DOI] [PubMed] [Google Scholar]
- 14.Smith WL, Lands WE. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry. 1972;11:3276–3285. doi: 10.1021/bi00767a024. [DOI] [PubMed] [Google Scholar]
- 15.Bergstrom S, Danielsson H, Klenberg D, Samuelsson B. The enzymatic conversion of essential fatty acids into prostaglandins. J Biol Chem. 1964;239:PC4006–4008. [PubMed] [Google Scholar]
- 16.Watanabe K. Prostaglandin F synthase. Prostaglandins Other Lipid Mediat. 2002;68–69:401–407. doi: 10.1016/s0090-6980(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 17.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 18.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 19.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59:207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 20.Flanagan JU, Smythe ML. Sigma-class glutathione transferases. Drug Metab Rev. 2011;43:194–214. doi: 10.3109/03602532.2011.560157. [DOI] [PubMed] [Google Scholar]
- 21.Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy RC, Barkley RM, Berry KZemski, Hankin J, Harrison K, Johnson C, Krank J, McAnoy A, Uhlson C, Zarini S. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem. 2005;346:1–42. doi: 10.1016/j.ab.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 24.Masoodi M, Nicolaou A. Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3023–3029. doi: 10.1002/rcm.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waugh RJ, Morrow JD, Roberts LJ, 2nd, Murphy RC. Identification and relative quantitation of F2-isoprostane regioisomers formed in vivo in the rat. Free Radic Biol Med. 1997;23:943–954. doi: 10.1016/s0891-5849(97)00133-0. [DOI] [PubMed] [Google Scholar]
- 26.McKnight K, Hoang HD, Prasain JK, Brown N, Vibbert J, Hollister KA, Moore R, Ragains JR, Reese J, Miller MA. Neurosensory perception of environmental cues modulates sperm motility critical for fertilization. Science. 2014;344:754–757. doi: 10.1126/science.1250598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoang HD, Prasain JK, Dorand D, Miller MA. A heterogeneous mixture of F-series prostaglandins promotes sperm guidance in the Caenorhabditis elegans reproductive tract. PLoS Genet. 2013;9:e1003271. doi: 10.1371/journal.pgen.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edmonds JW, Prasain JK, Dorand D, Yang Y, Hoang HD, Vibbert J, Kubagawa HM, Miller MA. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev Cell. 2010;19:858–871. doi: 10.1016/j.devcel.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubagawa HM, Watts JL, Corrigan C, Edmonds JW, Sztul E, Browse J, Miller MA. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol. 2006;8:1143–1148. doi: 10.1038/ncb1476. [DOI] [PubMed] [Google Scholar]
- 30.Edmonds JW, McKinney SL, Prasain JK, Miller MA. The gap junctional protein INX-14 functions in oocyte precursors to promote C. elegans sperm guidance. Dev Biol. 2011;359:47–58. doi: 10.1016/j.ydbio.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golovko MY, Murphy EJ. An improved LC-MS/MS procedure for brain prostanoid analysis using brain fixation with head-focused microwave irradiation and liquid-liquid extraction. J Lipid Res. 2008;49:893–902. doi: 10.1194/jlr.D700030-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Prasain JK, Hoang HD, Edmonds JW, Miller MA. Prostaglandin extraction and analysis in C. elegans. J Vis Exp. 2013;76:e50447. doi: 10.3791/50447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trufelli H, Palma P, Famiglini G, Cappiello A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom Rev. 2011;30:491–509. doi: 10.1002/mas.20298. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Lawson JA, Reilly M, Adiyaman M, Hwang SW, Rokach J, FitzGerald GA. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F(2)-isoprostanes in human urine. Proc Natl Acad Sci U S A. 1999;96:13381–13386. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song WL, Paschos G, Fries S, Reilly MP, Yu Y, Rokach J, Chang CT, Patel P, Lawson JA, Fitzgerald GA. Novel eicosapentaenoic acid-derived F3-isoprostanes as biomarkers of lipid peroxidation. J Biol Chem. 2009;284:23636–23643. doi: 10.1074/jbc.M109.024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Faouder P, Baillif V, Spreadbury I, Motta JP, Rousset P, Chene G, Guigne C, Terce F, Vanner S, Vergnolle N, Bertrand-Michel J, Dubourdeau M, Cenac N. LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;932:123–133. doi: 10.1016/j.jchromb.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Priddy AR, Killick SR, Elstein M, Morris J, Sullivan M, Patel L, Elder MG. Ovarian follicular fluid eicosanoid concentrations during the pre-ovulatory period in humans. Prostaglandins. 1989;38:197–202. doi: 10.1016/0090-6980(89)90082-8. [DOI] [PubMed] [Google Scholar]
- 38.Miller JE, Ahn SH, Monsanto SP, Khalaj K, Koti M, Tayade C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget. 2016 doi: 10.18632/oncotarget.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 40.Boots CE, Jungheim ES. Inflammation and Human Ovarian Follicular Dynamics. Semin Reprod Med. 2015;33:270–275. doi: 10.1055/s-0035-1554928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasiri N, Moini A, Eftekhari-Yazdi P, Karimian L, Salman-Yazdi R, Arabipoor A. Oxidative Stress Statues in Serum and Follicular Fluid of Women with Endometriosis. Cell J. 2017;18:582–587. doi: 10.22074/cellj.2016.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob KD, Hooten NNoren, Trzeciak AR, Evans MK. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech Ageing Dev. 2013;134:139–157. doi: 10.1016/j.mad.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright K, Luborsky-Moore JL, Behrman HR. Specific binding of prostaglandin F2 alpha to membranes of rat corpora lutea. Mol Cell Endocrinol. 1979;13:25–34. doi: 10.1016/0303-7207(79)90073-x. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto Y, Hasumoto K, Namba T, Irie A, Katsuyama M, Negishi M, Kakizuka A, Narumiya S, Ichikawa A. Cloning and expression of a cDNA for mouse prostaglandin F receptor. The Journal of biological chemistry. 1994;269:1356–1360. [PubMed] [Google Scholar]
- 46.Kitanaka J, Hasimoto H, Sugimoto Y, Negishi M, Aino H, Gotoh M, Ichikawa A, Baba A. Cloning and expression of a cDNA for rat prostaglandin F2 alpha receptor. Prostaglandins. 1994;48:31–41. doi: 10.1016/0090-6980(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 47.Abramovitz M, Boie Y, Nguyen T, Rushmore TH, Bayne MA, Metters KM, Slipetz DM, Grygorczyk R. Cloning and expression of a cDNA for the human prostanoid FP receptor. The Journal of biological chemistry. 1994;269:2632–2636. [PubMed] [Google Scholar]
- 48.Brambaifa N, Schillinger E. Binding of prostaglandin F2 alpha and 20 alpha-hydroxysteroid-dehydrogenase activity of immature rat ovaries throughout pseudopregnancy. Prostaglandins Leukot Med. 1984;14:225–234. doi: 10.1016/0262-1746(84)90206-3. [DOI] [PubMed] [Google Scholar]
- 49.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 50.Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 51.Farhat A, Philibert P, Sultan C, Poulat F, Boizet-Bonhoure B. Hematopoietic-Prostaglandin D2 synthase through PGD2 production is involved in the adult ovarian physiology. J Ovarian Res. 2011;4:3. doi: 10.1186/1757-2215-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


