Abstract
The goal of this series is to offer a survey of the latest literature for clinicians and scientists alike, providing a list of important recent advances relevant to the broad field of allergy and immunology. This particular assignment was to cover the topic of eosinophils. In an attempt to highlight major ideas, themes, trends and advances relevant to basic and clinical aspects of eosinophil biology, a search of papers published since 2015 in JACI and other high impact journals was performed. Manuscripts were then reviewed and organized, and then key findings were summarized. Given space limitations, many outstanding papers could not be included, but the hope is that what follows provides a succinct overview of recently published work that has significantly added to our knowledge of eosinophils and eosinophil-associated diseases.
Keywords: Eosinophilopoiesis, granule biogenesis, eosinophil subsets, apoptosis, adipose tissue, immunoregulation, asthma, CRSwNP, biologicals, EGID
Introduction
While definitively identified using the aniline dye eosin by Paul Ehrlich in 1879, eosinophils had likely been described by others even prior to this report. This underappreciated history of the early characterization of the eosinophil was conveyed by A.B. Kay in a review entitled, “The early history of the eosinophil.”1 For example, Wharton Jones depicted “coarsely granular cells” that resembled eosinophils in a number of species, including the lamprey, frog, fowl, horse, and elephant, as well as in humans prior to the production of eosin. Stacy and Raskin have carried on his legacy by using Wright–Giemsa staining to study eosinophils from a number of reptilian species, showing remarkable morphological beauty and diversity among and, interestingly, even within species.2 The fact that evolutionary pressures over many millennia have maintained the eosinophil lineage in vertebrates is irrefutable evidence of their importance in health. Yet the role of this cell in human wellbeing and disease remains controversial and inexactly defined. A monumental advance in this regard occurred in 2015 and 2016 with the approval of two drugs, both biologicals, whose mechanism of action is to neutralize an essential, selective eosinophilopoietic cytokine, IL-5, providing a critical pharmacologic tool to begin to dissect the contribution of eosinophils to disease. The purpose of this Fundamentals of Allergy and Immunology essay is to highlight key advances in molecular, cellular, biochemical and clinical aspects of eosinophil biology that provide new insight into their role in shaping homeostatic, immune and disease-related responses.
Eosinophil lineage and basic eosinophil biology
Transcriptional control of development
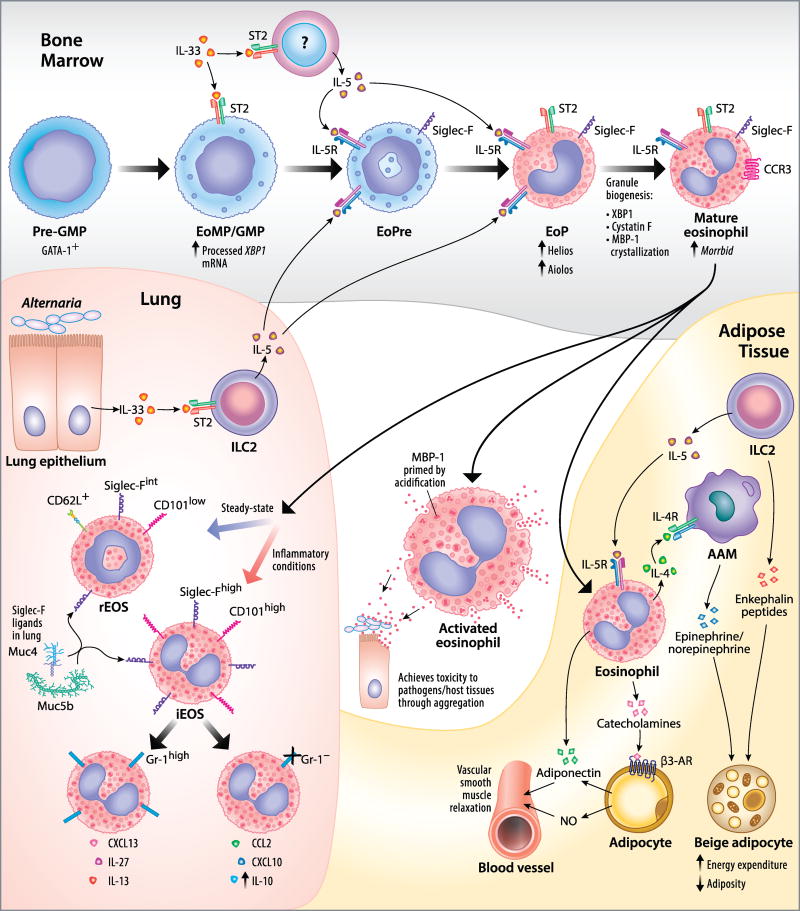
Current data from mice indicate that eosinophils develop from populations of progenitor cells that give rise to all myeloid cells: the pre–granulocyte-macrophage progenitor (pre-GM) and the granulocyte-macrophage progenitor (GMP). However, lymphoid-primed multi-potent progenitors, which give rise to lymphoid and myeloid cells but not megakaryocyte or erythroid lineages, and common myeloid progenitors, which give rise to myeloid, megakaryocyte and erythroid lineages but not lymphoid cells, have been proposed to generate myeloid lineages through the same pre-GM population. Drissen et al. utilized gene profiling of the pre-GM population to segregate these cells according to lineage potential.3 They found that expression of the transcription factor GATA-1 distinguished cells restricted to the mast cell, eosinophil, megakaryocyte, and erythroid lineages (GATA-1+ pre-GMs) from those restricted to the monocyte, neutrophil, and lymphoid lineages (GATA-1− pre-GMs and GMPs) (Figure 1). This result implies that there is an early developmental divergence between these families of lineages that challenges the current dogma.
Fig 1.
Recent advances in our understanding of eosinophil development, subset diversity, and functions in peripheral tissues. Bone Marrow: Eosinophils develop from GATA-1+ pre– granulocyte-macrophage progenitors (pre-GMPs) in the bone marrow. These pre-GMPs give rise to GMPs that (at least in mice) respond to IL-33 through the ST2 receptor, which promotes eosinophil development and IL-5Rα expression. GMPs express higher levels of processed XBP1 mRNA, which is essential later in development. These GMPs give rise to Siglec-F+ IL-5Rα+ mouse eosinophil precursors (EoPres). IL-33 additionally promotes eosinophil development by inducing IL-5 expression from other bone marrow cells, acting on EoPres and eosinophil lineage-committed progenitor cells (EoPs), which then follow EoPres in lineage development. EoPs express higher levels of Helios and Aiolos, members of the Ikaros family of transcription factors, which may play a role in regulating gene expression during eosinophil development and remain highly expressed in mature mouse eosinophils. Proper granule maturation requires expression of the transcription factor XBP1, the inhibition of cysteine protease activity by cystatin F, and the crystallization of the granule protein MBP-1 in a non-toxic form. Improper granule maturation can lead to the loss of cell viability and a blockade of eosinophil development. The long noncoding RNA Morrbid is highly expressed in eosinophils and other short-lived myeloid cells and has been found to prevent cell death by inhibiting the transcription of the pro-apoptotic Bcl2 family member BimActivated eosinophil: In the periphery, activation of the eosinophil leads to granule acidification, thereby priming MBP-1 by altering its conformation. Upon release, MBP-1 exerts a toxic effect on pathogens and host tissues via aggregation. Lung: Distinct eosinophil subsets exist in the mouse lung distinguishable by surface marker expression: lung-resident eosinophils (rEos) that traffic to the lung under steady-state conditions and recruited inflammatory eosinophils (iEos), which may be further subdivided by Gr-1 expression that corresponds to distinct sets of chemokine and cytokine products. rEos appear to possess regulatory properties, such as inhibiting the maturation of type 2-biased allergen-loaded DCs, that iEos do not. Sialosides on the mucins Muc4 and Muc5b bind to Siglec-F to induce eosinophil apoptosis in the mouse airway. ILC2s in the lung, in response to IL-33 signaling due to Alternaria exposure, produce IL-5 that promotes eosinophilopoiesis. Adipose Tissue: IL-5–activated mouse eosinophils indirectly promote energy expenditure in beige adipocytes by inducing the release of epinephrine and norepinephrine from alternatively activated macrophages (AAM) through IL-4 secretion. ILC2s produce IL-5 but also directly and independently act on beige adipocytes via the release of enkephalin peptides. Eosinophils directly and indirectly cause blood vessel relaxation in perivascular adipose tissue through adiponectin and catecholamine release, respectively. The catecholamines signal through β3-adrenergic receptors (β3-AR) on adipocytes to cause vessel relaxation via nitric oxide (NO) and adiponectin. Illustration by Jacqueline Schaffer.
To assess global transcriptomic changes that occur during homeostatic eosinophil development, Bouffi et al. sorted GMPs, eosinophil lineage-committed progenitors (EoPs), and mature resting eosinophils from mouse bone marrow and analyzed them via RNA sequencing.4 Associated with eosinophil lineage commitment (between the GMP and EoPs stages) and eosinophil maturation (between the EoP and Eos stages) were substantial changes in 490 genes and 1199 genes, respectively. Included among the genes that were expressed by eosinophils but not GMPs were 56 transcription factors, including two Ikaros family members, Helios and Aiolos, that were expressed by both EoPs and eosinophils and that have not previously been associated with the eosinophil lineage previously (Figure 1).
Granule biogenesis
During their development, eosinophils synthesize large amounts of toxic granule proteins that must be post-translationally modified and sequestered to maintain cell viability and ensure proper function. Three recent studies have highlighted novel points of regulation of granule biogenesis and its importance in eosinophil development and survival. The transcription factor XBP1 is generally associated with highly secretory cells, such as plasma cells, Paneth cells, or pancreatic acinar cells, and plays a role in regulating the unfolded protein response by promoting the transcription of genes encoding stress-response factors. XBP1 was not known previously to play a role in hematopoietic stem cells. However, after deleting Xbp1 in the hematopoietic lineage, Bettigole et al. discovered that this transcription factor is uniquely essential for eosinophil development.5 The presence of the active, spliced form of Xbp1 mRNA was found to peak during the GMP stage but remained prevalent until eosinophil maturation (Figure 1). Deletion of Xbp1 in the hematopoietic lineage did not affect the proportion of GMPs but significantly reduced the proportion of EoPs and completely eliminated mature eosinophils. This effect appears to be due to defects in the post-translational maturation of granule proteins MBP and EPX, disrupted granule formation, and downstream effects on GATA-1.
In addition to XBP1, the endogenous cysteine protease inhibitor cystatin F, also called leukocystatin, is necessary for proper granule biogenesis and eosinophil survival. Loss of cystatin F in mice uniquely affected the eosinophil compartment by leading to impaired granule formation and reduced cell viability (Figure 1).6 The effect on eosinophils could be reversed using pharmacologic inhibitors of cysteine proteases, suggesting that the regulation of protease activity was necessary for the proper maturation of granule proteins. Major basic protein (MBP) forms the electron-dense core of the secondary granules. Previously, it was not known how MBP was stored or mobilized in such a way to protect the eosinophil from its toxic effects. Using X-ray-free electron laser crystallography and granule core isolation, it was demonstrated that MBP is sequestered in a non-deleterious form as a nanocrystal and is mobilized during degranulation by acidification of the granule (Figure 1).7
Role of IL-33 in eosinophilopoiesis
The IL-1 family cytokine IL-33 signals through its receptor, ST2, which is expressed on a number of cell types involved in type 2 immunity, to initiate inflammatory responses. While IL-33 has previously been shown to activate eosinophils, several recent studies examined the role played by IL-33 in promoting eosinophilopoiesis. Anderson et al. examined how exposure of naïve BALB/c mice to a common fungal aeroallergen, Alternaria alternata, not only causes eosinophil recruitment to the lung but accelerates eosinophilopoiesis in the bone marrow.8 This phenomenon was ablated when the increased circulating IL-5 was neutralized or when the experiment was performed with ST2-deficient or ILC2-deficient mice. Together, these data suggest that ILC2s in the context of fungal allergen exposure respond to IL-33 by secreting IL-5 that promotes eosinophilopoiesis (Figure 1). However, the importance of IL-33 in eosinophilopoiesis extends to a direct role under steady-state conditions. Johnston et al. discovered that loss of IL-33 responsiveness in ST2-deficient mice reduced the rate of homeostatic eosinophilopoiesis.9 In contrast, IL-33 administration promoted eosinophil development both by increasing IL-5 production in the bone marrow and by expanding the number of IL-5Rα+ eosinophil precursors (Figure 1).
Regulation of eosinophil longevity
Beyond the modulation of eosinophil production in the bone marrow, eosinophil numbers can be regulated in the periphery in both cell-intrinsic and cell-extrinsic ways. Kotzin et al. showed that the long noncoding RNA Morrbid is present at high levels in mature eosinophils and other short-lived myeloid cells and that its deletion in mice leads to a loss of these cells via apoptosis in a cell-intrinsic manner (Figure 1).10 Furthermore, Morrbid levels were increased by pro-survival cytokine signaling in mouse eosinophils and in human eosinophils from donors with hypereosinophilic syndrome. In addition to their effects on Morrbid expression, another recent report found that pro-survival cytokines promoted eosinophil survival by upregulating Bcl-xL in a manner that is prevented by an inhibitor of NF-κB signaling.11 Accordingly, eosinophil-specific deletion of the endogenous inhibitor IκBα resulted in constitutively active NF-κB and promoted Bcl-xL expression and eosinophil survival. Bcl-xL was both necessary and sufficient for eosinophil survival through overexpression studies. Thus, pro-survival cytokines regulate eosinophil survival via several pathways that together modulate the balance of pro-survival and pro-apoptotic Bcl2 family members.
In a cell-extrinsic manner, eosinophil longevity can be regulated through the engagement of Siglec-F in the mouse. Previous reports indicated the presence of endogenous lung Siglec-F ligands that were cytokine-inducible and protease- and sialidase-sensitive. Kiwamoto et al. identified two glycoproteins from mouse lung, the mucins Muc4 and Muc5b, that display ligands for Siglec-F (Figure 1).12 They additionally showed that mucins isolated from mouse tracheal epithelial cells bind to unmasked Siglec-F on mouse eosinophils and induce their death. Finally, exacerbated eosinophilic airway inflammation was demonstrated in conditional Muc5b–deficient mice, indicating that this mucin contains Siglec-F sialoside ligands capable of resolving eosinophilic inflammation in vivo. Another lectin with previously acknowledged immunomodulatory activities on other cell types, galectin-1, can also help resolve eosinophilic inflammation in the mouse. Galectin-1 binds to LacNAc residues in O- or N-linked glycans on the cell surface. A recent study found that galectin-1 expression was induced in response to airway inflammation and bound to eosinophil cell-surface N-linked glycans. At low concentrations (≤0.25 µM), galectin-1 promoted eosinophil adhesion to VCAM-1 and inhibited migration toward eotaxin-1. However, at higher concentrations (≥1 µM), galectin-1 induced eosinophil apoptosis in a MEK-dependent and caspase-independent manner.
The molecule CRTh2, also known as the prostaglandin D2 (PGD2) receptor, can be used as a marker of a number of type 2 immune cells, including eosinophils. Because asthma pathogenesis involves more than a single cell type or inflammatory mediator, Huang et al. focused on CRTh2 as a therapeutic target to deplete these cells.14 They developed a mouse expressing human CRTh2 on eosinophils, basophils, and ILC2s and an antibody with enhanced antibody-dependent cell cytotoxic activity against the protein. They showed that treatment of these mice with the antibody depletes hCRTh2-expressing cells and reduces type 2 immunity in response to N. brasiliensis helminth infection.
Molecular profiling and phenotypic diversity of eosinophils
Molecular profiling can be used to compare eosinophil populations from different conditions or patients with one another in an unbiased manner. This more complete picture of molecules present in eosinophils can also be used to generate new hypotheses or help answer existing questions. Previously, relatively few of the proteins present in eosinophils had been annotated. Hence, Wilkerson et al. sought to define the proteome of the resting peripheral blood eosinophil and analyze changes in response to IL-5 stimulation by phosphoproteomics.15 Their analyses expanded the number of proteins identified in the eosinophil to 7,086 and ranked them according to estimated relative abundance, providing a wealth of information to mine for future studies. The phosphoproteomics data detected 220 phospho-isoforms that were significantly changed after 5 minutes of IL-5 stimulation, including some that were unanticipated and have not yet been characterized in eosinophils.
By flow sorting peripheral blood eosinophils from patients with asthma or other eosinophilic disorders or healthy subjects and performing RNA microarrays, Barnig et al. aimed to determine whether there were transcriptional differences that defined the eosinophil populations from these patients.16 They found that the transcriptional profile of eosinophils from patients with asthma was similar to those from patients with peripheral eosinophilia related to dermatological disease, parasitosis, or pulmonary aspergillosis. The eosinophils from patients with asthma were found to have higher mRNA levels for IL2RA, IL10RA, and LIPA, for example, and lower levels for IL8, CCL3, and AQP9, all of which were validated by real-time RT-PCR.
Beyond the distinctions between eosinophil populations in different individuals and disease states, there have been several recent studies highlighting the phenotypic diversity of eosinophils within an organism. Although lung-resident eosinophils (rEos) had been acknowledged before, no reports had fully characterized this population. A report by Mesnil et al. showed that this population in mice can be distinguished from recruited inflammatory eosinophils (iEos) on the basis of surface marker expression (rEos are Siglec-Fmt CD62L+ CD101low whereas iEos are Siglec-Fhigh CD62L− CD101high) (Figure l).17 An eosinophil population that resembled the lung-resident population could also be identified in the blood. Additional experiments supported a regulatory role for the rEos subset via suppressive effects on DC maturation and function. They found evidence of a similar lung-resident population in humans (Siglec-8+ CD62L+ IL-3Rl0W rEos vs. Siglec-8+ CD62Llow IL-3Rhigh iEos) but the function of these eosinophils, compared to others, remains to be defined. Another group examined mouse eosinophil subsets in the lung in response to allergen sensitization and challenge.18 On the basis of this inflammatory context, these subsets are depicted in Fig. 1 as probable subdivisions of the iEos subset, although this has not been definitively demonstrated. While Gr-1 expression is typically associated with neutrophils, Percopo et al. found a distinct subset of eosinophils in the lung that co-express this marker with Siglec-F, are absent in eosinophil-deficient AdblGATA mice, and that produce a set of chemokines and cytokines that differs from their more numerous Grl− counterparts. Finally, Abdala Valencia et al. examined the phenotypes of eosinophils that are recruited to distinct lung compartments (parenchyma versus airways) in response to allergen sensitization and successive challenges.19 They found an intriguing accumulation of a Siglec-Fhigh CD1 lclow eosinophil population (compared to an initial Siglec-Fint CD1lc− phenotype) in the lung tissue and that only the CD1 lc-bearing eosinophils localized to the airway, suggesting a possible role for this integrin in migration to this specific lung compartment.
Eosinophils and mechanisms of disease pathogenesis in pre-clinical models
Diseases of the skin and airways
Eosinophils may play a role in contactant-induced itch based on results from trimellitic anhydride (TMA) treatment of WT or eosinophil-deficient PHIL mice.20 The authors of this study found that in the absence of eosinophils, inflammatory cell infiltration, tissue remodeling, increased skin innervation, and itching due to TMA treatment were all reduced, indicating a role, either direct or indirect, of eosinophils in this response. While eosinophils are not normally present in healthy skin, they are abundant in a variety of skin diseases. The mechanism of eosinophil recruitment to the skin in an IgE-dependent model of eosinophilic dermatitis was investigated by Cheng et al. in a recent study.21 Eosinophil skin infiltration in response to passive sensitization and cutaneous antigen exposure was unaffected in mast cell–deficient mice but was ablated in basophil-deficient mice. The role of basophils in this response was corroborated in an active sensitization model as well, and was shown to be dependent on basophil-derived IL-4. In response to IL-4, endothelial cells express VCAM-1, which is important for eosinophil entry.
It has been assumed that eosinophil degranulation plays a role in the airway hyperresponsiveness and tissue remodeling observed in asthma. However, Jacobsen et al. found little evidence for this using a mouse model of chronic Th2 lung inflammation in which peripheral T cells constitutively express IL-5 and the central airway epithelium expresses human eotaxin-2 to drive eosinophil recruitment.22 They reported that, in this model, EPX is partially responsible for mucin induction in the airway but neither EPX nor MBP play major roles in inflammatory cell infiltration, collagen deposition, or methacholine-induced airway hyperresponsiveness. Instead, they showed that IL-13 is necessary for these eosinophil-dependent effects.
Diseases of the digestive tract
While eosinophils home in large numbers to the gut during steady-state conditions, their role in promoting inflammatory conditions such as chronic colitis was not yet well defined. Griseri et al. showed that eosinophils, but not neutrophils, were required for maximal inflammation in a Helicobacter hepaticus– and IL-10R blockade–dependent model of colitis.23 GM-CSF produced in response to the infection was responsible for the influx of inflammatory eosinophils, which released IL-13, TNF-α, and EPX, the last of which promoted colitis. In a dextran sulfate sodium–induced mouse model of colitis, Moshkovits et al. demonstrated that CD300f, expressed on eosinophils rather than on inflammatory monocytes, was partially responsible for disease severity.24 This receptor appeared to act in an activating or co-stimulatory fashion on eosinophils by promoting IL-6 and TNF-α production in response to stimulation with heat-killed E. coli. In contrast to the previously discussed roles of eosinophils in promoting disease in the skin, airways, and digestive tract, Sugawara et al. showed that small intestinal eosinophils uniquely (compared to blood or bone marrow eosinophils) expressed high levels of IL-1 receptor antagonist in response to GM-CSF signaling and thereby suppressed inflammatory Th17 responses in the intestine.25
Paired Ig-like receptor B (PIR-B) is an inhibitory receptor expressed on the surface of eosinophils and other cells. A study by Ben Baruch-Morgenstern et al. showed that PIR-B suppresses eosinophil accumulation and activation in the esophagus in a mouse model of EoE involving lung-specific and doxycycline-inducible IL-13 expression.26 The authors found that in PIR-B–deficient mice, eosinophils were present in the esophagus in greater numbers and tissue remodeling was enhanced, exacerbating the deleterious impact of eosinophils in this model.
Roles of eosinophils in cancer
In addition to inflammatory diseases, two new studies have shown diverse roles for eosinophils in tumor rejection and metastasis. The first, a study by Zaynagetdinov et al., showed that loss of IL-5 impeded tumor metastasis to the lung in a number of transplantable tumor models.27 This phenomenon was partially reversed by the administration of bone marrow–derived eosinophils back into IL-5–deficient mice. Tumor-induced CCL22 production by eosinophils in this model was necessary for the recruitment of regulatory T cells that established a suitable microenvironment for tumor metastasis, indicating that eosinophil activity can be exploited by tumors to promote metastasis. In contrast, another study demonstrated that eosinophils were important in anti-cancer immunity.28 Using an OVA-expressing B16 melanoma tumor model, the authors showed that Treg depletion led to tumor rejection in an eosinophil-dependent manner and that the adoptive transfer of OT-I OVA-reactive CD8 T cells with IFN-γ– and TNF-α–activated eosinophils was sufficient to cause tumor rejection whereas neither cell population alone was sufficient. The effect of the eosinophils in this model was likely due to the promotion of CD8+ T cell infiltration via the production of chemokines by eosinophils in the tumor microenvironment and the restoration of normal vascular architecture through an undefined mechanism.
Regulatory roles of eosinophils in pre-clinical disease models
In contrast to their expected role in allergic airway inflammation, Takeda et al. showed that eosinophils are (1) not necessary for the development of airway hyperresponsiveness in mice in response to repeated allergen challenges and (2) eosinophils are instead necessary for the resolution of such inflammation.29 This group arrived at this surprising finding by using an allergic sensitization and challenge model, employing assays after either seven or eleven challenges, in both wild type and eosinophil-deficient PHIL mice. The PHIL mice not only exhibited the same degree of airway hyperresponsiveness after seven challenges but a trend toward greater hyperresponsiveness after eleven challenges and significantly more mucin deposition. By restoring either wild type or IL-10–deficient eosinophils to the PHIL mice, the authors found that eosinophils, through their production of IL-10, exert a beneficial immunoregulatory function that helps resolve airway inflammation.
Inflammatory arthritis occurs through type 1– and type 17–polarized immune responses in the context of autoimmunity. Thus, the systemic introduction of stimuli that may skew immunity toward a type 2 response may assist with the resolution of such responses. A study by Chen et al. made use of N. brasiliensis helminth infection and systemic IL-5 overexpression to determine whether this may occur in two mouse models of inflammatory arthritis.30 The authors found that these stimuli reduced disease severity in association with an influx of eosinophils into the affected joints. The amelioration of joint disease was dependent on IL-4 and IL-13 signaling, and the use of eosinophil-deficient ΔdblGATA mice led to a partial reduction in this protective effect, implicating eosinophils directly in this protective effect.
Roles of eosinophils in adipose tissue
Recently, diverse roles for eosinophils in the homeostatic regulation of adipose tissue (AT) have come to light. Eosinophils and alternatively activated macrophages (AAMs) in AT have been implicated in the induction of a brown adipocyte phenotype in white adipocytes, a process known as browning or beiging. These “beige” adipocytes, due to expression of the uncoupling protein UCP1, increase energy expenditure and thermogenesis and reduce adiposity. A study by Brestoff et al. refined the relationships between the immune cells that regulate this process.31 The authors found that ILC2s, which are present in AT, regulate the recruitment and activity of eosinophils and AAMs through IL-33–induced production of IL-5 and IL-13. Eosinophils further act on AAMs by producing IL-4. Both AAMs and ILC2s produce signaling molecules that act directly on adipocytes to induce beiging, including AAM-derived epinephrine and norepinephrine and ILC2-derived enkephalin peptides (Figure 1). A study by Suárez-Zamorano et al. dealing with the same topic found that microbiome depletion, either through antibiotic treatment or by using germ-free mice, similarly leads to fat browning.32 Through an unknown mechanism, microbiome depletion leads to the local production of IL-4, IL-5, and IL-13 in the inguinal subcutaneous AT and the recruitment and conversion of eosinophils and AAMs in an IL-4Rα–dependent manner. These data suggest that eosinophils promote leanness, at least in mice. Beyond the role of eosinophils in regulating AT metabolism, eosinophils may play an important role in regulating the anti-contractile function of perivascular AT (PVAT). A study by Withers et al. showed that the constriction of small mesenteric arteries is reduced in the presence of PVAT in lean mice, whereas this effect is lost in obese mice, in which there are fewer PVAT eosinophils.33 In an ex vivo model of artery constriction, wild type or iNOS-deficient eosinophils, but not those lacking adiponectin expression, induced vessel relaxation. Finally, the authors showed that eosinophils contain tyrosine hydroxylase (TH) and catecholamines and find that an inhibitor of TH partially eliminates the relaxation effect induced by eosinophils. Together, these data indicate that eosinophils act directly on vascular smooth muscle through adiponectin expression and indirectly through adipocytes through catecholamine secretion to induce vessel relaxation (Figure 1).
Many studies of the relationship between eosinophils and AT have made use of models in which eosinophils are systemically absent or in excess. While eosinophils are present in the AT of lean WT mice and are reduced in the AT of obese WT mice, Bolus et al. noted augmented eosinophil levels in the AT of both lean and obese CCR2-deficient mice as well as the peritoneal cavity but not in the blood, spleen, liver, or bone marrow, providing a promising system to study the local effects of increased eosinophil levels.34
Eosinophilia, hypereosinophilia and hypereosinophilic syndromes
Eosinophilia is defined as an increase in blood eosinophil counts above the upper limit of normal, typically >0.5 × 109/L. The term hypereosinophilia is used to designate eosinophil counts ≥1.5 × 109/L, mainly because the differential diagnosis narrows. Hypereosinophilic syndromes (HES) are defined by persistent hypereosinophilia along with eosinophil-related organ involvement that is otherwise not attributable to any other diagnosis. The reader is directed to several excellent recent reviews focused on the diagnosis and treatment of patients with eosinophilia or hypereosinophilia.35,36
Important advances in the HES field included a retrospective analysis from the Mayo Clinic defining the risk of developing hematologic malignancies in patients with eosinophilia or hypereosinophilia, which fortunately was low over a 13-year period (0.2% of 2642 patients identified), and mainly consisted of T-cell malignancies in those with hypereosinophilia.37 Khoury et al. reported on four subjects with an extremely rare cyclic form of HES called Gleich syndrome or episodic angioedema with eosinophilia. They identified several novel features associated with the unique, roughly monthly periodicity of their recurrences: namely evidence for T-cell clonality with the presence of an abnormal CD3− CD4+ T cell population that is reminiscent of what is often seen in the lymphocytic variant of HES, except that there was a concomitant cycling increase of other cells besides eosinophils (e.g., lymphocytes and neutrophils) along with cyclic production of IL-5 and other type 2 cytokines that preceded each episode.38 What remains baffling is why this unique periodic pattern occurs. These same primary authors separately reported findings from a study treating HES patients with imatinib regardless of imatinib-responsive platelet-derived growth factor receptor-alpha (PDGFRA)-associated mutation status. As expected, they saw 100% response rates in those with the diagnosis of FIP1L1-PDGFRA-myeloid neoplasm. Interestingly, they observed ≈50% response rates in PDGFRA-negative HES with ≥ 4 criteria suggestive of a myeloid neoplasm, which strongly implicates the presence of other gain-of-function mutations responsive to tyrosine kinase inhibition. In marked contrast, none of the subjects with steroid-refractory, PDGFRA-negative HES with < 4 criteria suggestive of a myeloid neoplasm responded to imatinib. These data suggest that the presence of ≥ 4 myeloid features, even in the absence of the classic FIP1L1-PDGFRA deletion mutation, was the best predictor of imatinib responsiveness, and thus in whom a trial of imatinib may be warranted.39 Finally, while discussion in this particular section of the review may be premature, it was intriguing to read that the oral drug dexpramipexole (an enantiomer of pramipexole, a dopamine agonist approved for the treatment of Parkinson and restless legs syndrome), while being tested in clinical trials for efficacy in amyotrophic lateral sclerosis, did not have clinical efficacy, but on safety monitoring was noted to cause a slow-onset, sustained and selective eosinopenia.40 As a result of this unexpected finding, dexpramipexole is undergoing clinical trials based on its anti-eosinophil properties and, if safe and effective, could become the first oral agent to selectively reduce eosinophil numbers.
Two additional studies examined eosinophilic drug-induced reactions, one focusing on outpatient and the other on inpatient events. One was a prospective study of more than 800 former inpatients who continued to receive antibiotics in the outpatient setting, and revealed an unexpectedly high rate of eosinophilia (25%) and a nearly 1% rate of DRESS syndrome.41 Most episodes were attributable to the use of antibiotics, and those most commonly implicated were vancomycin, penicillin, rifampin, and linezolid. The development of eosinophilia was associated with an increased risk of developing rash and renal function abnormalities but not liver injury. In the other study, based at a single tertiary care hospital in Spain that followed patients prospectively during their hospitalization, found an incidence of eosinophil-associated drug reactions of 16.67 per 10,000 admissions and involved a wide range of drugs. Slightly more than half of the 274 cases in which the eosinophil count was ≥ 0.7 × 109/L were asymptomatic. But 44% of cases developed skin, soft tissue and/or organ involvement, with about half of these cases potentially being DRESS syndrome. The authors also concluded that the main predictors of severity were an earlier appearance and higher degree of eosinophila.42
The role of eosinophils in airways disease
The upper airway
Among eosinophilic diseases of the upper airways, chronic rhinosinusitis, especially the subset accompanied by nasal polyposis (CRSwNP), remains a particularly recalcitrant disorder. While our understanding of its pathophysiology continues to improve43, our ability to monitor disease activity and provide effective, long-lasting treatment remains unsatisfactory. Several recent publications found useful correlations between eosinophil-related biomarkers assessed in the upper airways and either upper or lower airways eosinophilic inflammation. Analysis of microparticles originating from various cellular sources, obtained by sampling nasal secretions in CRS subjects, provided unique signatures that appear to distinguish between subsets of CRS. For example, elevated numbers of microparticles from activated mast cells, basophils and platelets were seen more commonly in those with CRS with aspirin-exacerbated respiratory disease (AERD) than in CRS alone, while microparticle signatures consistent with epithelial injury were higher in AERD and CRS without NP than in CRSwNP.44 To examine local, mucosal anti-inflammatory responses, Jia et al. explored the presence of glycan ligands for the pro-apoptotic receptor Siglec-8 on eosinophils in normal and CRS upper airway tissue samples, and showed that specific, high-molecular-weight, sialic acid-containing ligands were abundant in submucosal glands and were increased in CRS.45 This could represent an endogenous pathway for controlling local eosinophilic inflammation. Proton pump inhibitors (PPIs) are commonly used to treat gastroesophageal reflux disease and some forms of eosinophilic esophagitis, so it was interesting to read that these agents, at physiologically relevant concentrations, also inhibit IL-13–induced epithelial expression of the important eosinophil chemoattractant CCL26 in vitro, and do so via the nongastric H,K-ATPase pathway. Additionally, CRS subjects on PPIs were found to have lower CCL26 levels compared to those not taking PPIs. One possible conclusion for this study is that PPIs might be beneficial in reducing eosinophilic inflammation via a pathway that is independent of its effects on gastric acidity.46 Experiments sampling the upper airway as a proxy for the lower airway included one study in which levels of eosinophil peroxidase in secretions obtained from the nasal passages and pharynx of inadequately controlled asthmatics correlated with sputum eosinophil counts.47 Finally, in the realm of novel therapeutic approaches, particularly promising was a study of the efficacy of dupilumab, an antibody to the IL-4Rα subunit that blocks the activity of both IL-4 and IL-13, in subjects with CRSwNP inadequately controlled with intranasal steroids.48 Compared to the nasal steroid–only treatment group, significant improvements were seen in polyp burden scores and sense of smell in the nasal steroid– plus dupilumab-treated group. While longer and larger studies are needed, these data clearly demonstrate the importance of the IL-4/IL-13 axis in CRSwNP.
The lower airway
Monitoring or predicting disease development, activity and response to therapy
Sverrild et al. reported fascinating correlative results related to the diversity of the airway microbiome in patients with eosinophil-low and eosinophil-high asthma based on the 16S rDNA sequences detected in the BAL fluid from each subject.49 The study found that microbial diversity was significantly lower in eosinophil-low asthmatics and that numerous statistically significant differences existed with respect to the abundance of several microbial genera. No such differences were found with respect to neutrophil-high or -low asthmatics. Neither the underlying reason for such differences nor their relationship to disease progression is clear. Wang et al. examined the influence of sputum mast cell subtypes on eosinophilia and asthma control in patients.50 They found that levels of the MCT/CPA3 subtype expressing both tryptase and carboxypeptidase 3 correlated with sputum eosinophil levels, elevated FeNO, and poorer asthma control. The role of this mast cell subtype in the establishment of eosinophilic asthma has not yet been defined.
Two IL-5–targeting therapies are now available for the treatment of eosinophilic asthma, namely mepolizumab and reslizumab (Table 1). The primary endpoint for approval of both agents was a reduction in asthma exacerbations. While we still do not know why neutralizing IL-5 (and by inference, lowering eosinophils) reduces asthma exacerbations, and while these two biologics have never been directly compared in the same clinical trial, what is emerging in the literature is that when used in the right population of asthmatics, they are indeed effective. Such studies are also giving us a better sense of how eosinophil counts in the blood relate to eosinophil counts in the airways in asthma, and how this information might influence treatment choice and outcomes.51 Several reports, including some that were retrospective, showed that blood eosinophil counts predict treatment outcomes with mepolizumab use, especially in severe asthma, including the observation that with a baseline blood eosinophil count ≥ 0.15 × 109/L, clinically meaningful reductions in exacerbation rates are achieved.52,53 Additional findings included the observation that elevated blood eosinophil counts ≥ 0.45 × 109/L, a higher cutoff than those used by the aforementioned clinical trials, predict sputum eosinophilia54, and that levels of IL-13 and IL-5 in the serum are useful in identifying the eosinophilic asthma subset.55 Revisiting the concept of using sputum eosinophils to assess asthma control, Demarche et al. followed sputum eosinophilia longitudinally in a clinical practice setting and reported that in the subgroup of intermittent or persistently eosinophilic asthmatics, a decrease of 3.4 fold or 4.3% in sputum eosinophils predicted improvement in asthma control, while an increase of 3.5% or 1.8 fold was associated with a deterioration of control.56 A prospective study done at Kaiser Permanente showed that in severe uncontrolled asthmatics age 12 or above, a blood eosinophil count ≥ 0.4 × 109/L was associated with a 1.55-fold risk of having ≥ 2 asthma exacerbations or asthma-related emergency department visit or hospitalization over a one-year period.57 Two studies examined blood eosinophil counts in COPD. One study from Copenhagen found that in subjects in the general population with COPD followed for a median of 3.3 years, those with a blood eosinophil count ≥ 0.34 × 109/L had a 1.76-fold greater risk of severe exacerbations58, while a separate multicenter study found no influence of blood eosinophil counts on COPD exacerbation rates among those receiving either indacaterol plus glycopyronium versus fluticasone plus salmeterol.59 Finally, several studies evaluated blood eosinophil counts in other unique circumstances. Anderson et al. reported that in children, evidence of aeroallergen sensitization and a blood eosinophil count ≥ 0.3 × 109/L at the age of two years were associated with a 3.1–3.3-fold increased risk of having asthma at age 6, but blood levels of periostin were not predictive, perhaps due to bone-derived sources related to bone turnover in this younger age group.60 In exacerbation-prone asthmatics followed in the National Heart Lung and Blood Institute’s Severe Asthma Research Program-3 study, blood eosinophils were positively associated with higher exacerbation frequency (1.6-fold for every log of eosinophil levels), and, reminiscent of comments above, the presence of CRS was independently associated with a 1.7-fold increase in asthma exacerbation frequency.61 One additional, retrospective, single-center report of 15 patients treated with bronchial thermoplasty for severe asthma found a 50% decline in blood eosinophil counts (from a pretreatment mean of 0.33 × 109/L to a post-treatment mean of 0.17 × 109/L). This was associated with a significant reduction in median number of emergency department and outpatient doctor visits but without any accompanying improvement in lung function.62 Taken together, these studies consistently report associations between eosinophils and CRS, asthma and asthma control, especially in certain cohorts of asthmatics, such as those at the severe end of the spectrum.
Table 1.
Newly approved drugs and others in development that directly or indirectly target eosinophils and that are mentioned in this article.
| Drug | Form | Drug Target | Approved indication (or recent clinical trial) |
|---|---|---|---|
| Mepolizumab | Humanized IgG1 given subcutaneously | IL-5 | Asthma age 12 and older (and others) |
| Reslizumab | Humanized IgG4 given intravenously | IL-5 | Asthma age 18 and older (and others) |
| Dupilumab | Human IgG4 given subcutaneously | IL-4Rα, blocking both IL-4 and IL-13 | Adults with atopic dermatitis (and asthma, CRSwNP) |
| Benralizumab | Humanized afucosylated IgG1 given subcutaneously | IL-5Rα | Asthma and others |
| Dexpramipexole | Oral small molecule | Unknown | CRSwNP and others |
| Lebrikizumab | Humanized IgG4 given intravenously | IL-13 | Asthma and others |
| Dectrekumab (QAX576) | Humanized IgG1 given intravenously | IL-13 | Asthma, EoE and others |
| SB010 | Inhaled small molecule | GATA3-specific DNAzyme | Asthma |
| Fevipiprant | Oral small molecule | CRTh2 (DP2, type 2 PDG2 receptor) | Asthma |
| Budesonide | Oral suspension; small molecule | Glucocorticoid receptor | EoE |
CRSwNP, chronic rhinosinusitis with nasal polyposis; EoE, eosinophilic esophagitis
Asthma studies involving eosinophil-related treatments that are not yet approved
The first study to be mentioned in this section is a successful trial of mepolizumab in eosinophilic granulomatosis with polyangiitis (EGPA, formerly known as Churg-Strauss syndrome).63 It is mentioned here because the study involved the subcutaneous administration of 300 mg every four weeks instead of the currently approved and available dose of 100 mg every four weeks. This multicenter, controlled trial enrolled 136 subjects with stable disease on a stable prednisolone or prednisone dose along with standard EGPA care. Treatment with mepolizumab led to a greater likelihood of having 24–48 weeks of disease remission (≈30% on active drug versus ≈3% on placebo), and 44% of the participants in the mepolizumab group, compared with 7% of those in the placebo group, ended up needing an average daily dose of prednisolone or prednisone of ≤ 4 mg per day during the last four weeks of this year-long study. Somewhat disappointing was the finding that remission did not occur in 47% of the participants in the treated group, although this was clearly better than rates of 81% for those on placebo.
Besides IL-5–targeting antibodies, other agents that directly or indirectly target eosinophils are in various stages of clinical development (Table 1). Particularly advanced are trials using benralizumab (anti-IL-5 receptor antibody), dupilumab and lebrikizumab (anti-IL-13 antibody). Various multicenter, international, phase 3 studies in severe, poorly controlled asthma with benralizumab consistently show marked, sustained reductions in eosinophils (and basophils), as well as safety and efficacy. Favorable outcomes included improvements in asthma control, reductions in asthma exacerbations and oral steroid-sparing effects, along with some, but inconsistent, evidence of improvement in lung function.64–66 When studied in mild-to-moderate persistent asthmatics receiving inhaled corticosteroids, a small but statistically significant improvement of 80 mL in the prebronchodilator FEV1 was seen at 12 weeks in the benralizumab treated group but not with placebo.67 Whether this is clinically significant and impactful on disease activity or progression is unknown, but the modest degree of improvement does not yet justify its use in this milder asthmatic population.
As a follow up to a successful initial trial of dupilumab in subjects with persistent, moderate-to-severe asthma and a blood eosinophil count ≥ 0.3 × 109/L or sputum eosinophils ≥ 3%68, a subsequent larger phase 2b study was completed examining the benefits of adding dupilumab to medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist in patients with uncontrolled persistent asthma. For this study, subjects were enrolled regardless of their eosinophil count. Particularly striking was the finding that on average, dupilumab improved FEV1 by 0.39 L and reduced severe exacerbations by 70%; this occurred irrespective of baseline eosinophil counts.69 The results with dupilumab are in contrast to disappointing results from two international phase 3 studies, each with over 1,000 subjects with uncontrolled asthma despite using inhaled corticosteroids and at least one controller medication, who were randomized to receive placebo or lebrikizumab. This study failed to meet its primary endpoint, which was a reduction in exacerbations over a one-year period in subjects with high blood eosinophil counts or periostin levels. This was due to the fact that one study reached statistical significance while the other one did not.70 Given the contrast between these dupilumab and lebrikizumab results, one is tempted to conclude that targeting both IL-4 and IL-13 is better than targeting IL-13 alone, and/or that targeting the receptor rather than the cytokine is more efficacious.
Two other agents in clinical trials that are not biologics and target very distinct pathways deserve mention due to recent publications. One is an inhaled agent that is an anti-sense DNA called SB010 designed to eliminate the master Th2 transcription factor GATA3. GATA3 is expressed not only in Th2 cells, but also in mast cells, eosinophils and other cells.71 When inhaled once daily for four weeks in subjects with mild allergic asthma with sputum eosinophilia prior to inhaled allergen provocation, Krug et al. showed that it reduced both the early- and late-phase asthmatic response.72 Further analyses in a subsequent report from the same group showed that protection from lung function decline in this model was more marked with increasing blood eosinophil counts and with levels of exhaled nitric oxide.73 The exact reason for the beneficial effects seen with SB010 on the acute and late response is unknown, but it did reduce serum IL-5 levels by a modest degree. So far, there are no published data from asthma clinical trials with SB010. Separately reported were the results of a phase 2 single-center trial in subjects with moderate to severe persistent asthma and sputum eosinophil counts ≥ 2% of twice-daily oral administration of fevipiprant (QAW039).74 This drug is a small molecule antagonist of the PGD2 receptor, also known as CRTh2 or DP2 and expressed on many cell types associated with type 2 inflammation, including eosinophils. After 12 weeks of treatment, which was well tolerated, there was a significant reduction in sputum eosinophils from a geometric mean of 5.4% to 1.1% in the fevipiprant group compared to a decline from 4.6% to 3.9% in the placebo-treated group. Whether or not this agent is beneficial in asthma remains to be seen.
Eosinophilic gastrointestinal disorders (EGID): diagnosis, pathophysiology and treatment
The term EGID refers to any disorder involving the accumulation of an abnormal number of eosinophils within a particular region of the gastrointestinal tract, and includes eosinophilic esophagitis (EoE), eosinophilic gastritis (EoG), eosinophilic colitis (EC) and combinations such as eosinophilic gastroenteritis. The prevalence of these disorders, and the role played by food in causing these disorders, decreases the further down one goes within the gastrointestinal tract. Therefore, most of the attention has been paid to EoE. Endoscopy with biopsy remains the mainstay of diagnosis and assessment of disease activity. EoE is also diagnosed partly on the basis of a failed proton pump inhibitor trial, which differentiates it from PPI-responsive esophageal eosinophilia (PPI-REE), a disorder with unknown etiology that otherwise shares many clinical characteristics with EoE. Wen et al. utilized transcriptomics to compare the inflammatory conditions in the esophagus underlying these two disorders as well as PPI-REE before and after PPI treatment.75 The study found that PPI-REE patients had transcriptomic profiles that were very similar to patients with EoE based on an analysis using an EoE diagnostic panel of 94 esophageal transcripts. However, these PPI-REE transcriptomic profiles almost entirely returned to a state similar to those of healthy control subjects following PPI treatment, indicating that PPI-REE mimics EoE at the transcriptome level as well as the eosinophilia and clinical features but that this too can be reversed by PPI treatment. While IL-5 and IL-13 are thought to drive eosinophilia and tissue remodeling in EoE, the cellular source of these cytokines had not been established. Doherty et al. examined the possibility that ILC2s in the esophagus may be expanded in the context of EoE and may therefore play a role in initiating these changes.76 They defined ILC2s in esophageal biopsies on the basis of their surface molecule expression (CD45+ Lin− CRTH2+), and found that ILC2s were overrepresented in patients with active EoE and that ILC2 levels strongly correlated with eosinophil levels, implicating them in eosinophil recruitment.
An international study published by Safroneeva et al. confirmed that in adult patients with EoE, monitoring symptoms as an assessment of disease remission, compared to the use of endoscopic and histologic findings, was only accurate about 60–65% of the time, underscoring the continued need for tissue monitoring to most accurately assess disease activity and remission.77 In children, however, the story may be different. Using a Pediatric Eosinophilic Esophagitis Symptom Score (PEESS v2.0), reported by parents of children with EoE, it was determined that this clinical measurement tool was effective at capturing important symptoms, and that the PEESS was useful and valid because values correlated well with histological and other tissue-derived parameters of disease activity, especially among those subjects with dysphagia.78 Nevertheless, one important goal in the management of such EoE patients, regardless of whether they are children or adults, would be advances in less invasive measures to monitor disease activity. One approach that avoids biopsies but still requires endoscopic sampling involved performing esophageal mucosal brushings to collect evidence of local eosinophil accumulation by measuring levels of eosinophil peroxidase in these samples. Indeed, a very strong correlation was seen between these levels and peak numbers of eosinophils in traditional biopsies.79 One additional potential advantage of this approach, as pointed out by the authors, is the ability of the brushing technique to sample a larger, more comprehensive surface area of the esophageal lumen. Taking a very different approach, Lingblom et al. sampled blood and performed extensive eosinophil flow cytometric phenotyping and mRNA analyses to look for any specific patterns that distinguished eosinophils in children and adults with or without EoE.80 Differences in surface levels of CD44, CD54 and/or CRTh2 on eosinophils, and intracellular levels of mRNA for galectin-10 (formerly called Charcot-Leyden crystal protein) and the FOXP3 transcription factor proved useful in distinguishing among the various groups, although some differences were age-related rather than disease-related. Whether this type of analysis will be useful in diagnosing patients with EoE (compared to other eosinophil-associated disorders) or assessing disease activity will require further testing. Regardless, the authors postulate that different eosinophil phenotypes in adult versus pediatric EoE might be the result of differing disease mechanisms or pathophysiology. In another study, a large cohort of children undergoing oral immunotherapy for food allergy were followed to determine if those who developed abdominal pain or vomiting (about 8% of all subjects) also developed blood eosinophilia, a finding that might suggest the development of EGID. They found an increased likelihood of developing vomiting and/or abdominal pain in subjects having a higher maximum blood eosinophil count.81 Employing receiver-operating characteristic analysis, a blood eosinophil count of 1.14 × 109/L yielded 85% sensitivity and 73% specificity of developing these gastrointestinal side effects. Because very few subjects underwent endoscopic evaluation, the risk of actually developing an EGID could not be determined, but it was of interest to note that when the food dosage was lessened or stopped, gastrointestinal symptoms subsided, and total eosinophil counts declined by >50%. The authors call these oral immunotherapy-induced gastrointestinal and eosinophilic responses, and highlight the possibility that oral immunotherapy has the potential, at least in some individuals, of inadvertently causing EGID-like disease.
Regarding recent advances in the treatment of EGID, again the primary focus has been on EoE. One particularly important advance came in the form of perfecting food elimination diets, where Kagawalla et al. found that in children with EoE, a four-food elimination diet (cow’s milk, wheat, egg and soy) resulted in a 60% remission rate.82 When compared to prior publications, including some by this same group, this approach was nearly as effective as the previous, commonly used six-food elimination diet that also avoided peanuts, tree nuts, fish and shellfish, so one conclusion is that these latter foods are rare triggers of EoE in children. Many of the other recent advances came in the form of studies looking at effectiveness of swallowed corticosteroids such as an oral budesonide suspension. These studies were separate multicenter trials, one in children, the other in both children and adults, as registered clinical trials to test the safety and efficacy of a new oral budesonide formulation being developed as a new agent that might one day receive FDA approval for the specific indication of treating EoE. Both studies showed this treatment to be safe. The pediatric study found drug- and dose-related improvements in histologic findings, but no difference in symptoms for those on drug versus placebo because all groups, including those on placebo unexpectedly showed improvement.83 In contrast, the study in both children and adults showed significant drug-related improvements in histology and symptoms.84 One additional study examined the effectiveness of the anti-IL-13 antibody dectrekumab (QAX576) in EoE.85 This was a 12-week trial involving monthly IV infusions, and the drug was well tolerated. Although the primary end point, which was based on the percentage of subjects showing a >75% decline in their endoscopic biopsy eosinophil counts, was not met, average eosinophil counts declined by 60% with drug compared to a 23% increase in the placebo group, a difference that was statistically significant. Particularly striking was the observation that the improvements in tissue eosinophils, mast cells, EoE-relevant transcript signatures and markers of barrier function persisted up to 6 months, months after the drug was stopped. Although this antibody is no longer in development, these results seem to underscore an important role for IL-13 in EoE pathogenesis.
Eosinophils and skin disease
While developments in recent years in eosinophils and dermatology were limited in scope, several important papers appeared. One examined the pathophysiology of bullous pemphigoid (BP), an autoimmune skin disease characterized by sub-epidermal blister formation, autoantibodies to hemi-desmosomal antigens, and blister eosinophilia with evidence of local eosinophil degranulation. Using an ex vivo human model, de Graauw et al. found that incubation of normal human skin sections with IL-5–activated eosinophils and BP autoantibodies was sufficient to induce dermal-epidermal junction separation pathognomonic of this disease.86 Whether targeting eosinophils in BP will be efficacious is an intriguing question that remains to be determined. Finally, a series of three landmark studies explored the safety and efficacy of dupilumab in adults with moderate-to-severe atopic dermatitis.87–89 Its benefit in these trials was substantiated in a subsequent meta-analysis90, and dupilumab received FDA approval in 2016 for this indication.
Summary Box 1: Advances in our understanding of eosinophils and eosinophil-associated diseases.
Examples of recent advances in our understanding of basic eosinophil biology involve
the role of IL-33 in eosinophilopoiesis,
the existence of multiple eosinophil subsets in the lung,
mechanisms of granule protein processing,
certain intrinsic and extrinsic pathways regulating eosinophil longevity,
and homeostatic and regulatory roles played by eosinophils in adipose tissue and the lung.
Recent clinical advances regarding eosinophils and eosinophil-associated diseases include
findings regarding the association between elevated eosinophil counts and asthma and COPD exacerbation risk;
the efficacy of biologicals targeting IL-5 in EGPA, targeting IL-5 or IL-5Rα in certain populations of asthma patients, and targeting IL-4Rα in CRSwNP and atopic dermatitis;
and intriguing and promising data regarding the use of PPIs, dexpramipexole, anti-sense DNA agent SB010, the CRTh2 antagonist fevipiprant and others.
Summary Box 2: What remains to be determined in eosinophils and eosinophil-associated diseases?
Key issues in basic eosinophil biology that remain incompletely defined/require further study:
the specific functions of the distinct eosinophil subsets,
the relevance of eosinophils located within the adipose tissue as opposed to elsewhere in the body,
whether these findings extend from mice to humans,
the mechanisms underlying some of the immunoregulatory activities of eosinophils,
the complete pathway of eosinophil hematopoietic development,
and how eosinophil identity and activity is transcriptionally regulated in the periphery
Key issues related to eosinophil-associated diseases that remain incompletely defined/require further study:
the mechanism underlying the periodicity of Gleich syndrome,
the identities of the other apparent gain-of-function mutations in imatinib-sensitive PDGFRα mutation-negative HES,
the mechanisms by which targeting eosinophils reduces asthma exacerbations,
which eosinophil-reducing or –depleting agent works best and most safely in which eosinophil-associated disease,
whether these agents favorably or adversely impact important homeostatic and regulatory functions associated with eosinophils,
the development of therapies to treat CRSwNP more efficaciously and durably,
and the invention of less invasive diagnostic tests for EGID.
Acknowledgments
We thank Jacqueline Schaffer for her contribution of Figure 1. This review is dedicated to Dr. James “Jamie” J. Lee, whose untimely death in 2017 caught the entire eosinophil community by surprise. Many of the experiments cited in this review could not have been accomplished without the generous, collaborative contributions of his intellect and the unique reagents and animals developed in his laboratory.
Declaration of all sources of funding: This work was supported by the National Heart, Lung, and Blood Institute (P01HL107151 to B.S.B.) and the National Institute of Allergy and Infectious Diseases (AI072265 to B.S.B.; T32AI083216 to J.A.O.).
Abbreviations used
- AAM
alternatively activated macrophages
- AERD
aspirin-exacerbated respiratory disease
- AT
adipose tissue
- BP
bullous pemphigoid
- CCL/CCR
C-C chemokine ligand/receptor
- COPD
chronic obstructive pulmonary disease
- CRS(wNP)
chronic rhinosinusitis (with nasal polyposis)
- CRTh2
chemokine receptor homologous molecule expressed on Th2 lymphocytes, also known as the PGD2 receptor or DP2
- DRESS
drug reaction with eosinophilia and systemic symptoms
- EC
eosinophilic colitis
- EGID
eosinophilic gastrointestinal disorder
- EGPA
eosinophilic granulomatosis with polyangiitis
- EoE
eosinophilic esophagitis
- EoG
eosinophilic gastritis
- EoP
eosinophil lineage-committed progenitors
- EPX
eosinophil peroxidase
- FeNO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GMP
granulocyte-macrophage progenitor
- HES
hypereosinophilic syndromes
- IgE
immunoglobulin E
- IκBα
NFκB inhibitor alpha
- ILC2
type 2 innate lymphoid cells
- MBP
major basic protein
- NFκB
nuclear factor of kappa light polypeptide gene enhancer in B cells
- OVA
chicken egg ovalbumin
- PDGFRA
platelet-derived growth factor receptor alpha
- PEESS
Pediatric Eosinophilic Esophagitis Symptom Score
- PGD2
prostaglandin D2
- PIR-B
paired immunoglobulin-like receptor B
- PPI
proton pump inhibitor
- PPI-REE
PPI-responsive esophageal eosinophilia
- Pre-GM
pre–granulocyte-macrophage progenitor
- PVAT
perivascular adipose tissue
- RT-PCR
reverse transcription polymerase chain reaction
- Siglec
sialic acid-binding immunoglobulin-like lectin
- TH
tyrosine hydroxylase
- TMA
trimellitic anhydride
- TNF-α
tumor necrosis factor alpha
- VCAM-1
vascular cell adhesion molecule 1
- XBP1
X-box binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kay AB. The early history of the eosinophil. Clin Exp Allergy. 2015;45:575–82. doi: 10.1111/cea.12480. [DOI] [PubMed] [Google Scholar]
- 2.Stacy NI, Raskin RE. Reptilian eosinophils: beauty and diversity by light microscopy. Vet Clin Pathol. 2015;44:177–8. doi: 10.1111/vcp.12246. [DOI] [PubMed] [Google Scholar]
- 3.Drissen R, Buza-Vidas N, Woll P, Thongjuea S, Gambardella A, Giustacchini A, et al. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat Immunol. 2016;17:666–76. doi: 10.1038/ni.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouffi C, Kartashov AV, Schollaert KL, Chen X, Bacon WC, Weirauch MT, et al. Transcription factor repertoire of homeostatic eosinophilopoiesis. J Immunol. 2015;195:2683–95. doi: 10.4049/jimmunol.1500510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettigole SE, Lis R, Adoro S, Lee AH, Spencer LA, Weller PF, et al. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol. 2015;16:829–37. doi: 10.1038/ni.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews SP, McMillan SJ, Colbert JD, Lawrence RA, Watts C. Cystatin F ensures eosinophil survival by regulating granule biogenesis. Immunity. 2016;44:795–806. doi: 10.1016/j.immuni.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soragni A, Yousefi S, Stoeckle C, Soriaga AB, Sawaya MR, Kozlowski E, et al. Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Mol Cell. 2015;57:1011–21. doi: 10.1016/j.molcel.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson EL, Kobayashi T, Iijima K, Bartemes KR, Chen CC, Kita H. IL-33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy. 2016;71:977–88. doi: 10.1111/all.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, et al. IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol. 2016;197:3445–53. doi: 10.4049/jimmunol.1600611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotzin JJ, Spencer SP, McCright SJ, Kumar DB, Collet MA, Mowel WK, et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016;537:239–43. doi: 10.1038/nature19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz C, Willebrand R, Huber S, Rupec RA, Wu D, Locksley R, et al. Eosinophil-specific deletion of IκBα in mice reveals a critical role of NF-κB-induced Bcl-xL for inhibition of apoptosis. Blood. 2015;125:3896–904. doi: 10.1182/blood-2014-10-607788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, et al. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 2015;135:1329–40. doi: 10.1016/j.jaci.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge XN, Ha SG, Greenberg YG, Rao A, Bastan I, Blidner AG, et al. Regulation of eosinophilia and allergic airway inflammation by the glycan-binding protein galectin-1. Proc Natl Acad Sci U S A. 2016;113:E4837–46. doi: 10.1073/pnas.1601958113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang T, Hazen M, Shang Y, Zhou M, Wu X, Yan D, et al. Depletion of major pathogenic cells in asthma by targeting CRTh2. JCI Insight. 2016;1:e86689. doi: 10.1172/jci.insight.86689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkerson EM, Johansson MW, Hebert AS, Westphall MS, Mathur SK, Jarjour NN, et al. The peripheral blood eosinophil proteome. J Proteome Res. 2016;15:1524–33. doi: 10.1021/acs.jproteome.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnig C, Alsaleh G, Jung N, Dembele D, Paul N, Poirot A, et al. Circulating human eosinophils share a similar transcriptional profile in asthma and other hypereosinophilic disorders. PLoS One. 2015;10:e0141740. doi: 10.1371/journal.pone.0141740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279–95. doi: 10.1172/JCI85664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Percopo CM, Brenner TA, Ma M, Kraemer LS, Hakeem RM, Lee JJ, et al. SiglecF+Gr1hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J Leukoc Biol. 2017;101:321–8. doi: 10.1189/jlb.3A0416-166R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdala Valencia H, Loffredo LF, Misharin AV, Berdnikovs S. Phenotypic plasticity and targeting of Siglec-F(high) CD11c(low) eosinophils to the airway in a murine model of asthma. Allergy. 2016;71:267–71. doi: 10.1111/all.12776. [DOI] [PubMed] [Google Scholar]
- 20.Lee JJ, Protheroe CA, Luo H, Ochkur SI, Scott GD, Zellner KR, et al. Eosinophil-dependent skin innervation and itching following contact toxicant exposure in mice. J Allergy Clin Immunol. 2015;135:477–87. doi: 10.1016/j.jaci.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng LE, Sullivan BM, Retana LE, Allen CD, Liang HE, Locksley RM. IgE-activated basophils regulate eosinophil tissue entry by modulating endothelial function. J Exp Med. 2015;212:513–24. doi: 10.1084/jem.20141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen EA, Ochkur SI, Doyle AD, LeSuer WE, Li W, Protheroe CA, et al. Lung pathologies in a chronic inflammation mouse model are independent of eosinophil degranulation. Am J Respir Crit Care Med. 2017;195:1321–32. doi: 10.1164/rccm.201606-1129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griseri T, Arnold IC, Pearson C, Krausgruber T, Schiering C, Franchini F, et al. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity. 2015;43:187–99. doi: 10.1016/j.immuni.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moshkovits I, Reichman H, Karo-Atar D, Rozenberg P, Zigmond E, Haberman Y, et al. A key requirement for CD300f in innate immune responses of eosinophils in colitis. Mucosal Immunol. 2017;10:172–83. doi: 10.1038/mi.2016.37. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara R, Lee EJ, Jang MS, Jeun EJ, Hong CP, Kim JH, et al. Small intestinal eosinophils regulate Th17 cells by producing IL-1 receptor antagonist. J Exp Med. 2016;213:555–67. doi: 10.1084/jem.20141388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben Baruch-Morgenstern N, Mingler MK, Stucke E, Besse JA, Wen T, Reichman H, et al. Paired Ig-like receptor B inhibits IL-13-driven eosinophil accumulation and activation in the esophagus. J Immunol. 2016;197:707–14. doi: 10.4049/jimmunol.1501873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 2015;75:1624–34. doi: 10.1158/0008-5472.CAN-14-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–17. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Shiraishi Y, Ashino S, Han J, Jia Y, Wang M, et al. Eosinophils contribute to the resolution of lung-allergic responses following repeated allergen challenge. J Allergy Clin Immunol. 2015;135:451–60. doi: 10.1016/j.jaci.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Andreev D, Oeser K, Krljanac B, Hueber A, Kleyer A, et al. Th2 and eosinophil responses suppress inflammatory arthritis. Nat Commun. 2016;7:11596. doi: 10.1038/ncomms11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–6. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suarez-Zamorano N, Fabbiano S, Chevalier C, Stojanovic O, Colin DJ, Stevanovic A, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21:1497–501. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Withers SB, Forman R, Meza-Perez S, Sorobetea D, Sitnik K, Hopwood T, et al. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci Rep. 2017;7:44571. doi: 10.1038/srep44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolus WR, Gutierrez DA, Kennedy AJ, Anderson-Baucum EK, Hasty AH. CCR2 deficiency leads to increased eosinophils, alternative macrophage activation, and type 2 cytokine expression in adipose tissue. J Leukoc Biol. 2015;98:467–77. doi: 10.1189/jlb.3HI0115-018R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klion AD. Eosinophilia: a pragmatic approach to diagnosis and treatment. Hematology Am Soc Hematol Educ Program. 2015;2015:92–7. doi: 10.1182/asheducation-2015.1.92. [DOI] [PubMed] [Google Scholar]
- 36.Klion AD. How I treat hypereosinophilic syndromes. Blood. 2015;126:1069–77. doi: 10.1182/blood-2014-11-551614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin JJ, Butterfield JH, Weiler CR. Hematologic malignancies identified in patients with hypereosinophilia and hypereosinophilic syndromes. J Allergy Clin Immunol Pract. 2015;3:920–5. doi: 10.1016/j.jaip.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Khoury P, Herold J, Alpaugh A, Dinerman E, Holland-Thomas N, Stoddard J, et al. Episodic angioedema with eosinophilia (Gleich syndrome) is a multilineage cell cycling disorder. Haematologica. 2015;100:300–7. doi: 10.3324/haematol.2013.091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khoury P, Desmond R, Pabon A, Holland-Thomas N, Ware JM, Arthur DC, et al. Clinical features predict responsiveness to imatinib in platelet-derived growth factor receptor-alpha-negative hypereosinophilic syndrome. Allergy. 2016;71:803–10. doi: 10.1111/all.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dworetzky SI, Hebrank GT, Archibald DG, Reynolds IJ, Farwell W, Bozik ME. The targeted eosinophil-lowering effects of dexpramipexole in clinical studies. Blood Cells Mol Dis. 2017;63:62–5. doi: 10.1016/j.bcmd.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Blumenthal KG, Youngster I, Rabideau DJ, Parker RA, Manning KS, Walensky RP, et al. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol. 2015;136:1288–94. doi: 10.1016/j.jaci.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez E, Medrano-Casique N, Tong HY, Bellon T, Cabanas R, Fiandor A, et al. Eosinophilic drug reactions detected by a prospective pharmacovigilance programme in a tertiary hospital. Br J Clin Pharmacol. 2017;83:400–15. doi: 10.1111/bcp.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331–57. doi: 10.1146/annurev-pathol-052016-100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi T, Kato A, Berdnikovs S, Stevens WW, Suh LA, Norton JE, et al. Microparticles in nasal lavage fluids in chronic rhinosinusitis: Potential biomarkers for diagnosis of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.01.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, et al. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol. 2015;135:799–810. doi: 10.1016/j.jaci.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min JY, Ocampo CJ, Stevens WW, Price CP, Thompson CF, Homma T, et al. Proton pump inhibitors decrease eotaxin-3/CCL26 expression in patients with chronic rhinosinusitis with nasal polyps: Possible role of the nongastric H,K-ATPase. J Allergy Clin Immunol. 2017;139:130–41. doi: 10.1016/j.jaci.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rank MA, Ochkur SI, Lewis JC, Teaford HG, 3rd, Wesselius LJ, Helmers RA, et al. Nasal and pharyngeal eosinophil peroxidase levels in adults with poorly controlled asthma correlate with sputum eosinophilia. Allergy. 2016;71:567–70. doi: 10.1111/all.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469–79. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 49.Sverrild A, Kiilerich P, Brejnrod A, Pedersen R, Porsbjerg C, Bergqvist A, et al. Eosinophilic airway inflammation in asthmatic patients is associated with an altered airway microbiome. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.10.046. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Baines KJ, Fu JJ, Wood LG, Simpson JL, McDonald VM, et al. Sputum mast cell subtypes relate to eosinophilia and corticosteroid response in asthma. Eur Respir J. 2016;47:1123–33. doi: 10.1183/13993003.01098-2015. [DOI] [PubMed] [Google Scholar]
- 51.Casale TB. Biologics and biomarkers for asthma, urticaria, and nasal polyposis. J Allergy Clin Immunol. 2017;139:1411–21. doi: 10.1016/j.jaci.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Ortega H, Katz L, Gunsoy N, Keene O, Yancey S. Blood eosinophil counts predict treatment response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2015;136:825–6. doi: 10.1016/j.jaci.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 53.Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4:549–56. doi: 10.1016/S2213-2600(16)30031-5. [DOI] [PubMed] [Google Scholar]
- 54.Fowler SJ, Tavernier G, Niven R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J Allergy Clin Immunol. 2015;135:822–4. doi: 10.1016/j.jaci.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 55.Agache I, Strasser DS, Klenk A, Agache C, Farine H, Ciobanu C, et al. Serum IL-5 and IL-13 consistently serve as the best predictors for the blood eosinophilia phenotype in adult asthmatics. Allergy. 2016;71:1192–202. doi: 10.1111/all.12906. [DOI] [PubMed] [Google Scholar]
- 56.Demarche SF, Schleich FN, Paulus VA, Henket MA, Van Hees TJ, Louis RE. Asthma control and sputum eosinophils: a longitudinal study in daily practice. J Allergy Clin Immunol Pract. 2017 doi: 10.1016/j.jaip.2017.01.026. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Zeiger RS, Schatz M, Dalal AA, Chen W, Sadikova E, Suruki RY, et al. Blood eosinophil count and outcomes in severe uncontrolled asthma: a prospective study. J Allergy Clin Immunol Pract. 2017;5:144–53. doi: 10.1016/j.jaip.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 58.Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen general population study. Am J Respir Crit Care Med. 2016;193:965–74. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 59.Roche N, Chapman KR, Vogelmeier CF, Herth FJF, Thach C, Fogel R, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med. 2017;195:1189–97. doi: 10.1164/rccm.201701-0193OC. [DOI] [PubMed] [Google Scholar]
- 60.Anderson HM, Lemanske RF, Jr, Arron JR, Holweg CTJ, Rajamanickam V, Gangnon RE, et al. Relationships among aeroallergen sensitization, peripheral blood eosinophils, and periostin in pediatric asthma development. J Allergy Clin Immunol. 2017;139:790–6. doi: 10.1016/j.jaci.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195:302–13. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryan DM, Fowler SJ, Niven RM. Reduction in peripheral blood eosinophil counts after bronchial thermoplasty. J Allergy Clin Immunol. 2016;138:308–10. doi: 10.1016/j.jaci.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 63.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921–32. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–27. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 65.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–41. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 66.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–58. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 67.Ferguson GT, FitzGerald JM, Bleecker ER, Laviolette M, Bernstein D, LaForce C, et al. Benralizumab for patients with mild to moderate, persistent asthma (BISE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2017;5:568–76. doi: 10.1016/S2213-2600(17)30190-X. [DOI] [PubMed] [Google Scholar]
- 68.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–66. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 69.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 70.Hanania NA, Korenblat P, Chapman KR, Bateman ED, Kopecky P, Paggiaro P, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4:781–96. doi: 10.1016/S2213-2600(16)30265-X. [DOI] [PubMed] [Google Scholar]
- 71.Bochner BS, Schleimer RP. Out of the orphanage and into the clinic--therapeutic targeting of GATA3. N Engl J Med. 2015;372:2060–1. doi: 10.1056/NEJMe1502660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krug N, Hohlfeld JM, Kirsten AM, Kornmann O, Beeh KM, Kappeler D, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med. 2015;372:1987–95. doi: 10.1056/NEJMoa1411776. [DOI] [PubMed] [Google Scholar]
- 73.Krug N, Hohlfeld JM, Buhl R, Renz J, Garn H, Renz H. Blood eosinophils predict therapeutic effects of a GATA3-specific DNAzyme in asthma patients. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.02.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 74.Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MF, Bacher G, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4:699–707. doi: 10.1016/S2213-2600(16)30179-5. [DOI] [PubMed] [Google Scholar]
- 75.Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol. 2015;135:187–97. doi: 10.1016/j.jaci.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:792–4. doi: 10.1016/j.jaci.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Safroneeva E, Straumann A, Coslovsky M, Zwahlen M, Kuehni CE, Panczak R, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults With eosinophilic esophagitis. Gastroenterology. 2016;150:581–90. doi: 10.1053/j.gastro.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin LJ, Franciosi JP, Collins MH, Abonia JP, Lee JJ, Hommel KA, et al. Pediatric eosinophilic esophagitis symptom scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol. 2015;135:1519–28. doi: 10.1016/j.jaci.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saffari H, Leiferman KM, Clayton F, Baer K, Pease LF, Gleich GJ, et al. Measurement of inflammation in eosinophilic esophagitis using an eosinophil peroxidase assay. Am J Gastroenterol. 2016;111:933–9. doi: 10.1038/ajg.2016.184. [DOI] [PubMed] [Google Scholar]
- 80.Lingblom C, Kappi T, Bergquist H, Bove M, Arkel R, Saalman R, et al. Differences in eosinophil molecular profiles between children and adults with eosinophilic esophagitis. Allergy. 2017 doi: 10.1111/all.13140. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 81.Goldberg MR, Elizur A, Nachshon L, Appel MY, Levy MB, Golobov K, et al. Oral immunotherapy-induced gastrointestinal symptoms and peripheral blood eosinophil responses. J Allergy Clin Immunol. 2017;139:1388–90. doi: 10.1016/j.jaci.2016.09.053. [DOI] [PubMed] [Google Scholar]
- 82.Kagalwalla AF, Wechsler JB, Amsden K, Schwartz S, Makhija M, Olive A, et al. Efficacy of a 4-food elimination diet for children with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2017.05.048. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13:66–76. doi: 10.1016/j.cgh.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 84.Dellon ES, Katzka DA, Collins MH, Hamdani M, Gupta SK, Hirano I, et al. Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology. 2017;152:776–86. doi: 10.1053/j.gastro.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 85.Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135:500–7. doi: 10.1016/j.jaci.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 86.de Graauw E, Sitaru C, Horn M, Borradori L, Yousefi S, Simon HU, et al. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy. 2017;72:1105–13. doi: 10.1111/all.13131. [DOI] [PubMed] [Google Scholar]
- 87.Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–9. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 88.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–48. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 89.Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 90.Han Y, Chen Y, Liu X, Zhang J, Su H, Wen H, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: A meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.04.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]