To the Editor
Most exacerbations of asthma in children are initiated by viral infections, most commonly due to rhinoviruses (RV). RV-A and RV-C species are more likely to cause moderate to severe illnesses and wheezing in infants, and exacerbations of asthma leading to hospitalization.1, 2 Bacterial pathogens such as Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis have also been linked to acute wheezing illnesses and exacerbations of asthma in young children.3, 4 In a prospective study of school-aged children during peak RV season in September (“RhinoGen”), we previously reported that viral infections often precede increased detection of these bacteria.5 Furthermore, viral infections with concurrent or subsequent detection of bacterial pathogens were more likely to be associated with symptomatic illnesses and exacerbations of asthma.5 These studies suggest that RV-A and RV-C infections could lead to more severe wheezing illnesses by promoting the proliferation of bacterial pathogens.
To test this hypothesis, we obtained weekly nasal mucus samples during both September and April as part of the RhinoGen study, and analyzed them for RV species (RT-PCR and partial sequencing)6 and the bacterial pathogens S. pneumoniae, M. catarrhalis and H. influenzae catarrhalis (quantitative PCR; full methods in online supplement).5 Children with the help of their parents were instructed to record daily common cold and asthma symptoms, morning peak expiratory flow, and albuterol use on calendars during their enrollment in the study. This study was approved by the University of Wisconsin Human Subjects Committee, written informed consent was obtained from the parents, and written assent obtained from children ages 7 years and older.
The study population consisted of 167 outpatient children with asthma and 143 children without asthma ages 4–12 years (Supplemental Table I and online supplement). Children scored cold and asthma symptom severity based on a 4-point scoring system (Supplemental Table II),5 and moderate asthma exacerbations were defined in accordance with ATS and NHLBI guidelines (see online supplement).
During the two-month monitoring period, there were 755 respiratory illnesses reported in the 310 children. In the 3028 nasal mucus samples, RV was the virus most commonly detected, and RV-A (n=566 solo, 674 total) was detected over twice as often as either RV-B (n=203 solo, 250 total) or RV-C (n=195 solo, 251 total). RV-B was detected more frequently in September, while RV-A and RV-C were detected more often in April (Supplemental Table III). For each RV species, we also determined the number of infections (one or more consecutive weeks with detection of the same viral type, Supplementary Table IV). The mean duration of viral infection varied by species (p<0.001); RV-B was longest (mean 2.09 weeks, 95% CI [1.92, 2.25]), followed by RV-A (1.77 weeks [1.68, 1.86]) and then RV-C (1.37 weeks [1.23, 1.50]).
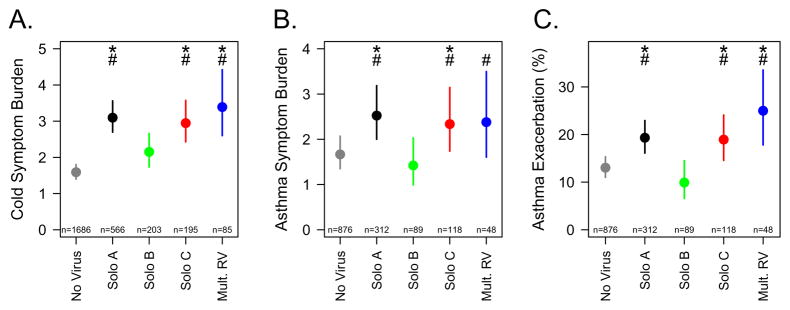
Compared to weeks with no virus detected, weeks that were positive for any RV species had significantly greater cold symptom scores (Figure 1A). Both RV-A and C were associated with increased cold symptoms compared to RV-B (p<0.02). Similarly, RV-A and RV-C were associated with greater asthma symptoms (Figure 1B) and a greater probability of exacerbation (Figure 1C) compared to RV-B. Relative to no virus detection, RV-B detection was not associated with increased cold or asthma symptoms or exacerbations of asthma.
Figure 1.
Association of viral detection with clinical outcomes. Detection of RV species in weekly samples were compared to contemporaneous reports of cold symptom scores (A), asthma symptom scores (B), and risk for exacerbation of asthma (C). Points and lines represent means and 95% confidence intervals. *p<0.05 vs no virus; #p<0.05 vs RV-B.
Of the 310 participating children, 276 had at least one clinically defined respiratory illness (see online data supplement for criteria) during the study period, with a total of 755 illnesses. Compared to periods with no virus detected, RV-A, RV-C, and multiple RV were each associated with increased risk for illness (OR = 2.35, 2.61, and 2.41, p<0.0005) while RV-B was not (OR = 0.99, p=0.95, Supplemental Figure 1).
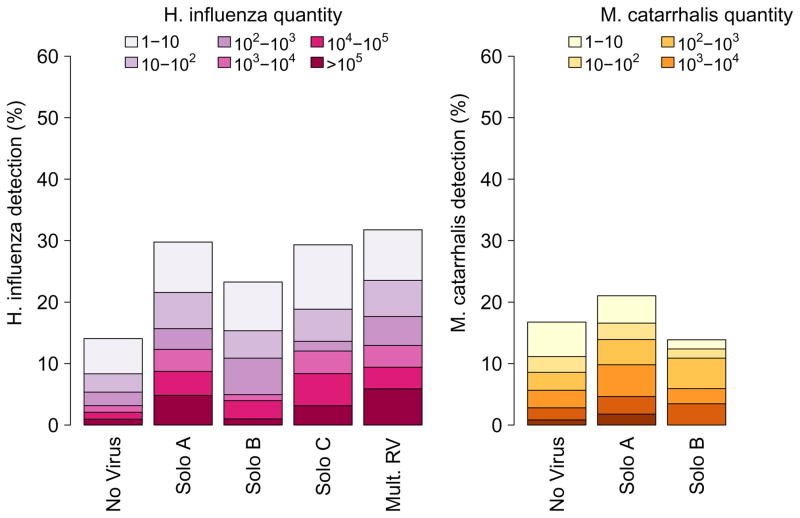
We next tested whether detection of bacterial pathogens was differentially related to infection with specific RV species. S. pneumoniae, H. influenzae and M. catarrhalis were detected in 45%, 21% and 19% of samples respectively, and were more often detected in younger children (Supplemental Figure 2). RV-C was associated with increased detection and quantity of H. influenzae (detection: OR 2.36 [1.65, 3.38]; quantity: ordinal OR 2.48 [1.64, 3.75]) and M. catarrhalis (OR 1.52 [1.04, 2.22]; ordinal OR 1.52 [1.01, 2.30]), RV-A was associated with increased H. influenzae (OR 2.43 [1.90, 3.10]; ordinal OR 2.60 [1.96, 3.45]) and S. pneumoniae (OR 1.52 [1.23, 1.89]; ordinal OR 1.97 [1.59, 2.43]), while RV-B was not associated with increased bacterial detection (Fig. 2).
Figure 2.
Association of RV species with quantity of bacterial pathogens.
We previously reported that during sampling of nasal mucus obtained in September, detection of both RVs and bacteria contributed to the probability of respiratory illness.5 In an analysis including samples from April and September (Supplemental Table V), detection of RV together with either M. catarrhalis (OR 1.79 [1.16, 2.78]) or H. Influenzae (OR 1.44 [1.01, 2.05]), but not S. pneumoniae (OR 1.22 [0.78, 1.62]), significantly increased the probability of respiratory illness compared to infection with RV alone. When detected in the absence of virus, none of the bacterial pathogens were significantly associated with respiratory illness.
The results of this study verify that infections with RV-A and RV-C species cause more severe illnesses and are more likely associated with exacerbations of asthma than infections with RV-B. We also demonstrated that RV-A and RV-C are more likely than RV-B to be associated with detection of bacterial respiratory pathogens. Strengths of this study include the large sample size, inclusion of children with and without asthma, and prospective collection of samples during periods of illness and health. Limitations include sample collection for only two months out of the year; however, these months were selected for the highest prevalence of RV infections and asthma morbidity. In addition, there may be effects of other seasonal factors such as allergens that were not measured in this study.
These findings suggest that RV virulence could be partly related to secondary effects on the overgrowth of bacterial pathogens. This relationship is plausible, as these bacteria can stimulate inflammatory responses that might lead to airway narrowing and acute exacerbations.7 Accordingly, two randomized studies have demonstrated that treatment with azithromycin improves outcomes of wheezing illnesses in high-risk preschool children.8, 9 Mechanisms for these effects are unclear, however, and use of antibiotics for respiratory illnesses could have unintended adverse effects including selecting for drug-resistant organisms, and killing beneficial microbes. Additional studies are needed to test whether other types of treatments (e.g. vaccines, probiotics) can prevent overgrowth of bacterial pathogens during viral respiratory infections and reduce the risk of more severe illnesses and exacerbations of asthma.
Supplementary Material
Clinical Implications.
Rhinovirus infections may cause clinical symptoms in part by promoting the proliferation of bacterial pathogens in the airway, suggesting that preventive treatments might target the bacterial pathogens.
Acknowledgments
This study was funded by NIH grants T32 AI007635, U19 AI070503-01, U19 AI104317 and P01 HL070831; and the project was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427.
Abbreviations
- RV
rhinovirus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee WM, Lemanske RF, Jr, Evans MD, Vang F, Pappas T, Gangnon R, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186:886–91. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bizzintino J, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011;37:1037–42. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisgaard H, Hermansen MN, Bonnelykke K, Stokholm J, Baty F, Skytt NL, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–15. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–7. e3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52:2461–71. doi: 10.1128/JCM.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA. 2015;314:2034–44. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19–26. doi: 10.1016/S2213-2600(15)00500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.