Abstract
Many women with epilepsy experience perimenstrual seizure exacerbation, referred to as catamenial epilepsy. There is no effective treatment for this condition, proposed to result from withdrawal of neurosteroid-mediated effects of progesterone. A double-blind, multicenter, phase III, clinical trial of catamenial epilepsy has failed to find a beneficial effect of progesterone. The neurosteroid-mediated effects of progesterone have been extensively studied in relation to catamenial epilepsy; however, the effects mediated by progesterone receptor activation have been overlooked. We determined whether progesterone increased excitatory transmission in the hippocampus via activation of progesterone receptors, which may play a role in regulating catamenial seizure exacerbation. In a double-blind study using a rat model of catamenial epilepsy, we found that treatment with RU-486, which blocks progesterone and glucocorticoid receptors, significantly attenuated neurosteroid withdrawal-induced seizures. Furthermore, progesterone treatment as well as endogenous rise in progesterone during estrous cycle increased the expression of GluA1 and GluA2 subunits of AMPA receptors in the hippocampi, and enhanced the AMPA receptor-mediated synaptic transmission of CA1 pyramidal neurons. The progesterone-induced plasticity of AMPA receptors was blocked by RU-486 treatment and progesterone also failed to increase AMPA receptor expression in progesterone receptor knockout mice. These studies demonstrate that progesterone receptor activation regulates AMPA receptor expression and may play a role in catamenial seizure exacerbation.
Keywords: Catamenial seizures, Progesterone, progesterone receptor, RU-486, AMPA receptor, CA1 neurons
Introduction
Approximately 30% of women of reproductive age with epilepsy experience cyclical exacerbation of seizures related to periodic changes in the serum progesterone and estrogen levels during the menstrual cycle (catamenial epilepsy) and its predominant form is perimenstrual seizure exacerbation (Herzog et al., 2015; Frye, 2008). Currently there are no scientifically-tested effective treatments of catamenial exacerbation. This period of seizure exacerbation coincides with a decline in progesterone levels at the end of the cycle following the mid-luteal peak levels.
Progesterone is a sedative and exerts an anticonvulsant action via conversion to the neurosteroid allopregnanolone, which binds to γ-amino-butyric acid (GABA) type A (GABAA) receptors, and enhances GABA action on the receptor (Reddy & Rogawski, 2012; Joshi et al., 2013). It has long been proposed that perimenstrual seizure exacerbation is related to neurosteroid withdrawal, which would impair GABAergic inhibition. Based on the premise that maintaining high progesterone and allopregnanolone levels during the perimenstrual period would alleviate seizure exacerbation, a large-scale phase 3 clinical trial was conducted. However, in this trial, progesterone replacement therapy was not different than placebo to protect against this exacerbation (Herzog, 2015).
Progesterone also activates the progesterone receptors, isoforms A and B, which are ligand-activated transcription factors encoded by a single gene (Conneely et al., 1987). Upon activation, progesterone receptors localize to the nucleus and regulate gene expression (Mani & Oyola, 2012; Singh & Su, 2013). The mRNA and protein of progesterone receptors are present in the principal neurons of the hippocampus, which is involved in seizure generation and propagation (Guerra-Araiza et al., 2003; Guerra-Araiza et al., 2000). The immunoreactivity of progesterone receptors is present over the soma, axon terminals, and dendritic spines of hippocampal principal neurons (Mitterling et al., 2010). While the allopregnanolone-mediated effects of progesterone have been extensively characterized, whether progesterone receptor activation also regulates seizures is not known. The mid-luteal rise in progesterone could activate progesterone receptors and increase excitatory neurotransmission. This would be counterbalanced as long as high allopregnanolone levels maintain the GABAergic neurotransmisison. However, the excitation/inhibition balance would be affected during perimenstrual period due to the decline in allopregnanolone levels and result in seizure exacerbation.
We tested the hypothesis that progesterone has two effects: an anticonvulsant action through neurosteroids and a slower excitatory action that occurs following the activation of progesterone receptors and worsens seizures. This hypothesis is informed by the homeostatic scaling theory, which states that there is a precise tuning of neuronal excitability and synaptic strength to maintain a neuron’s target firing rate (Marder & Goaillard, 2006; Turrigiano, 2008). We tested whether excitatory action contributes to the seizure exacerbation observed during progesterone withdrawal after prolonged treatment (perimenstrual exacerbation).
Materials and methods
All animals were handled according to a protocol approved by the University of Virginia Animal Care and Use Committee, and efforts were made to minimize animal stress and discomfort. A majority of the experiments were performed on adult Sprague-Dawley female rats (200–220 g) with intact ovaries. In addition, adult female mice (20–25 g) lacking progesterone receptor expression (PR−/−)(Hashimoto-Partyka et al., 2006), and C57Bl/6 and wild-type littermates (PR+/+) were also used.
Materials
PMSG, β-HCG, finasteride, nestorone, as well as all the common chemicals were purchased from Sigma-Aldrich. Mouse monoclonal anti-GluA1 subunit (1:1000, clone RH95, Millipore), anti-GluA2 subunit (1:1000, clone 6C4, Millipore), and anti-β-actin antibody (1:5000, clone AC74, Sigma-Aldrich) were used. The specificity of these antibodies was confirmed using JCN criteria (Saper, 2005). HRP-tagged anti-mouse antibody (1:5000) was obtained from BioRad. Hybond-P PVDF membranes were obtained from GE Healthcare.
Induction of TLE
SE was induced using lithium-pilocarpine method as described before using lithium-pilocarpine (Lawrence et al., 2010). Two weeks after SE, animals were implanted with cortical and hippocampal electrodes and EEG recording was performed as described previously (Lawrence et al., 2010). The animals were monitored by continuous video-EEG recording following a week of recovery and were designated as epileptic after they had experienced at least 2 spontaneous seizures. Electrographic seizures irrespective of the associated behavior were counted to determine seizure frequency.
Treatment of epileptic animals
The animals were monitored for 14 days to determine the basal seizure frequency. The animals were divided into two groups (vehicle and RU-486 treated groups, see below) such that each group included animals with high frequency, low frequency, and clustered seizures, and they were then treated with PMSG (20 IU in saline, intraperitoneal, ip), followed 48 hrs later by β-HCG (10 IU in saline, ip) as described previously (Lawrence et al., 2010). All treatments were performed between 9 and 11 AM. During the course of PMSG and β-HCG treatment, progesterone receptors were blocked by a daily injection of RU-486 (10 mg/kg, ip) in half of the animals; the remaining animals were treated daily with vehicle (10% β-cyclodextrin). Neurosteroid (allopregnanolone and tetrahydrodeoxycorticosterone in females and allopregnanolone, tetrahydrodeoxycorticosterone, and androstanediol in males) withdrawal was induced by the administration of finasteride (100 mg/kg in 30% cyclodextrin, ip) on the 8th day of β-HCG injection (Lawrence et al., 2010).
Serum progesterone levels were determined using a Progesterone ELISA kit (Immunobiological Laboratories Inc, USA, #IB79105)(Xiao et al., 2017) at the Reproductive Core Facility of the University of Virginia as described previously (Lawrence et al., 2010). The detection range of the kit was 0.3 to 40 ng/mL and the sensitivity was 0.045 ng/mL. Progesterone standards at different concentrations were run with the experimental samples to confirm that the concentration in the sample is within a detectable range. The samples with progesterone concentrations higher than the detectable range were diluted and reassayed.
Treatment of non-epileptic animals
Non-epileptic female animals were also treated with PMSG and β-HCG as above, saline-treated animals were used as controls. In a separate cohort of animals, a single dose of progesterone (50 mg/kg, ip) or vehicle (20% cyclodextrin, ip) was administered, and the experiments were performed 2 days later. Progesterone receptors were blocked by the administration of RU-486 (30 mg/kg). RU-486 or vehicle were administered 30 min before progesterone and one dose the next day. Progesterone receptors were also activated by treatment with a synthetic agonist nestorone (3 mg/kg in 10% β-cyclodextrin, subcutaneous). The animals were administered a single dose of nestorone and the experiments were performed 2 days later; animals treated with vehicle were used as controls.
Mice lacking progesterone receptor expression
PR floxed mice were kindly provided by Dr Iruela-Arispe (UCLA, Los Angeles, California) (Hashimoto-Partyka et al., 2006; Stephens et al., 2015; Janzen et al., 2013). These mice were bred with CMV-Cre mice (B6.C-Tg(CMV-cre)1Cgn/J, Jackson laboratories) to generate progesterone receptor knockout mice (PR−/−). C57Bl/6 and wild-type littermates were used as controls (PR+/+). Adult female mice were monitored for estrous cycle; progesterone (100 mg/kg, ip) or vehicle (20% cyclodextrin) was administered to mice in diestrus and AMPAR expression was determined 24 hours later.
Brain slicing
Acutely isolated hippocampal slices were prepared as described before (Rajasekaran et al., 2012). The animals were anesthetized with halothane and decapitated. The brain was immersed in oxygenated (95% O2/5% CO2) ice-cold (2–4°C) slicing buffer (in mM, 65.5 NaCl, 2 KCl, 5 MgSO4, 25 NaHCO3, 1.1 KH2PO4, 1 CaCl2, 10 glucose, and 113 sucrose; 300 mOsm), and horizontal 350-μm slices were prepared using a Vibratome (Leica VT1200S, Germany). The slices were collected in oxygenated artificial CSF (aCSF, containing in mM, 127 NaCl, 2 KCl, 1.5 MgSO4, 25.7 NaHCO3, 10 dextrose, and 1.5 CaCl2; 300 mOsm). The slices were then used for biochemistry or electrophysiology.
Electrophysiology
AMPAR-mediated currents from CA1 pyramidal neurons were recorded using a whole-cell patch clamp technique as described previously (Sun & Kapur, 2012). The slices were perfused with oxygenated aCSF containing DL-AP5 (50 μM) and picrotoxin (100 μM), to block NMDA and GABAA receptors, respectively, at a rate of 2–3 ml/min. The patch electrode was filled with an internal solution containing in mM, cesium methane sulfonate 115, cesium chloride 20, KCl 10, HEPES 10, sodium-EGTA 0.5, MgCl2 2.5, Mg-ATP 5 and lidocaine 5, pH 7.3; 285 mOsm. The neurons were voltage clamped to −65 mV. Electrode capacitance was electronically compensated. Access resistance was continuously monitored, and if the series resistance increased by 20% at any time, the recording was terminated. Currents were filtered at 2 kHz, digitized using a Digidata 1322 digitizer (Molecular Devices, Sunnyvale, CA, USA), and acquired using Clampex 10.2 software (Molecular Devices). All recordings were performed at 30 °C. The currents were analyzed as described before using the MiniAnalysis software (Sun & Kapur, 2012).
BS3 cross-linking assay and western blotting
A BS3 cross-linker was used to cross-link surface proteins that were eliminated from detection. Experiments were performed on the 8th day of β-HCG administration. Saline-treated animals were processed simultaneously as controls. Briefly, four to six hippocampal slices from one hemisphere were incubated in ice-cold (2–4°C) artificial CSF (aCSF) containing BS3 (1 mg/ml) at 4°C for 40 min with constant shaking. The slices from the other hemisphere were simultaneously incubated in ice-cold aCSF without BS3. Following incubation, the slices were washed twice with ice-cold aCSF containing 10 mM Tris-Cl (pH 7.4) to stop the cross-linking reaction and remove the remaining BS3 reagent. BS3- or aCSF-incubated slices from one animal were pooled together and formed a single replicate. The tissue was lysed in RIPA lysis buffer as described previously and resolved by SDS-PAGE. The blots were exposed on a Chemidoc Touch imaging system (BioRad), and the optical density (OD) of the signal was analyzed using ImageLab software (BioRad). The subunit expression was expressed as a ratio of OD of GluA1 or GluA2 subunits normalized to the OD of β-actin.
Real-time PCR
The GluA1 and GluA2 subunit mRNA expression was determined using a real-time PCR assay using SYBR green dye using ΔΔCT method (Schmittgen & Livak, 2008). Primer pairs spanning an intron in the GluA1 and GluA2 subunits described in a prior study were used (Table 1) (Priya et al., 2014). The specificity of the primers was first confirmed in a simple PCR assay using cDNA prepared from a naïve animal. Each primer pair amplified a single DNA fragment of the predicted size (data not shown). The mRNA was isolated using Trizol reagent (Invitrogen) and treated with DNase (New England Biolabs). The mRNA was converted to cDNA using a cDNA synthesis kit (BioRad). The real-time PCR was carried out using 1 μl of cDNA, 4 μM primers each and SensiFAST SYBR Loc mix (Bioline). The PCR cycle consisted of 95 °C for 5 min, and 40 cycles of 95 °C for 15 sec followed by 60 °C for 15 sec. The expression of GAPDH was determined in each sample as an internal control. Each cDNA was amplified in triplicate. No-RT samples were run in parallel to confirm specificity of the primers (data not shown).
Table 1.
Primers used in the real-time PCR assay.
Determination of estrous stage
Vaginal swabs were obtained daily between 9 and 11 AM for 8 days, and their cytology was studied as described before (Goldman et al., 2007).
Statistical analysis
The normally distributed data are presented as the mean ± SEM. Statistical significance was determined by using Student’s t test if the data were normally distributed or with Mann-Whitney test if the data did not pass normality test. Two or more groups were compared using Friedman test with post hoc Dunnet’s or Dunn’s multiple comparison, Kruskal-Wallis test with post hoc Dunn’s multiple comparison test.
Results
RU-486 treatment suppressed neurosteroid withdrawal-induced seizure exacerbation
Catamenial seizure exacerbation was induced using a well-characterized pseudopregnancy protocol; the animals were treated with PMSG followed 2 days later by β-HCG (referred to as PMSG-β-HCG) to increase serum progesterone levels (Fig. 2D) (Reddy et al., 2001). On the 10th day of PMSG administration, animals were treated with finasteride to induce neurosteroid withdrawal (catamenial seizures). During the duration of PMSG-β-HCG treatment, half of the animals received daily injection of RU-486 (n=9) and the remaining animals received vehicle (10% β-cyclodextrin, n=9). Two blinded investigators performed the experiment; the one who administered the drugs was blinded to the seizure frequency, and the one who observed the seizure frequencies was blinded to the drug treatment received by animals (double-blind design).
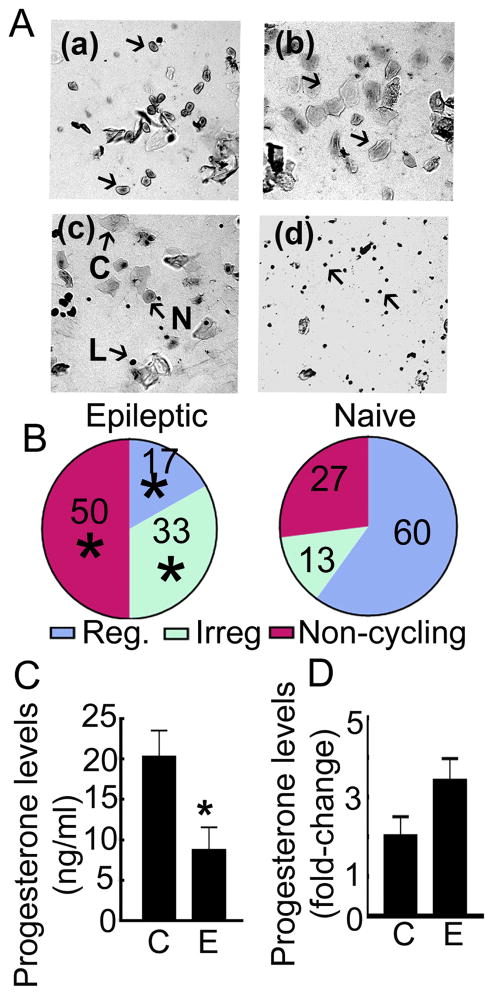
Figure 2. Estrous cyclicality was impaired in epileptic animals.
A: Cytology of vaginal swabs illustrating the four stages of estrous cycle. Presence of nucleated cells (arrows) marked the proestrus stage (a), the estrus stage (b) was characterized by a smear consisting of non-nucleated cornified cells (arrows), metestus stage (c) had all 3 cell types (N-nucleated, C-cornified, L-lymphocytes), and diestrus stage (d) was marked by the presence of lymphocytes. B: Percentage of animals with a regular cycle or irregular cycle (>2 days in one stage) and acyclic animals, n=8 epileptic and 10 naïve animals, * p<0.05 vs corresponding fraction in the naïve animals, Chi-square test. C: Serum progesterone levels in epileptic animals (8.7 ± 2.8 ng/ml, n= 7) compared to those in non-epileptic animals (20.3 ± 2.3, n= 6, * p= 0.02, student’s t-test (t=2.73 df=11)). D: Comparison of the rise in progesterone levels in non-epileptic (2.0 ± 0.5) and epileptic animals (3.4 ± 0.5) treated with PMSG and β-HCG. The serum progesterone levels on the 8th day of β-HCG administration were expressed relative to those before PMSG administration (n= same as in C).
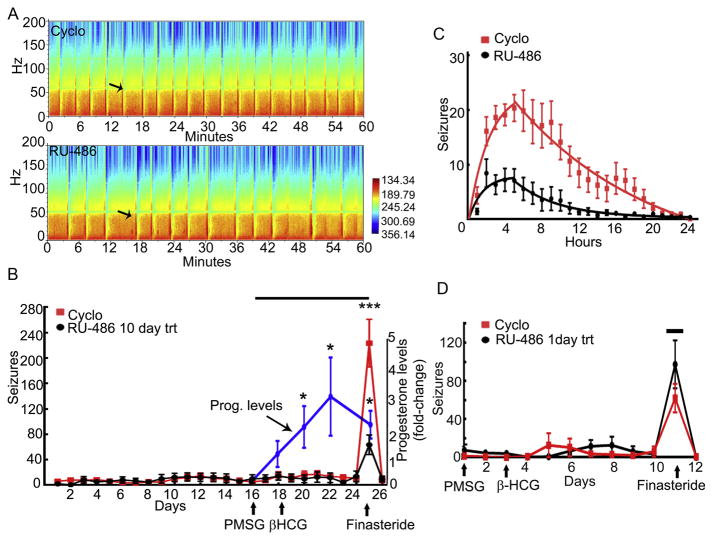
Catamenial seizures are prevalent in women with temporal lobe epilepsy (TLE) (Quigg et al., 2009), in which hippocampus is a major structure affected (Engel J Jr., 1997). Multielectrode recordings have revealed seizure onset in the hippocampi in the pilocarpine model of TLE used here (Toyoda et al., 2013; Bower & Buckmaster, 2008; Fujita et al., 2014). The cfos immunoreactivity, which is a surrogate for neuronal activity, also marks hippocampal neurons following spontaneous seizures (Peng & Houser, 2005). Therefore, electrographic seizures recorded from hippocampi irrespective of the associated behavior were counted. The seizure frequency during the baseline monitoring week in the animals allocated to RU-486 treatment group was 12 ± 6 and that in the animals assigned to vehicle treatment was 10 ± 3 (n=9 for both the groups). The seizure frequency remained stable during 10 days of PMSG-β-HCG treatment in the RU-486 or vehicle treatment groups, 11 ± 6 and 12 ± 4 respectively. The EEG power spectrum analysis revealed that the administration of finasteride, which inhibits neurosteroid synthesis and blocks the anticonvulsant action of progesterone, triggered more seizures in one group of animals than in the other group (Fig. 1A). Unblinding of EEG data revealed that RU-486-treated animals experienced fewer seizures compared to vehicle-treated animals (Fig. 1A, B). RU-486-treated animals experienced 63 ± 15 seizures following finasteride treatment whereas vehicle-treated animals experienced 233 ± 37 seizures after administration of finasteride. Thus, finasteride treatment increased the daily seizure frequency by 6 ± 1-fold in the RU-486-treated animals compared to a 22 ± 4-fold increase in the vehicle-treated animals (n= 9 each, p<0.0005, Mann-Whitney test). Seizures returned to baseline frequency much faster in the RU-486-treated animals than in the controls (Fig. 1C).
Figure 1. RU-486 treatment reduced the severity of neurosteroid withdrawal-induced seizures.
A: Power of EEG recorded from vehicle (20% cyclodextrin) and RU-486-treated animals 4 hrs after the administration of finasteride. Arrows mark a representative seizure in each animal. B: Daily seizure frequency in RU-486 (black line) or vehicle (red line)-treated animals. The arrows indicate the day of PMSG (20 IU, ip), β-HCG (10 IU, ip), and finasteride (100 mg/kg, ip) administration. The black bar at the top of the graph represents the duration of daily RU-486 or vehicle treatment (n=9 each, *** p<0.0001 Mann-Whitney test). The blue line illustrates the increase in progesterone levels (fold-change over baseline levels) following PMSG-β-HCG treatment of non-epileptic animals (n= 6, * p<0.05, Kruskal-Wallis test with post hoc Dunnet’s multiple comparison). C: Hourly seizure frequency for 24 hrs following finasteride administration in the RU-486- and vehicle-treated animals. The rise and decay of seizure frequency following finasteride administration were each fitted to exponential function and the half-life of decay of the seizures from the peak frequency was 3.3 hr in the RU-486 treatment group and 10.2 hr in the cyclodextrin-treated group. D: Daily seizure frequency in PMSG and β-HCG-treated animals that received a single injection of cyclodextrin or RU-486 30 min before finasteride on the 8th day of β-HCG treatment (n= 5 RU-486-treated animals and 3 cyclodextrin-treated animals). The black bar at the top of the graph represents the day of RU-486 treatment.
The seizure protection seen in the above studies could be due to a chronic blockade of progesterone or glucocorticoid receptor-mediated signaling or an acute anti-convulsant effect of RU-486. To distinguish between these effects, epileptic animals were treated with PMSG-β-HCG, and a single dose of RU-486 was administered 30 min prior to finasteride. The vehicle- and RU-486-treated animals experienced a similar, 15- to 20-fold, rise in seizure frequency following finasteride treatment (Fig. 1D). Thus, the acute administration of RU-486 did not suppress the finasteride-triggered seizure exacerbation.
We tested whether endogenous hormone cycling contributed to the increase in progesterone levels or withdrawal in epileptic animals. Two lines of evidence suggested that this was not the case. The basal progesterone levels were significantly lower in the epileptic animals than in the non-epileptic animals (Fig. 2C). This suggested that the estrous cycle may be impaired in epileptic animals. We confirmed this by studying the cytology of vaginal smears. Naïve animals typically had a 4-day-long estrous cycle (Fig. 2A, B). In contrast, many of the epileptic animals were acyclic and remained in persistent diestrus (Fig. 2A, B). Some of the animals also had irregular cycles, with a single stage lasting longer than 2 days (Fig. 2B). Thus, only a small fraction of epileptic animals were cycling regularly, as reported previously (Scharfman et al., 2009). PMSG-β-HCG treatment increased the serum progesterone levels by a similar extent in epileptic and naïve animals (Fig. 2D). Thus, endogenous progesterone cycling did not interfere with pharmacologically induced progesterone elevation and withdrawal.
Progesterone enhanced AMPA receptor (AMPAR)-mediated synaptic transmission in epileptic animals
Effect of PMSG-β-HCG treatment on AMPAR-mediated synaptic transmission of CA1 pyramidal neurons
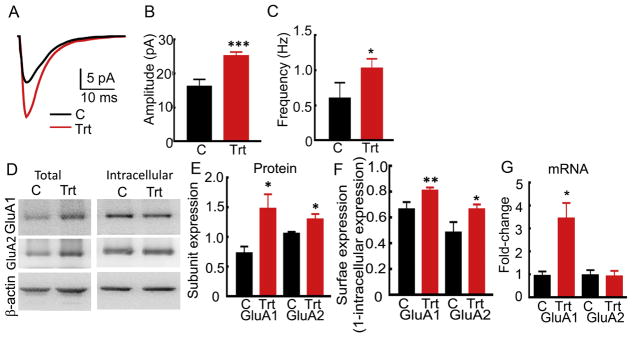
We hypothesized that elevation of progesterone in female epileptic animals enhances AMPAR-mediated glutamatergic synaptic transmission to compensate for the sedative effect of progesterone derivative neurosteroids (Turrigiano, 2008). We determined whether the AMPAR-mediated synaptic transmission of CA1 pyramidal neurons was enhanced in the PMSG-β-HCG-treated epileptic animals. The action potential-dependent AMPAR-mediated synaptic currents (sEPSCs) recorded from CA1 pyramidal neurons on the 8th day of β-HCG administration were larger than those recorded from the neurons in vehicle-treated epileptic animals (Fig. 3A–C, 25 ± 1 pA, n= 16 cells from 6 hormone-treated animals and 16 ± 2 pA, n= 8 cells from 6 vehicle-treated animals, p= 0.0003, Student’s t-test), and their frequency was higher (1.03 ± 0.14 Hz, n= 16 cells, 6 hormone-treated animals and 0.60 ± 0.22 Hz, n= 8 cells, 6 vehicle-treated animals, p= 0.023, Mann-Whitney test).
Figure 3. AMPAR-mediated neurotransmission of CA1 pyramidal neurons was enhanced and AMPAR expression was increased in PMSG-β-HCG-treated epileptic animals.
A: Average action potential-dependent excitatory post-synaptic currents (sEPSCs) recorded from CA1 neurons of vehicle-treated (C, black) and hormone-treated (Trt, red) animals. B: Mean of the median sEPSC amplitude recorded from CA1 neurons of the hormone-treated (n= 16 cells from 6 animals) and vehicle-treated animals (n= 8 cells from 6 animals, *** p<0.0001, Student’s t-test). C: Average frequency of sEPSCs from the same cells as in B (* p<0.005, Mann-Whitney test). D: Total and intracellular expression of GluA1 and GluA2 subunit protein in the hippocampi of hormone-treated (Trt) and vehicle-treated (C) animals. E: OD of GluA1 and GluA2 subunit signal normalized to the β-actin signal, n= 5, * p<0.05, Student’s t-test. F: Surface expression of GluA1 and GluA2 subunits, (n= 7 for GluA1 and n= 5 for GluA2, * p<0.05, ** p<0.005, Student’s t-test). G: Change in the expression of GluA1 and GluA2 subunit mRNA in the hormone-treated animals compared to that in the vehicle-treated animals, n= 7, * p<0.05, Mann-Whitney test.
Surface expression of GluA1 and GluA2 subunits in the hippocampi of PMSG-β-HCG-treated animals
The increased AMPAR-mediated transmission of CA1 pyramidal neurons in the PMSG-β-HCG-treated epileptic animals predicted an increased cell surface expression of AMPARs. Hippocampal AMPARs are primarily composed of GluA1/GluA2 heterodimers. We tested the cell surface expression by using a BS3 cross-linking assay, in which surface proteins are cross-linked and are eliminated from detection during the subsequent western blotting procedure (Fig. 3D) (Joshi et al., 2017). The surface expression of AMPAR subunits was determined from intracellular to total expression ratios and compared between the hormone-treated and vehicle-treated epileptic animals (Fig. 3F). The intracellular to total expression ratio of GluA1 subunit in the hormone-treated animals was smaller, 0.19 ± 0.03 than that in the vehicle-treated controls (0.34 ± 0.06, n= 7, p= 0.0074, Student’s t-test). The intracellular expression of GluA2 subunits in the hormone-treated animals was also lower (0.34 ± 0.04) than that in the controls (0.51 ± 0.08, n= 5, p= 0.044, Student’s t-test). Thus, the surface expression of GluA1 and GluA2 subunits was increased in the hormone-treated animals.
Hippocampal expression of GluA1 and GluA2 subunit mRNA and protein following PMSG-β-HCG treatment
PMSG-β-HCG treatment increased total GluA1 and GluA2 subunit expression in epileptic animals. Western blotting revealed stronger GluA1 and GluA2 signals in the proteins isolated from the hormone-treated animals compared to that in the vehicle-treated control animals (Fig. 3D, E). The ratio of GluA1 to β-actin expression in the hormone-treated animals was 1.48 ± 0.24 and that in the controls was 0.73 ± 0.12 (n= 5, p= 0.029, Student’s t-test). This ratio for GluA2 subunits was 1.32 ± 0.07 in the treated animals and 1.01 ± 0.05 in the controls (n= 5, p= 0.041, Student’s t-test). Finally, we determined whether the hormone treatment altered the transcription of GluA1 and GluA2 subunits; the expression of mRNA encoding the GluA1 and GluA2 subunits was determined using ΔΔCT RT-qPCR method (Fig. 3G). The GluA1 subunit mRNA expression in the treated epileptic animals was larger (treated: 3.45 ± 0.66 and controls: 0.94 ± 0.20, n= 7, p= 0.0012, Mann-Whitney test). However, the expression of GluA2 subunit mRNA in the treated animals was similar to controls (treated: 0.93 ± 0.23 and controls: 0.98 ± 0.22, n=5, p= 0.90, Mann-Whitney test).
Progesterone increased the AMPAR-mediated synaptic transmission in non-epileptic animals
Effect of PMSG-β-HCG treatment on AMPAR-mediated synaptic transmission and AMPAR expression
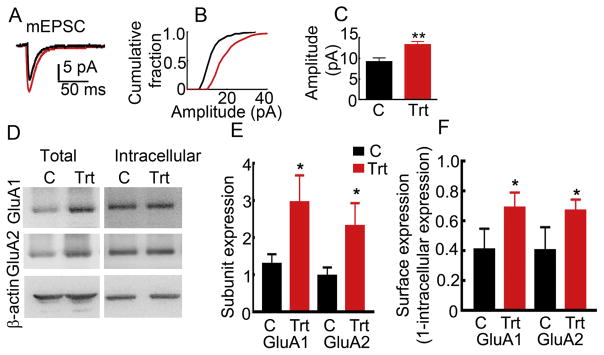
In epileptic animals, hormone treatment enhanced both AMPAR-mediated transmission and the expression of GluA1 and GluA2 subunits, whereas treatment with RU-486, which blocks progesterone receptors, attenuated withdrawal seizures in these animals. We determined whether the progesterone triggered the plasticity of AMPARs in non-epileptic, normally cycling female rats. The action potential-independent synaptic currents (mEPSCs) of CA1 pyramidal neurons of the PMSG-β-HCG-treated animals were also larger than those of vehicle-treated control animals (Fig. 4A–C, 13.35 ± 0.74, n= 10 cells from 6 treated animals and 9.21 ± 0.91, n= 6 cells from 5 vehicle-treated animals, p= 0.004, Student’s t-test). Consistent with the augmented AMPAR-mediated synaptic transmission of CA1 pyramidal neurons, the expression of GluA1 and GluA2 subunits in the hippocampi of the PMSG-β-HCG-treated animals was more than that in vehicle-treated animals processed in parallel (Fig. 4D, E). The ratio of GluA1 subunit to β-actin in the treated and control animals was 2.96 ± 0.73 and 1.30 ± 0.26 respectively (n= 9, p= 0.021, Student’s t-test). Similarly, the GluA2 subunit expression normalized to the β-actin expression in the treated animals was 2.32 ± 0.62 and that in the controls was 0.98 ± 0.23 (n= 9, p= 0.013 Student’s t-test). The surface expression of GluA1 and GluA2 subunits was also increased in the hormone-treated animals compared to that in the vehicle-treated controls (Fig. 4F). The intracellular expression of GluA1 subunits in the hormone-treated animals was 0.31 ± 0.10 and 0.59 ± 0.14 in the control animals (n= 9, p= 0.012, Student’s t-test). The intracellular expression of GluA2 subunits in the hormone-treated and control animals was 0.33 ± 0.07 and 0.6 ± 0.15 respectively (n= 9, p= 0.027, Student’s t-test). Thus, the PMSG-β-HCG treatment augmented the AMPAR-mediated neurotransmission of CA1 pyramidal neurons and increased GluA1 and GluA2 subunit expression in naïve cycling females.
Figure 4. AMPAR-mediated neurotransmission of CA1 pyramidal neurons was also enhanced in non-epileptic animals treated with PMSG-β-HCG.
A: Averaged action potential-independent excitatory synaptic current (mEPSCs) recorded from CA1 pyramidal neuron from a control (vehicle-treated, black) and a PMSG-β-HCG-treated animal (red). B: Cumulative amplitude distribution plot of mEPSCs recorded from the representative neurons shown in A. C: Mean of the median mEPSC amplitude in the treated animals (n= 10 cells/6 hormone-treated animals and 6 cells/5 vehicle-treated animals, ** p<0.001, Student’s t-test). D: Western blots illustrating the expression of GluA1 and GluA2 subunits in the total proteins and intracellular proteins isolated from hippocampi of PMSG-β-HCG-treated animals. E: Ratio of OD AMPAR subunit expression to that of the β-actin expression, n= 9, * p<0.05, Student’s t-test). F: The surface expression of GluA1 and GluA2 subunits in the treated animals compared to that in the controls (n= 9, * p<0.05, Student’s t-test). These animals were not matched for estrous cycle.
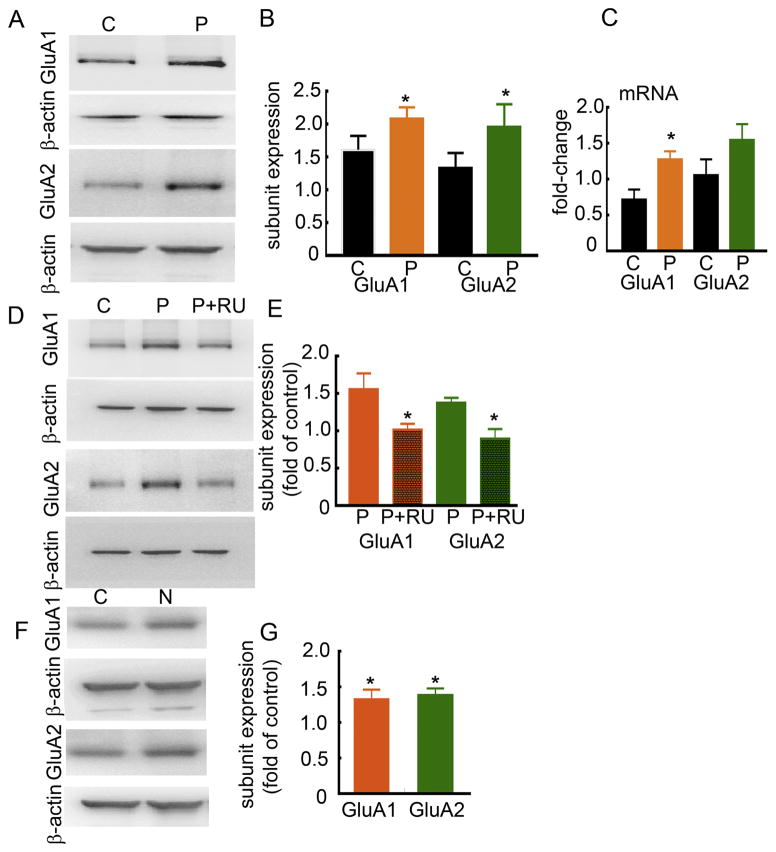
The GluA1 and GluA2 subunit mRNA and protein expression in progesterone-treated animals
PMSG-β-HCG treatment may have effects beyond the elevation of serum progesterone levels; therefore, we confirmed that the observed increase in AMPAR expression was due to elevated progesterone levels. Animals were treated with a single dose of progesterone (50 mg/kg, ip), and the expression of GluA1 and GluA2 subunits was determined 2 days later. The expression of GluA1 and GluA2 subunits was higher in the progesterone-treated animals than in vehicle (20% cyclodextrin)-treated animals (Fig. 5A, B). GluA1 subunit expression in the progesterone-treated and vehicle-treated animals was 2.08 ± 0.18 and 1.62 ± 0.21, respectively (n= 6, p= 0.003, Student’s t-test). The GluA2 subunit expression in the progesterone-treated and vehicle-treated animals was 1.96 ± 0.34 and 1.34 ± 0.22 (n= 6, p= 0.024 Student’s t-test). Progesterone treatment also increased the expression of GluA1 subunit mRNA, but not that of GluA2 subunit mRNA (Fig. 5C). The GluA1 subunit mRNA expression in the progesterone-treated animals was 1.28 ± 0.12 and that in the controls was 0.72 ± 0.13 (n= 9 control and 10 progesterone-treated, p= 0.006, Mann-Whitney test). The GluA2 subunit mRNA expression in the treated animals was 1.55 ± 0.22 (n=10,) and in the controls was 1.06 ± 0.22 (n=9, p= 0.165, Mann-Whitney test).
Figure 5. Progesterone increased AMPAR expression via activation of PRs.
A: Expression of GluA1 and GluA2 subunits in hippocampal proteins from vehicle- and progesterone (50 mg/kg)-treated animals. The animals received a single injection of progesterone and the proteins were isolated 2 days later. B: Ratio of OD of GluA1 or GluA2 subunits to that of β-actin (n= 6, *p<0.05, Student’s t-test). C: Expression of GluA1 and GluA2 subunit mRNAs in progesterone-treated animals relative to that in controls (n= 9 control and 10 progesterone-treated, * p<0.05, Mann-Whitney test). D: GluA1 and GluA2 subunit expression in animals treated with progesterone and RU-486 (30 mg/kg, Progesterone+RU 486) or progesterone and vehicle (P). E: Subunit expression in Progesterone+RU-486-treated or in Progesterone-treated animals normalized to that in the vehicle-treated animals run in parallel (n= 5, * p<0.05, Mann-Whitney test). F: The AMPAR expression in animals treated with a single dose of nestorone (3 mg/kg, sc) and the subunit expression was determined 2 days later. G: Subunit expression in the treated animals normalized to that in the vehicle-treated animals (n= 6, * p<0.05, Mann-Whitney test). All control and treated animals were matched for their estrous cycle stage.
Effect of RU-486 treatment on progesterone-induced AMPAR plasticity
We determined whether RU-486 could abolish the effects of progesterone on AMPAR expression. The animals were treated with RU-486 (30 mg/kg) or vehicle, progesterone was administered 30 min later and the subunit expression was determined after 2 days. The expression of GluA1 and GluA2 subunits in the progesterone and RU-486-treated animals was less than that in the progesterone and vehicle-treated animals (Fig. 5D, E). The GluA1 subunit expression in progesterone-treated animals was 1.56 ± 0.21 and that in progesterone and RU-486-treated animals was 1.02 ± 0.07 (n= 5, p= 0.032, Mann-Whitney test). The GluA2 subunit expression in progesterone-treated animals was 1.38 ± 0.07 and that in progesterone and RU-486-treated animals was 0.88 ± 0.11 (n= 5, p= 0.016, Mann-Whitney test).
Effect of synthetic progesterone receptor agonist nestorone on AMPAR expression
The specificity of progesterone receptor-mediated action was further confirmed by administering nestorone (3 mg/kg, subcutaneous), a synthetic progesterone receptor agonist (Kumar et al., 2000) (Fig. 5F). Animals were treated with nestorone and the receptor expression was determined 2 days later. In nestorone-treated animals, GluA1 and GluA2 subunit expression was more than that in vehicle-treated controls (Fig. 5F, G, GluA1: 1.33 ± 0.14-fold of controls, n= 6, p= 0.002 and GluA2: 1.39 ± 0.09-fold of controls, n= 6, p= 0.002, Mann-Whitney test).
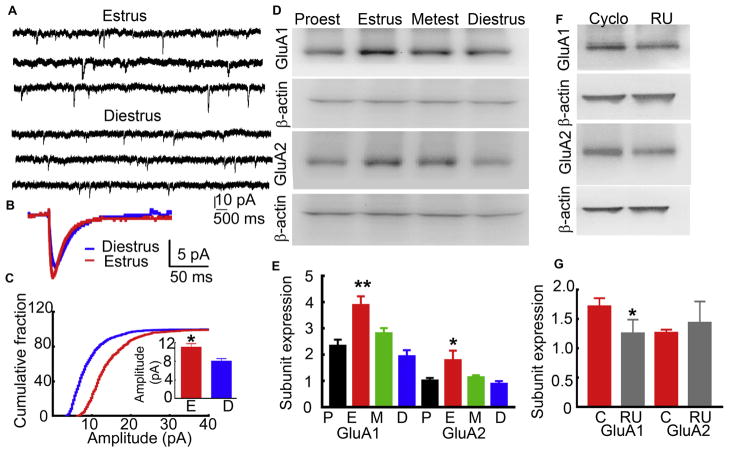
AMPAR expression and AMPAR-mediated neurotransmission fluctuated during estrous cycle
We determined whether the endogenous rise in progesterone during the late proestrus phase also increased AMPAR-mediated neurotransmission and enhanced AMPAR expression. The animals were monitored for 2 consecutive estrous cycles, and subsequent studies were performed on normally cycling animals (4–5 days). We measured AMPAR-mediated mEPSCs from CA1 pyramidal neurons of animals in diestrus and estrus phases. These phases were selected because they are temporally separated and precede and follow the rise in progesterone. The amplitude of mEPSCs recorded from CA1 neurons of animals in the estrus stage was larger than those in the diestrus stage (Fig. 6A–C; estrus: 10.95 ± 0.72, n= 16 CA1 neurons from 8 animals and diestrus: 8.12 ± 0.53, n= 10 neurons from 6 animals, p=0.009, Student’s t-test). The frequency of mEPSCs, 10–90% rise-time, and weighted decay were not different between animals in estrus and diestrus stages.
Figure 6. Estrous cycle-linked changes in the synaptic AMPAR-mediated transmission and GluA1 and GluA2 subunit expression.
A: Traces from representative animals in estrus and diestrus phases illustrating mEPSCs recorded from CA1 pyramidal neurons. B: Averaged mEPSC from an animal in estrus (red) and diestrus (blue) stages. C: Distribution of mEPSC amplitudes recorded from the cell shown in C. The inset shows the mean of the median mEPSC amplitude (n= 16 CA1 neurons from 8 animals in estrus and 10 neurons from 6 animals in diesturs, *p<0.05, Student’s t-test. The frequency of mEPSCs (0.89 ± 0.26 Hz vs 0.74 ± 0.13 Hz), 10–90% rise-time (4.56 ± 0.31 ms vs 3.81 ± 0.27 ms) and weighted decay (11.29 ± 0.72 ms vs 11.56 ± 1.07 ms) were not different between animals in estrus and diestrus stages. D: Expression of GluA1 and GluA2 in the hippocampal proteins isolated from animals in the four stages of estrous cycle. E: Subunit expression in animals in various stages of estrous cycle normalized to that of β-actin (n= 7, * p<0.05 vs diestrus, Friedman’s test followed by Dunn’s multiple comparison test). F: GluA1 and GluA2 subunit expression in RU-486 (30 mg/kg, daily) or vehicle-treated animals. The treatment was started when animals were in diestrus stage and continued until the following estrus stage. G: The subunit expression normalized to that of β-actin (n= 6 for GluA1 and 7 for GluA2, * p<0.05, paired t-test).
GluA1 and GluA2 subunit protein expression in the hippocampus was higher in animals in estrus than in those in the proestrus stage (Fig. 6D, E). The GluA1 subunit expression in the animals in different stages of estrous cycle was proestrus stage: 2.36 ± 0.22, estrus: 3.90 ± 0.33, metestrus: 2.83 ± 0.18 and diestrus: 1.96 ± 0.23 (n= 7 each, p= 0.0024 estrus vs diestrus, Friedman’s test followed by Dunn’s multiple comparison test). The GluA2 subunit expression in the animals in estrus stage was also more than that in animals in diestrus stage (proestrus: 1.04 ± 0.09, estrus: 1.79 ± 0.37, metestrus: 1.14 ± 0.08, and diestrus: 0.90 ± 0.10, n=7 each, p= 0.011 estrus vs diestrus, Friedman test followed by Dunn’s multiple comparison test). We then treated animals with RU-486 and tested whether doing so prevented the increase in the expression of AMPARs during estrus phase. RU-486 was administered daily to animals in the diestrus phase, and the proteins were isolated when animals were in the subsequent estrus phase. The expression of GluA1 subunit was lower in the RU-486-treated animals than in the animals treated daily with vehicle between the diestrus and estrus phases (Fig. 6F, G, RU-486-treated: 1.26 ± 0.23 and vehicle-treated: 1.72 ± 0.13, n= 6, p=0.02, Student’s t-test). In contrast, the GluA2 subunit expression in the RU-486-treated animals and vehicle-treated animals was similar (RU-486-treated: 1.44 ± 0.36 and vehicle-treated: 1.27± 0.05, n= 7, p= 0.678 Student’s t-test).
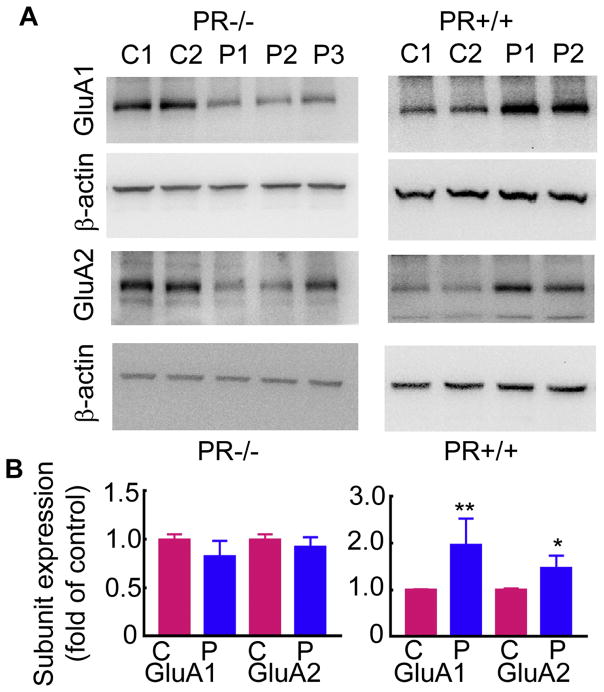
Progesterone-triggered increase in AMPAR expression was blocked in mice lacking progesterone receptor expression
Above studies using pharmacological blockade of progesterone receptors suggested that progesterone via progesterone receptor activation regulates the plasticity of AMPARs. However, RU-486 also blocks glucocorticoid receptors (Gagne et al., 1985). To confirm that progesterone receptor activation regulated the observed plasticity of AMPARs PR−/− mice were used. Progesterone treatment increased the hippocampal expression of GluA1 and GluA2 subunits in PR+/+ mice (Fig. 7A). In contrast, progesterone failed to increase the AMPAR expression in PR−/− mice (Fig. 7A). The GluA1 and GluA2 subunit expression in the PR+/+ mice was 2 ± 0.6 and 1.5 ± 0.2-fold of that in the vehicle-treated PR+/+ mice respectively (n= 8, p<0.05 Mann-Whitney test). On the other hand, the GluA1 and GluA2 subunit expression in progesterone-treated PR−/− mice was 0.83 ± 0.16 and 0.9 ± 0.10 respectively (n= 9), similar to that in the vehicle-treated mice (p>0.05, Mann-Whitney test).
Figure 7. Progesterone-induced increase in the GluA1 and GluA2 subunit expression was blocked in PR−/− mice.
A: Western blots showing the GluA1 and GluA2 subunit expression in the hippocampal proteins isolated from 2 vehicle-treated (lanes C1 and C2) and 3 progesterone-treated (100 mg/kg, 24 hours, lanes P1 to P3) PR−/− female mice. The expression of GluA1 and GluA2 subunits in the hippocampi of 2 vehicle or progesterone treated PR+/+ mice is shown for the comparison. The mice were matched for estrous cycle stage. B: The subunit expression in progesterone-treated animals expressed as fold-of vehicle-treated controls (n=8 vehicle-treated and 9 progesterone-treated PR−/− mice and n= 8 vehicle-treated and progesterone-treated PR+/+ mice, ** p<0.0005 and * p<0.05, Mann-Whitney test).
Discussion
The major findings of this study are 1) endogenous rise as well as exogenously triggered elevation in progesterone levels increased AMPAR subunit expression in the hippocampus and enhanced AMPAR-mediated synaptic transmission of CA1 pyramidal neurons, 2) progesterone receptors regulated the progesterone-induced plasticity of AMPARs, and 3) RU-486, which blocks progesterone and glucocorticoid receptors reduced the catamenial seizure exacerbation.
The findings of this study shift the dominant paradigm of progesterone as a sedative that enhances brain inhibition via conversion to neurosteroids to a hormone that has dual actions on the brain: inhibition via neurosteroids and excitatory action via progesterone receptor activation. Furthermore, this double-blind study in an experimental model of catamenial epilepsy revealed that RU-486 treatment attenuated progesterone withdrawal seizures. This action of RU-486 could result from inhibition of progesterone and/or glucocorticoid receptors.
Corticosteroids also regulate the excitability of hippocampal neurons through glucocorticoid receptors (GRs), and the RU-486 used in these studies targets these receptors as well (Gagne et al., 1985). GRs are abundantly expressed in the hippocampus; the strongest expression is on CA1 neurons (Morimoto et al., 1996; Patel & Bulloch, 2003). Corticosteroids regulate the glutamatergic synaptic transmission of hippocampal neurons, which requires the activation of GRs; however, these effects occur within minutes (Joéls et al., 2012). A single day treatment of RU-486 did not affect the intensity of finasteride-induced seizures; however, whether the effects of chronic RU-486 treatment involved GRs is not known. Since daily injection of RU-486 or vehicle can also cause stress and activate glucocorticoid receptor signaling, additional studies in mice lacking progesterone receptor expression will be important to confirm the role of progesterone receptors in regulation of catamenial seizures. The findings of a prior study, which found impaired limbic epileptogenesis in progesterone receptor knockout mice or after infusion of antisense oligos targeting progesterone receptor mRNA also support the pro-convulsant effect of progesterone receptor activation (Reddy & Mohan, 2011).
The findings of the current study may help explain the failure of progesterone replacement therapy for treating catamenial seizure exacerbation and identify potential alternate therapies. Progesterone is historically known to exert inhibitory, anticonvulsant effects via conversion to allopregnanolone, which enhances GABAA receptor-mediated neurotransmission (Selye H, 1942; Frye, 2008; Joshi et al., 2013). A perimenstrual drop in progesterone and allopregnanolone levels is proposed to cause catamenial seizure exacerbation (Herzog, 2015). In prior uncontrolled, observational, clinical studies, progesterone therapy was reported to be beneficial in suppressing catamenial seizure exacerbation in a limited number of patients and an intravenous infusion of progesterone decreased spike frequency in women with focal epilepsy (Herzog, 1999; Herzog, 1995; Bäckström et al., 1984). These observations in experimental animals and patients suggested that catamenial epilepsy is an allopregnanolone tolerance/withdrawal syndrome and that the replenishment of progesterone, the metabolic source of allopregnanolone, would alleviate withdrawal seizures. This led to a large well-controlled, multicenter, double-blind clinical trial of 14-day twice daily progesterone treatment of catamenial epilepsy (Herzog, 2015). In that trial, 294 subjects were randomized 2:1 to progesterone or placebo, stratified by catamenial and non-catamenial status. There was no significant difference in the proportions of responders between progesterone and placebo in the catamenial and non-catamenial strata. A post-hoc analysis has revealed reduction in the seizures of women with severe (3-fold) perimenstrual exacerbation of seizure exacerbation (Herzog & Frye, 2014). Furthermore, nestorone, a synthetic progesterone receptor agonist also increased AMPAR expression. Synthetic progestins are commonly used in contraceptives for women; thus, contraceptives could interfere with seizure management via mechanisms involving AMPARs.
The current study demonstrating an excitatory action of progesterone is also significant for the treatment of other neurological disorders, such as traumatic brain injury, spinal cord injury, and ischemic injury; progesterone was neuroprotective against these injuries in an extensive array of studies in experimental animals (Deutsch et al., 2013; Gibson & Bath, 2015). However, clinical trials of progesterone therapy for traumatic brain injury and spinal cord injury have also failed to find a beneficial effect over placebo treatment (Skolnick et al., 2014; Wright et al., 2014; Aminmansour et al., 2016). The increased AMPAR-mediated transmission following progesterone treatment shown here could contribute to excitotoxicity, which in turn could counter balance any other protective effects of progesterone.
Premenstrual dysphoric disorder (PMDD), which affects millions of women worldwide, is associated with negative mood, impaired cognitive performance, and changes in sleep (Hantsoo & Epperson, 2015). Treatment with a gonadotropin releasing hormone (GnRH) analog to suppress ovarian function alleviates these symptoms (Hantsoo & Epperson, 2015). The neurosteroid and benzodiazepine sensitivity of GABARs expressed in patients of PMDD is distinct from that of healthy women (Wihlbäck et al., 2006). The progesterone receptor-induced plasticity of AMPARs may also have implications in the treatment of PMDD.
Inhibitory action of progesterone, through conversion to allopregnanolone is fast, occurring over minutes; whereas, its excitatory action occurs more slowly, suggesting that this excitatory action provides balance to rapid inhibitory action. Evidence from in vivo and in vitro studies suggest that many central neurons maintain their firing rate by tightly regulating excitatory/inhibitory (E/I) balance. This homeostatic rebalancing of E/I is slow, taking hours to days (Turrigiano, 2011). Studies on homeostatic plasticity suggest that neurons sense their activity by cell firing and calcium influx leading to increased accumulation of GluA2 subunit-containing AMPA receptors in the synapse (Gainey et al., 2009). The progesterone-induced plasticity of excitatory transmission seen here appears to be distinct as it is triggered by progesterone receptors and it involves upregulation of both GluA1 and GluA2 subunits.
We propose that excitatory action of progesterone receptors counterbalances the memory impairing effect of allopregnanolone. Progesterone derivative allopregnanolone has a sedative anxiolytic action (Yoshizawa et al., 2017; Pinna, 2010). However, it also impairs spatial memory performance on Morris water maze task without impairing non-spatial learning (Johansson et al., 2002; Matthews et al., 2002). Such impairment of spatial learning is not observed with progesterone; progesterone did not impair (or improve) spatial learning on radial maze (Tanabe et al., 2004; Sato et al., 2004). Dynamic remodeling of dendritic spine morphology and density plays a key role in learning and memory (Kasai et al., 2010; Bhatt et al., 2009) and progesterone enhances dendritic spine density on CA1 pyramidal neurons (Woolley & McEwen, 1993).
Progesterone receptors are widely expressed in the brain and their expression is regulated by estrogen (Guerra-Araiza et al., 2003). Estrogen regulates the density of dendritic spines, which harbor glutamatergic synapses, on CA1 pyramidal neurons (Woolley & McEwen, 1993; Liu et al., 2008; Tada et al., 2015). This estrogen effect is amplified by treatment with progesterone (McEwen & Woolley, 1994). The findings of the current study taken together with those of prior studies show a synergistic action of progesterone and estrogen to regulate excitability in the brain
Many of the epileptic animals were acyclic, and the influence of endogenous hormonal fluctuations on AMPAR expression seen here was likely minimal. However, the influence of endogenous hormonal fluctuations cannot be ruled out in non-epileptic animals, which were matched for the stage of estrous cycle but were not ovariectomized. Estrogen treatment also increases GluA1 subunit expression via the activation of ERs (Liu et al., 2008); however, these effects are rapid and occur within minutes to hours of the treatment.
Findings of other studies also support the excitatory action of progesterone reported here. Progesterone via progesterone receptors increases the density of dendritic spines, which are sites of glutamatergic receptors, on hippocampal CA1 pyramidal neurons and cerebellar Purkinje cells (McEwen & Woolley, 1994; Sakamoto et al., 2001; Woolley & McEwen, 1993). Progesterone also upregulates the expression of GluA1 subunit of AMPARs in the anteroventral periventricular nucleus of hypothalamus (Gu et al., 1999). Furthermore, progesterone treatment increases the expression of Fos protein, which is a marker of neuronal activity, in the progesterone receptor-expressing neurons of medial preoptic area and ventromedial nucleus of hypothalamus (Auger & Blaustein, 1997).
In conclusion, to our knowledge, this is the first study to demonstrate that progesterone increases AMPAR expression via activation of progesterone receptors. This excitatory action of progesterone may play a role in catamenial seizure exacerbation. The findings presented here suggest that RU-486 which blocks progesterone and glucocorticoid receptors may be efficacious in preventing perimenstrual seizure exacerbation.
Highlights.
RU-486 treatment suppressed catamenial seizure exacerbation
Progesterone increased AMPAR-mediated neurotransmission of CA1 neurons
Progesterone increased hippocampal expression of GluA1 subunit mRNA and protein
Progesterone receptor activation mediated the progesterone effects on AMPARs
Acknowledgments
We thank David Breen and Crystal Passmore for technical assistance.
Funding:
This study was supported by NIH grants RO1 NS 040337 and RO1 NS 044370 to JK.
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- GABAR
γ-aminobutyric acid type A receptor
- PMSG
Pregnant meyer’s serum gonadotropin
- β-HCG
β subunit of human chorionic gonadotropin
- TLE
temporal lobe epilepsy
Footnotes
Conflict of interest:
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aminmansour B, Asnaashari A, Rezvani M, Ghaffarpasand F, Amin Noorian SM, Saboori M, et al. Effects of progesterone and vitamin D on outcome of patients with acute traumatic spinal cord injury; a randomized, double-blind, placebo controlled study. The Journal of Spinal Cord Medicine. 2016;39:272–280. doi: 10.1080/10790268.2015.1114224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström Tr, Zetterlund B, Blom S, Romano M. Effects of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurologica Scandinavica. 1984;69:240–248. doi: 10.1111/j.1600-0404.1984.tb07807.x. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- Bower MR, Buckmaster PS. Changes in Granule Cell Firing Rates Precede Locally Recorded Spontaneous Seizures by Minutes in an Animal Model of Temporal Lobe Epilepsy. J Neurophysiol. 2008;99:2431. doi: 10.1152/jn.01369.2007. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Maxwell BL, Toft DO, Schrader WT, O’Malley BW. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987;149:493–501. doi: 10.1016/0006-291x(87)90395-0. [DOI] [PubMed] [Google Scholar]
- Deutsch ER, Espinoza TR, Atif F, Woodall E, Kaylor J, Wright DW. Progesterone’s role in neuroprotection, a review of the evidence. Brain Res. 2013;1530:82–105. doi: 10.1016/j.brainres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Epps T, Carlen PL, MacLusky J. Progestin receptors mediate progesterone suppression of epileptiform activity in tetanized hippocampal slices in vitro. Neuroscience. 2000;101:895–906. doi: 10.1016/s0306-4522(00)00439-5. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, WPWH . Epilepsy: a comprehensive textbook. Philadelphia: Lippincott-Raven; 1997. Mesial temporal lobe epilepsy. [Google Scholar]
- Frye CA. Chapter 3 Hormonal Influences on Seizures: Basic Neurobiology. In: Gidal Barry E, Cynthia, editors. International Review of Neurobiology Epilepsy in Women The Scientific Basis for Clinical Management. Academic Press; 2008. pp. 27–77. [DOI] [PubMed] [Google Scholar]
- Fujita S, Toyoda I, Thamattoor AK, Buckmaster PS. Preictal Activity of Subicular, CA1, and Dentate Gyrus Principal Neurons in the Dorsal Hippocampus before Spontaneous Seizures in a Rat Model of Temporal Lobe Epilepsy. The Journal of Neuroscience. 2014;34:16671. doi: 10.1523/JNEUROSCI.0584-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne D, Pons M, Philibert D. RU 38486: a potent antiglucocorticoid in vitro and in vivo. J Steroid Biochem. 1985;23:247–251. doi: 10.1016/0022-4731(85)90401-7. [DOI] [PubMed] [Google Scholar]
- Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci. 2009;29:6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Bath PM. Feasibility of progesterone treatment for ischaemic stroke. Journal of Cerebral Blood Flow & Metabolism. 2015;36:487–491. doi: 10.1177/0271678X15616782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Villamar-Cruz O, González-Arenas A, Chavira R, Camacho-Arroyo I. Changes in Progesterone Receptor Isoforms Content in the Rat Brain During the Oestrous Cycle and After Oestradiol and Progesterone Treatments. Journal of Neuroendocrinology. 2003;15:984–990. doi: 10.1046/j.1365-2826.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Cerbón MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrotts cycle. Life Sciences. 2000;66:1743–1752. doi: 10.1016/s0024-3205(00)00497-5. [DOI] [PubMed] [Google Scholar]
- Hantsoo L, Epperson CN. Premenstrual Dysphoric Disorder: Epidemiology and Treatment. Curr Psychiatry Rep. 2015;17:87. doi: 10.1007/s11920-015-0628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Partyka MK, Lydon JP, Iruela-Arispe ML. Generation of a mouse for conditional excision of progesterone receptor. genesis. 2006;44:391–395. doi: 10.1002/dvg.20227. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Catamenial epilepsy: Update on prevalence, pathophysiology and treatment from the findings of the NIH Progesterone Treatment Trial. Seizure. 2015;28:18–25. doi: 10.1016/j.seizure.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with epilepsy: A 3-year follow-up. Neurology. 1999;52:1917–191a. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Sperling MR, Massaro JM Progesterone Trial Study Group. Distribution of seizures across the menstrual cycle in women with epilepsy. Epilepsia. 2015;56:e58–e62. doi: 10.1111/epi.12969. [DOI] [PubMed] [Google Scholar]
- Janzen DM, Rosales MA, Paik DY, Lee DS, Smith DA, Witte ON, et al. Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy. Cancer Res. 2013;73:4697–4710. doi: 10.1158/0008-5472.CAN-13-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Sarabdjitsingh RA, Karst H. Unraveling the Time Domains of Corticosteroid Hormone Influences on Brain Activity: Rapid, Slow, and Chronic Modes. Pharmacol Rev. 2012;64:901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- Johansson IM, Birzniece V, Lindblad C, Olsson T, Backstrom T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002;934:125–131. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- Joshi S, Rajasekaran K, Kapur J. GABAergic transmission in temporal lobe epilepsy: The role of neurosteroids. Exp Neurol. 2013;244:36–42. doi: 10.1016/j.expneurol.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Rajasekaran K, Sun H, Williamson J, Kapur J. Enhanced AMPA receptor-mediated neurotransmission on CA1 pyramidal neurons during status epilepticus. Neurobiol Dis. 2017 doi: 10.1016/j.nbd.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kumar N, Koide SS, Tsong YY, Sundaram K. Nestorone®: a progestin with a unique pharmacological profile. Steroids. 2000;65:629–636. doi: 10.1016/s0039-128x(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol. 2010;67:689–693. doi: 10.1002/ana.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, et al. Activation of estrogen receptor-[beta] regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Mani S, Oyola MG. Progesterone signaling mechanisms in brain and behavior. Frontiers in Endocrinology. 2012;3:1–8. doi: 10.3389/fendo.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL, Tokunaga S, McDaniel JR. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin Exp Res. 2002;26:1747–1751. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol. 1994;29:431–436. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, et al. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neuroscience Research. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Patel A, Bulloch K. Type II glucocorticoid receptor immunoreactivity in the mossy cells of the rat and the mouse hippocampus. Hippocampus. 2003;13:59–66. doi: 10.1002/hipo.10045. [DOI] [PubMed] [Google Scholar]
- Peng Z, Houser CR. Temporal Patterns of Fos Expression in the Dentate Gyrus after Spontaneous Seizures in a Mouse Model of Temporal Lobe Epilepsy. The Journal of Neuroscience. 2005;25:7210–7220. doi: 10.1523/JNEUROSCI.0838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G. In a mouse model relevant for post-traumatic stress disorder, selective brain steroidogenic stimulants (SBSS) improve behavioral deficits by normalizing allopregnanolone biosynthesis. Behav Pharmacol. 2010;21:438–450. doi: 10.1097/FBP.0b013e32833d8ba0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya A, Johar K, Nair B, Wong-Riley MTT. Specificity protein 4 (Sp4) regulates the transcription of AMPA receptor subunit GluA2 (Gria2) Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2014;1843:1196–1206. doi: 10.1016/j.bbamcr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG On behalf of the NIH Progesterone Trial Study Group. Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology. 2009;73:223–227. doi: 10.1212/WNL.0b013e3181ae7adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran K, Todorovic M, Kapur J. Calcium-permeable AMPA receptors are expressed in a rodent model of status epilepticus. Ann Neurol. 2012;72:91–102. doi: 10.1002/ana.23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroids - Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy. In: Jeffrey L, Noebels MAMARRWOaAVD-E, editors. Jasper’s basic mechanisms of epilepsies. Bethesda, MD, USA: Oxford University Press; 2012. pp. 984–1002. [Google Scholar]
- Reddy DS, Mohan A. Development and Persistence of Limbic Epileptogenesis Are Impaired in Mice Lacking Progesterone Receptors. The Journal of Neuroscience. 2011;31:650–658. doi: 10.1523/JNEUROSCI.4488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;493:477–478. doi: 10.1002/cne.20839. [DOI] [PubMed] [Google Scholar]
- Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T. Effects of estradiol and progesterone on radial maze performance in middle-aged female rats fed a low-calcium diet. Behav Brain Res. 2004;150:33–42. doi: 10.1016/S0166-4328(03)00249-3. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Malthankar-Phatak GH, Friedman D, Pearce P, McCloskey DP, Harden CL, et al. A Rat Model of Epilepsy in Women: A Tool to Study Physiological Interactions between Endocrine Systems and Seizures. Endocrinology. 2009;150:4437–4442. doi: 10.1210/en.2009-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Selye H. Antagonism between anesthetic steroid hormones and pentamethylene tetrazol (Metrazol) J Lab Clin Med. 1942;27:1051–1053. [Google Scholar]
- Singh M, Su C. Progesterone and neuroprotection. Hormones and Behavior. 2013;63:284–290. doi: 10.1016/j.yhbeh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, et al. A Clinical Trial of Progesterone for Severe Traumatic Brain Injury. N Engl J Med. 2014;371:2467–2476. doi: 10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- Stephens SBZ, Tolson KP, Rouse J, Poling MC, Hashimoto-Partyka MK, Mellon PL, et al. Absent Progesterone Signaling in Kisspeptin Neurons Disrupts the LH Surge and Impairs Fertility in Female Mice. Endocrinology. 2015;156:3091–3097. doi: 10.1210/en.2015-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Kapur J. M-type potassium channels modulate Schaffer collateral–CA1 glutamatergic synaptic transmission. The Journal of Physiology. 2012;590:3953–3964. doi: 10.1113/jphysiol.2012.235820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Koide M, Ara W, Shibata Y, Funabashi T, Suyama K, et al. Estrous Cycle-Dependent Phasic Changes in the Stoichiometry of Hippocampal Synaptic AMPA Receptors in Rats. PLoS ONE. 2015;10:e0131359. doi: 10.1371/journal.pone.0131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, Hashimoto M, Sugioka K, Maruyama M, Fujii Y, Hagiwara R, et al. Improvement of spatial cognition with dietary docosahexaenoic acid is associated with an increase in Fos expression in rat CA1 hippocampus. Clin Exp Pharmacol Physiol. 2004;31:700–703. doi: 10.1111/j.1440-1681.2004.04068.x. [DOI] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early Activation of Ventral Hippocampus and Subiculum during Spontaneous Seizures in a Rat Model of Temporal Lobe Epilepsy. The Journal of Neuroscience. 2013;33:11100. doi: 10.1523/JNEUROSCI.0472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The Self-Tuning Neuron: Synaptic Scaling of Excitatory Synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wihlbäck AC, Sundström-Poromaa I, Bäckström Tr. Action by and sensitivity to neuroactive steroids in menstrual cycle related CNS disorders. Psychopharmacology. 2006;186:388–401. doi: 10.1007/s00213-005-0185-2. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, et al. Very Early Administration of Progesterone for Acute Traumatic Brain Injury. N Engl J Med. 2014;371:2457–2466. doi: 10.1056/NEJMoa1404304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584. doi: 10.1038/ncomms14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa K, Okumura A, Nakashima K, Sato T, Higashi T. Role of allopregnanolone biosynthesis in acute stress-induced anxiety-like behaviors in mice. Synapse. 2017:71. doi: 10.1002/syn.21978. [DOI] [PubMed] [Google Scholar]