Abstract
Background
Several countries have implemented vaccination against human papillomavirus (HPV) for adolescent girls, and must decide whether and how to adapt cervical cancer screening for these low-risk women. We aimed to identify the optimal screening strategies for women vaccinated against HPV infections and quantify the amount that could be spent to identify vaccination status among women and stratify cervical cancer screening guidelines accordingly.
Methods
We used a mathematical model reflecting HPV-induced cervical cancer in Norway to project the long-term health benefits, resources and costs associated with 74 candidate screening strategies that varied by screening test, start age and frequency. Strategies were considered separately for women vaccinated with the bivalent/quadrivalent (2/4vHPV) and nonavalent (9vHPV) vaccines. We used a cost-effectiveness framework (i.e. incremental cost-effectiveness ratios and net monetary benefit) and a commonly-cited Norwegian willingness-to-pay threshold of a €75,000 per quality-adjusted life-year gained.
Results
The most cost-effective screening strategy for 9vHPV- and 2/4vHPV-vaccinated women involved HPV testing once and twice per lifetime, respectively. The value of stratifying guidelines by vaccination status was €599 (2/4vHPV) and €725 (9vHPv) per vaccinated woman. Consequently, for the first birth cohort of ~22,000 women who were vaccinated in adolescence in Norway, between €10.5–13.2 million over their lifetime could be spent on identifying individual vaccination status and stratify screening while remaining cost-effective.
Conclusion
Less intensive strategies are required for cervical cancer screening to remain cost-effective in HPV-vaccinated women. Moreover, screening can remain cost-effective even if large investments are made to identify individual vaccination status and stratify screening guidelines accordingly.
Keywords: cervical cancer, mass screening, cost-effectiveness analysis, mathematical model, human papillomavirus
INTRODUCTION
Cervical cancer is the fourth most common cancer in women worldwide, with the greatest burden in low- and middle-income countries (1). Following the implementation of prophylactic human papillomavirus (HPV) vaccination, the risk of developing cervical cancer (CC) in vaccinated women is expected to decrease considerably, which will increase the heterogeneity of CC risk in the population. Currently available vaccines include the first-generation bivalent and quadrivalent HPV vaccines (‘2/4vHPV’), targeting HPV-16 and -18 high-risk infections (with or without the addition of HPV-6 and -11 low-risk infections) that contribute to ~75% of all CCs, and the second-generation nonavalent vaccine (‘9vHPV’), targeting HPV-6/11/16/18/31/33/45/52/58 infections that cumulatively contribute to ~90% of all CCs (2). In clinical trials, the vaccines have demonstrated >90% efficacy against persistent HPV infections and precancers among HPV-negative individuals who completed the three-dose schedule (3–6). The vaccines are most effective when administered to young individuals prior to HPV exposure (7), and national immunization programmes for adolescent girls have been implemented in most developed countries. In Norway, all three HPV vaccines are available, and the 2vHPV was recently selected to replace the 4vHPV in the vaccination programme (8). In order to prevent CC caused by non-vaccine-targeted genotypes, screening may still be required for HPV-vaccinated women. The first cohort of Norwegian girls vaccinated with the 4vHPV at age 12 years in 2009 will become eligible for CC screening in 2022; however, no countries have yet adapted CC screening guidelines according to individual vaccination status, which may be required for screening to remain cost-effective and balance benefit-harm trade-offs for these low-risk women.
Previous model-based analyses have indicated that cost-effective CC screening strategies for HPV-vaccinated women involve primary HPV testing starting at later ages and occurring less frequently (9–13), and that cost-effective guidelines may differ between settings (10). Within the context of Norway, we aimed to identify the most cost-effective CC screening strategy for women vaccinated against HPV infections in adolescence. Moreover, as stratifying guidelines based on vaccination status may require additional resources (e.g. registry linkage to identify individual vaccination status) we enumerated the maximum amount of money that could be spent to obtain individual vaccination status and stratify guidelines while remaining cost-effective.
MATERIALS AND METHODS
Analytic overview
We used a previously developed mathematical simulation model of HPV-induced CC (14), adapted to reflect Norwegian epidemiologic data using 50 good-fitting parameter sets (described previously (11, 15, 16), Supplementary Appendix), to project the health and economic consequences of candidate CC prevention strategies for women vaccinated against HPV infections at age 12 years. The model simulates and tracks the disease history, clinical events and resource use for a hypothetical cohort of four million individual women from age 9 years until death. Women progress through the model at monthly transitions between health states, including HPV infection status, precancer and CC (by stage). Analyses were considered separately for women vaccinated with the 2/4vHPV and the 9vHPV vaccines. In addition to ‘no intervention’ and ‘vaccination only’ scenarios, we considered 74 candidate screening strategies that varied by the primary screening test (cytology or HPV), age to start screening (ages 25–34 years), and screening frequency (once/twice per lifetime and 3-yearly to 20-yearly). We also evaluated Norwegian-specific guidelines currently-in-use, including triennial cytology for women aged 25–69 years (‘current guidelines’) and a strategy under consideration in a pilot study (17) (‘proposed guidelines’) involving five-yearly HPV testing starting at age 34 years (with triennial cytology for ages 25–33 years).
Using a societal analytic perspective, we projected the lifetime risk of developing CC compared to no intervention, the number of colposcopy referrals and screening (cytology and HPV) tests per 1,000 women screened over their lifetime, the quality-adjusted life-years (QALYs), life expectancy, and the total lifetime cost (expressed in 2014 Euros (€EUR1.00=NOK8.35) (18)) per woman associated with each strategy. Costs and QALYs were discounted by 4% per year as recommended in Norway (19). We also considered a 0% discount rate as a lower bound for discount rates across European countries.
Parameter values for costs and utilities used to estimate QALYs have been previously described (15). Briefly, we included the direct medical, transportation and patient time costs associated with screening, diagnostic work-up, treatment of precancer and invasive cancer, and vaccination. We assumed a cost per vaccine dose (including administration cost) of €132 for the 2/4vHPV and €147 for the 9vHPV based on current market price in Norway (20, 21) (Supplementary Appendix). Cost assumptions were varied in uncertainty analysis to include (i) direct medical costs only, and (ii) productivity losses associated with sick leave after precancer and cancer treatment.
We used a cost-effectiveness framework to identify optimal screening strategies for HPV-vaccinated women and the value of stratified guidelines. We identified efficient strategies using the incremental cost-effectiveness ratio (ICER), defined as the additional cost per additional QALY, of a strategy compared to the next most costly strategy. The ‘most cost-effective’ strategy was identified using the commonly-cited Norwegian willingness-to-pay threshold of a €75,000 per QALY gained (15, 22). We calculated the incremental net monetary benefit (INMB) (per vaccinated woman over her lifetime) of each strategy compared to no intervention, and used this metric to identify the efficiency gains of stratifying screening guidelines according to HPV vaccination status. Specifically, we estimated the maximum amount that could be spent (per vaccinated woman) to identify a woman’s vaccination status and screen her according to a separate set of guidelines (from unvaccinated women), while remaining cost-effective (Supplementary Appendix).
Preventions strategies and assumptions
For 2/4vHPV, we assumed a 3-dose schedule and 100% lifelong efficacy (4–6) against vaccine-targeted HPV types; for 9vHPV we assumed 100% efficacy for HPV-16/18 infections and 96% efficacy for the five additional high-risk HPV types included in the vaccine (3). In uncertainty analysis, we used 90% efficacy against all HPV types targeted by the vaccines as a lower bound. We also performed a scenario analysis that reflected the 2vHPV with lifelong cross-protection against non-vaccine-targeted HPV types, using estimates from a recent meta-analysis (Supplementary Table 1) (23).
We evaluated 74 candidate screening strategies based on adaptations of the current and proposed Norwegian CC screening policies as well as discussions with experts (Table 1). We considered variations of the current guidelines, including current and delayed start ages (i.e. ages 25/28/31/34 years), as well as current and less intensive screening frequencies (i.e. 3-/5-/7-/10-/15-/20-yearly); these start ages and frequencies were considered for both cytology- and HPV-based strategies. We also included variations of the proposed guidelines (i.e. delayed screening start age and less frequent screening after switching to HPV testing). Finally, we considered HPV testing once-only or twice per lifetime (15 years apart), starting at ages 25, 30, 35, and 40 years. Women with a positive primary cytology or HPV test were managed according to current and proposed guidelines, respectively (17, 24). Consistent with Norwegian guidelines, screening ended at age 69 years, yet the implied stop age and the number of lifetime screens varied across strategies due to algorithm variations (Supplementary Table 2).
Table 1.
Candidate cervical cancer screening strategies for HPV-vaccinated women.*
| Primary screening test |
Screening start age | Screening interval |
Age of switching to primary HPV testing |
Interval after switching |
|---|---|---|---|---|
| Cytology† | 25, 28, 31, 34 | 3-, 5-, 7-, 10-, 15-, 20-year | -- | -- |
| Cytology† | 25, 28, 31 | 3-year | 34 | 3-, 5-, 7-, 10-, 15-, 20-year |
| HPV‡ | 25, 28, 31, 34 | 3-, 5-, 7-, 10-, 15-, 20-year | -- | -- |
| HPV‡ | 25, 30, 35, 40 | 2-times (15 years apart) | -- | -- |
| HPV‡ | 25, 30, 35, 40 | 1-time | -- | -- |
All strategies were considered separately for women vaccinated with the bivalent/quadrivalent and the nonavalent HPV vaccine.
We assumed that follow-up of screen-positive women was consistent with current cytology-based guidelines in Norway; i.e. delayed HPV and cytology co-test in 12 months for women with minor cervical lesions and diagnostic colposcopy with biopsy for women with high-grade cervical lesions.
We assumed that follow-up screen-positive women was consistent with proposed HPV-based guidelines in Norway; i.e. reflex cytology for HPV-positive with repeat HPV testing at 12 months for women with a normal cytology and diagnostic colposcopy with biopsy for women detected with atypical squamous cells of undetermined significance (ASC-US) or more severe.
Similar to previous analyses (11, 15), we assumed that HPV test sensitivity reflected the most sensitive HPV DNA assays; that is, the probability of having a positive test given presence of HPV infection was 100%, but we reduced this value to 90% in uncertainty analysis (Supplementary Appendix). Finally, we assumed perfect compliance to screening and follow-up procedures in our primary analysis, but also varied this assumption in uncertainty analysis (Supplementary Appendix).
RESULTS
Cost-effective screening for HPV-vaccinated women
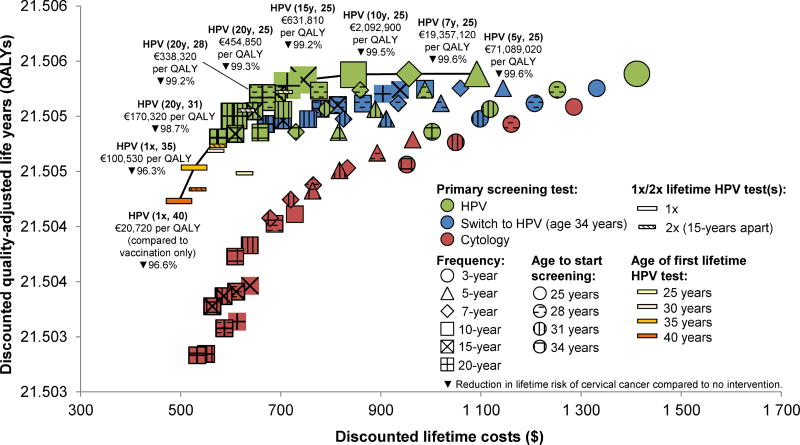
For women vaccinated with any of the three HPV vaccines, the current and proposed Norwegian screening guidelines were more costly and less effective than candidate strategies (i.e. inefficient). Efficient screening strategies involved primary HPV testing, of which most involved 1–3 screens per lifetime, while screening every ≥10 years exceeded €400,000 per QALY gained (Figures 1–2, Supplementary Tables 3–4). For 2/4vHPV-vaccinated women, the most cost-effective strategy involved two lifetime screens at ages 31 and 51 years using HPV testing (€53,570 per QALY) (Figure 1). However, for moderate increases in the willingness-to-pay, the preferred strategies involved HPV testing at ages 30 and 45 years (€77,570 per QALY) or at ages 28, 48 and 68 years (€90,810 per QALY). For other European settings with a lower willingness-to-pay (e.g. €30,000 per QALY), the optimal strategy involves once-only HPV testing at age 35 years (€25,220 per QALY). For women vaccinated with the 9vHPV, the preferred strategy involved once-only HPV testing at age 40 years (€20,720 per QALY), or at age 35 years, provided a higher willingness-to-pay threshold of €100,530 per QALY (Figure 2).
Figure 1.
Cost-effectiveness results for women vaccinated against HPV infections with the bivalent/quadrivalent vaccine (2/4vHPV). Efficient strategies are accentuated with a larger symbol and connected by the solid line (i.e. efficiency frontier). Parentheses for the efficient strategies indicate screening frequency (e.g. “20-yearly” or “1x/2x” indicate one or two lifetime screens) and age to start screening. All costs are expressed in 2014 Euros (EUR€ = NOK8.35).
Abbreviations: HPV, human papillomavirus; QALY, quality-adjusted life-years.
Figure 2.
Cost-effectiveness results for women vaccinated against HPV infections with the nonavalent vaccine (9vHPV). Efficient strategies are accentuated with a larger symbol and connected by the solid line (i.e. efficiency frontier). Parentheses for the efficient strategies indicate screening frequency (e.g. “20-yearly” or “1x/2x” indicate one or two lifetime screens) and age to start screening. All costs are expressed in 2014 Euros (EUR€ = NOK8.35).
Abbreviations: HPV, human papillomavirus; QALY, quality-adjusted life-years.
Cancer benefit and resource use trade-offs
For the strategies identified as efficient, cancer benefit and resource use generally increased with more intensive screening strategies (Figure 3). For both 2/4vHPV- and 9vHPV-vaccinated women, the strategies with an ICER just below or above €75,000 per QALY provided lower cancer benefits than the current and proposed guidelines; however, the strategies associated with greater cancer benefits also required more colposcopy referrals (Figure 3, panels A–B). In contrast, nearly all efficient strategies (for either vaccine) required fewer screening tests than both the current and proposed guidelines (Figure 3, panels C–D).
Figure 3.
Model-based estimates of health benefit (i.e. reduction in lifetime cervical cancer risk) and resource use (i.e. colposcopy referrals and screening tests) trade-offs associated with the current/proposed Norwegian guidelines and efficient strategies for women vaccinated with the bivalent/quadrivalent vaccine (2/4vHPV) and nonavalent vaccines (9vHPV). Red bars in panels A and B represent the number colposcopy referrals per 1,000 women screened over their lifetime (left axes) for 2/4vHPV (A) and 9vHPV (B). A single woman may have multiple colposcopies over her lifetime (e.g. due to repeated surveillance). Blue bars in panels C and D represent the number of screening tests (i.e. all HPV and cytology tests, including reflex tests and potential follow-up tests) per 1,000 women screened over their lifetime (left axes) for 2/4vHPV (C) and 9vHPV (D). In all panels, green diamonds represent the reduction in lifetime risk of developing cervical cancer compared to no screening (right axes). X-axes represent screening strategies, including the current and proposed Norwegian guidelines (see Methods) and efficient strategies for women vaccinated with 2/4vHPV and 9vHPV, respectively (see Results).
The value of stratified guidelines
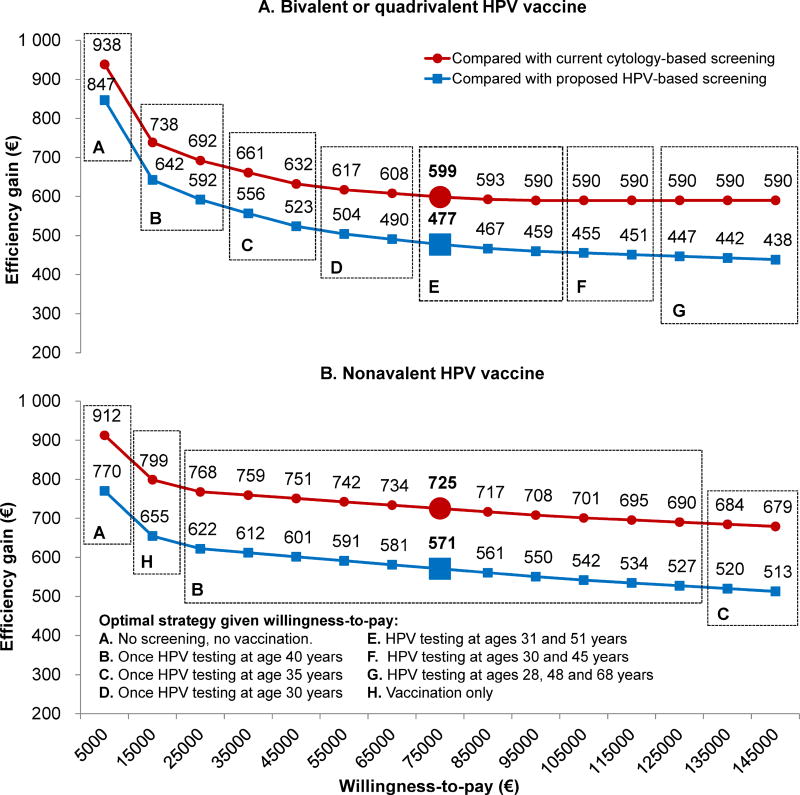
The efficiency gains (i.e. value) of stratifying screening guidelines according to HPV vaccination status were greater for lower willingness-to-pay thresholds, and for women vaccinated with the 9vHPV compared to the 2/4vHPV (Figure 4). For example, for a willingness-to-pay threshold of a €75,000 per QALY, the value of screening 2/4vHPV-vaccinated women involving HPV testing at ages 31 and 51 years (rather than continuing screening these women according to the current guidelines) was €599 per woman over her lifetime. This amount increased to €725 if 9vHPV-vaccinated women were screened using once-only HPV testing at age 40 years. Consequently, for the first 22,000 women fully vaccinated in Norway in 2009 with the 2/4vHPV (or hypothetically with the 9vHPV), between €10.5–13.2 (€12.6–16.0) million could be allocated over their lifetime to identify a woman’s vaccination status and screen vaccinated women according to a separate set of guidelines (e.g. primary HPV testing twice per lifetime) rather than continuing with the current triennial cytology-based screening guidelines, while remaining cost-effective (Supplementary Table 5).
Figure 4.
The value of stratifying screening guidelines for women vaccinated with the bivalent/quadrivalent vaccine (2/4vHPV) (panel A) and nonavalent vaccines (9vHPV) (panel B). Efficiency gains (EUR), i.e., the incremental net monetary benefit (INMB) of the optimal screening strategy for a vaccinated woman minus the INMB associated with current (red lines) and proposed (blue lines) Norwegian screening guidelines, for a given willingness-to-pay (WTP) threshold. The efficiency gains for a WTP of a €75,000 per QALY gained (a commonly-cited Norwegian threshold) is highlighted in bold. Dashed boxes and letter indicate the optimal screening strategy given the WTP threshold.
Uncertainty analysis
In univariate uncertainty analysis, results for the 2/4vHPV were most sensitive to assuming cross-protection against non-vaccine-targeted HPV genotypes and imperfect screening compliance, medical costs only, and 0% discounting (Supplementary Table 6). For example, less frequent screening was preferred when we assumed the 2vHPV conferred cross-protection, involving once-only HPV testing at ages 35 or 30 years (€45,980 and €79,110 per QALY, respectively). For 9vHPV, once-only HPV testing at age 40 years remained the preferred strategy across all uncertainty analyses, except when we reduced the vaccine efficacy to 90% or assumed 0% discounting (i.e. HPV testing at ages 31 and 51 years was most cost-effective) (Supplementary Table 7). For both vaccines, the rank order of the efficient strategies remained the same across all 50 good-fitting parameter sets (Supplementary Appendix).
DISCUSSION
For women vaccinated against HPV infections in adolescence, CC screening strategies that are less intensive than those currently-recommended for unvaccinated women are required in order for screening to remain cost-effective. For Norwegian women vaccinated with the 9vHPV or 2/4vHPV, the preferred strategies involved screening once or twice per lifetime using HPV testing, respectively. These strategies are expected to reduce lifetime risk of CC by >94% compared to no intervention, and require fewer screening tests and colposcopy referrals than continuing with current or proposed Norwegian guidelines. When the first Norwegian birth cohort of women who were vaccinated in adolescence enter screening in 2022, between €10.5–13.2 million could be spent over their lifetime to identify individual vaccination status (e.g. investing in infrastructure to link screening and vaccination registries) and implement stratified screening guidelines.
Our findings support previous studies, including a Norwegian study evaluating optimal screening for 2/4vHPV-vaccinated women (11)) suggesting less intensive HPV-based screening for HPV-vaccinated women (9–13) To our knowledge, no studies have comprehensively evaluated strategies (e.g. screening intervals >5 years) for women vaccinated with either the 2/4vHPV or the 9vHPV in Norway, and no studies (in any setting) have evaluated the cost-effectiveness of strategies involving only 1–2 lifetime screens for 2/4vHPV-vaccinated women. However, a recent model-based analysis from England projected the appropriate number of lifetime screens for HPV-vaccinated women when benchmarking on the proportion of cancers prevented per additional screen in unvaccinated women, suggesting two and three lifetime screens for 9vHPV- and 2vHPV-vaccinated women, respectively (25). Across uncertainty analyses, we found that optimal strategies for 2/4vHPV-vaccinated women may involve screening 1–3 times per lifetime, 15–20 years apart, starting at age ~30 years. For 9vHPV-vaccinated women, a single HPV test at age 40 years was the most cost-effective strategy across all uncertainty analyses, except for when assuming a lower vaccine efficacy or no discounting, suggesting two lifetime screens. Of note, 9vHPV with no screening may provide a higher CC risk reduction (i.e., 79%) than current screening with current adherence in Norway (projected to be 73% in a previous policy analysis (26)).
We evaluated optimal screening conditioned on a woman being fully vaccinated in adolescence, yet there are several barriers to implementing stratified guidelines, such as obtaining accurate information about individual vaccination status, ensuring compliance (more frequent screening may be easier to remember), and communicating differential guidelines to both women and providers. For countries with national vaccine registries (including many European countries such as Norway, Denmark and Scotland), stratified guidelines may be feasible by linking vaccine and screening registries. Alternatively, screening guidelines may be stratified for birth cohorts that have been offered the vaccine based on vaccination coverage and cohort-level herd immunity, in which case a slightly more intensive strategy may be optimal. For example, a recent cost-effectiveness analysis evaluating screening in cohorts offered the 9vHPV in USA, New Zealand, Australia and England (assuming country-specific vaccination and screening coverage) concluded that optimal guidelines involved 4, 5, 2 and 4 lifetime screens, respectively. As an increasing number of vaccinated cohorts initiate screening, a universal program may be considered; for example, a Dutch study (12) found that when >50% population-level herd immunity is reached, the screening programme may be universally adapted to the CC risk level for vaccinated women. Given the inherent challenges in determining when that level of herd immunity has been reached, stratified screening guidelines may help ensure that the benefits of screening outweigh the harms. We did not evaluate optimal screening among women who have received HPV vaccination under ‘catch-up’ programs, which is an important area for future research. Finally, vaccination costs may be unilaterally reduced (e.g. 2-dose vaccine schedules (27) or lower negotiated tender prices) or increased (e.g. additional booster doses); however, this will not necessarily impact the relative cost differences between screening strategies, or the maximum amount that could be spent to identify individual vaccination status.
CONCLUSION
Our findings suggest that de-intensifying CC screening in women confirmed to have been fully vaccinated against HPV in adolescence can result in efficiency gains and that a considerable amount could be spent towards implementing separate CC screening guidelines according to individual HPV vaccination status. Stratified screening is important for screening to balance benefits, harms and resource use, at least for the next few decades when there will remain a large amount of heterogeneity in CC risk in the population since most screen-eligible women were not vaccinated in adolescence.
Supplementary Material
Highlights.
-
▪
To remain cost-effective CC screening for HPV-vaccinated women should be de-intensified
-
▪
HPV-vaccinated women may be screened once or twice per lifetime
-
▪
A considerable amount can be spent to allow stratified screening guidelines
-
▪
Adapting screening becomes even more important with the 9vHPV
Acknowledgments
Funding: This work was supported in part by The Norwegian Research Council (grant number 238042 (EAB and KP)); and by the U.S. National Cancer Institute (R01CA160744 (PI: JJK)). The funders had no role in the conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: None declared.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. [on December 4, 2017]. Accessed at http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp. [Google Scholar]
- 2.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. International journal of cancer Journal international du cancer. 2012;131(10):2349–59. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 3.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. The New England journal of medicine. 2015;372(8):711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 4.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet (London, England) 2009;374(9686):301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 5.Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Human vaccines & immunotherapeutics. 2014;10(8):2147–62. doi: 10.4161/hv.29532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. The New England journal of medicine. 2007;356(19):1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 7.Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. British journal of cancer. 2011;105(1):28–37. doi: 10.1038/bjc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norwegian Institute of Public Health. [Last updated May 4, 2017];HPV-vaccination: Changes in the childhood immunization program from the fall of 2017. Accessed at https://fhi.no/sv/vaksine/barnevaksinasjonsprogrammet/endring-for-hpv-vaksine-i-barnevaksinasjonsprogrammet/on June 2, 2017.
- 9.Kim JJ, Burger EA, Sy S, Campos NG. Optimal Cervical Cancer Screening in Women Vaccinated Against Human Papillomavirus. Journal of the National Cancer Institute. 2017;109(2) doi: 10.1093/jnci/djw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simms KT, Smith MA, Lew JB, Kitchener HC, Castle PE, Canfell K. Will cervical screening remain cost-effective in women offered the next generation nonavalent HPV vaccine? Results for four developed countries. International journal of cancer Journal international du cancer. 2016;139(12):2771–80. doi: 10.1002/ijc.30392. [DOI] [PubMed] [Google Scholar]
- 11.Burger EA, Ortendahl JD, Sy S, Kristiansen IS, Kim JJ. Cost-effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. British journal of cancer. 2012;106(9):1571–8. doi: 10.1038/bjc.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naber SK, Matthijsse SM, Rozemeijer K, Penning C, de Kok IM, van Ballegooijen M. Cervical Cancer Screening in Partly HPV Vaccinated Cohorts - A Cost-Effectiveness Analysis. PloS one. 2016;11(1):e0145548. doi: 10.1371/journal.pone.0145548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldhaber-Fiebert JD, Stout NK, Salomon JA, Kuntz KM, Goldie SJ. Cost-effectiveness of cervical cancer screening with human papillomavirus DNA testing and HPV-16,18 vaccination. Journal of the National Cancer Institute. 2008;100(5):308–20. doi: 10.1093/jnci/djn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos NG, Burger EA, Sy S, Sharma M, Schiffman M, Rodriguez AC, et al. An updated natural history model of cervical cancer: derivation of model parameters. American journal of epidemiology. 2014;180(5):545–55. doi: 10.1093/aje/kwu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen K, Burger EA, Sy S, Kristiansen IS, Kim JJ. Cost-effective management of women with minor cervical lesions: Revisiting the application of HPV DNA testing. Gynecologic oncology. 2016 doi: 10.1016/j.ygyno.2016.08.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norwegian Technical Appendix 2017. [on May 10, 2017];Harvard Cervical Cancer Natural History Model Calibration and Costing Approach for Norway. Accessed at http://www.med.uio.no/helsam/english/research/projects/preventive-strategies-hpv/17-harvardmodel-norway-technicalappendix.pdf.
- 17.Nygard M, Andreassen T, Berland J, Hagen B, Hagmar B, Iversen O-E, et al. Kontrollert implementering og evaluering av forbedret helsetjeneste. The Norwegian Directorate of Health; Oslo: 2013. HPV-test i primærscreening mot livmorhalskreft. [Google Scholar]
- 18. [Accessed: January 19, 2016];The Central Bank of Norway: Average exchange rates 2014. 2014 Available at: http://www.norgesbank.no/Statistikk/Valutakurser/valuta/EUR/
- 19.Økonomisk evaluering av helsetiltak - en veileder. Norwegian Directorate of Health; 2012. URL: http://helsedirektoratet.no/publikasjoner/okonomisk-evaluering-av-helsetiltak--en-veileder/Publikasjoner/IS-1985.pdf. [Google Scholar]
- 20.Norwegian Medicines Agency. [on May 5, 2017];Medicine database: Cervarix. 2017 Accessed at https://www.legemiddelsok.no/sider/Legemiddelvisning.aspx?pakningId=12f12e28-0e1a-49cc-b409-3f5015ed2c2a&searchquery=cervarix&f=Han;MtI;Vir;ATC;Var;Mar;Mid;Avr;gen;par;&pane=0.
- 21.Norwegian Medicines Agency. [on May 5, 2017];Medicine database: Gardasil 9. 2017 Accessed at https://www.legemiddelsok.no/sider/Legemiddelvisning.aspx?pakningId=30aecd9cc6be-4494-850de9f9d7bc8cee&searchquery=gardasil%209&f=Han;MtI;Vir;ATC;Var;Mar;Mid;Avr;gen;par;&pane=0.
- 22.Norwegian Directorate of Health. Health effects of socio-economic analyses. Oslo: Norwegian Directorate of Health; 2007. [Google Scholar]
- 23.Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. The Lancet Infectious diseases. 2012;12(10):781–9. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 24.Cancer Registry of Norway. Annual Report of the Norwegian Cervical Cancer Screening Program, 2013–14. Oslo: Cancer Registry of Norway; [on October 16, 2015]. 2015. Accessed at https://wwwkreftregisteretno/globalassets/publikasjoner-og-rapporter/livmorhalskreft/arsrapport/livmorhals_2015pdf. [Google Scholar]
- 25.Landy R, Windridge P, Gillman MS, Sasieni PD. What cervical screening is appropriate for women who have been vaccinated against high risk HPV? A simulation study. International journal of cancer Journal international du cancer. 2017 doi: 10.1002/ijc.31094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen K, Burger EA, Sy S, Kristiansen IS, Kim JJ. Cost-effective management of women with minor cervical lesions: Revisiting the application of HPV DNA testing. Gynecologic oncology. 2016;143(2):326–33. doi: 10.1016/j.ygyno.2016.08.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Evidence based recommendations on Human Papilloma Virus (HPV) Vaccines Schedules. [on May 6, 2017];Background paper for SAGE discussions. 2014 Accessed at http://www.who.int/immunization/sage/meetings/2014/april/1_HPV_Evidence_based_recommendationsWHO_with_Appendices2_3.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.