Abstract
Optimal salvage therapy for primary refractory peripheral T-cell lymphomas (PTCL) and the role of hematopoietic stem cell transplant remain poorly defined. We conducted a retrospective review of clinical outcomes and prognostic factors in a single-center cohort of 93 patients with primary refractory PTCL, defined as progression during first-line therapy or relapse within 6 months of its completion. Clinical outcomes were poor in this population, with median event-free survival (EFS) of 3.5 months, median overall survival (OS) of 9.1 months, and 34% 3-year survival. Outcomes were comparable in patients who progressed through first-line therapy and patients who achieved CR/PR and subsequently relapsed within 6 months. A majority exhibited high-risk features and had intermediate to high risk IPI, which correlated with inferior outcomes. There was no difference in outcomes between patients who received single-agent salvage regimens and patients who underwent traditional, multi-agent salvage regimens. Thus, participation in well-designed clinical trials should be encouraged in this population. Additionally, there may be a trend toward improved EFS and OS in patients who underwent autologous or allogeneic SCT compared to patients who achieved CR or PR but were not transplanted.
Keywords: Peripheral T-cell lymphoma, salvage therapy, primary refractory, chemotherapy resistance, PTCL-NOS
INTRODUCTION
Peripheral T-cell lymphoma (PTCL) accounts for 10–15% of all non-Hodgkin’s lymphomas and 15–20% of aggressive lymphomas in western countries [1]. Despite anthracycline-based front-line treatment (usually CHOP or CHOEP), only a minority (~30%) of patients with the most common PTCL subtypes achieve a durable remission and long-term disease-free survival [1–3]. Although response rates and outcomes with currently available salvage therapies are poor, response to initial therapy is predictive of improved survival [4]. Primary refractory disease and early relapse upon completion of initial therapy are not uncommon. For example, the Nordic Lymphoma Group prospectively examined the role of dose-dense induction therapy consolidated with high-dose therapy and autologous stem-cell transplantation in first remission [5]. Among evaluable patients enrolled, primary refractory disease was observed in 16% and early relapses (within two years of treatment), presumably secondary to chemotherapy resistant minimal residual disease, was observed in 18%.
Front-line therapy for PTCL is largely extrapolated from the experience in management of aggressive B-cell NHL, most commonly anthracycline-based multi-drug regimens (e.g. CHOP, CHOEP). Similarly, conventional salvage regimens (e.g. ICE, DHAP) are widely utilized in the management of primary refractory or relapsed disease, particularly in fit or transplant eligible patients. Unfortunately, complete and durable response rates are disappointingly low with these regimens [2, 3]. In a cohort of 153 patients from British Columbia with relapsed or refractory PTCL after initial therapy, 26% achieved a complete remission with salvage therapy, with a median overall survival (OS) of 5.5 months and median progression-free survival of 3.1 months after failing first line therapy [2]. Similarly, Briski et al. described event-free survival of 3 months and OS of 6 months in relapsed/refractory PTCL patients with stage III or IV disease, and in all relapsed/refractory patients who did not undergo hematopoietic stem cell transplant [3]. Novel agents, including the novel antifolate pralatrexate [6], the HDAC inhibitors romidepsin [7–9] and belinostat [10, 11], and brentuximab vedotin [12–14], have been approved for the treatment of relapsed/refractory PTCL. With few exceptions, complete and durable responses are rarely achieved with these novel agents. The optimal sequencing of either conventional or novel agents, and the role of either autologous or allogeneic stem-cell transplantation, particularly in the setting of primary refractory disease remain uncertain and understudied. Therefore, we sought to investigate clinical outcomes and prognostic factors influencing progression and survival in patients with primary refractory PTCL.
METHODS
Study Population
Patients 18 years of age or older with biopsy-confirmed PTCL of all subtypes who were evaluated at the University of Michigan Health System between 1988 and 2016 were considered for study inclusion. Patients who received multi-agent, anthracycline-based first-line treatment and were subsequently found to have primary refractory disease, defined as disease progression during initial therapy or relapse within 6 months of completing initial therapy, were included in the study. Study approval was granted by the Institutional Review Board at the University of Michigan, in accordance with US federal regulations and the Declaration of Helsinki. Clinical characteristics were retrospectively collected.
Data analysis
Event free survival (EFS) was defined as time from confirmation of primary refractory PTCL to time of progression or relapse following salvage/second-line therapy, time of initiation of third-line therapy, or time of death, whichever occurred first. Overall survival (OS) was defined as time from confirmation of primary refractory disease to time of death. Patients were censored at time of last follow-up.
Statistical analysis was carried out using JMP Pro, version 13. Survival analyses were performed using the Kaplan-Meier method with pair-wise comparisons between treatment groups using the log-rank test, with p-value of <0.05 considered to be statistically significant. The Cox proportional hazards model was used to evaluate dichotomized variables.
RESULTS
The median age at diagnosis among patients with primary refractory PTCL (n = 93) was 52 years (range, 18–80 years). Median follow-up following diagnosis was 7 months for all patients, and 26 months for surviving patients. As summarized in Table I, the most frequently observed histological subtypes were PTCL, NOS (43%) and angioimmunoblastic T-cell lymphoma (AITL; 18%). A majority of patients had high-risk features, including an elevated LDH (61%), advanced Ann Arbor stage (84%), and extranodal involvement (75% with at least one site of extranodal disease, and 38% with at least two sites of extranodal disease). Consequently, 51% of patients were considered intermediate-risk (scores of 2–3) by the International Prognostic Index (IPI), and 22% were high-risk (scores of 4–5) at time of progression or relapse. Fifty-eight percent of patients were considered intermediate-risk (scores of 1–2) by the Prognostic Index for T-cell Lymphoma (PIT) and 20% were high-risk (scores of 3–4). Most patients (92%) received CHOP/CHOEP (cyclophosphamide, doxorubicin, vincristine, prednisone, +/− etoposide) as their first-line therapy. In this cohort with primary refractory disease, most patients (63%) received a conventional multiagent salvage regimen (most commonly ICE), and 16% received alternative, single-agent systemic therapies, including gemcitabine monotherapy, retinoids, pralatrexate, denileukin diftitox, the proteasome inhibitor ixazomib, brentuximab vedotin, the histone deacetylase inhibitor romidepsin, and cyclosporine. Twenty percent received local radiation, corticosteroids, supportive care, or had unknown salvage treatment status (Table I).
Table I.
Patient characteristics at diagnosis with primary refractory PTCL (n = 93).
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| > 60 | 33 | 35 |
| Gender | ||
| Male | 63 | 68 |
| Histology | ||
| PTCL, NOS | 40 | 43 |
| AITL | 17 | 18 |
| ALK(+) ALCL | 8 | 9 |
| ALK(−) and ALK-unknown ALCL | 16 | 17 |
| Other* | 12 | 13 |
| IPI | ||
| 0–1 | 17 | 18 |
| 2–3 | 47 | 51 |
| 4–5 | 20 | 22 |
| Missing | 9 | 9 |
| PIT | ||
| 0 | 11 | 12 |
| 1–2 | 54 | 58 |
| 3–4 | 19 | 20 |
| Missing | 9 | 9 |
| First line therapy | ||
| CHOP/CHOEP | 86 | 92 |
| Salvage therapy | ||
| Multi-agent regimens | 59 | 63 |
| ICE | 24 | 26 |
| DHAP | 6 | 6 |
| ESHAP | 10 | 11 |
| Other multi-agent | 19 | 20 |
| Single-agent regimens | 15 | 16 |
| Gemcitabine | 1 | 1 |
| Retinoid | 1 | 1 |
| Antimetabolite | 3 | 3 |
| Denileukin diftitox | 1 | 1 |
| Proteasome inhibitor | 4 | 4 |
| Brentuximab vedotin | 3 | 3 |
| HDAC inhibitor | 1 | 1 |
| Cyclosporine | 1 | 1 |
| Non-systemic therapies** | 19 | 20 |
| Stem-cell transplant | ||
| Autologous | 15 | 16 |
| Allogeneic | 20 | 22 |
PTCL, NOS, peripheral T-cell lymphoma, not otherwise specified; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; IPI, International Prognostic Index; ECOG, Eastern Cooperative Oncology Group performance status; CHOP/CHOEP, cyclophosphamide, doxorubicin, vincristine, prednisone, +/− etoposide; ICE, ifosfamide, carboplatin, and etoposide; DHAP, dexamethasone, high-dose cytarabine, and cisplatin; ESHAP, etoposide, methylprednisolone, cytarabine, and cisplatin.
Other histologies include extranodal NK/T-cell lymphoma (n=6), hepatosplenic T-cell lymphoma (n=2), and enteropathy-associated T-cell lymphoma (n=4).
Includes patients treated with local radiation alone, steroids alone, or observation/supportive care, as well as patients with unknown salvage therapy status.
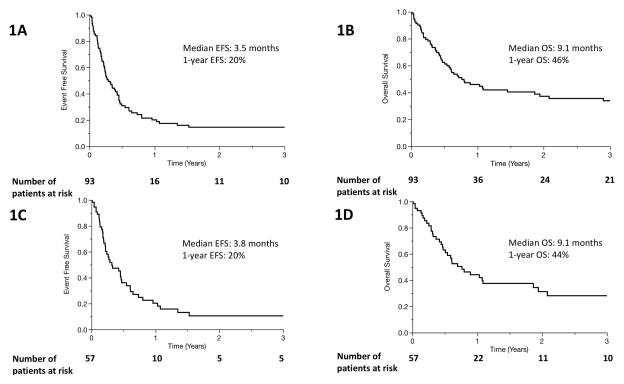
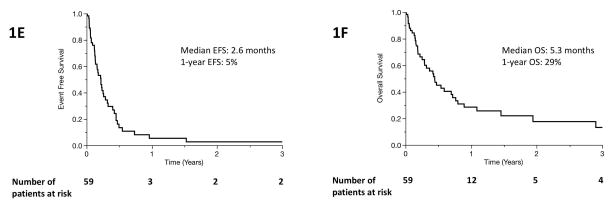
As expected, clinical outcomes in primary refractory PTCL were generally poor. Sixty-nine events and 55 deaths were observed in our cohort. The median EFS after relapse/progression on first-line therapy was 3.5 months (2.6–5.2, 95% confidence interval [CI]) (Figure 1A). The median OS was 9.1 months (6.1–22.3, 95% CI) (Figure 1B). The estimated 3-year OS was 34%. Among patients diagnosed with the two most common subtypes of PTCL (PTCL, NOS and AITL), the median EFS was 3.8 months (2.5–5.6, 95% CI) (Figure 1C). The median OS was 9.1 months (5.6–22.3, 95% CI), and the estimated 3-year OS was 28% (Figure 1D). Excluding cases of extranodal NK/T-cell lymphoma (n=6) did not significantly change the results of this study (Supplemental figures 3–7). Among patients who received systemic salvage therapy after progression or relapse, outcomes were somewhat improved: median EFS was 4.2 months (3.0–5.5, 95% CI), median OS was 12.4 months (7.3–53, 95% CI), and estimated 3-year survival was 39%. Among patients who did not receive either autologous or allogeneic stem cell transplant as part of consolidation, survival was poorer: median EFS was 2.6 months (1.6–3.5, 95% CI), median OS was 5.3 months (3.0–8.6, 95% CI), and 3-year OS was 13% (Figure 1E and 1F).
Figure 1.
Event free survival (A) and overall survival (B) of entire patient cohort after diagnosis with primary refractory PTCL. (C, Event free survival) and (D, overall survival) include only patients with the two most common histological subtypes of PTCL, PTCL-NOS and AITL. (E, Event free survival) and (F, overall survival) include only patients who did not undergo SCT.
Two subpopulations existed in our cohort of patients with primary refractory PTCL: 44 experienced progression of disease during first-line therapy, while the remaining 49 patients achieved complete remission (CR) or partial remission (PR) prior to relapsing within 6 months of completing first-line therapy. These patients had similar EFS (progression: median EFS 2.9 months v. relapse: 4.2 months, p = 0.35) and OS (median OS, 7.1 v. 9.7 months, p = 0.19; Figures S1A and S1B). When only PTCL, NOS and AITL cases were included, EFS (progression: median 3.1 months v. relapse: 5.4 months; p = 0.9) and OS (7.3 v. 9.2 months; p = 0.8) were comparable between these groups as well. Therefore, these subpopulations were combined as one cohort in further analyses.
The IPI at time of relapse or progression was prognostic in patients with primary refractory PTCL, and patients with higher risk IPI scores had progressively inferior EFS and OS (Figures S2A and S2B). Similar trends were observed when this analysis was confined to only patients receiving salvage therapies (Figures S2C and S2D), and when patients were stratified by PIT (Figures S2E and S2F). The prognostic significance of the components of the IPI and PIT at time of relapse or progression, which include age, Eastern Cooperative Oncology Group (ECOG) performance status, LDH, Ann Arbor stage, number of extranodal sites, and bone marrow involvement, were assessed on univariate analysis. Age greater than 60 years and elevated LDH were associated with inferior OS (p = 0.03 and 0.0003 respectively) (Supplemental Table I). On multivariate analysis with age, LDH, and number of extranodal sites as prognostic factors for OS, only elevated LDH was associated with inferior OS (p = 0.0008).
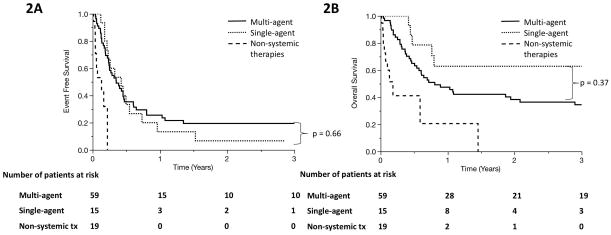
Single-agent regimens showed no difference in EFS or OS compared to traditional multi-agent regimens such as ICE, DHAP, and ESHAP in our cohort. Median EFS was 4.2 months in the traditional regimen group (95% CI, 2.9–5.6 months) v. 5.0 months for single-agent regimens (95% CI, 2.2–6.6 months) (p = 0.66), and median OS was 9.7 months (95% CI, 6.1–25 months) v. 53 months (95% CI, 5.6–71 months) (p = 0.37) (Figure 2A and 2B). As expected, patients who received best supportive care rather than systemic therapies had significantly poorer outcomes with median EFS 1.6 months and median OS 2.2 months. Following second-line therapy, patients underwent a median of one additional line of therapy (range, 0–7). Patients who underwent multi-agent salvage therapy received a mean of 1 additional line of therapy (95% CI, 0.7–1.3), whereas patients who underwent single-agent salvage therapy received an average of 2.1 additional lines (95% CI, 1.5–2.6; p = 0.001). Among 74 patients who underwent systemic salvage therapy, 18 (24%) received one of the currently approved novel agents (romidepsin, belinostat, brentuximab, or pralatrexate) as one of their lines of salvage therapy, and 12 (16%) received an HDAC inhibitor (romidepsin or belinostat). Between those who received HDACi at any point in their treatment course and those who never underwent HDACi therapy, there is no difference in EFS (median EFS HDACi 3.4 months v. no HDACi 4.6 months, p = 0.16) or OS (median OS 53 months [95% CI 4.4–71] v. 10.7 months [95% CI 6.8–35], p = 0.97).
Figure 2.
Event-free survival (A) and overall survival (B) in primary refractory PTCL patients who received traditional, multi-agent salvage regimens (solid line), single-agent salvage regimens (dotted line), and non-systemic therapeutic modalities (dashed line).
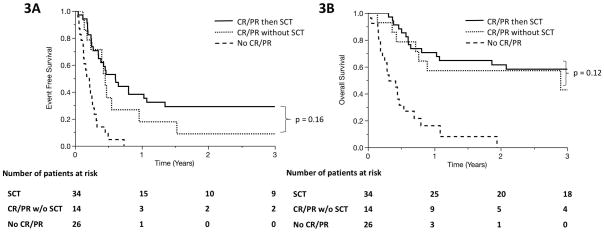
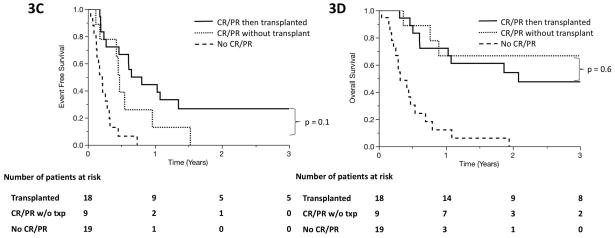
Forty-six percent of patients (n = 34) who underwent systemic salvage therapy went on to receive autologous stem cell transplant (auto-SCT; n = 15) or allogeneic stem cell transplant (allo-SCT; n = 20). One patient received auto-SCT, relapsed, then underwent allo-SCT. There was a trend suggestive of improved outcomes when patients undergo SCT compared to patients who achieved CR or PR during salvage therapy but did not undergo SCT (median EFS 7.3 months v. 5.4 months, p = 0.16; median OS NR v. 35 months, p = 0.12; Figures 3A and 3B). The trend persisted in PTCL, NOS/AITL patients (median EFS 8.8 months v. 5.6 months, p = 0.1; median OS 25 v. 53 months, p = 0.6; Figures 3C and 3D). Among the 20 patients in the cohort who survived for more than 3 years, 17 had undergone SCT. The 3 remaining patients either had slowly progressive disease managed with multiple palliative therapies or achieved a durable remission following therapy.
Figure 3.
Event-free survival (A) and overall survival (B) in primary refractory PTCL patients who achieved complete remission (CR) or partial remission (PR) at any time in response to salvage therapy and underwent autologous or allogeneic SCT (solid line), those who achieved CR or PR but never underwent SCT (dotted line), and those who never achieved PR or CR during salvage therapy (dashed line). (C, D) show event-free survival and overall survival among the subset of patients with PTCL, NOS or AITL.
DISCUSSION
In contrast to previous studies describing the natural history of relapsed/refractory PTCL [2, 3], we sought to investigate outcomes in patients with chemotherapy-resistant disease, as demonstrated by disease progression while on therapy or soon (within 6 months) after its completion. Outcomes were dismal in our cohort of primary refractory PTCL patients, with median EFS of 3.5 months, median OS of 9.1 months, and a 3-year survival rate of 34%. Inferior survival was seen in primary refractory patients who did not undergo SCT, with median EFS 2.6 months, median OS 5.3 months, and 3-year survival 13%, comparable to survival rates in a previously reported cohort of 153 relapsed/refractory PTCL patients who did not receive SCT [2]. Patients who relapsed soon after completion of first-line therapy had similar outcomes to those completely refractory to initial therapy. As anticipated, most patients in our cohort had high-risk features, including advanced age, poor performance status, advanced-stage disease, elevated LDH, and multiple extranodal sites of disease, which correlated with inferior outcomes. Patients salvaged with single-agent therapies (16% of cohort) had comparable EFS and OS to those who underwent traditional, multi-agent therapies (63% of cohort). Lastly, there was a trend toward improved survival for patients who achieved CR/PR and went on to receive SCT compared to those who achieved remission but were not transplanted, although this was not statistically significant. The observation that novel agents and single-agent therapies achieved outcomes comparable to traditional and more intense regimens (e.g. ICE, DHAP) in our cohort, despite older age and more frequent BM involvement in the single-agent group (Supplemental Table IIA), supports current recommendations encouraging participation in well-designed clinical trials among patients with primary refractory PTCL. Our findings are similar to findings from a cohort of 1020 cases of refractory and relapsed PTCL prospectively collected through the international T-Cell Project, in which median OS was 5.8 months and 3-year OS was 21% in the primary refractory setting, and auto-SCT was associated with improved outcomes [15].
The mechanisms of chemotherapy resistance and the inferior outcomes we observed are poorly understood. For example, IDH-mutated AITL is characterized by global DNA hypermethylation and downregulation of various tumor suppressor genes, including negative regulators of T-cell receptor (TCR) signaling [16]. Conversely, activating mutations in signaling intermediates required for TCR signaling (e.g. PLC-g1, PI3K, or CARD11) are recurrently observed in AITL [17] and in other T-cell malignancies [18]. The genetic landscape in AITL, particularly evidence implicating a pathogenic role for TCR engagement, is consistent with findings reported by Wang et. al. demonstrating that TCR engagement activated NF-κB and conferred resistance to chemotherapy in malignant T cells [19]. Gene expression profiling studies performed in PTCL, NOS demonstrate that this heterogeneous PTCL subtype is comprised of at least two molecularly and clinically distinct subtypes characterized by transcriptional programs orchestrated by the transcription factors T-bet and GATA-3, respectively [20, 21]. Dismal overall survival and a high-rate (>50%) of primary refractory disease was observed in GATA-3 expressing PTCL, NOS [19–21]. Loss-of-function studies performed in GATA-3-expressing T-cell lymphoma cells suggest that GATA-3 may confer resistance to chemotherapy in both a non-cell-autonomous and cell-autonomous manner [19, 21]. In addition, relevant tumor suppressor genes (including p53, PTEN, and CDKN2A) are recurrently lost in these [22] and other [23, 24] T-cell lymphomas. Further, GATA-3 expression was associated with increased expression of c-myc target genes [20], which may also promote chemotherapy resistance. The available evidence suggests that chemotherapy resistance in PTCL is likely driven by the genetic landscape, the cell-of-origin, and the tumor microenvironment. Improved understanding of the mechanisms driving chemotherapy resistance will be needed for the development of novel therapeutic strategies that may overcome the challenge of chemotherapy resistance.
Among patients with relapsed and refractory PTCL, particularly those who failed autologous SCT, allogeneic SCT remains a viable option to achieve durable remission [25]. Our study suggests that patients who undergo SCT (of any type) may have improved survival compared to those who also achieve a remission with salvage therapy but never undergo SCT. Of course, this contention is tentative, as the limited sample size in our cohort precludes a definitive conclusion, and there may be fundamental clinical differences between non-transplanted and transplanted patients (e.g. older age, more aggressive disease; Supplemental Table IIB). Nonetheless, our findings align with current recommendations for the use of autologous and allogeneic SCT for consolidation in transplant eligible patients with chemotherapy-sensitive disease. In addition, our study is also limited by its retrospective nature, including provider assessments of disease response, and a paucity of patients treated with recently approved novel, or investigational, agents.
In summary, this cohort of 93 patients with primary refractory PTCL is one of the largest reported in the literature, and as such, may provide a useful benchmark for future studies and clinical trials including patients with primary refractory PTCL. Most long-term disease-free survivors in this cohort had undergone high dose therapy and auto-SCT or allo-SCT, which are currently recommended consolidation strategies among patients with chemo-sensitive disease. We were unable to discern a significant difference in outcomes between traditional, multi-agent salvage regimens compared to novel, single-agent regimens in this cohort. This supports current recommendations for patients with relapsed/refractory PTCL to participate in rational and well-designed clinical trials.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Leukemia & Lymphoma Society (6503-16), the American Cancer Society (129084-RSG-16-045-01-LIB), and the NIH-NCI (K08CA172215).
References
- 1.Vose J, Armitage J, Weisenburger D, et al. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 3.Briski R, Feldman AL, Bailey NG, et al. Survival in patients with limited-stage peripheral T-cell lymphomas. Leuk Lymphoma. 2015;56:1665–1670. doi: 10.3109/10428194.2014.963078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramson JS, Feldman T, Kroll-Desrosiers AR, et al. Peripheral T-cell lymphomas in a large US multicenter cohort: prognostication in the modern era including impact of frontline therapy. Ann Oncol. 2014;25:2211–2217. doi: 10.1093/annonc/mdu443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.d’Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pro B, Horwitz SM, Prince HM, et al. Romidepsin induces durable responses in patients with relapsed or refractory angioimmunoblastic T-cell lymphoma. Hematol Oncol. 2016 doi: 10.1002/hon.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coiffier B, Pro B, Prince HM, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11. doi: 10.1186/1756-8722-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foss F, Advani R, Duvic M, et al. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015;168:811–819. doi: 10.1111/bjh.13222. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33:2492–2499. doi: 10.1200/JCO.2014.59.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 13.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32:3137–3143. doi: 10.1200/JCO.2013.54.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellei M, Foss FM, Horwitz SM, et al. The Outcome of Patients with Primary Refractory or Relapsed Peripheral T-Cell Lymphoma: Analysis of 1020 Cases Registered in the Prospective T-Cell Project. American Society of Hematology; San Diego, CA: 2016. [Google Scholar]
- 16.Wang C, McKeithan TW, Gong Q, et al. IDH2R172 mutations define a unique subgroup of patients with angioimmunoblastic T-cell lymphoma. Blood. 2015;126:1741–1752. doi: 10.1182/blood-2015-05-644591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallois D, Dobay MP, Morin RD, et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell-derived lymphomas. Blood. 2016;128:1490–1502. doi: 10.1182/blood-2016-02-698977. [DOI] [PubMed] [Google Scholar]
- 18.Vaque JP, Gomez-Lopez G, Monsalvez V, et al. PLCG1 mutations in cutaneous T-cell lymphomas. Blood. 2014;123:2034–2043. doi: 10.1182/blood-2013-05-504308. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Lu Y, Polk A, et al. T-cell Receptor Signaling Activates an ITK/NF-kappaB/GATA-3 axis in T-cell Lymphomas Facilitating Resistance to Chemotherapy. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123:2915–2923. doi: 10.1182/blood-2013-11-536359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Feldman AL, Wada DA, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2014;123:3007–3015. doi: 10.1182/blood-2013-12-544809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heavican TB, Yu J, Bouska A, et al. Molecular Subgroups of Peripheral T-Cell Lymphoma Evolve by Distinct Genetic Pathways [abstract] American Society of Hematology; San Diego, CA: 2016. [Google Scholar]
- 23.Elenitoba-Johnson KS, Wilcox R. A new molecular paradigm in mycosis fungoides and Sezary syndrome. Semin Diagn Pathol. 2017;34:15–21. doi: 10.1053/j.semdp.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox RA. A three-signal model of T-cell lymphoma pathogenesis. Am J Hematol. 2016;91:113–122. doi: 10.1002/ajh.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reimer P. Impact of autologous and allogeneic stem cell transplantation in peripheral T-cell lymphomas. Adv Hematol. 2010;2010:320624. doi: 10.1155/2010/320624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.