Abstract
Introduction
Dopaminergic therapy in Parkinson’s disease (PD) can be associated with both motoric (e.g., dyskinesias) and neuropsychiatric adverse effects. Examples of the latter include Dopamine Dysregulation Syndrome (DDS) and impulse control disorder (ICD), which are separate but related behavioral/psychiatric complications of treatment in PD. Dysregulation of volition characterizes both dyskinesias and DDS/ICD; thus, we analyzed potential disease-related correlates in a large PD cohort.
Methods
We analyzed cross-sectional data from 654 participants collected through the NINDS Parkinson’s Disease Biomarkers Program. DDS/ICD symptoms and dyskinesias were assessed using the Movement Disorders Society (revised) Unified Parkinson’s Disease Rating Scale. Potential associated variables were selected from PD-validated or PD-specific scales of neuropsychiatric or motoric status. Multivariable models with DDS/ICD or dyskinesia presence outcomes were produced with backward stepwise regression to identify factors independently associated with DDS/ICD and/or dyskinesias.
Results
Fifty-three (8.1%) participants endorsed DDS and/or ICD symptoms and 150 (22.9%) were dyskinetic. In multivariable analysis, psychosis was independently associated with both dyskinesias (p=0.006) and DDS/ICD (p<0.001). Unpredictable motor fluctuations (p=0.026) and depression (p=0.023) were also associated with DDS/ICD; female sex (p=0.025), low tremor score (p=0.001) and high akinesia-rigidity score (p<0.001) were associated with dyskinesias.
Conclusions
Our findings suggest that psychosis may be an important marker of impaired volition across motor and cognitive domains. Unpredictable motor fluctuations, psychosis, and depression may together comprise a phenotypic profile of patients at increased risk for DDS/ICD. Similarly, dyskinetic PD patients should be closely monitored for psychotic symptoms and treated appropriately.
Keywords: Parkinson’s disease, dyskinesias, dopamine dysregulation syndrome, DDS, impulse control disorders, ICD, hallucinations, depression, motor subtypes
Introduction
Parkinson’s disease (PD) is characterized by progressive deterioration of both motor and cognitive function. Dopamine (DA) replacement therapy can alleviate most motor symptoms, but tends to be complicated by a variety of unintended effects. Half of levodopa-treated PD patients develop dyskinesias within 6 years of initiation, although recent evidence demonstrates that duration of levodopa exposure is less relevant to dyskinesia onset than disease duration itself.[1] DA is a key modulator of motivation, impulsivity, and reward-oriented behavior;[2] thus, medications that facilitate DA neurotransmission can modify action selection in PD.
DA medications can engender a range of compulsive abnormalities related to negative reinforcement dysfunction, habit formation, incentive sensitization, and impulsivity, including multiple specific behaviors: (1) abuse of DA medications (DDS); (2) impulse control disorder (ICD) behaviors (excessive gambling or shopping, hypersexuality, hyperphagia, and kleptomania); and (3) punding.[2,3] The pathophysiology of DDS is unclear; potential positive reinforcement mechanisms include increased novelty-seeking and incentive sensitization.[3] Aversion to the “off” state may also predispose to excessive medication usage.[4] The relative preservation of DA neurons in the ventral tegmental area—compared to the substantia nigra—may facilitate DDS and ICD by enabling hyperdopaminergic neurotransmission in the mesolimbic system.[5] Recent evidence has also highlighted the significance of impaired cognitive volition in PD patients with ICDs.[6] Due to commonalities between DDS and ICD more generally, the Movement Disorders Society’s (revised) Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) assesses both using a single item (Part 1, question 6).
At a broader level, there is conceptual similarity between behavioral disorders marked by impaired cognitive volition (such as ICD and likely DDS) and involuntary bodily movements like dyskinesias. It has been proposed that these phenomena occupy a continuum of pathophysiologically similar mechanisms relating to topographically distinct basal ganglia circuits.[7] Hypersensitization of dorsal striatum neurons receiving coincident input from glutamatergic and nigrostriatal DA projections—in the context of pulsatile DA receptor stimulation—remains the current paradigm for dyskinesia etiology.[8] For DDS and ICD, the focus is shifted to mesocorticolimbic projections and the ventral striatum as the site of hyperdopaminergic dysregulation. Activity in signaling cascades that are implicated in substance use disorders—such as the ERK and DARPP-32/PPP1R1B pathways—are also altered in dyskinetic patients, suggesting molecular overlap between altered reward-processing and dyskinesias.[7] Similar considerations inspired a recent study demonstrating that PD patients with moderate to severe dyskinesias—assessed by the Unified Dyskinesia Rating Scale—more frequently had ICDs or related behaviors (such as DDS, hobbyism, and punding) than patients with only mild dyskinesias.[9]
We hypothesized that dyskinesias may share similar markers with DDS and ICD given the analogous impairment of volition and similarities in theoretical origins (hyperdopaminergic dysregulation). Shared clinical associations would strengthen the hypothesis of shared or parallel etiologies. To test this hypothesis, we performed a risk-factor analysis for potential neuropsychiatric and/or motoric markers of dyskinesias and DDS/ICD.
Methods
Database and Participants
Data were extracted from the NINDS Parkinson’s Disease Biomarker Program (PDBP) dataset on December 28, 2016. Baseline data from participants enrolled in the PDBP study at seven academic centers in the United States were used in the present study. Each participating center’s local IRB has approved the PDBP protocol and all participants provided informed consent.
Participant data were extracted for analysis only if a diagnosis of “probable or possible idiopathic Parkinson’s disease” was made per UK Brain Bank criteria.[10] Other inclusion criteria were complete demographic and medication information and complete data for the following PD-validated or PD-specific scales: MDS-UPDRS; the 17 item Hamilton Depression Rating Scale (HAM-D); the 14 item Hamilton Anxiety Rating Scale (HAM-A); and the Montreal Cognitive Assessment (MoCA). Additionally, patients with a MoCA score ≤ 17 were excluded to remove participants with probable dementia.[11]
Measures
Supplementary table 1 provides an overview of components from the MDS-UPDRS utilized in the present study. The primary outcome variables of interest were MDS-UPDRS question 1.6—which assesses the impact of DDS and/or ICD symptoms on patient and caregiver/family life, scored using a Likert scale of 0–4—and the presence of dyskinesias, assessed using Part-IV (complications of therapy) of the MDS-UPDRS. Dyskinesias were deemed present if a patient reported a score of ≥ 1 on Q4.1, which assesses the daily duration of dyskinesias.
To determine the presence of clinically significant anxiety and depression, we used PD-validated cutoffs for the HAM-A (> 11) and HAM-D (> 9).[12,13] Medication data were used to calculate Levodopa Equivalent Daily Dosage (LEDD, mg/day) per convention.[14] LEDD from DA agonists was recorded separately (“DA-LEDD”) and not included in the total LEDD; these were independent measures. Akinesia-rigidity (AR) and tremor motor impairment scores were calculated based on updated methodology for the MDS-UPDRS Motor Examination; however, rather than dividing mean AR score by mean tremor score to derive subtype classification, we utilized these mean scores separately for regression analysis to standardize and facilitate interpretation of odds ratios.[15]
Statistics
We used univariable methods to characterize differences among participants grouped by DDS/ICD and dyskinesia presence, including Kruskal-Wallis (nonparametric ANOVA) tests for continuous or ordinal data and Fisher’s exact tests for nominal data (Table 2). For regression, the distributions of the primary outcome variables (MDS-UPDRS questions 1.6 for DDS/ICD, 4.1 for dyskinesias) were first evaluated. As they did not follow either a Poisson or a normal distribution, we dichotomized them as binary variables, such that any response ≥ 1 was scored as 1. LEDD values (mg/day) were divided by 100 in regression to improve interpretation of odds ratios. For all regression analyses, the MDS-UPDRS items assessing DDS/ICD and psychotic symptoms were treated as binary variables (≥ 1 scored as 1) due to the low prevalence of scores over 1.
Table 2.
Patient Characteristics by DDS/ICD and Dyskinesia presence (n=654)
| Variable | No DDS/ICD or dyskinesia (n=469) | Dyskinesias only (n=132) | DDS/ICD only (n=35) | DDS/ICD and dyskinesia (n=18) | p |
|---|---|---|---|---|---|
|
| |||||
| Demographic and Medication Information Variables | |||||
|
| |||||
| Age, years | 64.8 (64.0–65.6) | 66.2 (64.6–67.8) | 63.3 (60.4–66.3) | 64.0 (60.4–67.5) | 0.219 |
|
| |||||
| Age at diagnosis, years | 59.4 (58.5–60.2) | 56.5 (54.5–58.5) | 56.9 (53.6–60.1) | 52.8 (48.8–56.9) | 0.002** |
|
| |||||
| Disease duration, years | 5.4 (5.0–5.8) | 9.7 (8.8–10.6) | 6.5 (4.8–8.1) | 11.2 (8.6–13.7) | <0.001*** |
|
| |||||
| Total levodopa equivalent daily dosage (LEDD) mg/day | 599 (551–647) | 889 (800–978) | 687 (487–887) | 994 (734–1254) | <0.001*** |
|
| |||||
| Dopamine agonist usage | 228 (48.6%) | 67 (50.8%) | 23 (65.7%) | 13 (72.2%) | 0.063 |
|
| |||||
| Dopamine agonist LEDD (mg/day) | 112 (98–125) | 123 (96–150) | 130 (87–173) | 149 (82–216) | 0.214 |
|
| |||||
| Hoehn & Yahr stage | |||||
| &smap:(1) | 81 (17.3%) | 13 (9.8%) | 5 (14.3%) | 0 | |
| &smap:(2) | 334 (71.2%) | 88 (66.7%) | 25 (71.4%) | 14 (77.8%) | |
| &smap:(3) | 48 (10.2%) | 27 (20.5%) | 4 (11.4%) | 3 (16.7%) | <0.001*** |
| &smap:(4) | 5 (1.1%) | 4 (3.0%) | 1 (2.9%) | 1 (5.6%) | |
| &smap: (5) | 1 (0.2%) | 0 | 0 | 0 | |
| Mean Hoehn & Yahr stage | 2.0 (1.9–2.0) | 2.2 (2.1–2.3) | 2.0 (1.8–2.2) | 2.3 (2.0–2.5) | |
|
| |||||
| Sex, male | 296 (63.1%) | 75 (56.8%) | 23 (65.7%) | 9 (50%) | 0.380 |
|
| |||||
| PD-Validated Scales | |||||
|
| |||||
| Montreal Cognitive Assessment (MoCA) | 25.4 (25.2–25.7) | 25.1 (24.6–25.6) | 25.3 (24.3–26.3) | 24.8 (23.1–26.5) | 0.594 |
|
| |||||
| Clinically significant anxiety (HAM-A > 12) | 29 (6.2%) | 15 (11.4%) | 6 (17.1%) | 3 (16.7%) | 0.013* |
|
| |||||
| Clinically significant depression (HAM-D-17 > 10) | 32 (6.8%) | 21 (15.9%) | 6 (17.1%) | 7 (38.9%) | <0.001*** |
|
| |||||
| MDS-UPDRS | |||||
|
| |||||
| Tremor items total score (Part III) | 5.0 (4.7–5.4) | 4.1 (3.5–4.8) | 6.2 (4.8–7.7) | 5.2 (2.4–7.9) | 0.016* |
|
| |||||
| Akinesia-rigidity items total score (Part III) | 2.8 (2.5–3.1) | 4.5 (3.8–5.1) | 4.2 (3.1–5.3) | 4.4 (3.1–5.8) | <0.001*** |
|
| |||||
| Psychotic symptoms present (Q1.2) | 28 (6.0%) | 17 (12.9%) | 7 (20%) | 9 (50%) | <0.001*** |
|
| |||||
| Wearing-off present (Q4.3) | 108 (23.0%) | 85 (64.4%) | 13 (37.1%) | 17 (94.4%) | <0.001*** |
|
| |||||
| Functional impact of motor fluctuations (Q4.4) | 83 (17.7%) | 71 (53.8%) | 9 (25.7%) | 16 (88.9%) | <0.001*** |
|
| |||||
| Unpredictable motor fluctuations (Q4.5) | 102 (21.7%) | 82 (62.1%) | 12 (34.3%) | 17 (94.4%) | <0.001*** |
Means and 95% confidence intervals are presented for demographic and medication information variables and PD-validated scale variables. Patient scores on MDS-UPDRS items in parts I & IV are presented as proportions of total responses. P-values were produced using Kruskal-Wallis (nonparametric ANOVA) tests for continuous or ordinal data and Fisher’s exact test for nominal data (sex, dopamine agonist usage, and primary source of MDS-UPDRS information) across all groups. LEDD from DA agonists is not included in total LEDD.
p<0.05;
p<0.01;
p<0.001
We used backward stepwise logistic regression to identify independent variables for multivariable modeling. Sixteen of the variables in Table 2—all except age to avoid collinearity—were included for the DDS/ICD outcome model, plus a variable measuring the sum of the two dyskinesia items of the MDS-UPDRS IV (daily duration and functional impact of dyskinesias; q4.1 and 4.2 respectively). These seventeen variables were included in a logistic regression (with binary DDS/ICD scores as the outcome variable), which was then subjected to backward stepwise variable removal guided by corrected Akaike’s information criterion (AICc). In parallel, a model for stepwise input with dyskinesia presence as the outcome variable was constructed, using the same variables, except with the DDS/ICD item substituting for the dyskinesia predictor. No measures of motor fluctuations were included, as fluctuations are intrinsic to certain classes of dyskinesias that are not differentiated by the MDS-UPDRS.[16] Each model also included an interaction term between AR motor score and disease duration to account for the observation that conversion to the AR phenotype over time is typical in PD.[17] An interaction term between LEDD and disease duration was tested but did not reach significance. We report odds ratios with corresponding 95% confidence intervals and p-values for our multivariable logistic regression model. Odds ratios were calculated as the exponentiated regression coefficients and confidence interval endpoints. Statistical significance was set at p < 0.05.
Results
Demographic and clinical characteristics data for the 654 participants in our study are presented in Table 1. The age of participants ranged from 35 to 87, with an average of 65.0 ± 9.0 years (±, standard deviation) and an average age of disease onset of 58.5 ± 10.0 years. Half of the participants (50.6%) were using DA agonist medications at the time of their assessment and two-thirds (61.6%) of the participants were male. Fifty-three participants had clinically significant anxiety (8.1%) and 66 (10.1%) had clinically significant depression. Responses to select items from parts I (Non-motor Aspects of Experiences of Daily Living) and IV (Motor Complications) of the MDS-UPDRS scale were also characterized for our sample. Fifty-three participants (8.1%) scored ≥ 1 on q1.6, which assesses DDS/ICD symptom impact on daily functioning, while 150 participants (22.9%) scored ≥ 1 on question q4.1, which measures the daily duration of dyskinesia presence on average over the past week. For all remaining analyses, these participants were considered to have DDS/ICD and/or dyskinesias, respectively.
Table 1.
Sample Characteristics (N=654)
| Demographic and Medication Information | Mean | SD | Range | |
|---|---|---|---|---|
|
| ||||
| Age, years | 65.0 | 9.0 | 35.8–87.5 | |
|
| ||||
| Age at diagnosis, years | 58.5 | 10.0 | 31–82 | |
|
| ||||
| Disease duration, years | 6.5 | 5.1 | 0.1–38.3 | |
|
| ||||
| Levodopa equivalent daily dosage (LEDD), mg/day | 673 | 549 | 0–3396 | |
|
| ||||
| Dopamine agonist LEDD (mg/day) | 116 | 151 | 0–900 | |
|
| ||||
| Dopamine agonist usage | 331 (50.6%) | |||
|
| ||||
| Clinically significant anxiety (HAM-A ≥ 12) | 53 (8.1%) | |||
|
| ||||
| Clinically significant depression (HAM-D-17 ≥ 10) | 66 (10.1%) | |||
|
| ||||
| Hoehn & Yahr stage | ||||
| (1) | 99 (15.4%) | |||
| (2) | 461 (68.2%) | |||
| (3) | 82 (13.0%) | |||
| (4) | 11 (2.2%) | |||
| (5) | 1 (1.2%) | |||
|
| ||||
| Sex, male | 403 (61.6%) | |||
|
| ||||
| PD-Validated Scales | Mean | SD | Range | |
|
| ||||
| Montreal Cognitive Assessment (MoCA) | 25.3 | 3.0 | 18–30 | |
|
| ||||
| MDS-UPDRS Part III (Motor Examination) | 24.8 | 13.2 | 2–81 | |
|
| ||||
| MDS-UPDRS | Patient Scores (0/1/2/3/4) | Scores > 0, n (%) | ||
|
| ||||
| Q1.2 Hallucinations and Psychosis | 90.7 / 8.0 / 1.4 / 0 / 0 | 61 (9.3%) | ||
|
| ||||
| Q1.6 DDS/ICD | 91.9 / 4.9 / 2.8 / 0.3 / 0.2 | 53 (8.1%) | ||
|
| ||||
| Q4.1 Time spent with Dyskinesias | 77.1 / 16.5 / 4.1 / 1.7 / 0.6 | 150 (22.9%) | ||
|
| ||||
| Q4.2 Functional Impact of Dyskinesias | 88.5 / 7.5 / 2.6 / 1.2 / 0.2 | 75 (11.5%) | ||
|
| ||||
| Q4.3 Time spent in the off state | 65.9 / 27.7 / 5.8 / 0.5 / 0.2 | 223 (34.1%) | ||
|
| ||||
| Q4.4 Functional Impact of Fluctuations | 72.6 / 15.3 / 6.6 / 4.7 / 0.8 | 179 (27.4%) | ||
|
| ||||
| Q4.5 Unpredictability of Motor Fluctuations | 67.4 / 24.5 / 3.5 / 2.6 / 2.0 | 213 (32.6%) | ||
SD, Standard deviation. Patient scores on MDS-UPDRS items in parts I & IV are presented as proportions of total responses. Question 1.6 (“Dopamine Dysregulation Syndrome”) assesses the interference of either DDS or ICD behaviors with patient functionality and quality of life. Clinically significant depression and anxiety were considered present for participants with HAM-A > 12 and HAM-D > 10, respectively.
We next performed univariable comparisons of patients grouped by the presence of DDS/ICD symptoms and/or dyskinesias (Table 2). Participants with both dyskinesias and DDS/ICD had the earliest disease onset age of any group (p=0.002), whereas dyskinesias were specifically associated with longer disease duration (p<0.001). All groups with DDS/ICD and/or dyskinesias were taking higher total non-DA agonist LEDD (p<0.001), but dopamine agonist use frequency and DA-LEDD were not significantly different among groups. Depression was most common in participants with both DDS/ICD and dyskinesias (38.9%), but also more frequent in the presence of either alone compared to participants with neither DDS/ICD nor dyskinesias (p<0.001). Participants with dyskinesias alone had the lowest tremor scores (p=0.016), whereas AR scores were lowest in participants with neither dyskinesias nor DDS/ICD (p<0.001) Participants with both dyskinesias and DDS/ICD had the highest frequency of psychotic symptoms, wearing-off, and motor fluctuations measures (p<0.001 for all).
Backward stepwise regression—conducted separately for the outcomes of dyskinesia presence and DDS/ICD presence—identified an optimal multivariable model for either outcome (Table 3). Collinearity among independent variables was assessed using correlation estimates for each unique independent variable pair; no pairwise correlation coefficient (Kendall’s tau) was greater than 0.30 in magnitude. However, we included an interaction term for AR motor impairment and disease duration, motivated by prior reports showing conversion to the AR disease phenotype over time.[17]
Table 3.
Markers of DDS/ICD symptoms or dyskinesias in logistic regression (n=654)
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Outcome | Independent Variable | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p |
| DDS/ICD symptom presence | Psychotic symptom presence | 5.34 (2.71–10.22) | <0.001*** | 4.30 (2.07–8.62) | <0.001*** |
| Depression (clinically significant) | 3.36 (1.64–6.54) | <0.001*** | 2.37 (1.09–4.86) | 0.023* | |
| Complexity of fluctuations score | 1.55 (1.19–1.97) | <0.001*** | 1.36 (1.02–1.79) | 0.026* | |
| DA-LEDD (mg/day)/100 | 1.10 (1.01–1.28) | 0.067 | 1.07 (0.98–1.22) | 0.180 | |
| Age at diagnosis | 0.97 (0.94–0.996) | 0.024* | 0.97 (0.94–1.00) | 0.0504 | |
| Dyskinesia presence | Disease duration (years) | 1.18 (1.13–1.22) | <0.001*** | 1.25 (1.17–1.33) | <0.001*** |
| Mean akinesia-rigidity score | 1.22 (0.88–1.67) | 0.222 | 2.43 (1.42–4.17) | 0.002** | |
| Interaction: Disease duration and mean akinesia-rigidity score | — | 0.92 (0.87–0.96) | <0.001*** | ||
| Psychotic symptom presence | 2.81 (1.62–4.83) | <0.001*** | 2.43 (1.27–4.57) | 0.006** | |
| Mean tremor items score | 0.47 (0.27–0.80) | 0.007** | 0.49 (0.28–0.83) | 0.0096** | |
| LEDD (mg/day)/100 | 1.10 (1.06–1.14) | <0.001*** | 1.05 (1.01–1.09) | 0.013* | |
| Male sex | 0.74 (0.51–1.07) | 0.107 | 0.62 (0.40–0.94) | 0.025* | |
| Depression (clinically significant) | 2.81 (1.65–4.76) | <0.001*** | 1.75 (0.94–3.20) | 0.074 | |
| DA-LEDD (mg/day)/100 | 1.01 (0.92–1.09) | 0.816 | 0.91 (0.80–1.01) | 0.166 | |
Independent variables for regression were identified through backward stepwise regression. Two separate models were produced, one with DDS/ICD symptom presence as the outcome, the other with dyskinesia presence as the outcome. Clinically significant depression was considered present if a score of ≥ 10 was reached on the HAM-D-17.
p<0.05;
p<0.01;
p<0.001
For DDS/ICD, the final model included five covariates. Of these, three were significantly associated with DDS/ICD in multivariable regression: psychosis (OR: 4.30; 95% CI: 2.07–8.62; p<0.001), clinically significant depression (OR: 2.37; 95% CI: 1.09–4.86; p=0.023), and unpredictability of motor fluctuations score (MDS-UPDRS q4.4; OR: 1.36; 95% CI: 1.02–1.79; p = 0.0326). Younger age at diagnosis was only significantly associated in univariable. The final model using dyskinesias as the outcome of interest included nine covariates. Corroborating previous reports,[1] we found that disease duration was the strongest marker of dyskinesias in multivariable analysis (OR: 1.25; 95% CI: 1.17–1.33; p<0.001). Like DDS/ICD, dyskinesias were positively associated with psychosis (OR: 2.43; 95% CI: 1.27–4.57; p=0.006), but depression was not. Lower mean tremor motor impairment scores were associated with dyskinesia presence (OR: 0.49; 95% CI: 0.28–0.83; p=0.0096). Conversely, higher mean AR score was significantly associated with dyskinesias (OR: 2.43; 95% CI: 1.42–4.17; p=0.002). Additionally, there was a significant negative interaction between mean AR score and disease duration (OR: 0.92; 95% CI: 0.87–0.96; p<0.001). Finally, increased LEDD was associated with dyskinesias (OR: 1.05; 1.01–1.09; p=0.013), but not DA-LEDD. Male sex was associated with a lower likelihood of dyskinesias (OR: 0.62; 95% CI: 0.40–0.94; p=0.025). In summary, the main overlapping factor associated with both DDS/ICD and dyskinesias was the presence of psychotic symptoms.
To better characterize the temporal relationship between DDS/ICD and associated markers, we graphed the frequencies of unpredictable fluctuations, DA agonist usage, and psychotic symptoms among the 53 DDS/ICD cases as a function of disease duration (Figure 1). Unpredictable fluctuations were more common in participants with a five-year or greater PD history, whereas there was no clear temporal grouping of psychotic symptoms among DDS/ICD cases. Of 35 DDS/ICD cases with a PD duration over 5 years, 25 (71.4%) reported unpredictable fluctuations.
Figure 1. Unpredictable fluctuations accompany DDS/ICD later in PD than hallucinations or clinically significant anxiety.
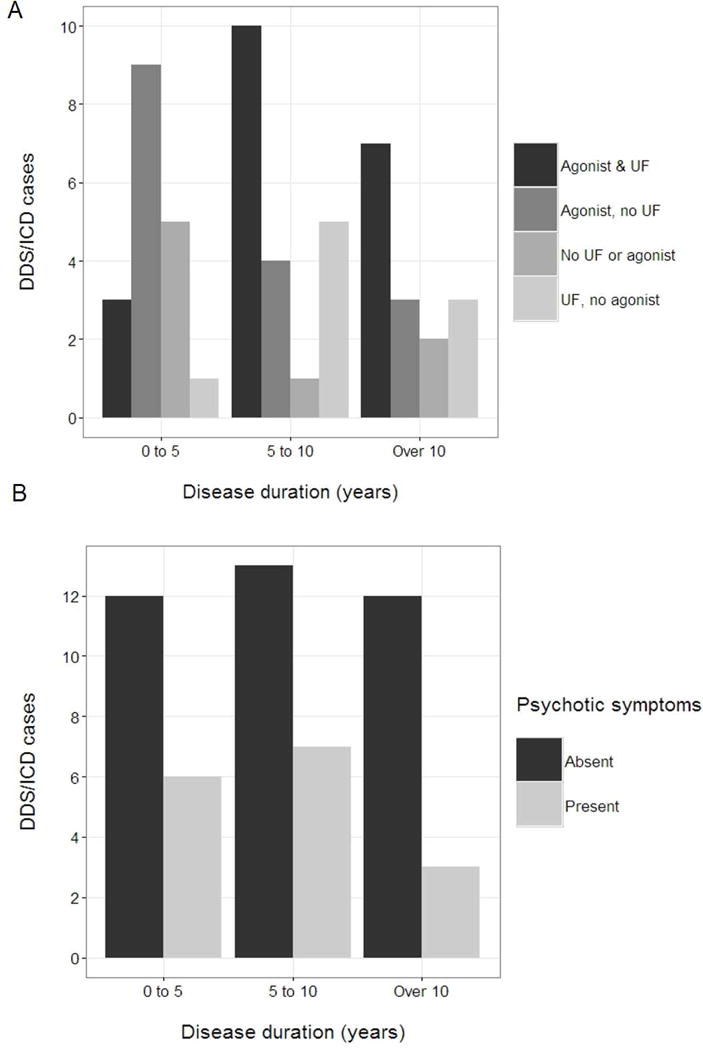
We compared the relative frequencies of (A) unpredictable fluctuations and dopamine agonist use and (B) hallucinations in participants reporting DDS/ICD symptoms (n=53). Scores ≥ 1 on q1.2 and q4.5 of the MDS-UPDRS were considered indicative of the presence of hallucinations and unpredictable fluctuations, respectively. The number of DDS/ICD cases in the “0–5”, “5–10”, and “over 10” disease duration groups were 18, 20, and 15, respectively.
Discussion
With respect to our stated hypothesis of shared clinical correlates between DDS/ICD and dyskinesias in PD, we observed that psychotic symptoms were strongly and independently associated with both disorders in a cross-sectional analysis of a large PD cohort. Clinically significant depression and motor fluctuations characterized as irregular or unpredictable were associated specifically with DDS/ICD. DDS is not a highly prevalent complication of PD,[18] making it difficult to investigate risk factors, although previous smaller studies have suggested mood and perceptual alterations to be implicated in both DDS and ICD.[18,19] Our observed prevalence for DDS and ICD (8.1%) is difficult to compare to prior reports of DDS, as no study has utilized the MDS-UPDRS scale for this purpose. Existing studies have estimated prevalence at 3–4% for DDS[18] and 14% for ICD.[20] Importantly, PD patients are often more reticent than their caregivers to discuss DDS and ICD symptoms, which constitutes a substantial barrier to disclosure in a patient-reported context and may lower sensitivity.[21]
Our findings suggest that psychosis may be an important marker for disruption of motor and cognitive volition. The intrinsic disease processes of PD predisposing patients to psychotic symptoms may affect dopaminergic neurotransmission in a manner that also increases vulnerability to DDS and/or ICD. Indeed, a recent study linked low dopamine transporter (DAT) availability in the ventral striatum of PD patients to the later development of visual hallucinations, [22] an association previously observed for ICDs.[23] Similarly, disease duration has emerged as the principal risk factor for dyskinesias in PD,[1] but an association with psychosis has not been previously observed. Dyskinesias and psychosis are often considered a complication of DA therapy, even though the relative contributions of PD treatment and the disease process itself to the onset of psychotic symptoms in individual patients remain unclear.[7] Serotonergic dysfunction in PD has been linked to both dyskinesias and psychosis, suggesting serotonin networks modulate dopaminergic circuits related to these disorders.[24] A prior investigation[25] found no association between psychotic symptoms and compulsive behaviors, including hypersexuality, gambling, and shopping. However, the most frequent psychotic symptom reported in that study was altered dream phenomena, which are not included in the recent NINDS/NIMH criteria for PD psychosis.[26] The MDS-UPDRS emphasizes the presence of illusions, hallucinations, and delusions, which are key components of the NINDS/NIMH criteria.
Previous evidence of an association between motor fluctuations and compulsive disorders has been indirect. A case-control study[4] showed that DDS participants had more severe motor impairment and decreased positive affect than non-DDS participants when both were rated during the “off” state. Patient aversion to debilitating “off” state symptoms may be important for DDS etiology and trigger compulsive dopaminergic medication overuse. Although other factors, such as pulsatile DA levodopa therapy, genetic vulnerability, and personality[2] are likely more relevant causes of DDS in early PD before fluctuation onset, we show that motor fluctuations perceived as being unpredictable or complex are themselves associated with altered reward-oriented behavior. Generally, motor fluctuations signal declining satisfaction with dopamine replacement efficacy, which may induce patients to use higher doses of medications than previously required, initiating an addictive response to increasing dosages of reinforcing dopaminergic medications.[27]
The MDS-UPDRS assesses ICD and DDS behaviors together based on the theory that although these are discrete clinical entities, they share some etiological elements of reward system dysfunction and compulsivity, including altered positive reinforcement.[27] For example, DDS is linked to potentiated levodopa-induced DA release in the ventral striatum of PD patients[3] and a prospective study showed that PD patients who go on to develop ICDs have lower baseline DAT availability in the ventral striatum,[23] either of which would bias striatal reinforcement pathways toward positive outcome-coding. Notably, we did not identify DA-LEDD as a significant marker of DDS/ICD. The association between ICDs and DA agonists is well-recognized,[2,20] whereas DDS is most frequently seen with pulsatile levodopa exposure.[2] Because of this distinction, our approach may not capture the differential associations of DDS and ICD with different types of DA medication. Our analysis is mainly relevant for identifying general markers shared by these conditions of impaired cognitive volition and compulsion and is likely not sensitive enough to capture all factors associated with ICD or DDS specifically. Additionally, our data only records prescribed dosages and thus would not accurately model abnormal medication use related to DDS.
We found a novel inverse association between current dyskinesias and tremor in PD after adjusting for disease duration and LEDD, among other factors; a previous study[28] in a smaller cohort identified resting tremor at the time of diagnosis to be a negative predictor of eventual dyskinesia onset, but no association between current dyskinesia and tremor was noted. Additionally, we identified AR as a positive marker of dyskinesias and observed a significant negative interaction between AR motor impairment and disease duration. This suggests that marked AR impairment, especially in early PD, may portend a high likelihood of dyskinesias. Bradykinesia and rigidity—but not resting tremor—are correlated with nigrostriatal dopamine depletion and are more responsive to levodopa therapy than tremor.[29] Our findings are consistent with the increasingly compelling evidence that while dyskinesias are a dopaminergic phenomenon, tremor has extranigral origins.
There are several limitations to our analysis. The MDS-UPDRS item (1.6) used in our analysis does not differentiate ICD and DDS, which may not share an identical pathophysiology. However, because ICDs and medication abuse may share similar dopaminergic pathology as discussed above, grouping these behaviors together seems appropriate and facilitated our objective of identifying characteristics of patients with impaired cognitive volition. The developers of the MDS-UPDRS scale released a statement in 2012 acknowledging that the original item loading value of the DDS/ICD question did not replicate during a re-analysis. However, this appears to be more relevant to the MDS-UPDRS scale itself than to the validity of the DDS/ICD question specifically. Interestingly, the DDS/ICD question is one of the few non-motor questions that independently correlates with a validated quality of life measurement, the PDQ-39, validating the clinical relevance of this question alone in PD cohorts.[30] Finally, only single MDS-UPDRS items were used to measure psychosis and DDS/ICD. However, a validation study[31] demonstrated high correlation (0.86) of the MDS-UPDRS psychosis item with the Parkinson’s Psychosis Rating Scale.
Overlapping mechanisms of DDS and ICD in general remain an area for further investigation. The fact that psychosis was a strong marker of both DDS/ICD and dyskinesias may speak to disease-specific commonalities between DDS/ICD and dyskinesias, although the pathological basis of this nexus is unclear. Additionally, our findings and other emerging evidence linking ICDs and psychosis[22] prompt re-evaluation of potential pathophysiological connections. Fluctuations are already recognized as being intrinsic to the experience of dyskinesias for many PD patients. However, our findings may speak to a common pathophysiology in patients who are experiencing disabling fluctuations, DDS/ICD, and psychosis. PD patients with this phenotype may be at risk when using DA agonists (for ICD) or pulsatile levodopa therapy (for DDS). We suggest that clinicians ensure that patients who have begun to experience motor fluctuations are counseled on the addictive properties of dopaminergic medications and their potential for adverse behavioral effects. Similarly, dyskinesias should indicate evaluation for changes in neuropsychiatric status.
Supplementary Material
Highlights.
In PD, dyskinesias and DDS or ICD may have analogous pathophysiologies.
Shared correlates of dyskinesias, DDS, and ICD were investigated.
Psychosis was a prominent marker of both dyskinesias and DDS/ICD.
Depression and complex motor fluctuations were associated with DDS/ICD.
High akinesia-rigidity—but low tremor—impairment was associated with dyskinesias.
Acknowledgments
Data used in the preparation of this manuscript were obtained from the Parkinson’s Disease Biomarkers Program (PDBP) Consortium, part of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health. Investigators include: Roger Albin, Roy Alcalay, Alberto Ascherio, DuBois Bowman, Alice Chen-Plotkin, Ted Dawson, Richard Dewey, Dwight German, Xuemei Huang, Rachel Saunders-Pullman, Liana Rosenthal, Clemens Scherzer, David Vaillancourt, Vladislav Petyuk, Andy West and Jing Zhang. The PDBP Investigators not included in the author list have not participated in reviewing the data analysis or content of the manuscript.
Financial Disclosures
J.H.: Receives tuition and stipend support through the Medical Scientist Training Program at the Johns Hopkins School of Medicine (NIH/NIGMS 5 T32 GM007309).
K.P.: No disclosures to report.
L.R.: Dr. Rosenthal has received support from NIH/NINDS P50NS038377, Marilyn and Edward Macklin Foundation, and the Michael J. Fox Foundation. She also received an honorarium from the Edmond J. Safra Foundation and Functional Neuromodulation.
C.B.: Supported by P50 NS38377.
K.M.: Dr. Mills receives salary support through the NIH NCATS (KL2TR001077, PI Daniel Ford). He has received funding from Northwestern University.
AP: Dr. Pantelyat is supported for this project by NIH/NINDS U01 NS082133 and P50 NS38377.
Z.M.: Dr. Mari is supported by the National Parkinson’s foundation with a Center of Excellence Grant and is supported by NIH/NINDS U01 NS082133.
L.T.: No disclosures to report
J.B.: No disclosures to report
M.G.: No disclosures to report
N.Y.: Salary support through NIH/NINDS U01NS082133-04
A.B.: No disclosures to report
C.B.: Supported by P50 NS38377.
V.J.: Supported by P50 NS38377.
E.M.: No disclosures to report
T.M.D.: Dr. Dawson acknowledges the Adrienne Helis Malvin and Diana Henry Helis Medical Research Foundations and their research partnership with The Johns Hopkins Hospital, The Johns Hopkins University School of Medicine, and the Foundation’s Parkinson’s Disease Programs. He is supported by NIH/NINDS P50NS038377, NIH/NINDS U01NS082133, NIH/NINDS R37NS067525, NIH/NIDA P50 DA00266 and the JPB Foundation. Dr. Dawson is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases, chair of the Dystonia Prize committee of the Bachmann Strauss Dystonia and Parkinson’s Disease Foundation and the Michael J. Fox Foundation. He is on the Board of Directors of the Bachmann Strauss Dystonia and Parkinson’s Disease Foundation and on the Scientific Advisory Board of CurePSP and a member of American Gene Technologies International Inc. advisory board. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies. Dr. Dawson is a founder of Valted, LLC and holds an ownership equity interest in the company.
GP: Dr. Pontone receives funding through the NIH/NIA as part of a K23 award (AG044441-01A1). He is also co-sponsored by Acadia Pharmaceuticals, Inc., for writing a book on psychosis in Parkinson’s that was co-written by the National Parkinson’s Foundation.
Author Roles
1) Research Project: A. Conception, B. Organization, C. Execution
2) Statistical analysis: A. Design, B. Execution, C. Review and Critique
3) Manuscript: A. Writing of the first draft, B. Review and Critique
J.T.H.: 2A, B; 3A
K.P.: 3B
L.R.: 1A, B, C; 3B
K.M.: 2C; 3B
A.P.: 1C; 3B
Z.M.: 3B
L.T.: 1C
J.B.: 1C
M.G.: 1C
N.Y.: 1C
A.B.: 3B
C.B.: 3B
V.J.: 3B
E.M.: 3B
T.M.D.: 1A, B; 3B
G.P.: 1A, B; 2C; 3B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Cilia R, Akpalu A, Sarfo FS, Cham M, Amboni M, Cereda E, Fabbri M, Adjei P, Akassi J, Bonetti A, Pezzoli G. The modern pre-levodopa era of Parkinson’s disease: Insights into motor complications from sub-Saharan Africa. Brain. 2014;137:2731–2742. doi: 10.1093/brain/awu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katzenschlager R. Dopaminergic dysregulation syndrome in Parkinson’s disease. J Neurol Sci. 2011;310:271–275. doi: 10.1016/j.jns.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, Brooks DJ, Lees AJ, Piccini P. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59:852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- 4.Evans AH, Lawrence AD, Cresswell SA, Katzenschlager R, Lees AJ. Compulsive use of dopaminergic drug therapy in Parkinson’s disease: Reward and anti-reward. Mov Disord. 2010;25:867–876. doi: 10.1002/mds.22898. [DOI] [PubMed] [Google Scholar]
- 5.Evans AH, Lees AJ. Dopamine dysregulation syndrome in Parkinson’s disease. Mov Disord. 2004:393–398. doi: 10.1097/01.wco.0000137528.23126.41. [DOI] [PubMed] [Google Scholar]
- 6.Ricciardi L, Haggard P, de Boer L, Sorbera C, Stenner MP, Morgante F, Edwards MJ. Acting without being in control: Exploring volition in Parkinson’s disease with impulsive compulsive behaviours. Park Relat Disord. 2017;40:51–57. doi: 10.1016/j.parkreldis.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E. Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009;8:1140–1149. doi: 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- 8.Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with Parkinson’s disease: Filling the bench-to-bedside gap. Lancet Neurol. 2010;9:1106–1117. doi: 10.1016/S1474-4422(10)70218-0. [DOI] [PubMed] [Google Scholar]
- 9.Biundo R, Weis L, Abbruzzese G, Calandra-Buonaura G, Cortelli P, Jori MC, Lopiano L, Marconi R, Matinella A, Morgante F, Nicoletti A, Tamburini T, Tinazzi M, Zappia M, Vorovenci RJ, Antonini A. Impulse control disorders in advanced Parkinson’s disease with dyskinesia: The ALTHEA study. Mov Disord. 2017;0:1–9. doi: 10.1002/mds.27181. [DOI] [PubMed] [Google Scholar]
- 10.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease : a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefanova E, Ziropadja L, Petrović M, Stojković T, Kostić V. Screening for anxiety symptoms in Parkinson disease: a cross-sectional study. J Geriatr Psychiatry Neurol. 2013;26:34–40. doi: 10.1177/0891988713476368. [DOI] [PubMed] [Google Scholar]
- 13.Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, Weintraub D, Poewe W, Rascol O, Sampaio C, Stebbins GT, Goetz C. Depression Rating Scales in Parkinson’s Disease: Critique and Recommendations. Mov Disord. 2007;22:1077–1092. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 15.Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov Disord. 2013;28:668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 16.Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA. Levodopa-Induced dyskinesias in Parkinson’s disease: Clinical and pharmacological classification. Mov Disord. 1992;7:117–124. doi: 10.1002/mds.870070204. [DOI] [PubMed] [Google Scholar]
- 17.Kotagal V. Is PIGD a legitimate motor subtype in Parkinson disease? Ann Clin Transl Neurol. 2016;3:473–477. doi: 10.1002/acn3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pezzella FR, Colosimo C, Vanacore N, Di Rezze S, Chianese M, Fabbrini G, Meco G. Prevalence and clinical features of hedonistic homeostatic dysregulation in Parkinson’s disease. Mov Disord. 2005;20:77–81. doi: 10.1002/mds.20288. [DOI] [PubMed] [Google Scholar]
- 19.Pontone G, Williams J, Yeager S, Bassett S, Marsh L. Clinical features associated with impulse control disorders in Parkinson’s disease. Mov Disord. 2006;21:S102–S102. doi: 10.1212/01.wnl.0000238401.76928.45. [DOI] [PubMed] [Google Scholar]
- 20.Weintraub D, Siderowf AD, Whetteckey J. Impulse Control Disorders in Parkinson Disease. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 21.Baumann-Vogel H, Valko PO, Eisele G, Baumann CR. Impulse control disorders in Parkinson’s disease: Don’t set your mind at rest by self-assessments. Eur J Neurol. 2015;22:603–609. doi: 10.1111/ene.12646. [DOI] [PubMed] [Google Scholar]
- 22.Jaakkola E, Joutsa J, Mäkinen E, Johansson J, Kaasinen V. Ventral striatal dopaminergic defect is associated with hallucinations in Parkinson’s disease. Eur J Neurol. 2017;0:1–7. doi: 10.1111/ene.13390. [DOI] [PubMed] [Google Scholar]
- 23.Vriend C, Nordbeck AH, Booij J, van der Werf YD, Pattij T, Voorn P, Raijmakers P, Foncke EMJ, van de Giessen E, Berendse HW, van den Heuvel OA. Reduced dopamine transporter binding predates impulse control disorders in Parkinson’s disease. Mov Disord. 2014;29:904–911. doi: 10.1002/mds.25886. [DOI] [PubMed] [Google Scholar]
- 24.Huot P, Fox SH. The serotonergic system in motor and non-motor manifestations of Parkinson’s disease. Exp Brain Res. 2013;230:463–476. doi: 10.1007/s00221-013-3621-2. [DOI] [PubMed] [Google Scholar]
- 25.Verbaan D, van Rooden SM, Visser M, Marinus J, Emre M, van Hilten JJ. Psychotic and compulsive symptoms in Parkinson’s disease. Mov Disord. 2009;24:738–744. doi: 10.1002/mds.22453. [DOI] [PubMed] [Google Scholar]
- 26.Ravina B, Marder K, Fernandez HH, Friedman JH, McDonald W, Murphy D, Aarsland D, Babcock D, Cummings J, Endicott J, Factor S, Galpern W, Lees A, Marsh L, Stacy M, Gwinn-Hardy K, Voon V, Goetz C. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22:1061–1068. doi: 10.1002/mds.21382. [DOI] [PubMed] [Google Scholar]
- 27.Merims D, Giladi N. Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson’s disease. Park Relat Disord. 2008;14:273–280. doi: 10.1016/j.parkreldis.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Kipfer S, Stephan M, Schupbach M, Ballinari P, Kaelin-Lang A. Resting Tremor in Parkinson Disease: A Negative Predictor of Levodopa-Induced Dyskinesia. Arch Neurol. 2011;68:1037–1039. doi: 10.1001/archneurol.2011.147. [DOI] [PubMed] [Google Scholar]
- 29.Benamer HTS, Patterson J, Wyper DJ, Hadley DM, Macphee GJA, Grosset DG. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15:692–698. doi: 10.1002/1531-8257(200007)15:4<692::aid-mds1014>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Skorvanek M, Rosenberger J, Minar M, Grofik M, Han V, Groothoff JW, Valkovic P, Gdovinova Z, Van Dijk JP. Relationship between the non-motor items of the MDS-UPDRS and Quality of Life in patients with Parkinson’s disease. J Neurol Sci. 2015;353:87–91. doi: 10.1016/j.jns.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher DA, Goetz CG, Stebbins G, Lees AJ, Schrag A. Validation of the MDS-UPDRS Part I for nonmotor symptoms in Parkinson’s disease. Mov Disord. 2012;27:79–83. doi: 10.1002/mds.23939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


