Abstract
It has been suggested that microRNAs (miRs) are involved in the immune regulation of periodontitis. However, it is unclear whether and how miRs regulate the function of B cells in the context of periodontitis. This study is to explore the role of miR-146a on the inflammatory cytokine production of B cells challenged by Porphyromonas gingivalis (P. gingivalis) lipopolysaccharide (LPS). Primary B cells were harvested from mouse spleen. Quantitative real-time polymerase chain reaction (qPCR), enzyme-linked immunosorbent assay (ELISA) were used to detect the expression of inflammatory cytokines in B cells in the presence or absence of P. gingivalis LPS and/or miR-146a. Bioinformatics, luciferase reporter assay and overexpression assay were used to explore the binding target of miR-146a. Our results showed that miR-146a level in B cells was elevated by P. gingivalis LPS stimulation, and the mRNA expressions of interleukin (IL)-1β, 6 and 10, and IL-1 receptor associated kinase-1 (IRAK1), but not TNF receptor associated factor 6 (TRAF6), were also upregulated. The expression levels of IL-1β, 6, 10 and IRAK1 were reduced in the presence of miR-146a mimic, but were elevated by the addition of miR-146a inhibitor. MiR-146a could bind with IRAK1 3′ untranslated region (UTR) but not TRAF6 3′-UTR. Overexpression of IRAK1 reversed the inhibitory effects of miR-146a on IL-1β, 6 and 10. In summary, miR-146a inhibits inflammatory cytokine production in B cells through directly targeting IRAK1, suggesting a regulatory role of miR-146a in B cell-mediated periodontal inflammation.
Keywords: B cell, miR-146a, LPS, IRAK1
1. Introduction
Periodontitis is featured with gingival inflammation, alveolar bone resorption, tooth loosening and migration, eventually leading to tooth loss. A complex immune/inflammatory cascade plays an important role in the process of periodontitis, in which immune cells and cytokines are involved [1]. It is now clear that inflammatory responses to chronic periodontal infections involve activated immune B cells, which helps protect against microbial insults but also contribute to bone pathogenesis in periodontitis [2–4]. A pathogenic role of B cells in periodontitis has been confirmed in B cell-deficient mice whose alveolar bone loss was protected after bacterial infection [2]. Our previous study showed that Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans)-binding B cells triggered the resorption of alveolar bone [3].
MicroRNAs (miRs) are small molecular RNAs (about 22 nucleotides), which could downregulate gene expression via binding with 3′-untranslated region (UTR) of target genes. Studies have shown that miRs participate in the physiological and pathological process, playing a vital role in cancer, inflammation, immune responses, and metabolic disorders [5–7]. It has been demonstrated that miRs function as an important regulator in periodontitis [8, 9]. Our previous studies demonstrated that miR-146a regulated the cytokine secretion in human gingival fibroblasts and periodontal ligament cells [10, 11]. Recent studies from others delineated that miR-128 controlled the inflammatory response in macrophages after stimulated with porphyromonas gingivalis (P. gingivalis) [12], and miR-24 acted as a negative regulator in the polarization and plasticity of macrophages activated by P. gingivalis LPS or A. actinomycetemcomitans LPS [13].
It has been suggested that miR-146a could regulate the function of B cells in disease. Contreras et al reported that miR-146a could modulate B-cell oncogenesis through early growth response-1 [14]. Loss of miR-146a led to the accumulation of T follicular helper cells and the germinal center B cells, which enhanced the maturation of germinal centers response [15]. In myasthenia gravis, knockdown of miR-146a reduced numbers of memory B cells, B-1 cells and plasma cells, and decreased the activation of B cells [16]. However, the effect of miR-146a on B cells in periodontitis is unclear.
P. gingivalis, one of the key periodontal pathogens, could induce periodontal inflammation and bone resorption through activation and infiltration of T and B cells [17, 18]. P. gingivalis LPS has been confirmed to contribute to periodontal tissue destruction through the induction of inflammatory mediators by periodontal cells [10, 11]. The purpose of this study is to determine the effect of miR-146a on the cytokine production by P. gingivalis LPS-challenged B cells, and to elucidate the mechanism of miR-146a-mediated regulation of B cell function.
2. Material and methods
2.1. Mouse B cell extraction and culture
C57BL/6 mice (8–10 weeks), purchased from the Jackson Laboratory (USA), were sacrificed and dissected according to the guidelines of the Institutional Animal Care and Use at the Forsyth Institute. The spleens were harvested and gently grinded in complete medium (Iscove’s Modified Dulbecco’s Medium with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 0.25 μg/ml Amphotericin B) (Gibco by life technologies, Carlsbad, CA, USA). The cell suspension were collected and centrifuged at 1500 rpm for 5 min. The pellet was lyzed using 1 ml ACK lysis buffer (Gibco by life technologies, Carlsbad, CA, USA) to remove red blood cells. Single cell suspension was centrifuged and resuspended in phosphate buffer saline (PBS). Afterward, B cells were isolated by using Pan B cell isolation kit, filtered with LD column (Miltenyi Biotec, Somerville, MA, USA) and centrifuged at 1500 rpm for 5 min. Isolated B cells were resuspended in final culture medium (complete medium, 1×2-mercaptoethanol (Gibco by life technologies, Carlsbad, CA, USA)) and were seeded in 96-well plate at 1×106/well.
B cells were treated with miR-146a mimic, miR-146a inhibitor or scramble controls in the presence or absence of 1 μg/ml P. gingivalis LPS (Strain ATCC33277, InvivoGen, San Diego, CA, USA). After 24 or 48h, supernatant and B cells were harvested for the subsequent measurements.
2.2. Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs were extracted with PureLink® RNA Mini Kit (Invitrogen by Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instruction. Reverse transcription and qPCR were performed using SuperScript II reverse transcriptase (Invitrogen by Life Technologies, Carlsbad, CA, USA) and LightCycler 480 SYBR Green I Master (Roche Diagnostics, Mannheim, Germany). The primers used for these genes were as following. IL-1β: 5′-ATGCCTTCCCCAGGGCATGT-3′ (forward), 5′-CTGAGCGACCTGTCTTGGCCG-3′ (reverse); IL-6: 5′-TCCAGTTGCCTTCTTGGGAC-3′ (forward), 5′-GTACTCCAGAAGACCAGAGG-3′ (reverse); IL-10: 5′-GACCAGCTGGACAACATACTGCTAA-3′ (forward), 5′-GATAAGGCTTGGCAACCCAAGTAA-3′ (reverse), IRAK1: 5′-GCCCTTTGGCTCTATTTGGG-3′ (forward), 5′-TCTGAGGCTCATCCAGCAAAG-3′ (reverse); TRAF6: 5′-ATATGACAGCCACCTCCCCT-3′ (forward),5′-TTGGCGTCCATGACCTCTTC-3′ (reverse); glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-CCCCAGCAAGGACACTGAGCAA-3′ (forward), 5′-GTGGGTGCAGCGAACTTTATTGATG-3′ (reverse); Mmu-miR-146a: 5′-GGGTGAGAACTGAATTCCA-3′(forward), 5′-CAGTGCGTGTCGTGGAGT-3′ (universal reverse); U6 small nuclear RNA: 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward), 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse). GAPDH and U6 were taken as internal reference to mRNA and miR, respectively. The expression of genes was presented using the 2−ΔΔCT method.
2.3. Enzyme-linked immunosorbent assay (ELISA)
To analyze the secretion of IL-1β, IL-6 and IL-10, mouse ELISA MAX™ Standard sets were used (Biolegend, San Diego, CA, USA) and the assays were performed according to the manufacturer’s instruction. For intracellular IRAK1 and TRAF6, mouse IRAK1 and TRAF6 ELISA kit (LSBio, Seattle, WA, USA) were used. In brief, collected supernatant (for the detection of IL-1β, IL-6 and IL-10) or cell lysate (for the detection of IRAK1 and TRAF6) was added into the antibody pre-coated 96-well plates, incubated, and washed with PBS including 0.1% Tween. After reaction with primary antibody and secondary antibody, substrate solution was added and incubated for 15–30 min at 37°C. The stop solution was applied to each well and the optical density (OD value) of each well was immediately determined at 450 nm using a microplate reader (Bio-tek, Winooski, VT, USA).
2.4. Transfection
After seeding of 1×106 cells per well in 96-well plate, cells were cultured for 2 h at 37°C and 5% CO2. miR-146a mimic, inhibitor and negative controls (final concentration 10 nM) were mixed with HiPerFect transfection reagent (Qiagen, Germantown, MD, USA). For co-transfection of IRAK1 plasmids (100ng) and miR-146a mimic or scramble RNA (10nM), IRAK1 plasmids or vector only (100ng), Lipofectamine® 2000 reagent (Invitrogen, Carlsbad, CA, USA) were used. The mixture was incubated for 10 min at room temperature to allow the formation of transfection complexes. The cells were transfected with these complexes for 24 h. The cells were then treated with 1 μg/ml P. gingivalis LPS and the expressions of cytokines were detected 24 h after P. gingivalis LPS stimulation.
2.5. Luciferase reporter assay
We used miR database (miRNA.org) to explore the potential target genes of miR-146a in the NF-κB pathway, and found the two candidate targets as IRAK1 and TRAF6. BCL1 clone 5B1b (ATCC®TIB-197™, ATCC, Manassas, VA, USA) were cultured at 37°C with 5% CO2. The B cells were transfected with miR-146a mimic (final concentration 40 nM), scramble RNA (final concentration 40 nM), IRAK1 or TRAF6 luciferase plasmid (200 ng), control plasmid (200 ng) (GeneCopoeia, Rockville, MD, USA) with Lipofectamine® 2000 reagent (Invitrogen) according to the manufacturer’s protocol. After incubation for 24 h at 37°C, B cells were harvested and resuspended with PBS, then centrifuged at 3000 rpm for 5 min. The pellets were used for luciferase assay with Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s protocol. The data were normalized to the activity of Renilla luciferase in the same cells.
2.6 Mouse model of experimental periodontitis with oral P. gingivalis infection
C57BL/6J mice (8–10 weeks old) were randomly divided into 4 groups (n=6): 1) control uninfected, 2) P. gingivalis infection only, 3) P. gingivalis infection with gingival PBS injection, and 4) P. gingivalis infection with gingival miR-146a injection. For groups 2–4, mice were orally infected with live P. gingivalis bacteria (ATCC 33277) pre-mixed with an equal volume of sterile 2% (wt/vol) low-viscosity carboxymethylcellulose (CMC). The infection was administered by oral gavage using 1 × 108 live P. gingivalis bacteria per animal per day for four consecutive days (days 0 to 3). For groups 3–4, each mouse received palatal gingival injections of 1 μl/injection of PBS or 100nM miR-146a on the gingival papillae using a 28.5-gauge double-beveled MicroFine needle (Becton, Dickinson). The injections for animals were administered three times on days 5, 9, and 14. Experiments were terminated at day 28.
2.7 Bone morphometric analysis
The maxillae were removed and defleshed by a dermestid beetles colony. After bleaching with 3% hydrogen peroxide, the bone was stained with 1% toluidine blue. Bone resorption measurements were assessed under a microscope (Nikon SMZ745T, Nikon Instruments Inc, Japan). The polygonal area was measured using Image J (NIH) on buccal and palatal surfaces for each segment. The bone resorption area was enclosed coronally by the cemento-enamel junction (CEJ) of the molars, laterally by the exposed distal root of the first molar and the exposed mesial root of the third molar, and apically by the alveolar bone crest.
2.8. Statistical analysis
All experiments were performed at least twice. The data were presented as means ± standard deviation. Multi-grouped data were analyzed by one-way ANOVA, with post hoc test. Two-grouped data were analyzed by student t-test. Statistical significance was set at p < 0.05.
3. Results
3.1. P. gingivalis LPS stimulated inflammatory cytokine production in B cells
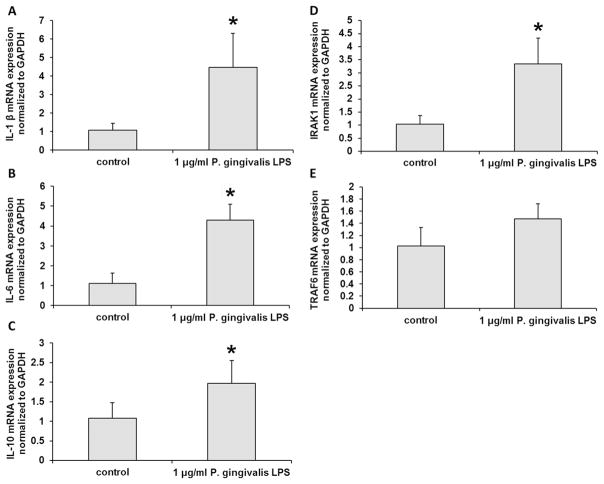
After P. gingivalis LPS treatment, mRNA levels of inflammatory cytokines including IL-1β, IL-6 and IL-10 were increased in B cells compared with non-LPS treated cells (p < 0.05) (Fig. 1A–C). To further explore the signaling pathway of inflammatory responses, we checked the expression of IRAK1 and TRAF6. As shown in Fig. 1D, IRAK1 mRNA level was increased after P. gingivalis LPS treatment (p < 0.05). However, TRAF6 mRNA level did not change significantly after P. gingivalis LPS treatment (Fig. 1E, p > 0.05). These data suggested that P. gingivalis LPS could induce inflammatory cytokine response in B cells.
Fig. 1. The inflammatory responses in B cells were activated byP. gingivalis LPS.
After P. gingivalis LPS treatment, mRNA levels of inflammatory cytokines including IL-1β (A), IL-6 (B) and IL-10 (C) were significantly increased in B cells. IRAK1 mRNA level was also increased (D). However, TRAF6 mRNA level was not changed (E). The experiments were performed in triplicate. n=6–8. Data are presented as mean±SD. Student’s t-test. * p < 0.05 indicates significant difference compared with control group. Control: non-P. gingivalis LPS treatment.
3.2. MiR-146a expression was induced in B cells by P. gingivalis LPS
To detect miR-146a expression in B cells, qRT-PCR was used after treatment with 1μg/ml P. gingivalis LPS. As shown in Fig. 2, miR-146a expression was increased 24 h and 48 h (p < 0.05) after P. gingivalis LPS stimulation compared with non-P. gingivalis LPS treatment. Up-regulation of miR-146a in P. gingivalis LPS-stimulated B cells indicates that miR-146a may be involved in the inflammatory response in B cells.
Fig. 2. The expression of miR-146a in B cells was induced by P. gingivalis LPS.
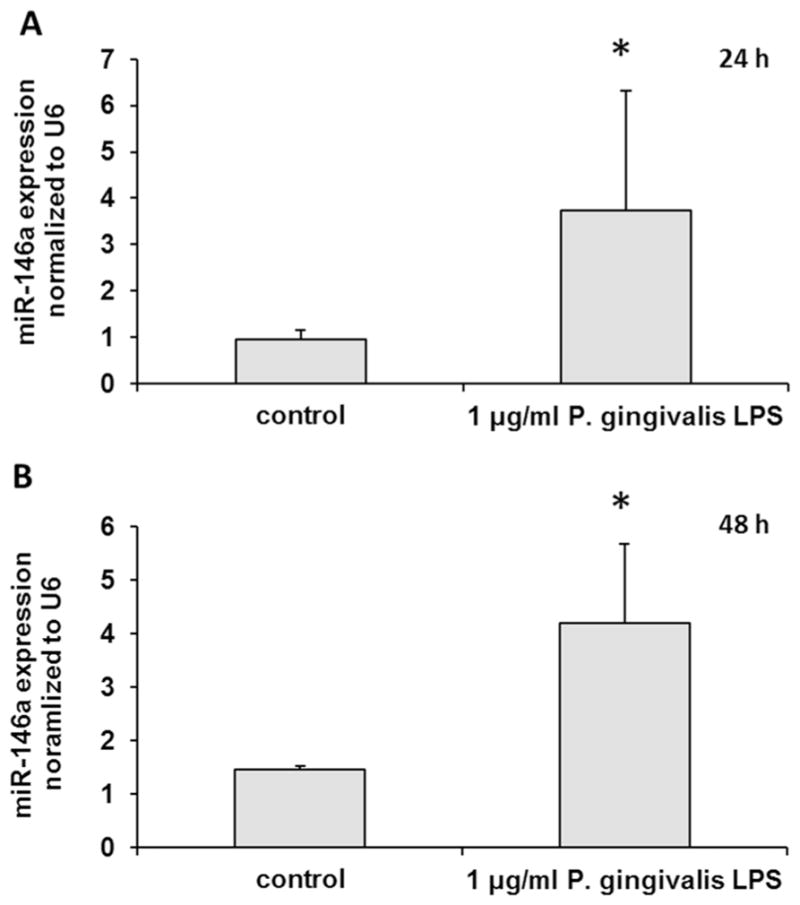
The expression of miR-146a in B cells was detected by qRT-PCR after P. gingivalis LPS-stimulation. The miR-146a expression was increased at both 24 h and 48 h. The experiments were performed in triplicate. n=6–8. Data are presented as mean±SD. Student’s t-test. * p < 0.05 indicates significant difference compared with control group. Control: non-P. gingivalis LPS treatment.
3.3. Addition of miR-146a inhibited inflammatory cytokine production in P. gingivalis LPS-challenged B cells
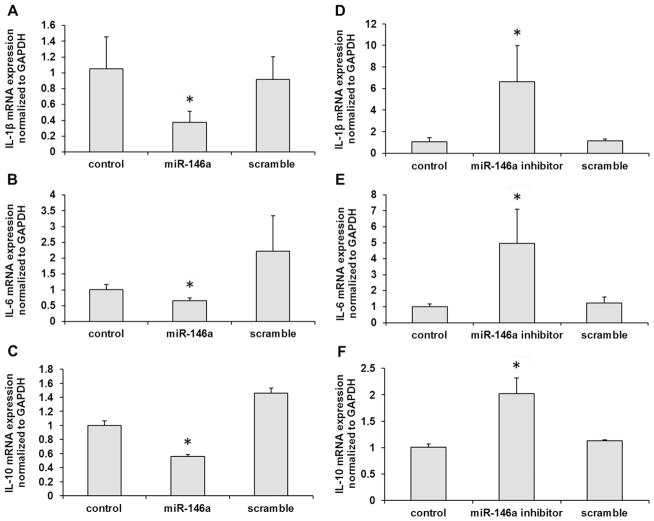
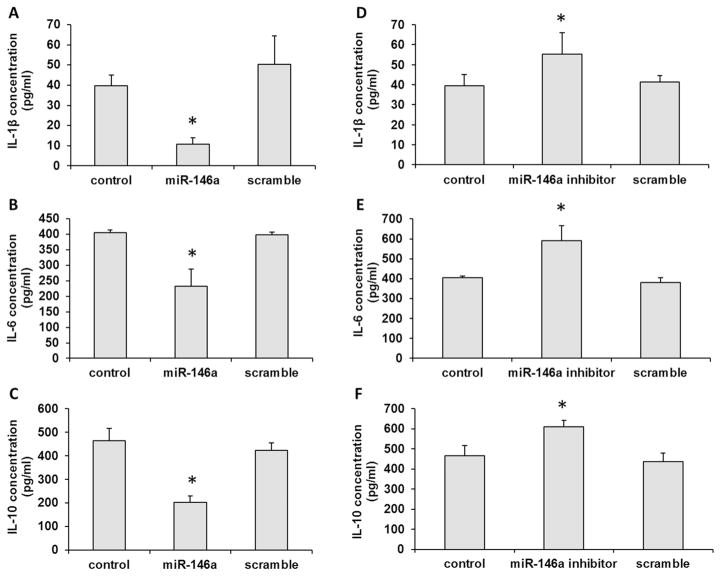
After transfection with miR-146a mimic or its inhibitor in B cells, the productions of IL-1β, IL-6 and IL-10 were measured following P. gingivalis LPS challenge (1μg/ml). As shown in Fig. 3A–C, miR-146a mimic inhibited IL-1β, IL-6 and IL-10 mRNA expression levels in B cells (p < 0.05). Meanwhile, miR-146a inhibitor could increase the mRNA expressions of IL-1β, IL-6 and IL-10 in B cells (p < 0.05, Fig. 3D–F). Furthermore, we detected the secreted protein levels of IL-1β, IL-6 and IL-10 in the presence of miR-146a mimic or its inhibitor. The secreted protein productions of IL-1β, IL-6 and IL-10 were inhibited by miR-146a mimic (p < 0.05, Fig. 4A–C) and enhanced by miR-146a inhibitor in B cells (p < 0.05, Fig. 4D–F) after challenged by P. gingivalis LPS. These data demonstrated that miR-146a could regulate the inflammatory cytokine productions in B cells.
Fig. 3. The mRNA expressions of inflammatory cytokines were inhibited by miR-146a in B cells after challenged with P. gingivalis LPS.
After overexpression of miR-146a in B cells, mRNA levels of IL-1β (A), IL-6 (B) and IL-10 (C) were significantly decreased. However, after blockade of miR-146a by its inhibitor, the mRNA levels of IL-1β (D), IL-6 (E) and IL-10 (F) were significantly increased. The experiments were performed in triplicate. n=6–8. Data are presented as mean±SD. One-way ANOVA. * p < 0.05. Control: no RNA transfection.
Fig. 4. miR-146a inhibited inflammatory cytokine secretion from in B cells after challenged with P. gingivalis LPS.
The secretions of IL-1β (A), IL-6 (B) and IL-10 (C) decreased in presence of miR-146a mimic. The productions of IL-1β (D), IL-6 (E) and IL-10 (F) were enhanced by miR-146a inhibitor. The experiments were performed in triplicate. n=6–8. Data are presented as mean±SD. One-way ANOVA. * p < 0.05. Control: no RNA transfection.
3.4. MiR-146a directly targeted IRAK1 through binding with its 3′-UTR
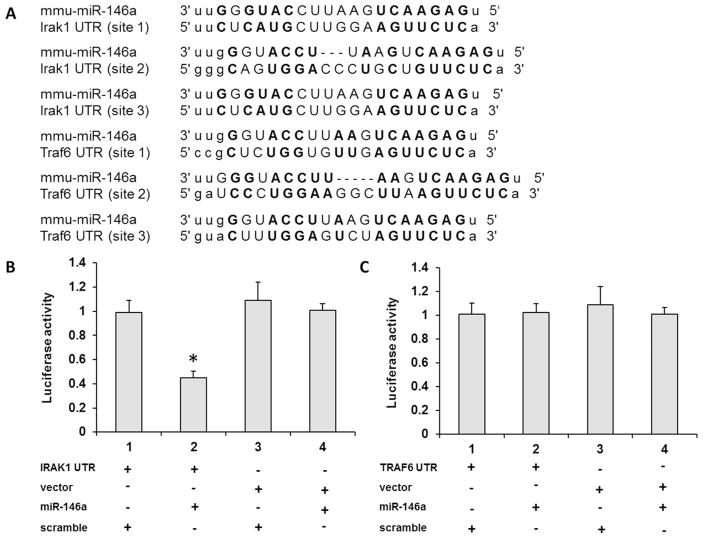
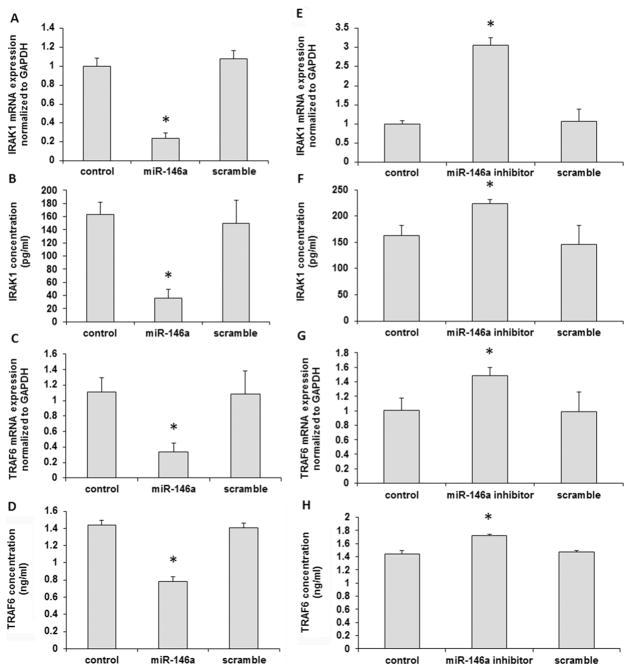
To explore the mechanism of miR-146a inhibitory effect on cytokine secretion, we analyzed the potential target genes of miR-146a from miR database. According to our previous study [10, 11], P. gingivalis LPS activated the Toll-like receptor-nuclear factor (NF)-κB signaling pathway. Based on the information, we found two key factors, IRAK1 and TRAF6, which were candidate targets of miR-146a involved in this pathway. Fig. 5A showed that 3′-UTR of IRAK1 and TRAF6 possessed the putative binding sequence for miR-146a. As shown in Fig. 5B and C, the addition of miR-146a could decrease the luciferase activity of IRAK1 luciferase plasmid (p < 0.05), but not that of TRAF6 luciferase plasmid (p > 0.05), which indicates that miR-146a could directly target IRAK1 3′-UTR.
Fig. 5. miR-146a directly targeted IRAK1 through binding with its 3′-UTR.
From miRNA target database, miR-146a has the binding sequence in 3′-UTR of IRAK1 and TRAF6 (A). In luciferase assay, miR-146a could decrease the luciferase activity of IRAK1 luciferase plasmid (B), however, not TRAF6 luciferase plasmid (C). The experiments were performed at least three times in triplicate. Data are presented as mean±SD. One-way ANOVA. * p < 0.05.
3.5. Addition of miR-146a inhibited IRAK1 expression in P. gingivalis LPS-challenged B cells
As shown in Fig. 6, addition of miR-146a mimic significantly reduced the mRNA and protein levels of IRAK1 expression (p < 0.05) in B cells challenged with P. gingivalis LPS, as detected by qRT-PCR (Fig. 6A) and ELISA (Fig. 6B) respectively. The addition of miR-146a mimic also reduced the mRNA (Fig. 6C) and protein levels (Fig. 6D) of TRAF6 expression (p < 0.05) in B cells pretreated with P. gingivalis LPS. Meanwhile, miR-146a inhibitor significantly increased the mRNA and protein levels of IRAK1 expression in P. gingivalis LPS-challenged B cells (p < 0.05) (Fig. 6E–F). The expression of TRAF6 was also elevated at both mRNA and protein levels in P. gingivalis LPS-challenged B cells in the presence of miR-146a inhibitor (Fig. 6G–H).
Fig. 6. IRAK1 expression was inhibited by miR-146a in B cells after challenged with P. gingivalis LPS.
miR-146a mimic could reduce the mRNA and protein levels of IRAK1 as detected by PCR (A) and ELISA (B). Also, addition of miR-146a inhibited TRAF6 expression in B cells at both mRNA (C) and protein level (D). Inhibition of miR-146a by its inhibitor significantly elevated both mRNA and protein levels of IRAK1 expression in B cells (E and F), as well as mRNA (G) and protein (H) levels of TRAF6 expression in B cells. The experiments were performed in triplicate. n=6–8. Data are presented as mean±SD. One-way ANOVA. * p < 0.05. Control: no RNA transfection.
3.6. Overexpression of IRAK1 reversed the inhibitory effect of miR-146a on cytokine production
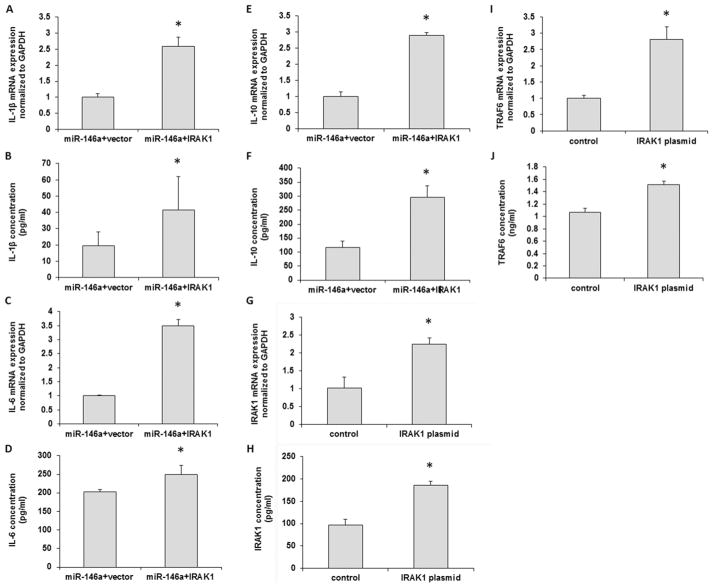
To further determine the effects of miR-146a on IRAK1 pathway, we transfected IRAK1 expression plasmid in B cells. After overexpression of IRAK1, the mRNA levels and the protein secretions of IL-1β, IL-6 and IL-10 were significantly increased in B cells even though in the presence of miR-146a (p < 0.05) (Fig. 7A–F). An increased IRAK1 expression at mRNA and protein level was observed by qPCR and ELISA respectively (Fig. 7G–H), confirming the stable transfection of IRAK1 plasmid, Also, the expression levels of TRAF6 were significantly increased after IRAK1 overexpression (p < 0.05) (Fig. 7I–J).
Fig. 7. Overexpression of IRAK1 reversed the inhibitory effect of miR-146a on cytokine production.
After overexpression of IRAK1, the mRNA and protein levels of IL-1β (A, B), IL-6 (C, D) and IL-10 (E, F) were significantly increased in B cells pretreated with miR-146a mimic. As expected, after transfection with IRAK1 plasmid, IRAK1 mRNA and protein levels were significantly increased in B cells (G, H). In addition, mRNA and protein levels of TRAF6 expression were also elevated in B cells (I, J). The experiments were performed in triplicate. n=6–8. Data are presented as mean±SD. Student’s t-test. p < 0.05 indicates significant difference compared with the cells transfected with miR-146a and/or blank vector. Control: cells transfected with blank vector.
3.7 Gingival injection of miR-146a significantly reduced inflammatory bone loss in P. gingivalis infection-associated experimental periodontitis
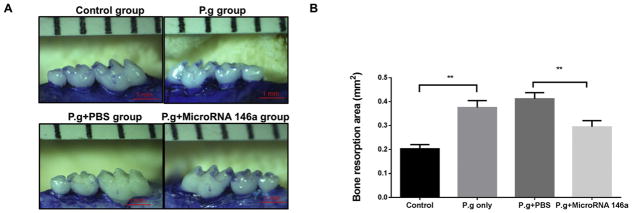
In order to investigate the effect of miR-146a in vivo, the mouse model of P. gingivalis infection-induced experimental periodontitis was used and miR-146a was injected into gingival tissues at days 5, 9 and 14 in the total 28 days period. The areas of bone loss around maxillary second molars were measured in each group (Fig. 8A). The resorption area was significantly increased after P. gingivalis infection as compared to the control (Fig. 8B). The resorption area on the miR-146a treatment group was significantly decreased when compared with the PBS treatment group, indicating that miR-146a significantly decreased periodontal bone loss in vivo (Fig. 8B).
Fig. 8. Gingival injection of miR-146a reduced bone loss in P. gingivalis infection-induced experimental periodontitis.
Mice were orally infected with live P. gingivalis bacteria for 4 consecutive days on day 0 to day 3. Gingival injection of miR-146a or PBS (as control) was performed on days 5, 9 and 14. Maxilla were collected on day 28. (A) The alveolar bone resorption areas around maxillary second molars were measured and (B) analyzed as bone resorption area/mm2 at the magnification of 30X (means ± SD, n=6, **p<0.01).
4. Discussion
B cell inflammatory infiltration is the hallmark of periodontitis [19], which participates in periodontal bone loss in periodontitis [4]. In this study, we determined whether miR-146a plays a role in B cell inflammatory response. We also explored the regulatory mechanism of miR-146a in B cells stimulated with P. gingivalis LPS. MiR-146a has been identified as a protective regulator in inflammation through regulating inflammatory cytokine secretion [10, 11]. In this study, P. gingivalis LPS could enhance miR-146a expression in B cells, which indicates that B cells might deploy miR-146a as a negative feedback mechanism for protective regulation of cytokine productions when they are activated.
IL-1β participates in the inflammatory responses in periodontitis. Increased IL-1β level in saliva and gingival crevicular fluid is associated with periodontitis progression [20, 21]. However, IL-1β has dual roles in which IL-1β at low doses that are close to physiologically healthy levels promotes the osteogenesis, but at higher doses inhibits osteogenesis of periodontal ligament stem cells which are the main cells to repair alveolar bone [22]. Also, IL-1β could enhance macrophage M1 phenotypes, which is responsible for bone resorption [23]. In our study, P. gingivalis LPS could enhance IL-1β expression from B cells (Fig. 1A). The upregulated IL-1β would impair the osteogenesis of periodontal ligament stem cells and accelerate bone resorption, which is not beneficial for bone repair and regeneration. Increased miR-146a could decrease the production of IL-1β (Fig. 4A), which suggested that miR-146a may play a protective role in B cell-mediated osteogenesis impairment and bone resorption through regulation of IL-1β.
As one of main pro-inflammatory cytokines, IL-6 plays an important role in periodontitis, which enhances periodontal inflammation at higher dose. IL-6 level in gingival crevicular fluid showed significant positive correlation with the clinical parameters in periodontitis [21, 24]. Meanwhile, Mahanonda et al mentioned that IL-6 was involved in local B cell responses and was detected in inflamed gingival tissues [19]. P. gingivalis LPS could enhance IL-6 secretion in human gingival fibroblasts and periodontal ligament cells [10, 11]. Also, we confirmed that P. gingivalis LPS could significantly up-regulate IL-6 in B cells (Fig. 1B), and miR-146a could reduce the production of IL-6 in B cells (Fig. 4B), which is consistent with other researches [10, 11]. These results suggested that miR-146a may protect periodontal tissue from inflammation partially through reducing IL-6 secretion by B cells.
Studies have demonstrated that IL-10 is a potent anti-inflammatory cytokine [25, 26]. IL-10 can maintain bone mass through inhibiting osteoclastic bone resorption and enhancing osteoblastic bone formation. From Zhang et al’s research, IL-10 levels were lower in the gingival crevicular fluid in patients with chronic periodontitis and aggressive periodontitis [24]. However, other reports demonstrated that anti-IL-10 antibody could cause Th1-like immune response and protect mice injected with P. gingivalis LPS from abscess induced by P. gingivalis [27]. Also, IL-10 was involved in local B cell response and detected in inflamed gingival tissues [19]. IL-10 could be secreted by T cells, B cells and other periodontal cells [10, 26, 28]. Studies demonstrated that miR-146a inhibition did not change IL-10 production in human gingival fibroblasts [10]. In this study, IL-10 production from B cells was inhibited by miR-146a mimic (Fig. 4C) and enhanced by miR-146a inhibitor (Fig. 4F), which indicates that miR-146a has multiple regulatory functions not only on the production of pro-inflammatory but also anti-inflammatory cytokines by B cells. Nonetheless, the overall anti-inflammatory effect of miR-146a was further suggested by the in vivo results showing the reduction of P. gingivalis-induced inflammatory bone loss after gingival injection of miR-146a (Fig. 8).
According to the previous studies, miR-146a was regulated by NF-κB in human gingival fibroblasts [29], meanwhile, inhibition of miR-146a could decrease toll-like receptor 4 and NF-κB on acetylcholine receptor-specific B cells in peripheral blood mononuclear cells from myasthenia gravis patients [16], which indicates that miR-146a is involved in NF-κB pathway. From bioinformatics analysis miR-146a might target 3′-UTR of IRAK1 and TRAF6. In human periodontal ligament cells and gingival cells, miR-146a targets IRAK1, not TRAF6 [10, 11]. In this present study, overexpression of miR-146a could downregulate IRAK1 and TRAF6 at both mRNA and protein levels (Fig. 6), which is inconsistent with Zhang et al’s results [16], showing that knockdown of miR-146a had no effect on IRAK1 and TRAF6 in B cells [16], although there was no LPS-induced activation of B cells in that study. This may suggest that the involvement of miR-146a in B cells is dependent on how cells are activated. We have further demonstrated that miR-146a could directly bind with 3′-UTR of IRAK1, not TRAF6 (Fig. 5). It is suggested that IRAK1 was the key signaling molecule of miR-146a regulatory pathway in B cells during inflammatory responses because overexpressed IRAK1 could reverse the inhibitory effect of miR-146a (Fig. 7). Also, we found that overexpressed IRAK1 could increase TRAF6 level, which explained, to some extent, that IRAK1 inhibition by miR-146a might be accompanied with TRAF6 decrease. However, the mechanism of TRAF6 pathway regulation need to be further investigated.
In conclusion, miR-146a could inhibit inflammatory cytokine secretion in P. gingivalis LPS-challenged B cell through directly targeting IRAK1, which may provide a novel immunotherapeutic method to treat periodontitis.
Highlights.
miR-146a inhibits inflammatory cytokine production in B cells.
miR-146a directly targets IRAK1 through binding its 3′ untranslated region.
The inhibitory effects of miR-146a on cytokines was neutralized by IRAK1 overexpression.
Acknowledgments
All affiliations, corporate or institutional, and all sources of financial support to this research are properly acknowledged. We certify that we do not have any commercial or associate interest that represents a conflict of interest in connection with the manuscript.
Formatting of funding sources
This project was supported by NIH NIDCR (R01DE025255), Forsyth Pilot Grant (FPILOT36) and Chinese Scholarship Council.
Abbreviations
- miRs
microRNAs
- P. gingivalis
Porphyromonas gingivalis
- LPS
lipopolysaccharide
- PBS
phosphate buffer saline
- IL
interleukin
- IRAK1
IL-1 receptor associated kinase-1
- TRAF6
TNF receptor associated factor 6
- UTR
untranslated region
- A. actinomycetemcomitans
Aggregatibacter actinomycetemcomitans
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- OD
optical density
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2014;64:57–80. doi: 10.1111/prd.12002. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver-Bell J, Butcher JP, Malcolm J, MacLeod MK, Adrados Planell A, Campbell L, Nibbs RJ, Garside P, McInnes IB, Culshaw S. Periodontitis in the absence of B cells and specific anti-bacterial antibody. Mol Oral Microbiol. 2014;30:160–169. doi: 10.1111/omi.12082. [DOI] [PubMed] [Google Scholar]
- 3.Harada Y, Han X, Yamashita K, Kawai T, Eastcott JW, Smith DJ, Taubman MA. Effect of adoptive transfer of antigen-specific B cells on periodontal bone resorption. J Periodontal Res. 2006;41:101–107. doi: 10.1111/j.1600-0765.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 4.Zouali M. The emerging roles of B cells as partners and targets in periodontitis. Autoimmunity. 2016;26:1–10. doi: 10.1080/08916934.2016.1261841. [DOI] [PubMed] [Google Scholar]
- 5.Rasko JE, Wong JJ. Nuclear microRNAs in normal hemopoiesis and cancer. J Hematol Oncol. 2017;10:8. doi: 10.1186/s13045-016-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomofuji T, Yoneda T, Machida T, Ekuni D, Azuma T, Kataoka K, Maruyama T, Morita M. MicroRNAs as serum biomarkers for periodontitis. J Clin Periodontol. 2016;43:418–425. doi: 10.1111/jcpe.12536. [DOI] [PubMed] [Google Scholar]
- 7.Meydan C, Shenhar-Tsarfaty S, Soreq H. MicroRNA regulators of anxiety and metabolic disorders. Trends Mol Med. 2016;22:798–812. doi: 10.1016/j.molmed.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Xie YF, Shu R, Jiang SY, Liu DL, Zhang XL. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int J Oral Sci. 2011;3:125–134. doi: 10.4248/IJOS11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoecklin-Wasmer C, Guarnieri P, Celenti R, Demmer M, Kebschull RT, Papapanou PN. MicroRNAs and their target genes in gingival tissues. J Dent Res. 2012;91:934–940. doi: 10.1177/0022034512456551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang SY, Xue D, Xie YF, Zhu DW, Dong YY, Wei CC, Deng JY. The negative feedback regulation of microRNA-146a in human periodontal ligament cells after Porphyromonas gingivalis lipopolysaccharide stimulation. Inflamm Res. 2015;64:441–451. doi: 10.1007/s00011-015-0824-y. [DOI] [PubMed] [Google Scholar]
- 11.Xie YF, Shu R, Jiang SY, Liu DL, Ni J, Zhang XL. MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J Inflamm (Lond) 2013;10:20. doi: 10.1186/1476-9255-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Na HS, Park MH, Song YR, Kim S, Kim HJ, Lee JY, Choi JI, Chung J. Elevated microRNA-128 in periodontitis mitigates tumor necrosis factor-α response via p38 signaling pathway in macrophages. J Periodontol. 2016;87:e173–182. doi: 10.1902/jop.2016.160033. [DOI] [PubMed] [Google Scholar]
- 13.Fordham JB, Naqvi AR, Nares S. miR-24 regulates macrophage polarization and plasticity. J Clin Cell Immunol. 2015 Oct;6(5) doi: 10.4172/2155-9899.1000362. pii: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contreras JR, Palanichamy JK, Tran TM, Fernando TR, Rodriguez-Malave NI, Goswami N, Arboleda VA, Casero D, Rao DS. MicroRNA-146a modulates B-cell oncogenesis by regulating Egr1. Oncotarget. 2015;6:11023–11037. doi: 10.18632/oncotarget.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratama A, Srivastava M, Williams NJ, Papa I, Lee SK, Dinh XT, Hutloff A, Jordan MA, Zhao JL, Casellas R, Athanasopoulos V, Vinuesa CG. MicroRNA-146a regulates ICOS-ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nat Commun. 2015;6:6436. doi: 10.1038/ncomms7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Jia G, Liu Q, Hu J, Yan M, Yang B, Yang H, Zhou W, Li J. Silencing miR-146a influences B cells and ameliorates experimental autoimmune myasthenia gravis. Immunology. 2015;144:56–67. doi: 10.1111/imm.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han X, Lin X, Yu X, Lin J, Kawai T, LaRosa KB, Taubman M. Porphyromonas gingivalis infection-associated periodontal bone resorption is dependent on receptor activator of NF-κB ligand. Infect Immun. 2013;81:1502–1509. doi: 10.1128/IAI.00043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Bi L, Yu X, Kawai T, Taubman MA, Shen B, Han X. Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infect Immun. 2014;82:4127–4134. doi: 10.1128/IAI.02084-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahanonda R, Champaiboon C, Subbalekha K, Sa-Ard-Iam N, Rattanathammatada W, Thawanaphong S, Rerkyen P, Yoshimura F, Nagano K, Lang NP, Pichyangkul S. Human memory B cells in healthy gingiva, gingivitis, and periodontitis. J Immunol. 2016;197:715–725. doi: 10.4049/jimmunol.1600540. [DOI] [PubMed] [Google Scholar]
- 20.Liukkonen J, Gürsoy UK, Pussinen PJ, Suominen AL, Könönen E. Salivary concentrations of interleukin (IL)-1β, IL-17A, and IL-23 vary in relation to periodontal status. J Periodontol. 2016;87:1484–1491. doi: 10.1902/jop.2016.160146. [DOI] [PubMed] [Google Scholar]
- 21.Stadler AF, Angst PD, Arce RM, Gomes SC, Oppermann RV, Susin C. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: a meta-analysis. J Clin Periodontol. 2016;43:727–745. doi: 10.1111/jcpe.12557. [DOI] [PubMed] [Google Scholar]
- 22.Mao CY, Wang YG, Zhang X, Zheng XY, Tang TT, Lu EY. Double-edged-sword effect of IL-1β on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-κB, MAPK and BMP/Smad signaling pathways. Cell Death Dis. 2016;7:e2296. doi: 10.1038/cddis.2016.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu T, Zhao L, Huang X, Ma C, Wang Y, Zhang J, Xuan D. Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in periodontal infection. J Periodontol. 2016;87:1092–1102. doi: 10.1902/jop.2016.160081. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Chen B, Zhu D, Yan F. Biomarker levels in gingival crevicular fluid of subjects with different periodontal conditions: A cross-sectional study. Arch Oral Biol. 2016;72:92–98. doi: 10.1016/j.archoralbio.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Chen B, Yan F, Guo J, Zhu X, Ma S, Yang W. Interleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseases. Biomed Res Int. 2014;2014:284836. doi: 10.1155/2014/284836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu P, Hu Y, Liu Z, Kawai T, Taubman MA, Li W, Han X. Local induction of B cell interleukin-10 competency alleviates inflammation and bone loss in ligature-induced experimental periodontitis in mice. Infect Immun. 2016;85 doi: 10.1128/IAI.00645-16. pii: e00645-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi R, Kono T, Bolerjack BA, Fukuyama Y, Gilbert RS, Fujihashi K, Ruby J, Kataoka K, Wada M, Yamamoto M, Fujihashi K. Induction of IL-10-producing CD4+ T-cells in chronic periodontitis. J Dent Res. 2011;90:653–658. doi: 10.1177/0022034510397838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herminajeng E, Sosroseno W, Bird PS, Seymour GJ. The effects of interleukin-10 depletion in vivo on the immune response to Porphyromonas gingivalis in a murine model. J Periodontol. 2001;72:1527–1534. doi: 10.1902/jop.2001.72.11.1527. [DOI] [PubMed] [Google Scholar]
- 29.Xie YF, Shu R, Jiang SY, Song ZC, Guo QM, Dong JC, Lin ZK. miRNA-146 negatively regulates the production of pro-inflammatory cytokines via NF-κB signalling in human gingival fibroblasts. J Inflamm (Lond) 2014;11:38. doi: 10.1186/s12950-014-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]