Abstract
INTRODUCTION
Existing studies predominantly consider the association of late-life lipid levels and subsequent cognitive change. However, midlife, rather than late-life risk factors, are often most relevant to cognitive health.
METHODS
We quantified the association between measured serum lipids in midlife and subsequent 20-year change in performance on three cognitive tests in 13,997 participants of the Atherosclerosis Risk in Communities (ARIC) study.
RESULTS
Elevated total cholesterol, low density lipoprotein cholesterol (LDL-c), and triglycerides were associated with greater 20-year decline on a test of executive function, sustained attention, and processing speed. Higher total cholesterol and triglycerides were also associated with greater 20-year decline in memory scores and a measure summarizing performance on all three tests. High density lipoprotein cholesterol (HDL-c) was not associated with cognitive change. Results were materially unchanged in sensitivity analyses addressing informative missingness.
DISCUSSION
Elevated total cholesterol, LDL-c and triglycerides in midlife were associated with greater 20-year cognitive decline.
Keywords: lipids, cognition, dementia, epidemiology, cohort, longitudinal, cognitive decline, cognitive change, cholesterol
1. INTRODUCTION
Despite sustained interest, the impact of lipid levels on late-life cognition remains unclear. Prior studies of elevated late-life lipids and subsequent risk of incident dementia report either null results or protective associations.[1, 2] Studies of elevated midlife total cholesterol and risk of dementia are mixed, with many[3–8] but not all[9, 10] reporting adverse associations. Accelerated cognitive decline is also itself a concern, even if it never progresses to dementia;[11] therefore, risk factors for cognitive change represent potential targets for intervention with the dual goal of improving quality of life and preventing dementia. Associations between risk factors and cognitive change are also less susceptible to reverse causation and confounding than associations between risk factors and cognitive status. While existing studies have examined the association between late-life lipids and near-term cognitive change,[12–16] the impact of midlife or decades-prior lipid levels on cognitive change remains unknown. In addition, questions remain about the influence of contextual factors, including race and apolipoprotein (APOE) ε4 allele status. Most prior studies of lipids and cognition have examined predominately white populations. While existing studies of midlife lipids and dementia collectively do not support a synergistic effect of APOE and lipids on dementia risk [1], there is evidence in other contexts to suggest that the combination of vascular risk factors and APOE may confer greater risk of cognitive deterioration than would be otherwise expected.[17, 18] Therefore, our goal was to consider the association between multiple lipid fractions in midlife and 20-year cognitive decline using data from the large and predominantly biracial Atherosclerosis Risk in Communities (ARIC) study, overall and within selected subgroups.
2. METHODS
2.1. Study Population
ARIC is a longitudinal cohort study of 15,792 persons recruited at ages 45 to 65 from four U.S. communities: Minneapolis suburbs, MN; Forsyth County, NC; Washington County, MD; and Jackson, MS.[19] All participants in Jackson, MS were black. Five study visits have been completed: Visit 1 (1987–1989), Visit 2 (1990–1992), Visit 3 (1993–1995), Visit 4 (1996–1998) and Visit 5/ARIC Neurocognitive Study (Visit 5/ARIC-NCS, 2011–2013). Visit 2, the time of the first cognitive testing, serves as study baseline.
For this analysis, we excluded ARIC participants who did complete cognitive testing at Visit 2 (n=1,752). We also excluded 43 individuals who were neither black nor white, were non-white from MD or MN, or who did not agree to use of their genetic data, to allow for adequate control of confounding by race-ethnicity and APOE e4 status. This study was approved by the institutional review boards of all participating institutions. All subjects provided written informed consent to participate at each study visit, based on local standards.
2.2. Lipid Measurements
We considered concentrations of total cholesterol, LDL-c, HDL-c, and triglycerides, regardless of fasting status, at Visit 2. Most participants (97.0%) had been fasting >8 hours at the time of blood draw. Details of blood collection, handling, storage, and lipid measurement are available elsewhere.[20] Briefly, plasma total cholesterol and triglycerides were measured using enzymatic methods[21, 22] while HDL-c concentrations were determined after precipitation of non-HDL lipoproteins.[23, 24] LDL-c was calculated using the Friedewald equation for those with triglycerides <400 mg/dL (4.52 mmol/L).[25] Blind-duplicate coefficients of variation ranged from 5–10%.[20]
2.3. Cognitive Assessment
At Visits 2, 4, and 5, trained study personnel administered three cognitive tests in a standard order in a quiet room: the Delayed Word Recall Test (DWRT),[26] the Digit-Symbol Substitution Test (DSST),[27] and the Word Fluency Test (WFT).[28]
The DWRT assesses verbal learning and memory. Participants are asked to learn 10 nouns, use them in sentences, and recall them 5 minutes later; the score is the number of correctly recalled nouns. The DSST is a test of executive function, sustained attention, and processing speed. Participants translate symbols to numbers using a key; the score is the number of correct translations within 90 seconds. The WFT is a test of phonemic fluency where participants are asked to generate words starting with a specific letter during a 60 second interval. We used the letters F, A, and S; the score is the number of correct words generated over all three trials.
All scores were roughly normally distributed. We created z-scores for each test using the mean and SD of scores at Visit 2. We also created a summary z-score by standardizing the sum of the three individual test z-scores in the same manner.
2.4. Covariate Assessment
Potential confounders accounted for in the analysis included age and its square (years), sex (male/female), race-center (MS-black/NC-black/NC-white/MN-white/MD-white), education (<high school/high school or equivalent/>high school), leisure time physical activity (inactive/active), health insurance status (yes/no, as a proxy for socioeconomic status and access to health care), body mass index (kg/m2), alcohol use (current/former/never), smoking status (current/former/never), diabetes (yes/no), hypertension (yes/no), lipid-lowering medication use (yes/no), APOE ε4 allele status (0, 1, or 2 ε4 alleles), and two indices of dietary pattern (continuous, z-scored). Demographics, leisure time physical activity, health insurance status, and dietary patterns were assessed at Visit 1, APOE was measured via genotyping, and all other covariates were defined using data at Visit 2. We defined leisure time physical activity as inactive if a participant had a sport activity score of <2[29, 30] from the Baecke Questionnaire.[31] We defined diabetes as self-reported physician diagnosis of diabetes, ≥126 mg/dL fasting glucose, ≥200 mg/dL non-fasting glucose, or use of diabetes medications. We defined hypertension as measured systolic blood pressure ≥140 mmHg, measured diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications. Medication use was determined through visual inspection of medications at the study visit and linkage to Medi-Span Therapeutic Classification codes. Dietary pattern indices were based on food frequency questionnaire data, derived using principal components analysis and z-transformed, and reflect degree of adherence to either a prudent or western dietary pattern.[32] Additional variables used in sensitivity or effect measure modification analyses include fasting status at Visit 2 blood draw (>8 hours, yes/no), race (black/white), stroke prior to Visit 5 (yes/no), and statin use at Visit 5 (yes/no).
2.5. Statistical Analyses
We used separate linear mixed models to consider the association between each Visit 2 lipid measure and changes on each cognitive outcome over Visits 2, 4, and 5. We report findings in terms of the difference in 20-year cognitive change for a given exposure contrast. We modeled the association of lipids with cognitive change using both continuous and, to promote interpretability, categorical lipid variables. Categories were defined as in the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults (ATP III).[33] We used time on study as the primary time scale, modeled using a linear spline with a knot at 6 years (approximately the time of Visit 4). All models were adjusted for the potential confounders listed above, as well as their interactions with each spline parameter. We specified an independence covariance matrix for the three random effects included: a random intercept and random slopes for each piecewise range of the linear spline. Exploration of potential nonlinearity of the association between continuous baseline lipid concentrations and subsequent cognitive change suggested inclusion of a quadratic term for lipid concentration (but not the interaction between this quadratic term and time) improved model fit for a subset of models; therefore, we included a quadratic lipid term (but not its interaction with time) in models considering lipids as a continuous variable. For triglycerides only, model diagnostics suggested a non-linear association between triglycerides and cognitive change driven by those in the highest category (>500 mg/dL or 5.65 mmol/L); therefore, we report linear association excluding the 0.7% of persons with >500 mg/dL (5.65 mmol/L) triglycerides. For our primary analyses, we used a complete case approach to deal with missing exposure and covariate data.
2.6. Sensitivity Analyses
To address questions about whether alternate lipid fractions are additionally informative we also assessed associations with non-HDL-c. As fasting status can impact lipid values, we repeated our primary analyses after restricting to the 97.0% of persons who were fasting >8 hours prior to blood draw. As stroke can significantly impact cognition, we repeated our primary analyses after excluding those with documented stroke during follow-up. Finally, to understand the impact of missing data and selective attrition, we multiply imputed missing exposure, covariate, and cognitive data using an adaptation of multiple imputation chained equations models (MICE) previously developed for this purpose.[34] We examined associations after (a) imputing exposure and covariate data only, (b) additionally imputing cognitive data at the time of each study visit for those known to be alive at the time of the study visit, and (c) additionally imputing cognitive data for those known to die during follow-up at a time 6 months prior to death. Imputation of cognitive data addresses the potential issue of selection bias, given the expectation that persons who are lost to follow-up are preferentially those with cognitive impariment [35, 36] and evidence less desirable lipid levels are associated with a slight increase in risk of attrition. For all MICE models, we used a burn-in of 25 iterations, and report results based on combined estimates from 20 to 25 imputations.
2.7. Effect Measure Modification
We evaluated whether there was support for effect measure modification of the reported associations between continuous lipids and cognitive change by race (black/white), sex, APOE (no ε4/any ε4) lipid-lowering medication use at Visit 2 (yes/no) and (only among those with complete follow-up) statin use at Visit 5 (yes/no) using multiplicative interaction terms and Wald tests of these combined interaction terms. We present stratified analyses where we found support for effect modification. Throughout we report 95% confidence intervals and consider a p-value of <0.05 to be statistically significant. We did not correct for multiple comparisons because such correction stands to be highly conservative considering substantial correlations among the various cognitive tests and lipid measures. Rather, we interpret our findings relative to our hypothesis that less desirable midlife lipid levels would be associated with excess 20-year cognitive change, and weigh whether our findings are consistent with those expected if cognitive decline were independent of lipids determinants. All analyses were completed in STATA, Version 14 or SAS, Version 9.3.
3. RESULTS
At the time of baseline cognitive testing (ARIC visit 2), all potentially eligible participants (N=13,997) were between the ages of 46 and 70, 92% were 65 or younger, 56% were female, and 23% were black. The mean age of those who provided cognitive data at Visit 5 was 76. On average, eligible study participants completed 2.2 follow-up visits. Additional characteristics are provided in Table 1 and Appendix Table 1.
Table 1.
Characteristics of the eligible ARIC sample at Visit 2 (1990–1992), n=13997
| Characteristic | n(%) or mean (SD)† |
|---|---|
| Age | 57.5 (5.7) |
| Female | 7788 (55.6%) |
| Education | |
| Less than high school | 2998 (21.4%) |
| High school or equivalent | 5831 (41.7%) |
| Greater than high school | 5168 (36.9%) |
| Race-Center | |
| White, MN | 3768 (26.9%) |
| White, MD | 3613 (23.3%) |
| White, NC | 3258 (23.3%) |
| Black, NC | 373 (2.7%) |
| Black, MS | 2985 (21.3%) |
| APOE | |
| 0 ε4 alleles | 9413 (67.3%) |
| 1 ε4 allele | 3817 (27.3%) |
| 2 ε4 alleles | 355 (2.5%) |
| Smoking | |
| Never | 5568 (39.8%) |
| Former | 5300 (37.9%) |
| Current | 3122 (22.3%) |
| Alcohol Use | |
| Current | 7907 (56.5%) |
| Former | 2935 (21.0%) |
| Never | 3147 (22.5%) |
| Hypertension | |
| No | 8987 (64.2%) |
| Yes | 4967 (35.5%) |
| Diabetes | |
| No | 11858 (84.7%) |
| Yes | 2076 (14.8%) |
| Lipid-lowering Medication Use | |
| No | 12387 (88.5%) |
| Yes | 743 (5.3%) |
| Physically Active | |
| No | 3838 (27.4%) |
| Yes | 10110 (72.2%) |
| Health Insurance at Visit 1 | |
| No | 1210 (8.6%) |
| Yes | 12765 (91.2%) |
| Body Mass Index (kg/m2) | 28.0 (5.4) |
| Prudent Diet Score (unitless)* | 0.01 (0.99) |
| Western Diet Score (unitless)* | −0.02 (0.99) |
| Total Cholesterol (mg/dL) ‡ | 210.0 (39.5) |
| LDL-c (mg/dL)‡ | 133.6 (36.8) |
| HDL-c (mg/dL) ‡ | 49.5 (16.7) |
| Triglycerides (mg/dL) ‡ | 136.1 (90.3) |
| Missing any lipid data | 322 (2.3%) |
| Missing any covariate data | 1738 (12.4%) |
| Alive but Missing Visit 4 Summary Z Score | 2566 (18.3%) |
| Alive but Missing Visit 5 Summary Z Score | 4108 (29.3%) |
| Deceased by Visit 4 | 511 (3.7%) |
| Deceased by Visit 5 | 4036 (28.8%) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; LDL-c, low density lipoprotein; HDL-c, high density lipoprotein.
Z-scores for adherence, higher values indicate greater adherence.
Categorical variable frequencies (%) may not add up to 13,997 (100%) due to missing data.
In mmol/L:Total cholesterol: 5.43(1.02), LDL-c: 3.45(0.95), HDL-c: 1.28(0.43), Triglycerides: 1.54(1.02)
Using linear parameterizations of lipid levels, higher total cholesterol and triglycerides at Visit 2 were associated with greater 20-year decline on all measures of cognition (Table 2). Higher LDL-c was associated with greater 20-year decline only on the DSST. HDL-c was not associated with cognitive change.
Table 2.
Average adjusted difference in 20-year decline in cognitive test z-scores per 10 mg/dL‡ higher plasma lipid levels at Visit 2 in participants of the Atherosclerosis Risk in Communities Study (1990–2013)
| Difference in 20-year cognitive change* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Summary Z-score | DWRT Z-score | DSST Z-score | WFT Z-score | |||||
| Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | |
| Total Cholesterol (n=12,232) | −0.007 (−0.012, −0.002) | 0.004 | −0.01 (−0.017, −0.002) | 0.02 | −0.006 (−0.009, −0.003) | 0.0006 | −0.004 (−0.009, 0.000) | 0.06 |
| LDL-c (n=12,017) | −0.004 (−0.009, 0.001) | 0.16 | −0.006 (−0.015, 0.002) | 0.14 | −0.004 (−0.008, −0.001) | 0.02 | −0.003 (−0.007, 0.002) | 0.24 |
| HDL-c (n=12,195) | −0.003 (−0.016, 0.009) | 0.59 | −0.005 (−0.026, 0.015) | 0.61 | 0.007 (−0.002, 0.016) | 0.15 | −0.003 (−0.014, 0.009) | 0.64 |
| Triglycerides† (n=12,151) | −0.006 (−0.009, −0.003) | <0.0001 | −0.006 (−0.011, −0.002) | 0.007 | −0.005 (−0.007, −0.003) | <0.0001 | −0.003 (−0.005, −0.000) | 0.03 |
Abbreviations: CI, Confidence interval; DSST, Digit-Symbol Substitution Test; DWRT, Delayed Word Recall Test; LDL-c, low density lipoprotein cholesterol; HDL-c, high density lipoprotein cholesterol; WFT, word fluency test
Adjusted for baseline age, age squared, education, sex, race-center, APOE ε4 status, body mass index, alcohol use, smoking status, diabetes, hypertension, physical activity, health insurance status, adherence to a western dietary pattern, adherence to a prudent dietary pattern, and lipid-lowering medication use, including both their main effects and their interactions with time on study.
Within the range of 0 to 500mg/dL (0 to 5.65 mmol/L)
In mmol/L:Total cholesterol, LDL-c, HDL-c: per 0.26 mmol/L, Triglycerides: per 0.11 mmol/L higher.
Taken as a whole, analyses considering lipid categories suggest an association between elevated total cholesterol, LDL-c, and triglycerides with greater 20-year decline in DSST scores, and between elevated total cholesterol and triglycerides with greater 20-year change in summary z-scores (Table 3). However, we note estimates for individual category contrasts were sometimes non-significant and/or did not perfectly reflect a linear-dose response pattern.
Table 3.
Average adjusted difference in 20-year decline in cognitive test z-scores across ATP-III lipid categories at Visit 2 in participants of the Atherosclerosis Risk in Communities Study (1990–2013)
| Difference in 20-year cognitive change* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Summary Z-score | DWRT Z-score | DSST Z-score | WFT Z-score | ||||||
| %† | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | |
| Total Cholesterol‡ (n=12,232) | |||||||||
| <200 mg/dL | 42% | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||
| 200 to 239 mg/dL | 38% | −0.051 (−0.09, −0.011) | 0.01 | −0.04 (−0.105, 0.025) | 0.22 | −0.04 (−0.068, −0.012) | 0.005 | −0.047 (−0.083, −0.011) | 0.01 |
| 240+ mg/dL | 20% | −0.046 (−0.095, 0.004) | 0.07 | −0.044 (−0.126, 0.038) | 0.29 | −0.051 (−0.087, −0.016) | 0.004 | −0.035 (−0.08, 0.011) | 0.13 |
| LDL-c‡ (n=12,017) | |||||||||
| <100 mg/dL | 16% | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||
| 100 to 129 mg/dL | 32% | −0.025 (−0.078, 0.027) | 0.35 | 0.014 (−0.072, 0.101) | 0.75 | −0.037 (−0.075, 0) | 0.05 | −0.034 (−0.082, 0.014) | 0.17 |
| 130 to 159 mg/dL | 30% | −0.032 (−0.087, 0.022) | 0.24 | −0.043 (−0.133, 0.046) | 0.34 | −0.04 (−0.079, −0.001) | 0.04 | −0.025 (−0.074, 0.025) | 0.33 |
| 160 to 189 mg/dL | 15% | −0.05 (−0.114, 0.014) | 0.13 | −0.041 (−0.146, 0.064) | 0.45 | −0.059 (−0.105, −0.014) | 0.01 | −0.023 (−0.082, 0.035) | 0.44 |
| 190+ mg/dL | 6% | −0.033 (−0.124, 0.059) | 0.48 | −0.087 (−0.236, 0.061) | 0.25 | −0.034 (−0.098, 0.031) | 0.31 | −0.028 (−0.11, 0.055) | 0.51 |
| HDL-c‡ (n=12,195) | |||||||||
| <40 mg/dL | 30% | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||
| 40 to 59 mg/dL | 47% | −0.005 (−0.051, 0.04) | 0.82 | 0.015 (−0.06, 0.09) | 0.70 | −0.002 (−0.034, 0.031) | 0.92 | −0.038 (−0.079, 0.004) | 0.08 |
| 60+ mg/dL | 24% | −0.02 (−0.077, 0.037) | 0.49 | −0.011 (−0.105, 0.083) | 0.82 | 0.018 (−0.023, 0.058) | 0.39 | −0.024 (−0.076, 0.028) | 0.37 |
| Triglycerides‡ (n=12,230) | |||||||||
| <150 mg/dL | 70% | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||
| 150 to 199 mg/dL | 16% | −0.038 (−0.089, 0.013) | 0.14 | −0.009 (−0.092, 0.075) | 0.83 | −0.045 (−0.081, −0.008) | 0.02 | −0.058 (−0.104, −0.011) | 0.02 |
| 200–500 mg/dL | 14% | −0.071 (−0.128, −0.014) | 0.01 | −0.073 (−0.166, 0.02) | 0.12 | −0.08 (−0.12, −0.04) | 0.0001 | −0.012 (−0.064, 0.039) | 0.63 |
| 500+ mg/dL | 1% | 0.007 (−0.239, 0.252) | 0.96 | 0.116 (−0.284, 0.515) | 0.57 | −0.125 (−0.298, 0.048) | 0.16 | 0.016 (−0.207, 0.24) | 0.88 |
Abbreviations: CI, Confidence interval; DSST, Digit-Symbol Substitution Test; DWRT, Delayed Word Recall Test; LDL-c, low density lipoprotein cholesterol; HDL-c, high density lipoprotein cholesterol; WFT, word fluency test
Adjusted for baseline age, age squared, education, sex, race-center, APOE ε4 status, body mass index, alcohol use, smoking status, diabetes, hypertension, physical activity, health insurance status, adherence to a western dietary pattern, adherence to a prudent dietary pattern, and lipid-lowering medication use, including both their main effects and their interactions with time on study.
Within the complete case sample.
Conversions to SI units - Total cholesterol: 200mg/dL = 5.17 mmol/L, 240 mg/dL = 6.21 mmol/L; LDL-c: 100 mg/dL = 2.59 mmol/L, 130 mg/dL = 3.36 mmol/L, 160 mg/dL = 4.14 mmol/L, 190 mg/dL = 4.91 mmol/L; HDL-c: 40 mg/dL = 1.03 mmol/L, 60 mg/dL = 1.55 mmol/L; Triglycerides: 150 mg/dL = 1.69 mmol/L, 200 mg/dL = 2.26 mmol/L, 500 mg/dL = 5.65 mmol/L
Sensitivity analyses considering associations with non-HDL-c were consistent with analyses considering total cholesterol and LDL-c (Appendix Table 2). Results of our sensitivity analyses were generally consistent with the primary findings, with the only substantial difference being the emergence of an association between higher LDL-c, parameterized as a linear term, and greater 20-year decline on the summary Z score in all three of sensitivity analyses imputing missing data (Appendix Tables 3–8).
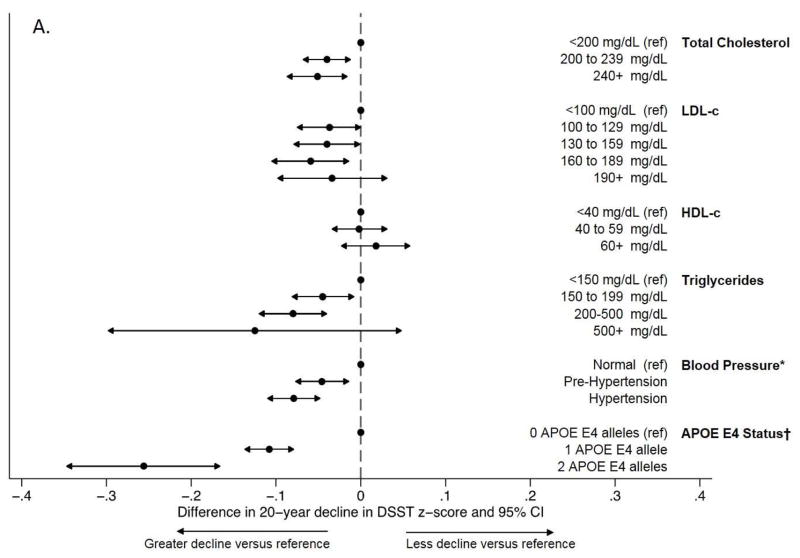
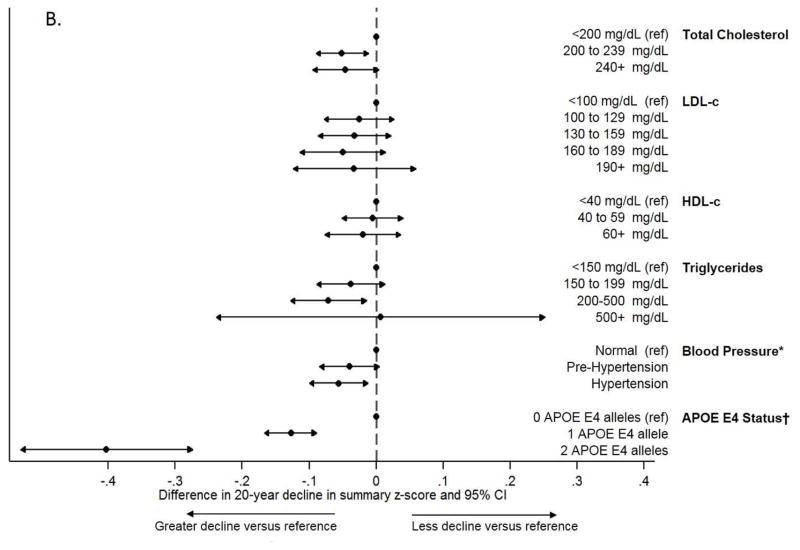
For context, the estimated impact of having high versus desirable total cholesterol, LDL-c, or triglycerides -- according to the ATPIII guidelines >240 versus <200 mg/dL (>6.21 versus <5.17 mmol/L) total cholesterol, 160–189 versus <100 mg/dL (4.14–4.91 versus <2.59 mmol/L) LDL-c, and 200–499 versus <150 mg/dL (2.26–5.65 versus < 1.69 mmol/L) triglycerides -- on 20-year cognitive change in DSST score was 47–74% of the estimated impact of having 1 APOE ε4 allele in our sample and was 65–101% of the previously reported estimated impact of having hypertension versus normal blood pressure at Visit 2 in previously reported analyses from substantially the same sample (Figure 1, Panel A).[37] The estimated impact of high versus desirable total cholesterol, LDL-c, and triglycerides on 20-year change in summary z-score was similarly comparable (Figure 1, Panel B).
Figure 1.
Comparison of the magnitude of adjusted associations across estimates of the added impact of midlife lipids, midlife hypertension, and APOE E4 status on 20-year cognitive decline in (a) DSST z-scores and (b) summary z-scores
Abbreviations: DSST, Digit-Symbol Substitution Test; LDL-c, low density lipoprotein cholesterol; HDL-c, high density lipoprotein cholesterol
*From Gottesman et al. (2014) JAMA Neurology
†From a model adjusting for total cholesterol and all other covariates included in the primary analyses
‡Conversions to SI units - Total cholesterol: 200mg/dL = 5.17 mmol/L, 240 mg/dL = 6.21 mmol/L; LDL-c: 100 mg/dL = 2.59 mmol/L, 130 mg/dL = 3.36 mmol/L, 160 mg/dL = 4.14 mmol/L, 190 mg/dL = 4.91 mmol/L; HDL-c: 40 mg/dL = 1.03 mmol/L, 60 mg/dL = 1.55 mmol/L; Triglycerides: 150 mg/dL = 1.69 mmol/L, 200 mg/dL = 2.26 mmol/L, 500 mg/dL = 5.65 mmol/L
There was no evidence of effect modification by sex, use of lipid-lowering medications at Visit 2, or, among those with complete follow-up, use of statins at Visit 5. There was significant effect modification by race and APOE ε4 allele status for a subset of the considered associations (Table 4). The associations between higher total cholesterol or LDL-c and greater decline in DSST, DWRT, or summary z-scores were present in white participants, but absent in black participants. The associations of total cholesterol, LDL-c, and triglycerides with greater decline in DWRT scores were limited to those with at least one APOE ε4 allele while the adverse association between triglycerides and 20-year change in WFT scores was present only in those lacking an APOE ε4 allele.
Table 4.
The adjusted association between a 10 mg/dL‡ change in plasma lipids at Visit 2 and 20-year cognitive decline in cognitive test z-scores by race or APOE ε4 status where there was support for effect measure modification
| Difference in 20-year cognitive change* | |||||
|---|---|---|---|---|---|
| White | Black | ||||
| Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | P-value for interaction | |
| Total cholesterol (n=12,232) | (n=9,496) | (n=2,736) | |||
| Summary Z-score | −0.011 (−0.017, −0.006) | 0.0001 | 0.006 (−0.004, 0.016) | 0.24 | 0.003 |
| DWRT Z-score | −0.014 (−0.023, −0.005) | 0.003 | 0.004 (−0.012, 0.020) | 0.62 | 0.05 |
| DSST Z-score | −0.009 (−0.013, −0.005) | <0.0001 | 0.005 (−0.002, 0.012) | 0.18 | 0.0005 |
| LDL-c (n=12,017) | (n=9,310) | (n=2,707 | |||
| Summary Z-score | −0.008 (−0.014, −0.002) | 0.007 | 0.009 (−0.001, 0.020) | 0.07 | 0.004 |
| DWRT Z-score | −0.012 (−0.022, −0.002) | 0.02 | 0.009 (−0.007, 0.026) | 0.27 | 0.03 |
| DSST Z-score | −0.007 (−0.011, −0.002) | 0.002 | 0.003 (−0.005, 0.010) | 0.49 | 0.03 |
| No APOE ε4 | At least one APOE ε4 | ||||
| Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | P-value for interaction | |
| Total cholesterol (n=12,232) | (n=8,526) | (n=3,706) | |||
| DWRT Z-score | −0.001 (−0.011, 0.008) | 0.77 | −0.030 (−0.044, −0.015) | 0.0001 | 0.001 |
| LDL-c (n=12,017) | (n=8,378) | (n=3,639) | |||
| DWRT Z-score | 0.001 (−0.009, 0.011) | 0.86 | −0.024 (−0.040, −0.009) | 0.002 | 0.007 |
| Triglycerides† (n=12,151) | (n=8,476) | (n=3,675) | |||
| DWRT Z-score | −0.004 (−0.009, 0.002) | 0.17 | −0.013 (−0.021, −0.005) | 0.002 | 0.05 |
| WFT Z-score | −0.004 (−0.007, −0.001) | 0.004 | 0.002 (−0.003, 0.006) | 0.51 | 0.03 |
Abbreviations: CI, Confidence interval; DSST, Digit-Symbol Substitution Test; DWRT, Delayed Word Recall Test; LDL-c, low density lipoprotein cholesterol; HDL-c, high density lipoprotein cholesterol; WFT, word fluency test
Adjusted for baseline age, age squared, education, sex, race-center, APOE ε4 status, body mass index, alcohol use, smoking status, diabetes, hypertension, physical activity, health insurance status, adherence to a western dietary pattern, adherence to a prudent dietary pattern, and lipid-lowering medication use, including both their main effects and their interactions with time on study.
Within the range of 0 to 500mg/dL (0 to 5.65 mmol/L)
In mmol/L:Total cholesterol, LDL-c, HDL-c: per 0.26 mmol/L, Triglycerides: per 0.11 mmol/L higher
4. DISCUSSION
In our data, elevated total cholesterol, LDL-c and triglycerides at baseline were associated with greater 20-year cognitive decline, with the strongest evidence in support of associations between lipids and decline on the DSST, a test of executive function, sustained attention, and processing speed. While this may suggest lipids are related to selective decline in these domains, we are hesitant to draw strong conclusions given differences in the psychometric properties of the three cognitive tests. The estimated magnitudes of effect are admittedly small, such that the mean impact of elevated lipid levels on 20-year decline would not be noticeable in an average individual in the context of typical age-related declines. The most extreme point estimates from Table 3 translate into a difference of 1.8 points on the DSST, 0.2 points on the DWRT, and 0.7 points on the WFT on their raw scales. However, even a small population-level impact could have noticeable impact on the number of persons ultimately affected with cognitive difficulties. For context, the estimated magnitude of excess cognitive decline on the DSST contrasting those with “bad” to “good” lipid levels is approximately 1/2 to 3/4 of what we observed for having one APOE ε4 allele, an established risk factor for Alzheimer’s disease dementia, and is in the range of the magnitude of effect previously reported in this cohort contrasting those with midlife hypertension to those without.[37]
Strengths of this study include the large sample size, consideration of multiple lipid fractions measured in midlife, the bi-racial cohort, and long follow-up. Our study also has limitations. Informative attrition may have introduced selection bias. However, sensitivity analyses imputing cognitive data, which were specifically designed to address this potential source of bias and which were previously shown to significantly alter associations between diabetes and 20-year change,[34] did not substantially change effect estimates. This suggests that the degree of bias due to attrition is likely small. Moreover, any remaining selection bias due to either death or non-death attrition would be expected to mute or reverse adverse findings under plausible causal structures supported by our data [38], but is unlikely to create the adverse associations we observed. As with any study, we cannot completely discount the potential for residual confounding. We use only three cognitive tests to assess cognition that do not cover the full range of cognitive domains and may be variably sensitive to change. Thus, our findings of strong, consistent associations with the DSST may reflect better sensitivity of the DSST to small changes in cognition, rather than selective decline in executive function, sustained attention, and processing speed.
Our results must be considered in the context of existing studies of lipids and dementia, lipids and cognitive decline, prior work in the ARIC cohort, and prior work on the impact of statin use on cognition. First, our results concur with prior studies suggesting an adverse association between higher lipid levels (typically total cholesterol) in midlife and increased risk of dementia.[3–5, 7, 8] However, it should be noted that other studies of midlife lipids and dementia report no association[9, 10] or association only among subgroups.[6] Second, in contrast to our study, prior studies of the association between lipids and cognitive change assessed lipids in late life and have short follow-up times. In the Longitudinal Aging Study Amsterdam, higher total cholesterol at baseline (mean age: 76 years) was associated with slower decline on the Mini-Mental State Examination (MMSE), processing speed, and memory performance tasks over 6 years.[12] Similarly, in both the Neurological Disorders in Central Spain (NEDICES) cohort[15] and a small sample of non-demented persons,[16] hypercholesterolemia in late life was associated with slower decline on the MMSE over 3 years. To the contrary, hypertriglyceridemia and low HDL-c at baseline (mean age: 73 years) were significantly associated with greater decline on the MMSE but not other cognitive tasks over a 2 to 4 year follow-up in the Three-City study.[13] Moreover, lipid levels at baseline (mean age: 76 years) were not associated with cognitive decline over up to 7 years of follow-up in the Washington Heights-Inwood Columbia Aging Project (WHICAP).[14] Potential explanations for the discordance between our findings and many of these prior studies include the potential for greater floor effects or selection bias in older cohorts,[39] differences in effect based on age at lipid assessment or amount of time since assessment,[40] or reverse causation.[6] Third, prior work in ARIC has considered the association between lipids and cognition. One prior study found no association between midlife total cholesterol and 6-year cognitive change (Visit 2 to 4).[41] This is consistent with our findings as most of the effect between Visit 2 lipids and cognition was the result of greater decline between Visits 4 and 5 in those with higher lipids. A second study in ARIC reported a strong, but non-significant association of total cholesterol and risk of dementia hospitalizations when total cholesterol was measured before age 55,[42] which is also consistent with our findings. Finally, despite evidence from our study and others linking midlife lipids to late life cognition, the current evidence suggests that use of statins does not protect against dementia, at least in the near term.[43] Our study cannot comment directly about the impact of statin use in midlife on risk of cognitive decline given statins had only been recently introduced at the time of our lipid measures. Only 2.5% of eligible persons were taking statins at Visit 2, resulting in insufficient numbers to address known issues of confounding by indication and healthy user effects. However, since Visit 2, the indications for statin use and its uptake have increased dramatically. At the time of Visit 5/ARIC-NCS, 51% of persons in our sample were using statins, and lipid levels were lower than those observed at Visit 2, likely reflecting the impact of older age and increased emphasis on lipid management. Thus, our findings are consistent with the notion that lowering lipids via medication use in late life does not have a near-term beneficial impact on cognition. It further implies that statin use or other lipid-lowering interventions in midlife may be effective for mitigating cognitive decline over the subsequent decades.
In our study, the association between lipids and cognitive decline appears absent in black participants, mirroring prior findings of a lack of association in black participants between hypertension and cognitive change in this cohort.[37] However, whether this represents true differences or is a result of unaccounted for bias or remains unclear. For example, our findings may reflect differences in the etiology driving cognitive change across individuals of differing racial groups in our sample. To the contrary, we cannot discount the possibility of a chance finding, greater unaccounted for selection bias among black participants, differential unaccounted for confounding, or the possibility that it is some other aspect of place, rather than race (the vast majority of black participants were from the Jackson, MS site), that accounts for the lack of association in this group. Notably, while the mean baseline cognitive scores were lower for black than white participants, we did not see strong evidence of floor effects that would preclude detection of cognitive change. Interestingly, we also observed much stronger associations between elevated lipids and accelerated change in DWRT scores among those carrying APOE ε4, consistent with the “double hit” hypothesis,[17] whereby APOE and vascular risk factors in combination confer greater excess risk of Alzheimer’s disease dementia than would be expected. Future work will be needed to further investigate this possibility.
Given the magnitude of the observed associations between accelerated cognitive decline and elevated total cholesterol, LDL-c and triglycerides is in the range of that observed for associations with established risk factors – namely midlife hypertension and the APOE ε4 allele - our study suggests that prior lipid levels are a notable risk factor for cognitive decline. As aggressive lipid management became standard medical practice during the course of follow-up, our study further suggests that lipid-lowering interventions in late life, including statins, cannot completely mitigate the adverse consequences of elevated lipid levels earlier in life. However, lipid management, beginning in midlife, may help lower risk of dementia. Future work is needed to understand the long-term impact of lipid management, including statin use, and to further potentially susceptible or resilient sub-groups.
Supplementary Material
HIGHLIGHTS.
Elevated midlife lipids were associated with 20-year decline in executive function.
Total cholesterol and triglycerides were associated with overall cognitive decline.
Results were similar in sensitivity analyses addressing informative missingness.
RESEARCH IN CONTEXT.
Systematic Review
The authors reviewed the literature using traditional (e.g. Pubmed) sources. Existing studies of lipids and cognitive change consider the association of lipid levels in late-life with subsequent cognitive change, despite a pervasive pattern where midlife, rather than late-life risk factors are most relevant to cognitive health. Thus, the impact of elevated lipids in midlife on cognition trajectories remains unknown.
Interpretation
Elevated total cholesterol, LDL-c and triglycerides at midlife were associated with greater decline in cognition over the subsequent 20 years. The magnitude of these associations is comparable to the magnitude of association between midlife hypertension or one APOE ε4 allele and 20-year cognitive change.
Future Directions
Future work is needed to understand the long-term impact of lipid management and identify susceptible sub-groups, including investigation of the “double hit” hypothesis postulating that APOE genotype interacts with vascular risk factors to confer excess risk of Alzheimer’s disease dementia.
Acknowledgments
We thank the staff and participants of the ARIC study for their important contributions. We also thank Dr. Pamela Lutsey for providing the dietary pattern scores.
Funding: Melinda C Power was supported by the National Institute of Aging (T32 AG027668). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 with previous brain MRI examinations funded by R01-HL70825.
Abbreviations
- APOE
apolipoprotein E
- ARIC
Atherosclerosis Risk in Communities study
- ARIC-NCS
ARIC Neurocognitive Study
- ATP III
Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults
- DWRT
Delayed Word Recall Test
- DSST
the Digit-Symbol Substitution Test
- HDL-c
high density lipoprotein cholesterol
- LDL-c
low density lipoprotein cholesterol
- MICE
multiple imputation chained equations models
- MMSE
Mini-Mental State Examination
- NEDICES
Neurological Disorders in Central Spain cohort
- WFT
Word Fluency Test
- WHICAP
Washington Heights-Inwood Columbia Aging Project
Footnotes
Conflicts of interest: Dr. Power and Dr. Mosley report grants from NIH, during conduct of the study; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Coresh reports grants from NIH and NKF, during the conduct of the study; grants from NIH, grants from NKF, outside the submitted work; In addition, Dr. Coresh has a patent PCT/US2015/044567 Provisional patent [Coresh, Inker and Levey] filed 8/15/2014 -- Precise estimation of glomerular filtration rate from multiple biomarkers issued. Dr. Gottesman reports no support from any organization for the submitted work; personal fees from Neurology journal, where she serves as Associate Editor, outside the submitted work; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Ballantyne, Dr. Sharrett, Dr. Rawlings, Dr. Alonso, Dr. Pokharel and Dr. Penman report no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. Dr. Michos reports no support from any organization for the submitted work; personal fees from Siemens Diagnostics, outside the submitted work; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Knopman reports no support from any organization for the submitted work; personal fees from DIAN study DSMB, personal fees from Lundbeck AD drug DSMB, outside the submitted work; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Bandeen-Roche reports grants from NIH, during the conduct of the study; grants from NIH, outside the submitted work; and no other relationships or activities that could appear to have influenced the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–54. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 2.Reitz C. Dyslipidemia and the risk of Alzheimer’s disease. Curr Atheroscler Rep. 2013;15:307. doi: 10.1007/s11883-012-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 4.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20:2255–60. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 6.Mielke MM, Zandi PP, Shao H, Waern M, Ostling S, Guo X, et al. The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology. 2010;75:1888–95. doi: 10.1212/WNL.0b013e3181feb2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137:149–55. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 8.Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 9.Schnaider Beeri M, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, et al. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004;63:1902–7. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- 10.Beydoun MA, Beason-Held LL, Kitner-Triolo MH, Beydoun HA, Ferrucci L, Resnick SM, et al. Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health. 2010;65:949–57. doi: 10.1136/jech.2009.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Kommer TN, Dik MG, Comijs HC, Fassbender K, Lutjohann D, Jonker C. Total cholesterol and oxysterols: early markers for cognitive decline in elderly? Neurobiol Aging. 2009;30:534–45. doi: 10.1016/j.neurobiolaging.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Raffaitin C, Feart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, et al. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76:518–25. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 14.Reitz C, Luchsinger J, Tang MX, Manly J, Mayeux R. Impact of plasma lipids and time on memory performance in healthy elderly without dementia. Neurology. 2005;64:1378–83. doi: 10.1212/01.WNL.0000158274.31318.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benito-Leon J, Vega-Quiroga S, Villarejo-Galende A, Bermejo-Pareja F. Hypercholesterolemia in elders is associated with slower cognitive decline: a prospective, population-based study (NEDICES) J Neurol Sci. 2015;350:69–74. doi: 10.1016/j.jns.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Wada T, Matsubayashi K, Okumiya K, Kimura S, Osaka Y, Doi Y, et al. Lower serum cholesterol level and later decline in cognitive function in older people living in the community, Japan. J Am Geriatr Soc. 1997;45:1411–2. doi: 10.1111/j.1532-5415.1997.tb02949.x. [DOI] [PubMed] [Google Scholar]
- 17.van Norden AGW, van Dijk EJ, de Laat KF, Scheltens P, OldeRikkert MGM, de Leeuw FE. Dementia: Alzheimer pathology and vascular factors: From mutually exclusive to interaction. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012;1822:340–9. doi: 10.1016/j.bbadis.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–38. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 20.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 1994;14:1098–104. doi: 10.1161/01.atv.14.7.1098. [DOI] [PubMed] [Google Scholar]
- 21.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–80. [PubMed] [Google Scholar]
- 22.Nagele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, et al. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. Journal of clinical chemistry and clinical biochemistry Zeitschrift fur klinische Chemie und klinische Biochemie. 1984;22:165–74. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 23.Patsch W, Brown SA, Morrisett JD, Gotto AM, Jr, Patsch JR. A dual-precipitation method evaluated for measurement of cholesterol in high-density lipoprotein subfractions HDL2 and HDL3 in human plasma. Clin Chem. 1989;35:265–70. [PubMed] [Google Scholar]
- 24.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–88. [PubMed] [Google Scholar]
- 25.McNamara JR, Cohn JS, Wilson PW, Schaefer EJ. Calculated values for low-density lipoprotein cholesterol in the assessment of lipid abnormalities and coronary disease risk. Clin Chem. 1990;36:36–42. [PubMed] [Google Scholar]
- 26.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–5. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale-Revised (WAIS-R) San Antonio, Texas: The Psychological Corporation; 1981. [Google Scholar]
- 28.Lezak M. Neuropsychological Assessment. New York: Oxford University Press; 1995. pp. 335–84. [Google Scholar]
- 29.Demerath EW, Lutsey PL, Monda KL, Linda Kao WH, Bressler J, Pankow JS, et al. Interaction of FTO and physical activity level on adiposity in African-American and European-American adults: the ARIC study. Obesity (Silver Spring) 2011;19:1866–72. doi: 10.1038/oby.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer AM, Evenson KR, Couper DJ, Stevens J, Pereria MA, Heiss G. Television, physical activity, diet, and body weight status: the ARIC cohort. The international journal of behavioral nutrition and physical activity. 2008;5:68. doi: 10.1186/1479-5868-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 32.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–61. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 33.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 34.Rawlings AM, Sang Y, Sharrett AR, Coresh J, Griswold M, Kucharska-Newton AM, et al. Multiple imputation of cognitive performance as a repeatedly measured outcome. Eur J Epidemiol. 2016 doi: 10.1007/s10654-016-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bassuk SS, Wypij D, Berkman LF. Cognitive impairment and mortality in the community-dwelling elderly. Am J Epidemiol. 2000;151:676–88. doi: 10.1093/oxfordjournals.aje.a010262. [DOI] [PubMed] [Google Scholar]
- 36.Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol. 2005;58:13–9. doi: 10.1016/j.jclinepi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, et al. Midlife Hypertension and 20-Year Cognitive Change: The Atherosclerosis Risk in Communities Neurocognitive Study. JAMA neurology. 2014 doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayeda E, Tchetgen Tchetgen E, Power M, Weuve J, Jacqmin-Gadda H, Marden J, et al. A simulation platform to quantify survival bias: an application to research on determinants of cognitive decline. American Journal of Epidemiology. 2016;184:378–87. doi: 10.1093/aje/kwv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weuve J, Proust-Lima C, Power MC, Gross AL, Hofer SM, Thiebaut R, et al. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement. 2015;11:1098–109. doi: 10.1016/j.jalz.2015.06.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Power MC, Tchetgen EJ, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: factors that may account for their inconsistent association. Epidemiology. 2013;24:886–93. doi: 10.1097/EDE.0b013e3182a7121c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–8. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 42.Alonso A, Mosley TH, Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80:1194–201. doi: 10.1136/jnnp.2009.176818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Power MC, Weuve J, Sharrett AR, Blacker D, Gottesman RF. Statins, cognition, and dementia-systematic review and methodological commentary. Nat Rev Neurol. 2015 doi: 10.1038/nrneurol.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.