Abstract
Purpose
To identify and compare predictors of local tumor progression free survival (LTPFS) after radiofrequency ablation (RFA) and microwave ablation (MWA) of colorectal liver metastases (CLM).
Methods
This is a retrospective review of CLM ablated from November 2009 to April 2015 (110 patients). Margins were measured on contrast-enhanced CT 6 weeks post-ablation. Clinical and technical predictors of LTPFS were assessed using a competing risk model adjusted for clustering.
Results
Technique effectiveness (complete ablation) was 93% (79/85) for RFA and 97% (58/60) for MWA (P=0.47). The median follow-up period was significantly longer for RFA versus MWA (56 versus 29 months) (P<0.001). There was no difference in the LTP rates between RFA and MWA (P=0.84). Significant predictors of shorter LTPFS for RFA on univariate analysis were ablation margin ≤5 mm (P<0.001) (HR: 14.6; 95% CI: 5.2–40.9) and peri-vascular tumors (P=0.021) (HR: 2.2; 95% CI: 1.1–4.3); both retained significance on multivariate analysis. Significant predictors of shorter LTPFS on univariate analysis for MWA were ablation margin ≤5 mm (P<0.001) (SHR: 11.6; 95% CI: 3.1–42.7) and no history of prior liver resection (P<0.013) (HR: 3.2; 95%: 1.3–7.8); both retained significance on multivariate analysis. There was no LTP for tumors ablated with margin over 10 mm (median LTPFS: not reached). Peri-vascular tumors were not predictive for MWA (P=0.43).
Conclusion
Regardless of the thermal ablation modality used, margins >5 mm are critical for local tumor control, with no LTP noted for margins over 10 mm. Unlike RFA, the efficiency of MWA was not affected for peri-vascular tumors.
Introduction
Radiofrequency has been the most studied ablation modality in the treatment of colorectal liver metastases (CLM) [1]. The wide range of local tumor progression (LTP) reported for the percutaneous approach (12–48 %) [2–4], when compared to resection, limited its use to highly selected patients with small well-positioned tumors [3–6] or with liver recurrences post-hepatectomy [7]. Ablation with sufficient radiographic margins [4, 5] and histologically proven necrosis significantly lowers LTP [6].
Microwave ablation (MWA) is gaining popularity in the ablation of liver tumors with the hope of capitalizing on its potential benefits over RFA, demonstrated mainly in animal studies [8–13]. These include no need for grounding pads, less susceptibility to the heat sink phenomenon, larger ablation zones, shorter ablation times, and possibly better local tumor control [8–13]. Prior clinical comparisons of MWA to RFA suggested an advantage for MWA with regards to local tumor control for CLM, however they did not evaluate outcomes based on the creation of ablation margin[14].
The aim of this study was to compare local tumor progression free survival (LTPFS) after RFA and MWA of CLM taking into account all possible predictors that can impact LTP and LTPFS.
Methods
Study population
This is an IRB-waived retrospective review of a HIPAA compliant clinical image-guided percutaneous ablation database of CLM patients. From December 2002 to April 2015, 260 patients were treated using either RFA (n=183) or MWA (n=77). As of November 2009, a mandatory uniform immediate post-ablation contrast enhanced CT (CECT) was implemented to evaluate adequate tumor coverage. Therefore, for the purpose of this study, only patients ablated from November 2009 to April 2015 were included (n=154); RFA (n=80) and MWA (n=74). This was done so that both the technical approach and endpoints were identical in both groups, including the fact that all ablations would have been uniformly assessed by an immediate and a 6 weeks post-ablation contrast enhanced CT (CECT). The final study population after exclusions, as presented in the diagram/Figure 1, consisted of 62 patients with 85 tumors ablated in 72 sessions using RFA, and 48 patients with 60 tumors ablated in 52 sessions using MWA.
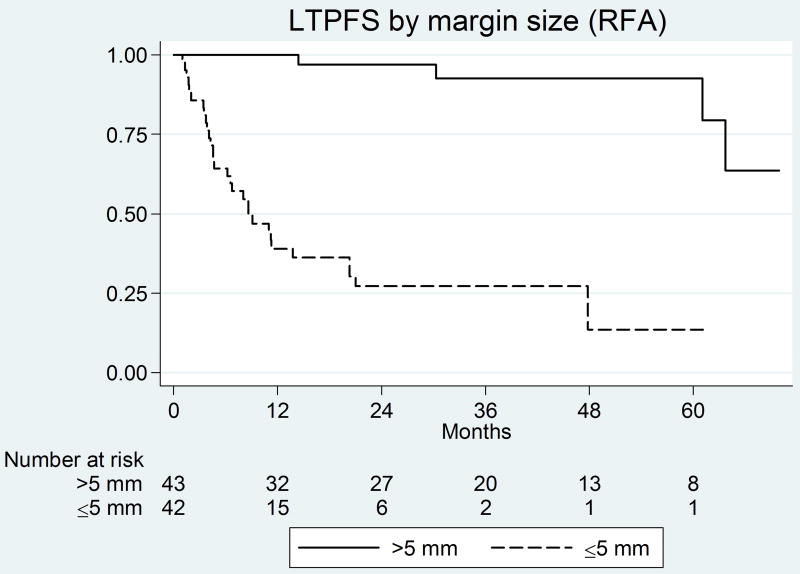
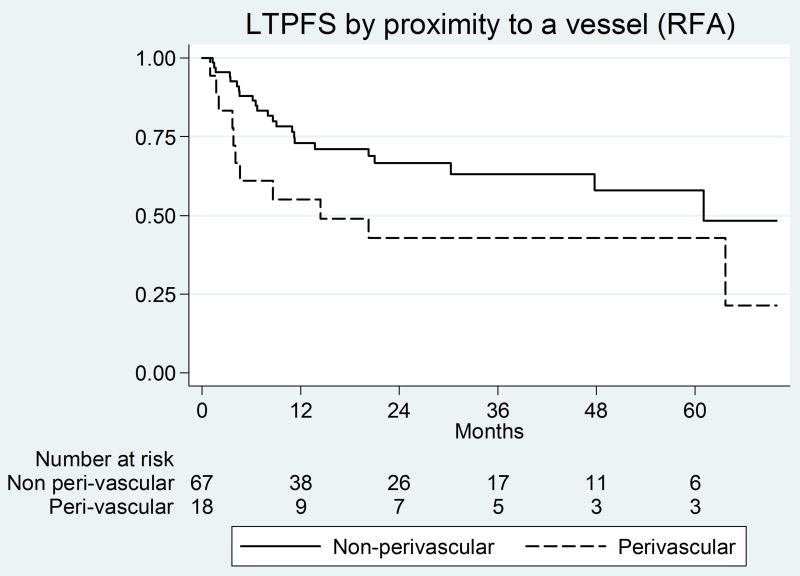
Figure 1.
Kaplan-Meier curves for margin size and peri-vascular tumors in the RF group
Baseline characteristics
The RFA and MWA groups had similar baseline characteristics, with the exception of age, number of liver tumors at presentation, and peri-vascular tumors (Table 1). There were significantly more peri-vascular tumors in the MWA (45%; 27/60) versus the RFA group (21%; 18/81) (P=0.002). The median distance between the vessel and the edge of the tumor was 3.4 mm (range: 0–9.3) for RFA and 2.5 mm (range: 0–9) for MWA (P=0.89) groups. Both groups had a similar distribution of patients with low versus high clinical risk score (CRS) (P=0.44) [Blinded 1, 4] and tumors with an ablation margin >5 mm (P=0.29). The median tumor size was 1.8 cm (range: 0.6–4.5) in the RFA and 1.7 cm (range: 0.7–3.7) in the MWA (P=0.67) groups. There was a slightly higher percentage of tumors >3 cm in the RFA (12%; 10/85) versus MWA (7%; 4/60) group that was not significant (P=0.40).
Table 1.
A comparison of the baseline patient/tumor characteristics
| Characteristic | RF group | MW group | P-value |
|---|---|---|---|
| Sex (Female) | 24/62 (39%) | 13/48 (27%) | 0.20 |
| Age (> 60 years) | 22/62 (35%) | 29/48 (60%) | 0.009 |
| Node positive primary | 38/62 (61%) | 31/48 (65%) | 0.77 |
| DFI* (<12 mn) | 51/62 (82%) | 39/48 (81%) | 0.89 |
| Prior liver resection | 50/62 (81%) | 35/48 (73%) | 0.34 |
| Prior HAIC | 33/62 (53%) | 31/48 (65%) | 0.23 |
| Tumor number at time of ablation (>1)* | 22/62 (35%) | 8/48 (17%) | 0.023 |
| CEA >5 ng/ml | 37/62 (60%) | 26/48 (54%) | 0.56 |
| Clinical risk score* | |||
| 0–2 | 32/62 (52%) | 29/48 (60%) | 0.44 |
| 3–5 | 30/62(48%) | 19/48 (40%) | |
| Extra-hepatic disease | 24/62 (39%) | 16/48 (33%) | 0.56 |
| Tumor size (median, range) | 1.8 cm (0.6–4.5) | 1.7 cm (0.7–3.7) | 0.67 |
| Sub-capsular location | 69/85 (81%) | 48/60 (80%) | 0.86 |
| Requiring Hydro/pneumo-dissection | 14/85 (16%) | 11/60 (18%) | 0.77 |
| Proximity to a vessel | 18/85 (21%) | 27/60 (45%) | 0.002 |
| Ablation margin | |||
| ≤5 mm | 42/85 (49%) | 35/60 (48%) | 0.29 |
| >5 mm | 43/85 (51%) | 25/60 (42%) |
Range was 1–3 tumors
Componants: CEA level >5 ng/ml, size of largest tumor >3 cm, tumor number >1, DFI >12 months, node positive primary. A CEA level >5 ng/ml was chosen instead of >30 ng/ml since only one patient in MWA group had a CEA level >30 ng/ml.
Ablation procedure
General anesthesia with continuous electrophysiologic monitoring was always used. CT (with CT fluoroscopy capabilities) ± ultrasonography was used for real time needle positioning, guidance, and ablation zone monitoring. Additionally, PET/CT was used in 6 sessions and split-dose PET/CT technique in 68 sessions. The radiofrequency electrodes used were: Cool-tip RF Ablation System (Medtronic, Minneapolis, MN) (n=57), RITA XL/XLI (Angiodynamics, Latham, NY) (n=24), LeVeen Needle Electrode (Boston Scientific, Natick, Mass) (n=4). The numbers of Cool-tip RF electrodes were: one in 12 cases, 2 in 18 cases, and 3 in 27 cases. The microwave antennae used were: Neuwave (NeuWave Medical, Madison, WI) (n=45), Amica (Mermaid Medical, Centennial, CO) (n=9), Microsulis (Angiodynamics, NY, NY) (n=4), and Emprint (Medtronic, Minneapolis, MN) (n=2). The numbers of microwave antenna used were: one for 78% of tumors (n=47/60) and two for 22% of tumors (n=13/60). Ablations were performed according to the manufacturer’s protocol with the aim of creating an ablation margin > 5 mm all around the tumor. Two interventional radiologists (IR) (X.X.X. and X.X.X), both with 9 years of experience with ablation at the start of the study, performed the majority of ablations (81%). The mean ablation time for each overlap, and the total ablation time per tumor were compared between RFA and MWA. The information regarding the number of overlaps for each tumor was available for 140/145 tumors and the ablation time for each overlap/total ablation time was available for 108/145 tumors.
Definitions
Technique effectiveness (complete ablation) was defined as an ablation defect completely covering the target tumor with no evidence of residual disease on the first post-ablation CECT (6 weeks). This was used as the new basis for future radiologic comparisons [15]. Subsequently, all patients underwent a CECT every 3 months. MRI or PET/CT was obtained for further evaluation of questionable findings. LTP was defined radiologically as a hypodense lesion or peripheral/nodular enhancement <1cm from the edge of the ablation zone on CECT or a new metabolically active area in close proximity on PET/CT (confirmed by Nuclear Medicine and IR clinic faculty notes). LTPFS was defined as the time period from ablation until radiologic evidence of LTP or latest follow-up.
Peri-vascular tumors were defined on the baseline CECT as being <10 mm from a vessel ≥ 3mm in diameter (Figure 1 online). According to location, tumors were also classified as sub-capsular (<10 mm from the liver edge) and non-subcapsular. Maneuvers required to protect adjacent structures during ablation (air and/or hydro-dissection) were noted.
Ablation margins were measured on a PACS workstation using the 1st pre- and post-ablation CECT scans and anatomic landmarks, as previously described [Blinded 1, Blinded 2] (Figure 2 online). In short, distances were measured from the edge of the tumor/ ablation defect to the anatomic landmarks chosen on the pre- and post-ablation CECT scans, respectively. For each landmark, the pre-ablation distance was subtracted from the post-ablation distance to render the margin at that site; the smallest value was considered the minimal margin. Margin size was classified as: 0 mm, 1–5 mm, >5–10 mm, and >10 mm. Ablation with margins over 10mm was defined as A0. These assessments were done in consensus (independently obtained then reconciled) by a minimum of 2 and up to 4 readers: X.X. (MD, IR Research fellow), X.X. (MD, Radiologist, IR Research fellow, acknowledged), X.X.X (MD, Hepato-biliary Radiologist), and X.X.X (MD, IR, senior author).
Complications
Complications were classified as minor (requiring no therapy) and major (requiring therapy and hospitalization) according to the guidelines of the Society of Interventional Radiology (SIR)[16]. The proportions of complications were compared between the RFA and MWA groups.
Statistical analysis
The proportions of baseline factors and complication rates were compared using a Chi-square or a Fischer exact test. The non-parametric Mann-Whitney U test was used to compare the time of each overlap and total ablation time between the RFA and MWA groups. LTPFS was calculated using Kaplan-Meier (KM) methodology. Univariate and multivariate analysis were done using a competing risk model (Fine and Gray) to account for patients that died before LTP. The model was adjusted for clustering to account for multiple tumors per patient. The sub-hazard ratios for statistically significant variables on univariate and multivariate analysis were reported. Multivariate analysis was performed by backward stepwise selection using variables with a P-value <0.15 on univariate analysis. Since no recurrences were noted in tumors ablated with margin over 10 mm, a regression analysis to allow inclusion of all margin categories (0 mm, 1–5mm, >5–10mm, and >10 mm) using Firth’s method was additionally performed. Statistical significance was defined as a P-value of <0.05. STATA version 12.0 (StataCorp LP, College Station, Texas) was used. The cumulative incidence of LTP was calculated using the cmprsk package using the software R version 3.2.3.
Results
Ablation procedure comparison
The mean ablation time for each overlap was significantly shorter for MWA (P<0.001) with a median of 7 (range: 2.7 – 20) versus 15 (range: 5.5 – 24) minutes for RFA. A higher percentage of tumors required >2 overlaps for MWA (63%; 38/60) versus RFA (31%; 25/80) (P<0.001). Despite this, the total ablation time per tumor was significantly shorter for MWA (P=0.044) with a median of 18 (range: 3.5 – 40) versus 23.5 (range: 7 – 77) minutes for RFA.
Complete ablation and LTP rates
Complete ablation was achieved in 93% of tumors (79/85) in the RFA group and in 97% of the tumors (58/60) in the MWA group (P=0.47). Patients lost to follow-up were eight for RFA with a median follow-up time of 14 months (range: 8–60 months), and five for MWA with a median follow-up time of 16 months (range: 12–21 months). The median follow-up period was significantly longer for RFA (56 months) versus MWA (29 months) (P<0.001). During the follow-up period there was no difference in the LTP rate between RFA (40%; 34/85) and MWA (38%; 23/60) (P=0.84). The LTP-free survival rates for RFA versus MWA at 12, 18 and 24 months were 69% versus 75%, 66% versus 66%, and 61% versus 60% respectively. The cumulative incidence of LTP for RFA and MWA at 12, 18, and 24 months were 30% vs 25%, 32% vs 33%, and 36% vs 38%, respectively.
Univariate and multivariate analysis
On univariate analysis predictors of shorter LTPFS for RFA were ablation margin ≤5 mm (Figure 2a) and peri-vascular tumors (Figure 2b) (See Table 2). On multivariate analysis, both ablation margin ≤5mm (P<0.001) and peri-vascular location (P<0.001) were independent predictors (Table 3). The LTP rate for tumors with a margin of ≤5mm, >5–10mm, and >10 mm (A0) were 71% (n=30/42), 14.8% (n=4/27), and 0% (n=0/16), respectively.
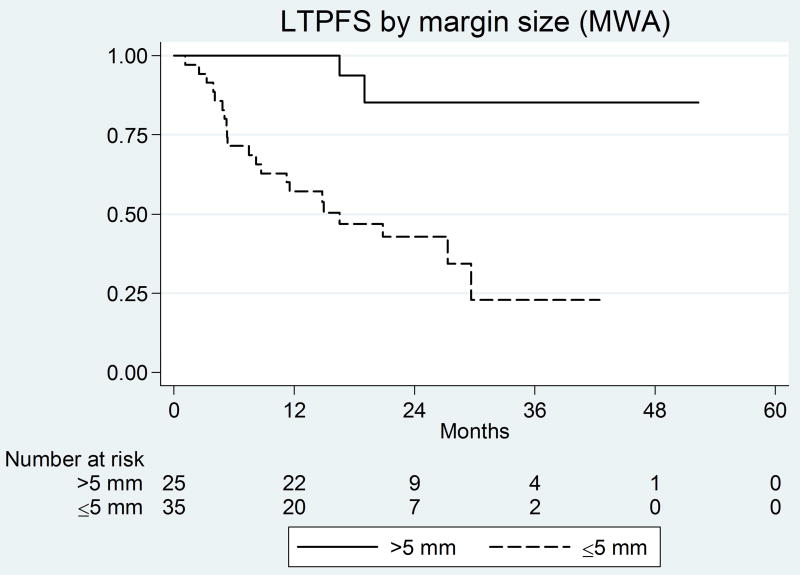
Figure 2.
Kaplan-Meier curves for margin size (significant predictor) and peri-vascular tumors (non-significant predictor) in the MW group.
Table 2.
Univariate analysis for the RF group and MW group
| RF group | MW group | |||||
|---|---|---|---|---|---|---|
| Predictor | P-value | SHR | 95% CI | P-value | SHR | 95% CI |
| Sex (Male vs Female) | 0.3 | 0.7 | 0.3–1.4 | 0.6 | 0.7 | 0.2–2.2 |
| Age (> 60 years) | 0.5 | 0.8 | 0.4–1.6 | 0.9 | 1.0 | 0.4–2.7 |
| Node positive primary | 0.8 | 1.1 | 0.6–2.1 | 0.6 | 0.8 | 0.3–2.1 |
| DFI* (<12 months) | 0.6 | 1.3 | 0.5–3.5 | 0.4 | 2.1 | 0.4–10.0 |
| Prior liver resection | 0.3 | 1.6 | 0.7–3.9 | 0.013 | 3.1 | 1.3–7.8 |
| Prior HAIC | 0.1 | 1.7 | 0.8–3.4 | 0.9 | 0.9 | 0.3–2.5 |
| Tumor number at time of ablation (2–3) | 0.6 | 0.8 | 0.4–1.7 | 0.3 | 1.8 | 0.7–4.8 |
| CEA >5 ng/ml | 0.5 | 1.3 | 0.6–2.7 | 0.4 | 1.5 | 0.6–4.0 |
| Extra-hepatic disease | 0.5 | 1.2 | 0.6–2.5 | 0.7 | 0.8 | 0.3–2.3 |
| Tumor size (each cm) | 0.09 | 1.3 | 1.0–1.9 | 0.7 | 1.1 | 0.7–2.0 |
| Sub-capsular location | 0.4 | 0.8 | 0.4–1.5 | 0.1 | 0.5 | 0.2–1.2 |
| Ablation margin size ≤5 mm | <0.001 | 14.6 | 5.2 – 40.9 | <0.001 | 11.6 | 3.1 – 42.7 |
| Requiring hydro/pneumo-dissection | 0.3 | 0.5 | 0.1–1.7 | 0.9 | 0.91 | 0.34–2.5 |
| Proximity to a vessel | 0.02 | 2.2 | 1.1–4.4 | 0.4 | 0.74 | 0.35–1.6 |
| CRS ≥3 | 0.6 | 1.2 | 0.6–2.6 | 0.1 | 2.1 | 0.8–5.5 |
Table 3.
Multivariate analysis for the RF and MW groups
| RF group | MW group | ||||||
|---|---|---|---|---|---|---|---|
| Predictor | P-value | SHR | 95% CI | Predictor | P-value | SHR | 95% CI |
| Ablation margin ≤5mm | <0.001 | 20.0 | 7.49–50.5 | Ablation margin ≤5mm | <0.001 | 13.7 | 3.2–58.6 |
| Proximity to a vessel | <0.001 | 4.1 | 2.0–8.6 | Prior surgical resection | 0.002 | 0.25 | 0.11–0.59 |
For MWA, the predictors of shorter LTPFS on univariate analysis were no history of prior liver resection and ablation margin ≤5 mm (Figure 3a) (See Table 2). Peri-vascular location was not a predictor (P=0.43) (Figure 3b). On multivariate analysis, both ablation margin size ≤5 mm (P<0.001) and no prior liver resection (P=0.002) remained independent predictors of LTP (Table 3). The LTP rate for tumors with a margin of ≤5mm, >5–10mm, and >10 mm (A0) were 60% (n=21/35), 10.5% (n=2/19), and 0% (n=0/6), respectively. Achieving a margin >10 mm had a protective effect on LTPFS for tumors treated with either RFA or MWA (HR: 0.002, 95%CI: 1 – 1.02, p<0.0001 and HR: 0.023, 95%CI: 1 – 1.6, p=0.016, respectively) when compared to margin 0 mm. A statistically significant difference was also noted when compared margin >10 mm to margin 1–5mm for the RFA group (p=0.006), but not for the MWA (p=0.176). Despite the lack of LTP in the over 10 mm margin (0% LTP) in both groups, the difference from the >5–10mm (6/46: 13% LTP) groups did not reach significance (p=0.278 for RFA and 0.968 for MWA).
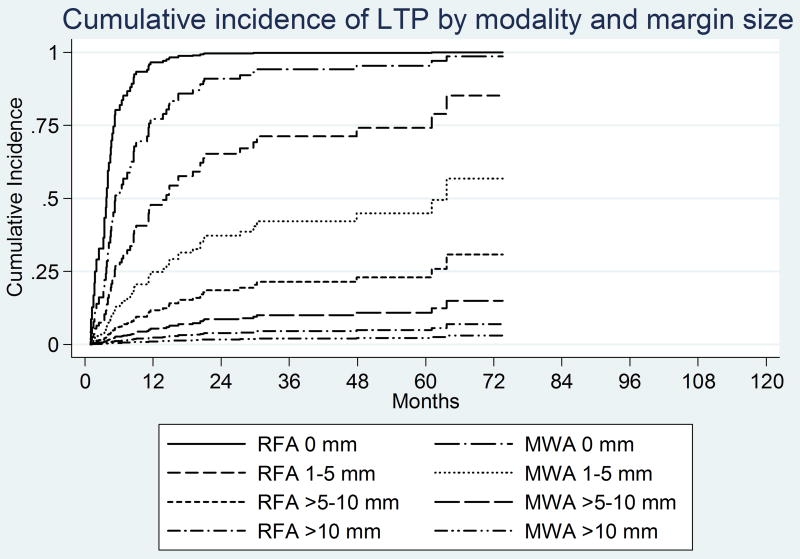
Figure 3.
Kaplan-Meier and cumulative incidence curves by modality (RF and MW) at each margin size.
The LTPFS Kaplan Meier and cumulative incidence curves across the margin categories for both RFA and MWA groups are displayed in figure 4 a–b, showing no significant difference between the groups.
Figure 4.
Complications
Table 4 displays the complications. There was no difference in complication rates between groups (P=0.35).
Table 4.
Complications in the RFA and MWA groups
| Major complications | |||||
|---|---|---|---|---|---|
| RF group | MW group | ||||
|
|
|
||||
| Complication (Class) | No. | Treatment | Complication (Class) | No. | Treatment |
| Pneumothorax (C) | 6 | chest tube | pneumothorax (C) | 4 | chest tube |
| Venous/pulmonary embolism (D) | 1 | Anticoagulation | hepatic artery-portal venous fistula (D) | 1 | Embolization |
| Anuria and bacteremia (BPH patient) (D) | 1 | Foley's catheter and IV antibiotics | bowel perforation (D) | 1 | Surgical repair |
| Intrahepatic hematoma (D) | 1 | Embolization | |||
|
| |||||
| Total (rate) | 9 (13%) | 6 (12%) | |||
| Minor complications | |||
|---|---|---|---|
| RF group | MW group | ||
|
|
|
||
| Complication (Class) | No. | Complication (Class) | No. |
| Pneumothorax (A) | 4 | Pneumothorax (A) | 7 |
| intra/perihepatic hematoma | 3 | Biloma (B) | 2 |
| AV fistula (B) | 1 | left portal vein thrombosis (B) | 1 |
| pain and delirium (A) | 1 | subcapsular hematoma (B) | 1 |
| pleural effusion (B) | 1 | subcutaneous emphysema (B) | 1 |
| pleural effusion (B) | 1 | ||
|
| |||
| Total (rate) | 10 (14%) | 13 (25%) | |
Discussion
Heat sink effect is an important limitation of RFA resulting in higher LTP rates for perivascular tumors (vessel ≥ 3mm in diameter) [17, 18]. Abolishing the heat sink effect using percutaneous balloon occlusion for tumors ≤ 3.5 cm directly adjacent to a major hepatic/portal vein (≥4 mm), reduced the LTP rate to that of non peri-vascular tumors; 11% vs 9%, respectively [19]. Because of the different mechanism and faster rate of tissue heating, MWA may be relatively resistant to the heat sink effect, as shown in animal studies [8, 9, 11, 20]. In this study, peri-vascular tumors had a significantly higher LTP after RFA, similar to prior reports [2, 17]. Peri-vascular location did not impact LTP after MWA. The results presented here validate the findings of animal studies [8, 9, 11, 20], regarding the diminished effect of heat sink on MWA zones. The heat sink effect may still affect MWA [9, 13, 21], however, based on the results of this study and those of animal studies, lower LTP rates and less susceptibility to the heat-sink for peri-vascular tumors can be expected with MWA when compared to RFA.
An interesting finding in the present study was that no history of prior liver resection led to shorter LTPFS in patients treated with microwave ablation. This is probably related to tumor biology. Resectable patients are very carefully selected so they have no extrahepatic disease or known factors related to high risk for subsequent recurrences. Conversely, non-resectable patients are usually in more advanced stages of disease or have high volume of liver disease making surgery impossible. Ablation margins are probably the most important predictor of LTP after RFA of liver tumors [4–6, 18]. In the present study, margin size was a significant predictor of LTP for both MWA and RFA, highlighting the importance of achieving sufficient ablation margins with MWA, as it is for RFA. Although there was no recurrence in the group of CLM ablated with margin over 10 mm (median LPFS: not reached), this difference did not reach significance when compared to the CLM ablated with margin 6–10 mm (LPFS: 64 months). The lack of significance may be due to the small samples in these 2 groups. Thus, based on these results, every effort should be taken to create at least margins >5 mm and ideally >10mm (A0) all around the tumor with either modality. LTP was not noted in any of the A0 ablations (n=22), regardless of modality.
Ablations with margins >5 mm and with histologically proven tumor-free ablation zones have LTP rates (3–15%) [4, 6] comparable to surgical site recurrences after wedge or anatomic resections (3.7–12.8%) [22–24]. Additionally, biopsies of the ablation zone can help detect patients at risk for LTP (by use of prolific or viability markers) and indicate the need for additional ablation or adjuvant therapy similarly to resection with positive margins [6, 25].
A recent meta-analysis of 16 studies (2295 tumors) compared MWA and RFA and concluded that LTP rates were similar [26]. The pooled LTP rate was 13.4% for MW and 11.8% for RF (P=0.91) [26]. The majority of these studies were for HCC; however, the 2 studies for liver metastases reported a significantly lower LTP rate for MWA. One study was for mixed primary tumors with an LTP rate of 8.6% for MWA and 20.3% for RFA (P=0.07) [27] did not clarify the timeline for use of each modality, thus device development and operator experience, that are prone to change over time, may be confounding factors. In the second study, the LTP rate for MWA was 6% versus 20% for RFA (P<0.01) [14]. The LTP rate for the RFA group was significantly higher in the first half of that study (2001–2005) at 33% versus the second half at 15% (P<0.01), likely a result of differences in the surgeons’ experience. MWA was used increasingly in that study from 2008 (20%) till 2011 (98%). The lack of stratification based on ablation margins is perhaps the most important shortfall in that study as it may well account for all observed differences. To avoid confounders of operator experience and differences in margin size, only RFA cases performed since November 2009 were included in the present study, when ablation margin creation and assessment was uniformly established resulting in improved LTP rates [Blinded 1]. In addition, the proportion of margins > 5mm was similar between RFA and MWA groups, and LTPFS was stratified by modality and margin size.
There was no difference in the complication rates between the two modalities in the present study. Previous studies reported similar complication rates for RFA [28] and for MWA[29].
The limitations of this study include the retrospective design, the shorter follow-up period for the MWA versus the RFA group, and the lack of pathologic confirmation of complete ablation with margins free of tumor cells at the end of ablation. Additionally, our study suffers from potential selection bias in regard to the ablation technology used, being at the discretion of the operating physician. As recently shown, biopsy of the ablation zone and tissue morphologic assessment with proliferation and viability markers may further help detect patients at risk for LTP [6]. The need to identify reliable biomarkers [30] and surrogate image biomarkers [31] is an important limitation of percutaneous ablation when compared to resection. This need has led investigators to design special needles that can ablate and sample the tumor with a single application [32]. This might allow pathologic and immunohistochemical confirmation of complete tumor ablation and tumor free margins while minimizing needle passes. Furthermore, the lack of molecular profiling and assessment of tumor biology is another important limitation of this study, as well as the IR literature in general[30, 33].
The manual method of margin assessment is time-consuming and prone to registration errors, as previously outlined[5]. On the other hand, this method is reproducible even in practices that do not have and sometimes cannot even afford software packages that allow 3D assessment of tumors and margins.
In conclusion, sufficient ablation margins remain the most important technical factor for complete tumor ablation. It also provides the most reliable predictor of LTP regardless of the thermal ablation modality used. The creation of sufficient ablation margins is as essential for MWA as it is for RFA. It is clear that A0 (margin >10 mm) is likely to provide complete tumor necrosis and therefore recommended whenever safe and feasible. A minimum margin of at least 5mm is mandatory when offering ablation with curative intent. MWA proved to be resilient to the heat sink effect when compared to RFA, offering good control for peri-vascular tumors. Even though the overall LTP rates for the small CLM treated in this cohort were similar between RFA and MWA, we favor the use of MWA due to its improved ability to treat perivascular tumors, as well as achieving ablation in significantly reduced times and with no need for grounding pads.
Supplementary Material
Acknowledgments
Funding:
This study was partially supported by the NIH/NCI through R21 CA131763-01A1.
Memorial Sloan Kettering Cancer Center is supported by the grant P30 CA008748 from the National Cancer Institute (NCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pathak S, Jones R, Tang JM, et al. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2011;13:e252–65. doi: 10.1111/j.1463-1318.2011.02695.x. [DOI] [PubMed] [Google Scholar]
- 2.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–71. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–68. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 4.Shady W, Petre EN, Gonen M, et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes--A 10-year Experience at a Single Center. Radiology. 2016;278:601–11. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2012 doi: 10.1007/s00270-012-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sotirchos VS, Petrovic LM, Gonen M, et al. Colorectal Cancer Liver Metastases: Biopsy of the Ablation Zone and Margins Can be Used to Predict Oncologic Outcome. Radiology. 2016:151005. doi: 10.1148/radiol.2016151005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. Journal of vascular and interventional radiology : JVIR. 2011;22:755–61. doi: 10.1016/j.jvir.2011.01.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT., Jr Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–9. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 9.Awad MM, Devgan L, Kamel IR, Torbensen M, Choti MA. Microwave ablation in a hepatic porcine model: correlation of CT and histopathologic findings. HPB. the official journal of the International Hepato Pancreato Biliary Association. 2007;9:357–62. doi: 10.1080/13651820701646222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. European journal of radiology. 2011;79:124–30. doi: 10.1016/j.ejrad.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Dodd GD, 3rd, Dodd NA, Lanctot AC, Glueck DA. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology. 2013;267:129–36. doi: 10.1148/radiol.12120486. [DOI] [PubMed] [Google Scholar]
- 12.Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovascular and interventional radiology. 2013;36:505–11. doi: 10.1007/s00270-012-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. Journal of vascular and interventional radiology : JVIR. 2008;19:1087–92. doi: 10.1016/j.jvir.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014;21:4278–83. doi: 10.1245/s10434-014-3817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pua BB, Sofocleous CT. Imaging to optimize liver tumor ablation. Imaging Med. 2010;2:433–43. doi: 10.2217/IIM.10.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241–60. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol. 2008;15:2757–64. doi: 10.1245/s10434-008-0043-7. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480–8. doi: 10.2214/AJR.05.2079. [DOI] [PubMed] [Google Scholar]
- 19.de Baere T, Deschamps F, Briggs P, et al. Hepatic malignancies: percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion. Radiology. 2008;248:1056–66. doi: 10.1148/radiol.2483070222. [DOI] [PubMed] [Google Scholar]
- 20.Bhardwaj N, Strickland AD, Ahmad F, Atanesyan L, West K, Lloyd DM. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology. 2009;41:168–72. doi: 10.1080/00313020802579292. [DOI] [PubMed] [Google Scholar]
- 21.Ringe KI, Lutat C, Rieder C, Schenk A, Wacker F, Raatschen HJ. Experimental Evaluation of the Heat Sink Effect in Hepatic Microwave Ablation. PloS one. 2015;10:e0134301. doi: 10.1371/journal.pone.0134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzzetti E, Pulitano C, Catena M, et al. Impact of type of liver resection on the outcome of colorectal liver metastases: a case-matched analysis. Journal of surgical oncology. 2008;97:503–7. doi: 10.1002/jso.20979. [DOI] [PubMed] [Google Scholar]
- 23.Elias D, Baton O, Sideris L, Matsuhisa T, Pocard M, Lasser P. Local recurrences after intraoperative radiofrequency ablation of liver metastases: a comparative study with anatomic and wedge resections. Ann Surg Oncol. 2004;11:500–5. doi: 10.1245/ASO.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–22. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sofocleous CT, Nascimento RG, Petrovic LM, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology. 2008;249:364–74. doi: 10.1148/radiol.2491071752. [DOI] [PubMed] [Google Scholar]
- 26.Huo YR, Eslick GD. Microwave Ablation Compared to Radiofrequency Ablation for Hepatic Lesions: A Meta-Analysis. J Vasc Interv Radiol. 2015;26:1139–46.e2. doi: 10.1016/j.jvir.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Li S, Wan X, et al. Efficacy and safety of thermal ablation in patients with liver metastases. Eur J Gastroenterol Hepatol. 2013;25:442–6. doi: 10.1097/MEG.0b013e32835cb566. [DOI] [PubMed] [Google Scholar]
- 28.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 29.Livraghi T, Meloni F, Solbiati L, Zanus G. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–74. doi: 10.1007/s00270-011-0241-8. [DOI] [PubMed] [Google Scholar]
- 30.Shady W, Petre EN, Vakiani E, et al. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget. 2017 doi: 10.18632/oncotarget.19806. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornelis F, Storchios V, Violari E, et al. 18F-FDG PET/CT Is an Immediate Imaging Biomarker of Treatment Success After Liver Metastasis Ablation. J Nucl Med. 2016;57:1052–7. doi: 10.2967/jnumed.115.171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wimmer T, Srimathveeravalli G, Silk M, et al. Feasibility of a Modified Biopsy Needle for Irreversible Electroporation Ablation and Periprocedural Tissue Sampling. Technology in cancer research & treatment. 2015 doi: 10.1177/1533034615608739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziv E, Erinjeri JP, Yarmohammadi H, et al. Lung Adenocarcinoma: Predictive Value of KRAS Mutation Status in Assessing Local Recurrence in Patients Undergoing Image-guided Ablation. Radiology. 2017;282:251–8. doi: 10.1148/radiol.2016160003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.