Abstract
The longitudinal pattern of lung function in children with sickle cell anemia (SCA) has shown a decrease in FEV1 % predicted, a risk factor for death in adults with SCA, but predictors for this decline are poorly characterized. In a prospective longitudinal multi-center cohort of children with SCA, we tested the hypotheses that: 1) FEV1 % predicted declines over time; and 2) SCA-specific characteristics and therapy predict this decline. At three clinical centers, children with SCA (HbSS or HbSβ0 thalassemia), unselected for respiratory disease, were enrolled in the Sleep and Asthma Cohort (SAC) study. Study-certified pulmonary function technicians performed spirometry and lung volumes. Each assessment was reviewed centrally. Predicted values were determined for TLC, FEV1, FVC, and FEV1/FVC ratio. A total of 197 participants, mean age 11.0 years at first testing (range 4–19.3 years), had a minimum of three spirometry measurements, over an average of 4.4 years (range 1.08–6.5 years) from baseline to endpoint. In a multivariable model, FEV1 % predicted declines by 0.3% for every additional year of age (95% CI −0.56 – −0.05, p=0.020). Sex, asthma history, hemoglobin, reticulocyte count, white blood cell count, incidence rate of severe acute pain and acute chest syndrome episodes, and hydroxyurea therapy were not associated with a decline in FEV1 % predicted. In a large, rigorously evaluated, prospective cohort of an unselected group of children with SCA, FEV1 % predicted declines minimally over an average of 4 years, and none of the examined disease features predict the decline.
Keywords: Lung function, sickle cell anemia, FEV1
Introduction
Pulmonary abnormalities are being increasingly recognized as a significant cause of morbidity and mortality in sickle cell anemia (SCA), including acute chest syndrome (ACS), asthma, recurrent wheezing, sleep disordered breathing, dyspnea, and pulmonary hypertension. Improved childhood survival has resulted in increased recognition of chronic lung disease in older patients with SCA. Studies are now beginning to explore the impact of pulmonary complications on lung function for adults and children with SCA.1 While most children with SCA have lung function that is within normal limits,2,3 lung growth is significantly reduced compared to age and race matched controls.4 By adulthood, restrictive defects are commonly reported.5,6 However, few studies have prospectively evaluated the longitudinal trajectory of lung function in children with SCA.
Decreased FEV1 % predicted has been associated with an increased risk for earlier death in the general population7–9 and in individuals with cystic fibrosis.10,11 Similarly, longitudinal data from the Cooperative Study of Sickle Cell disease showed that adults with SCA who had a decrease in expiratory volume in 1 second (FEV1) died earlier over the course of five years compared to those who did not.12 Only three studies have been performed in children with SCA to understand what factors, if any, contribute to a longitudinal decline in FEV1 % predicted. The three prior studies, two retrospective and one prospective, have shown variability in the magnitude of FEV1 % predicted decline over time (0.9–3.3% per year)13–15 leading to difficulty for providers in interpreting this change.
None of the previous studies have accounted for intra-individual variability of the test results using the coefficient of variation (CV), which gives an estimation of the expected participant biological and instrument variability.16 We tested the hypothesis that children with SCA have a decline in FEV1 % predicted and SCA-specific characteristics, treatment with hydroxyurea, or both, predict a decline in FEV1 % predicted. We also sought to interpret any observable change in lung function relative to a calculated age-based CV for children with SCA.
Methods
Study design and recruitment
The Sleep and Asthma Cohort (SAC) is a prospective cohort study of children and adolescents with SCA, unselected for respiratory disease. Participants ranged from 4 to 18 years of age and were enrolled and followed between 2005 and 2011 at three clinical centers: Washington University School of Medicine in St. Louis, Missouri; Case Western Reserve University in Cleveland, Ohio; and UCL Great Ormond Street Institute of Child Health in London, UK (which recruited from three London hospitals). Institutional review boards for each site approved the study protocol. Participants were either homozygous for sickle cell hemoglobin [HbSS (N=240)] or compound heterozygous for sickle β thalassemia zero [HbSβ0 (N=12)]. Children were not eligible for participation in the study if they received long term blood transfusion therapy, received overnight oxygen or continuous positive airway pressure (CPAP) support, had chronic lung disease other than asthma (such as sarcoidosis), or were known to be human immune deficiency virus (HIV) positive. After the start of the study, data from participants initiated on chronic blood transfusion therapy were censored from this point forward due to the known decrease in pain events following initiation of chronic transfusion therapy. Parents provided written informed consent for all participants and patients provided assent when appropriate. Full manual of operations for the study design can be provided upon request.
Pulmonary Function Testing
Pulmonary function tests were obtained when participants were at least 4 weeks after discharge from the hospital for SCA complications and at baseline health without current illness, pain, or acute respiratory symptoms. Pulmonary function testing (spirometry and body plethysmography) was done at study enrollment and then at least annually during the study period. Total number of pulmonary function tests was highly dependent on participants’ ability to perform the test with adequate quality control measures. Spirometry was performed using best maximal effort as selected by study certified pulmonary function technicians using a pneumotachograph-type spirometer interfaced with a personal computer system (Jaeger MasterScope; VIASYS, Hoechberg, Germany) as described in detail by Field.17 Spirometry was performed at least 4 hours after the use of a short-acting bronchodilator and 12 hours after use of a long-acting bronchodilator according to ATS standards for children.18,19 Measures obtained and validated through rigorous quality control include FEV1 and forced vital capacity (FVC). Static lung volume measurements were performed at the time of spirometry measurements using a Jaeger MasterScreen (Cleveland and London) or Sensor Medics VMAX (St. Louis) plethysmograph per ATS/ European Respiratory Society (ERS) standards.20 Results for spirometry and lung volumes from all three centers were over-read by a senior technician to ensure that results were valid.
Abnormal lung function definition
Predicted values were determined for each participant based on their age, sex, height, and race for FEV1, FVC, and FEV1/FVC ratio using the Global Lung Function 2012 multi-ethnic reference equations (GLI).21,22 Abnormal results for FEV1, FVC, and FEV1/FVC ratio were determined by comparison to their lower limits of normal (LLN) defined with a cut-off at the 5th percentile (LLN 5%, z-score −1.64).21 Reference equations for TLC were used23 with an African-American adjustment of 12%.24 Participants were categorized with a restrictive abnormality if their TLC was less than 80% predicted per ATS/ERS recommendations25 with a normal FEV1/FVC, an obstructive abnormality if their FEV1/FVC was less than LLN, and a mixed pattern if both TLC and FEV1/FVC were less than the LLN.25 Nonspecific pattern was defined as reduced FEV1 and/or FVC with a normal FEV1/FVC and normal TLC.26,27
Clinical Covariates
Postulated covariates were selected due to their proposed impact on lung disease in children with SCA and included: age at enrollment, sex, baseline hemoglobin level, baseline white blood cell count, baseline reticulocyte count, incidence rates of acute pain or ACS episodes, treatment with hydroxyurea at baseline and throughout the study, and a history of asthma.28 Asthma was defined as a clinical diagnosis made by a physician coupled with current use of asthma medication.29 ACS was defined as a new clinical or radiographic pulmonary infiltrate in the context of an acute illness characterized by respiratory symptoms (cough, wheezing, rales, chest pain, decreased oxygen saturation (>2%) from baseline, use of accessory muscles of respiration, or increased respiratory rate) with or without fever. Pneumonia was included in this definition. An acute pain episode was defined as a hospitalization for SCA-associated pain, excluding headaches, and requiring opioid treatment. All ACS and pain episodes in the first three years of the study were reviewed by a single investigator at each participating site with over-reading by the principal investigator (M.R.D), to ensure uniform definitions of ACS and pain in this multi-center study.
Coefficient of Variation
A separate sub-analysis to calculate the CV was performed. Participants with 2 spirometry measurements within a 6-month window were included in the sub-analysis. The CV was calculated from these paired spirometry measurements for FEV1 % predicted, FVC % predicted, and FEV1/FVC % predicted. For the group of paired measurements, the CV is the ratio of the standard deviation (SD) of the mean to the mean value of all measurements. The CV was calculated separately for paired measurements in participants below and above 16 years of age. We selected to have two separate CV for older and younger participants to allow for anticipated improvement in reproducible spirometry technique when comparing the effort of a 4 year old compared to a 16 year old.
Comparison to adult cohort of participants with SCA
To predict if children in our cohort would have a rate of decline in FEV1 % predicted comparable to what has been observed in adults with SCA, we included original data from two adult SCA cohorts. The Cooperative Study of Sickle Cell Disease (CSSCD) included physical examinations with spirometry evaluations from March 6, 1979 to August 13, 1993.30 We also analyzed a more contemporary cohort of adults with SCA followed at the Vanderbilt-Meharry Center for Excellence in Sickle Cell Disease in Nashville, TN from January 1, 2003 to January 31, 2016. Methods for data collection of the Vanderbilt-Meharry cohort are described in detail as per Chaturvedi et al.31
Statistical Analysis
Descriptive statistics are presented for demographic and clinical factors. A multivariable mixed model regression analysis was used to predict change over time in three spirometry parameter outcomes (FEV1 % predicted, FVC % predicted, and FEV1/FVC % predicted) with a pre-specified set of covariates. Hydroxyurea use was measured as a time varying covariate. Therefore, if an individual was started on hydroxyurea after first spirometry, the change in the remainder of their spirometry values would be analyzed including hydroxyurea use as a covariate. The mixed model approach was chosen to allow longitudinal data for participants to be combined into a single model while taking into account intra-individual correlations in spirometry values. As per mixed model methods, participants with missing data on a covariate are not excluded, and participants can have different numbers of spirometry measures. The models also allow for both random intercepts and slopes to assess the effect of age, which allow each participant to have their own intercept, slope, or both. The CV was calculated for spirometry measurements within six months of each other. The CV was then compared to actual change in each lung function parameter for each participant. Analyses were conducted using IBM SPSS Statistics (Version 24, Armonk, NY, IBM).
Results
Demographics
A total of 223 participants were enrolled and had spirometry measurements performed. Of these participants, 197 were eligible for the primary analysis as they had at least three (range 3–8) spirometry measurements performed over a minimum follow up of 1 year (range 1.1–6.5 years). The only statistical differences in demographic features were in age, as the excluded group were younger (median 8.8 years versus 10.7 years). The only differences in 16 baseline clinical or laboratory features were the excluded group had a higher proportion of bronchodilator response to albuterol and a slightly lower baseline FEV1 % predicted (mean 82.82 versus 88.48), Supplemental Table 1. Mean age of our final cohort at first testing was 11.0 years (range 4–19.3 years). Table 1 shows descriptive statistics of the final sample. Supplemental Figure 1 provides a schematic of our study design.
Table 1.
Characteristics of the Sleep and Asthma Cohort (SAC) study population (N = 197) that completed at least 3 spirometry over a minimum of 1 year
| Participant characteristic | N=197 |
|---|---|
|
| |
| Prospective follow-up (years) | Median 4.7; IQR 3.3–5.4 |
| Range 1.1 to 6.5 | |
|
| |
| Number of spirometry evaluations per patient, n (%) | |
|
| |
| 3 | 53 (26.9) |
|
| |
| 4 | 39 (19.8) |
|
| |
| 5 | 54 (27.4) |
|
| |
| 6 | 26 (13.2) |
|
| |
| 7 | 14 (7.1) |
|
| |
| 8 | 11 (5.6) |
|
| |
| Age at first spirometry (years) | Median 10.7, IQR 8.0–14.2 |
| Range 4 to 19.3 | |
|
| |
| Gender, male, N (%) | 94 (47.7) |
|
| |
| Hemoglobin (g/dl), mean (std. dev.) | 8.2 (1.2) |
|
| |
| WBC (k/mm3), mean (std. dev.) | 11.7 (3.6) |
|
| |
| Reticulocyte (%), mean (std. dev.) (N=196) | 11.4 (5.6) |
|
| |
| Has asthma, N (%) | 58 (29.4) |
|
| |
| On hydroxyurea at first spirometry, N (%), (N=194) | 24 (12.4) |
|
| |
| On hydroxyurea during study period, N (%), (N=194) | 64 (33.0) |
|
| |
| On ICS at first spirometry, N (%) | 40 (20.3) |
|
| |
| On ICS during study period, N (%) | 56 (28.4) |
|
| |
| BD response >=12.0, n (%), (N=191) | 32 (16.8) |
|
| |
| ACS rate during study period, per year, mean (std. dev.) | 0.24 (0.36) |
|
| |
| Pain rate during study period, per year, mean (std. dev.) | 0.86, (1.23) |
|
| |
| FEV1% predicted at first spirometry, mean (std. dev.) | 88.48 (13.04) |
|
| |
| FEV1% predicted at last spirometry, mean (std. dev.) | 87.88 (12.77) |
|
| |
| FVC% predicted at first spirometry, mean (std. dev.) | 92.78 (13.68) |
|
| |
| FVC% predicted at last spirometry, mean (std. dev.) | 92.96 (13.00) |
|
| |
| FEV1/FVC% predicted at first spirometry, mean (std. dev.) | 95.22 (7.23) |
|
| |
| FEV1/FVC% predicted at last spirometry, mean (std. dev.) | 94.27 (7.06) |
Age is the only covariate predictive of a significant decline in FEV1 % predicted
The multivariable linear regression mixed model demonstrated that FEV1 % predicted declines by 0.3% per year (95% CI −0.56, −0.05, p=0.02). Other than age, no postulated risk factors were associated with a decline in FEV1 % predicted over time (Table 2 and Figure 1). We directly tested the effect of duration of follow-up on decline in FEV1 % predicted by substituting that variable for age, and adding baseline age, but follow-up time was not significant (p=0.49). Age was not significantly associated with change in FVC % predicted (−0.20% per year, CI −0.45 – 0.06, p=0.13). In the multivariable model, no covariates were associated with a change in FEV1/FVC % predicted over time, including age (−0.07% per year, 95% CI −0.21– 0.08, p=0.35). In the multivariable model, any hydroxyurea use (at baseline and/or throughout the study) was associated with a higher FVC % predicted using time varying covariates (2.02% per year, 95% CI 0.04 – 4.0, p=0.05). Results are summarized in Table 2 and Figure 1.
Table 2.
Multivariable mixed regression model of longitudinal change in spirometry parameters over time in 197 children with sickle cell anemia enrolled between 4 and 18 years of age, and followed prospectively for a median of 4.7 years in the Sleep and Asthma Cohort (SAC) study
| 1a. Multivariable model of change in FEV1 %predicted# | |||
| Covariate | B | 95% CI | P value |
| Male sex | 0.20 | −3.15 – 3.56 | 0.91 |
| Age | −0.30 | −0.56 – −0.05 | 0.02 |
| Asthma | −2.29 | −6.05 – 1.48 | 0.23 |
| Hydroxyurea use | 1.14 | −0.88 – 3.16 | 0.27 |
| Hemoglobin at baseline | 0.99 | −0.46 – 2.44 | 0.18 |
| White blood cell count at baseline | 0.16 | −0.32 – 0.65 | 0.50 |
| Reticulocyte count at baseline | 0.13 | −0.19 – 0.46 | 0.41 |
| Pain incidence rate | −0.03 | −0.34 – 0.29 | 0.87 |
| ACS incidence rate | −0.05 | −0.87 – 0.77 | 0.91 |
| 1b. Multivariable model of change in FVC %predicted# | |||
| Male sex | 1.37 | −2.21 – 4.94 | 0.45 |
| Age | −0.20 | −0.45 – 0.06 | 0.13 |
| Asthma | −0.38 | −4.41 – 3.64 | 0.85 |
| Hydroxyurea use | 2.12 | 0.14 – 4.12 | 0.04 |
| Hemoglobin at baseline | 0.98 | −0.56 – 2.53 | 0.21 |
| White blood cell count at baseline | 0.22 | −0.29 – 0.74 | 0.40 |
| Reticulocyte count at baseline | 0.13 | −0.21 – 0.47 | 0.46 |
| Pain incidence rate | −0.03 | −0.34 – 0.27 | 0.83 |
| ACS incidence rate | −0.60 | −1.40 – 0.19 | 0.14 |
| 1c. Multivariable model of change in FEV1/FVC %predicted* | |||
| Male sex | −0.74 | −2.47 – 1.00 | 0.40 |
| Age | −0.07 | −0.21 – 0.07 | 0.35 |
| Asthma | −1.74 | −3.68 – 0.21 | 0.08 |
| Hydroxyurea use | −0.45 | −1.72 – 0.81 | 0.48 |
| Hemoglobin at baseline | 0.06 | −0.70 – 0.81 | 0.88 |
| White blood cell count at baseline | −0.04 | −0.29 – 0.21 | 0.73 |
| Reticulocyte count at baseline | 0.04 | −0.13 – 0.20 | 0.67 |
| Pain incidence rate | 0.01 | −0.20 – 0.21 | 0.96 |
| ACS incidence rate | 0.34 | −0.19 – 0.86 | 0.21 |
Abbreviations: ACS=acute chest syndrome; SCA=sickle cell anemia; IRR=incidence rate ratio; CI=confidence interval
Includes random intercepts and slopes
Includes random intercepts
Mann-Whitney U test
Mid-p exact test
Figure 1.

Multivariable mixed regression model of longitudinal change in FEV1 % predicted and FVC % predicted (with bars representing 95% CI) over time in 197 children with SCA between 4 and 18 years of age followed prospectively for a mean of 4 years in the Sleep and Asthma Cohort (SAC) study.
Calculated coefficient of variation was lower in children greater than 16 years of age
From the 197 participants in the analysis, 142 were included in the analysis to calculate the coefficients of variation for FEV1 % predicted, FVC % predicted, and FEV1/FVC % predicted as they had at least two spirometry measurements completed less than 6 months apart (Supplemental Figure 1). Among 142 participants, 116 pairs were analyzed before age 16 and 26 pairs at age 16 or later. The CV was calculated separately within each age subgroup to allow for change as participants age, as previous data has demonstrated an increase in variability in spirometry measurements in younger children as compared to older adults and adolescents.32 The mean time period between paired measurements was 5.4 months. Calculated CV for FEV1 % predicted before age 16 was 7.32% and 4.46% after age 16 and for other spirometry values with 95% confidence intervals listed in Supplemental Table 2.
Majority of participants did not have decline in spirometry values greater than coefficient of variation
To apply the CV to the individual change in our cohort of 197 participants, we used the age of the first spirometry (below or above/equal to 16 years of age) to determine which CV to use. We found that the majority of our cohort did not demonstrate a decline on any lung function parameter greater than the calculated CV (N=100, 50.8%). Fifty three participants (26.9%) had a decrease in FEV1 % predicted greater than the calculated CV while 51 participants (25.9%) showed an increase in FEV1 % predicted greater than the calculated CV. Supplemental Table 2 describes the number of participants with a decline in lung function greater than the CV for each spirometry parameter. Supplemental Figure 2 provides examples of the change in FEV1 % predicted for several patients to demonstrate the observed variation.
Higher baseline spirometry measurements are associated with a decrease in FEV1, FVC, FEV1/FVC % predicted values that are greater than the CV
Multivariable logistic regression models demonstrate that a larger baseline FEV1 % predicted was associated with a higher odds of having a decline in FEV1 % predicted that exceeds the CV (OR 1.06, 95% CI 1.03 – 1.09, p<0.001), such that those participants beginning with a 1% higher FEV1 at baseline were 6% more likely to have a decline in FEV1 % predicted that exceeds the CV. None of the other assessed covariates were associated with a decrease in FEV1 % predicted.
Similarly, only baseline FVC % predicted is associated with a larger decrease in FVC % predicted (OR 1.06, 95% CI 1.03–1.09, p<0.001). None of the other assessed covariates are associated with a decrease in FVC % predicted greater than the CV. Baseline FEV1/FVC % predicted is associated with a decline in FEV1/FVC % predicted greater than the CV (OR 1.18, 95% CI 1.10–1.26, p<0.001). Age also had a negative relationship to a decrease in FEV1/FVC % predicted (OR 0.89, 95% CI 0.81 – 0.98, p=0.012), such that participants who are younger at baseline were more likely to have a decline in the FEV1/FVC % predicted greater than the CV.
Baseline lung function pattern is not associated with future lung function pattern
Participants in our cohort did not demonstrate a consistent change in lung function pattern over time. A total of 81 participants had complete spirometry and TLC data available for categorization of lung function category at baseline and endpoint. Participants with complete TLC data were noted to be older at first spirometry measurement (mean age 12.7 years vs 9.6 years, p<0.001), and had longer prospective follow up (mean 4.6 years vs 3.8 years, p<0.001). The majority of the participants (N= 47, 58.0%) started the study with normal lung function and ended with study with normal lung function. Results are summarized in Supplemental Table 3.
Discordance between predicted and observed decline in FEV1 % in children and adults with SCA
In the CSSCD adult cohort, 430 participants had a mean age of 32.6 years, with a mean FEV1 % predicted of 77.4%.12 In the more contemporary adult Vanderbilt-Meharry SCA cohort, 197 adults with a mean age of 31.8 years, demonstrated a mean FEV1 % predicted of 77.2%.31 If we extrapolate the predicted decline in FEV1 % predicted of 0.3% per year in the SAC cohort, starting with an 11 year old participant, 19 years later (by 32 years of age) the predicted mean FEV1 % predicted would be 88%. This extrapolated result is discordant with the observed FEV1 % predicted of 77% measured in both CSSCD and the Vanderbilt –Meharry Sickle Cell Disease Center adult cohorts (Figure 2).
Figure 2.
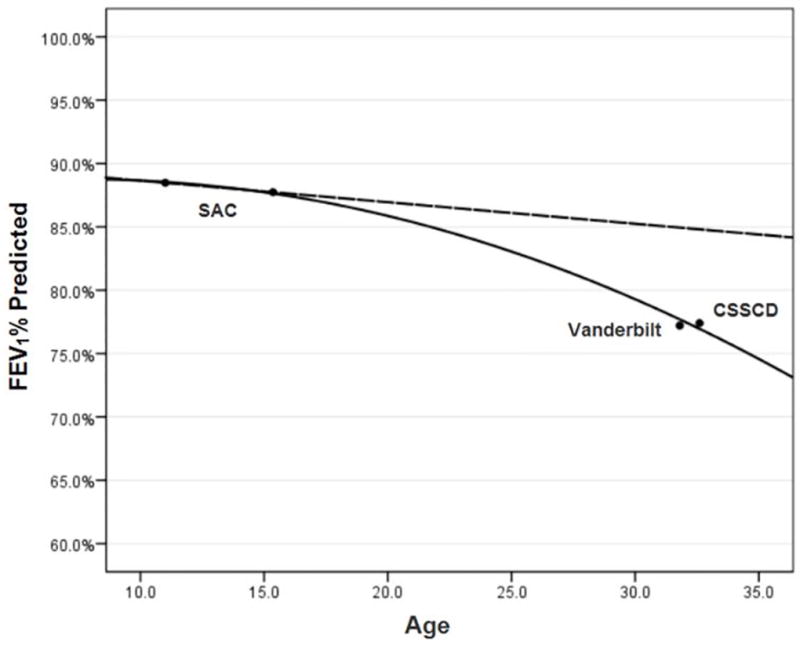
Decline in FEV1 % predicted in the Sleep and Asthma Cohort (SAC) (mean ages 11 and 15 years), and extrapolated decline at later ages, as compared to the Cooperative Study of Sickle Cell Disease (CSSCD) (mean age 33 years) and Vanderbilt Cohort (mean age 32 years).
Discussion
In a large prospective, rigorously evaluated study of 197 children with SCA who had undergone a minimum of three spirometry measurements over a median of 4.7 years, we have demonstrated that children have a very small decline in FEV1 % predicted over time (0.3% per year) and none of the expected covariates were associated with this decline. This observed change was considerably less than the predicted average yearly decline that has been previously reported.14,15,33 For the first time in a cohort of children with SCA, we present lung function change over time in the context of the coefficient of variation, such that 52.8% of our cohort had an absolute change (increase or decrease) in FEV1 that exceeded the coefficient of variation for one or more lung function parameters.
Our findings demonstrate a smaller decline in FEV1 % predicted per year when compared to other studies in children with SCA. In a retrospective analysis of 45 children with at least two spirometry measurements performed a minimum of a year apart, Koumbourlis et.al. showed a generally normal pattern of lung function but with a significant decrease in FEV1 % predicted from first to second measurement (87 +/− 21 vs. 80 +/− 15, p<0.001), with a decline over time of approximately 2% per year.33 A second retrospective study by MacLean et al. of 312 children and adolescents with SCA showed a significant decline in FEV1 % predicted of approximately 3% per year, which was variable by sex and hemoglobin phenotype.14 In the only prior prospective study, Lunt et al. demonstrated a decline of 1.7% per year in one cohort and 0.9% per year in a second cohort.15 Findings of the prior SCA cohort studies evaluating FEV1 % predicted are summarized in Supplemental Table 4.
The distribution of lung function categorization found in our cohort at baseline is similar to reports in other cohorts of children with SCA.15,33 While a change in lung function pattern was observed in some of our study population, this pattern was not significant or predictable over time. Our data suggest that the progression from normal to restrictive defects, may not begin in childhood.
A critical component in interpreting longitudinal changes in FEV1% predicted in the current study and all previous studies, is the coefficient of variation. Unfortunately none of the prior three studies estimating FEV1 % predicated determined the CV. Our calculated CV for FEV1 % predicted in our population of children with SCA less than and greater than 16 years of age, was 7.32% and 4.46%, respectively showing more variation in the younger children compared to adolescents. The CV for FEV1 % for the group in total was 6.91%. Studies of healthy adult individuals, using a similar instrument, have reported a CV for FEV1 of 2.74%.16 Studies in adult asthma, chronic obstructive pulmonary disease and CF have reported larger CV of 3.77% 34, 8.29% 34, and approximately 5% 35, respectively. Our results appear to be within range of other chronic illnesses, with as expected clinically, more variation in the CV being observed in the younger population. For the subset of participants who did demonstrate a decline in FEV1 % predicted larger than the CV, none of the postulated clinical covariates nor treatment with hydroxyurea were associated with this decline. Only individuals with a higher baseline FEV1 % predicted were more likely to show a significant decline greater than the CV and the result was likely due to regression to the mean, caused by random variation in observed values around the true mean.36
An unexpected finding in our cohort is the association of an increase in FVC % predicted (but not FEV1 % predicted) with hydroxyurea use shown in the multivariable model (2.02, 95% CI 0.04–4.0, p=0.05). However, this small change is well within the boundaries of the 95% confidence intervals of CV for FVC measured in this cohort(< 16 years of age: 6.21%, (5.56–7.03%) age ≥ 16 years of age: 3.76%, (3.36–4.26%), raising the question as to whether the measured increase in FVC% predicted is random or clinically significant. None of the other three studies evaluating change in FVC demonstrated an increase in FVC% predicated.
McLaren et al. demonstrated a decline an FEV1 and FVC over time with use of hydroxyurea; however, this decline was less in those on hydroxyurea when compared to those not on hydroxyurea.37 Further studies are needed to clarify the impact of hydroxyurea use on pulmonary function over time in children with SCA.
Our study is limited in that all factors contributing to a decline in lung function over time may not have been assessed. Our analysis focused on what has been perceived as the most clinically relevant risk factors in SCA based on the available literature at the time of study entry. Additionally, while we followed a large number of participants, the mean duration was approximately 4 years and the mean age was 11 years. Quite possibly our participants were too young and followed for too short of a period of time to demonstrate a large change in lung function during the study period. Based on the substantial discrepancy between observed and expected FEV1 % predicted in adults with SCA, we postulate that there is a greater change in lung function occurring beyond the average age in our cohorts, 11.0 years, and below the age of the CSSCD and Vanderbilt- Meharry Sickle Center adult cohorts, 32 years. We postulate our cohort simply did not have a sufficient long enough follow-up period to identify possible clinical risk factors that may result in significant decline in FEV1 % predicted observed in adults with SCA. Furthermore, as has been suggested in children with cystic fibrosis38, conventional spirometry may not be the best tool to test for lung disease in children with SCA. Consideration should be given to predictors of lung disease such as lung clearance index and assessment of lung structure to truly capture early lung disease in children.
In a large, rigorously evaluated, and prospective research cohort of children with SCA, FEV1 % predicted declined minimally over an average of 4 years and none of the SCA specific clinical characteristics, significantly predicted a decline in lung function. Additionally, we found that the small FEV1 % predicted decline in children was discrepant with the observed average FEV1 % predicted in two adult cohorts. New longitudinal studies that extend across the transition age group into adulthood and more refined pulmonary function assessment methods are needed to further explore the impact of sickle cell lung disease on pulmonary growth and function in young adults with SCA.
Supplementary Material
Acknowledgments
Supported in part by the National Heart, Lung, and Blood Institute: NIH 1R01HL079937 (DeBaun), UL1 RR024989 (CWRU CRU) and by Research and Development in the National Health Service (UK)
Footnotes
This article has an online data supplement, which is accessible from this issue's table of content online.
Author Contributions: S.M.W. analyzed and interpreted the data, drafted and revised the manuscript, and approved the version to be published. R.C. interpreted the data, drafted and revised the manuscript, and approved the version to be published. M.R. analyzed and interpreted the data, drafted and revised the manuscript, and approved the version to be published. S.S.R., C.L.R., and F.J.K. contributed to conception and design of the study, acquisition of the data, and critical revision of the manuscript, and approved the version to be published. M.R.D. conceived and designed the study, participated in acquisition and interpretation of the data and drafting and revision of the manuscript, and approved the final version to be published.
References
- 1.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84(6):363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 2.Cohen R, Strunk RC, Rodeghier M. Pattern of lung function is not associated with prior or future morbidity in children with sickle cell anemia. Ann Am Thorac Soc. 2016;13(8):1314–1323. doi: 10.1513/AnnalsATS.201510-706OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arteta M, Campbell A, Nouraie M, et al. Abnormal pulmonary function and associated risk factors in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol. 2014;36(3):185–189. doi: 10.1097/MPH.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field JJ, DeBaun MR, Yan Y, Strunk RC. Growth of lung function in children with sickle cell anemia. Pediatr Pulmonol. 2008;43(11):1061–1066. doi: 10.1002/ppul.20883. [DOI] [PubMed] [Google Scholar]
- 5.Klings ES, Wyszynski DF, Nolan VG, Steinberg MH. Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med. 2006;173(11):1264–1269. doi: 10.1164/rccm.200601-125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen N, Kozanoglu I, Karatasli M, et al. Pulmonary function and airway hyperresponsiveness in adults with sickle cell disease. Lung. 2009;187(3):195–200. doi: 10.1007/s00408-009-9141-y. [DOI] [PubMed] [Google Scholar]
- 7.Hole DJ, Watt GC, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorlie PD, Kannel WB, O’connor G. Mortality associated with respiratory function and symptoms in advanced age: the Framingham study. Am Rev Respir Dis. 1989;140(2):379–384. doi: 10.1164/ajrccm/140.2.379. [DOI] [PubMed] [Google Scholar]
- 9.Stavem K, Aaser E, Sandvik L. Lung function, smoking and mortality in a 26-year follow-up of healthy middle-aged males. Eur Respir J. 2005;25(4):618. doi: 10.1183/09031936.05.00008504. [DOI] [PubMed] [Google Scholar]
- 10.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326 (18):1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 11.Kerem E, Viviani L, Zolin A, et al. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS Patient Registry. Eur Respir J. 2014;43(1):125. doi: 10.1183/09031936.00166412. [DOI] [PubMed] [Google Scholar]
- 12.Kassim AA, Payne AB, Rodeghier M, et al. Low forced expiratory volume is associated with earlier death in sickle cell anemia. Blood. 2015;126(13):1544–1551. doi: 10.1182/blood-2015-05-644435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catanzaro T, Koumbourlis AC. Somatic growth and lung function in sickle cell disease. Paediatr Respir Rev. 2014;15(1):28–32. doi: 10.1016/j.prrv.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 14.MacLean JE, Atenafu E, Kirby-Allen M, et al. Longitudinal decline in lung volume in a population of children with sickle cell disease. Am J Respir Crit Care Med. 2008;178(10):1055–1059. doi: 10.1164/rccm.200708-1219OC. [DOI] [PubMed] [Google Scholar]
- 15.Lunt A, Mcghee E, Sylvester K, et al. Longitudinal assessment of lung function in children with sickle cell disease. Pediatr Pulmonol. 2016;723:717–723. doi: 10.1002/ppul.23367. [DOI] [PubMed] [Google Scholar]
- 16.Jensen RL, Teeter JG, England RD, et al. Sources of long-term variability in measurements of lung function: implications for interpretation and clinical trial design. Chest. 2007;132(2):396–402. doi: 10.1378/chest.06-1999. [DOI] [PubMed] [Google Scholar]
- 17.Field JJ, Stocks J, Kirkham FJ, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139(3):563–568. doi: 10.1378/chest.10-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Kirkby J, Welsh L, Lum S, et al. The EPICure study: comparison of pediatric spirometry in community and laboratory settings. Pediatr Pulmonol. 2008;43(12):1233–1241. doi: 10.1002/ppul.20950. [DOI] [PubMed] [Google Scholar]
- 20.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quanjer PH, Hall GL, Stanojevic S, et al. Age- and height-based prediction bias in spirometry reference equations. Eur Respir J. 2012;40 (1):190–197. doi: 10.1183/09031936.00161011. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal M, Cramer D, Bain SH, et al. Lung function in white children aged 4 to 19 years: II-Single breath analysis and plethysmography. Thorax. 1993;48(8):803–808. doi: 10.1136/thx.48.8.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkby J, Bonner R, Lum S, et al. Interpretation of pediatric lung function: impact of ethnicity. Pediatr Pulmonol. 2013;48(1):20–26. doi: 10.1002/ppul.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 26.Hyatt RE, Cowl CT, Bjoraker JA, Scanlon PD. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest. 2009;135(2):419–424. doi: 10.1378/chest.08-1235. [DOI] [PubMed] [Google Scholar]
- 27.Iyer VN, Schroeder DR, Parker KO, Hyatt RE, Scanlon PD. The nonspecific pulmonary function test: longitudinal follow-up and outcomes. Chest. 2011;139(4):878–886. doi: 10.1378/chest.10-0804. [DOI] [PubMed] [Google Scholar]
- 28.DeBaun MR, Rodeghier M, Cohen R, et al. Factors predicting future ACS episodes in children with sickle cell anemia. Am J Hematol. 2014;89(11):E212–E217. doi: 10.1002/ajh.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strunk RC, Cohen RT, Cooper BP, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. J Pediatr. 2014;164(4):821–826. e1. doi: 10.1016/j.jpeds.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaston M, Smith J, Gallagher D, et al. Recruitment in the cooperative study of sickle cell disease (CSSCD) Control Clin Trials. 1987;8(4 Suppl):131S–140S. doi: 10.1016/0197-2456(87)90016-x. [DOI] [PubMed] [Google Scholar]
- 31.Chaturvedi S, Labib Ghafuri D, Kassim A, Rodeghier M, DeBaun MR. Elevated tricuspid regurgitant jet velocity, reduced forced expiratory volume in 1 second, and mortality in adults with sickle cell disease. Am J Hematol. 2017;92(2):125–130. doi: 10.1002/ajh.24598. [DOI] [PubMed] [Google Scholar]
- 32.Cole TJ, Stanojevic S, Stocks J, et al. Age- and size-related reference ranges: a case study of spirometry through childhood and adulthood. Stat Med. 2009;28(5):880–898. doi: 10.1002/sim.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koumbourlis AC, Lee DJ, Lee A. Longitudinal changes in lung function and somatic growth in children with sickle cell disease. Pediatr Pulmonol. 2007;42(6):483–488. doi: 10.1002/ppul.20601. [DOI] [PubMed] [Google Scholar]
- 34.Timmins SC, Coatsworth N, Palnitkar G, et al. Day-to-day variability of oscillatory impedance and spirometry in asthma and COPD. Respir Physiol Neurobiol. 2013;185(2):416–424. doi: 10.1016/j.resp.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Nickerson BG, Lemen RJ, Gerdes CB, Wegmann MJ, Robertson G. Within-subject variability and per cent change for significance ofspirometry in normal subjects and in patients with cystic fibrosis. Am Rev Respir Dis. 1980;122(6):859–866. doi: 10.1164/arrd.1980.122.6.859. [DOI] [PubMed] [Google Scholar]
- 36.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2004;34(1):215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 37.McLaren A, Klingel M, Behera S, et al. Effect of hydroxyurea therapy on pulmonary function in children with sickle cell anemia. Am J Respir Crit Care Med. 2017;195(5):689–691. doi: 10.1164/rccm.201606-1119LE. [DOI] [PubMed] [Google Scholar]
- 38.Sly PD, Wainwright CE. Preserving lung function: the holy grail in managing cystic fibrosis. Ann Am Thorac Soc. 2017;14(6):833–835. doi: 10.1513/AnnalsATS.201703-254ED. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


