Abstract
Background
HIV-infected (HIV+) donor organs can be transplanted into HIV+ recipients under the HIV Organ Policy Equity (HOPE) Act. Quantifying HIV+ donor referrals received by Organ Procurement Organizations (OPOs) is critical for HOPE Act implementation.
Methods
We surveyed the 58 US OPOs regarding HIV+ referral records and newly discovered HIV+ donors. Using data from OPOs that provided exact records and CDC HIV prevalence data, we projected a national estimate of HIV+ referrals.
Results
Fifty-five (95%) OPOs reported HIV+ referrals ranging from 0–276 and newly discovered HIV+ cases ranging from 0–10 annually. Six OPOs in areas of high HIV prevalence reported more than 100 HIV+ donor referrals. Twenty-seven (47%) OPOs provided exact HIV+ referral records and 28 (51%) OPOs provided exact records of discovered HIV+ cases, totaling 1,450 HIV+ referrals and 39 discovered HIV+ donors in the prior year. These OPOs represented 67% and 59% of prevalent HIV cases in the US; thus, we estimated 2,164 HIV+ referrals and 66 discovered HIV+ cases nationally per year.
Conclusions
OPOs reported a high volume of HIV+ referrals annually, of which a subset will be medically-eligible for donation. Particularly in areas of high HIV prevalence, OPOs require ongoing support to implement the HOPE Act.
Keywords: HIV, public health, organ transplantation, deceased donors
INTRODUCTION
The prevalence of end-stage organ disease is increasing among HIV-infected (HIV+) individuals.1–4 Outcomes with organ transplantation among HIV+ recipients (HIV R+) are excellent5–11 however mortality on the waitlist is disproportionately higher in HIV+ transplant candidates compared to those who are HIV-uninfected (HIV−).12–15 Using organs from HIV+ donors (HIV D+) for HIV+ transplant candidates could address this disparity. In South Africa, 27 cases of HIV D+/R+ kidney transplants have been reported with good early outcomes.16,17 In the United States, HIV D+/R+ transplantation is now legal, under research protocols,18 as a result of the HIV Organ Policy Equity (HOPE) Act.19
Based on a national registry study20 and a retrospective study of HIV clinics in Philadelphia,21 the size of the medically-eligible national HIV D+ pool has been estimated to be between 300 and 600 annually. However, the number of HIV D+ referrals is likely to be much higher, as many donors will not be deemed suitable for organ recovery, due to donor age, organ quality, and/or HIV-related factors. Additionally, potential HIV D+ referrals might not be pursued further due to barriers including lack of brain death testing for potential donors and lack of knowledge about the safety and feasibility of HIV+ organ recovery at the donor hospital or Organ Procurement Organization (OPO) level. Understanding the total number of HIV D+ referrals is critical for HOPE Act implementation and for targeting OPOs that would benefit from additional support and training.
The objective of this study was to determine how many HIV+ referrals are received each year by OPOs nationally, as well as how many cases of HIV are discovered during donor evaluation. By federal mandate, OPOs receive referrals for all potential deceased donors, including HIV D+ referrals. Thus, in order to estimate the number of annual HIV D+ referrals, we surveyed all 58 US OPOs. For those OPOs that provided exact records of HIV D+ referrals, we analyzed these results in conjunction with Centers for Disease Control and Prevention (CDC) regional HIV prevalence data in order to better characterize the potential HIV D+ pool.
METHODS
Survey of OPO HIV Referrals
A survey was administered by email to all 58 OPO chief executive officers between November 2014 and August 2016. Respondents were asked to provide the number of referrals in the past 12 months in which the decedent was known by the hospital team to have HIV infection (known HIV D+) and the number of cases in which a potential donor was found to have a positive HIV antibody or nucleic acid test during the donor evaluation process (discovered HIV D+). The survey also asked whether the OPO supported the HOPE Act and if the OPO was willing to participate in a research protocol with Johns Hopkins University related to HIV+ organ donation. This study was approved by the Johns Hopkins University Institutional Review Board, study number IRB00049442.
Exact Records and Estimates of HIV D+ Referrals
OPOs were asked if responses of HIV+ donor referral and discovered cases were based on quantitative reports (exact records) or estimates. In the cases of estimates, respondents were asked to characterize the estimates as accurate or inaccurate. If OPOs provided a range of HIV D+ referrals, the midpoint of this range was used as the estimate. If OPOs provided an exact number of referrals over a range of years, the number of referrals was divided by the number of years to provide an average annual estimate.
Prevalent HIV Cases in OPO Donation Service Areas
The most recent CDC HIV prevalence data for each state were obtained from the CDC 2015 HIV/AIDS surveillance report.22 We calculated the number of cases of HIV within each county using the state HIV prevalence and the proportion of the state population within each county. To calculate cases of HIV within each OPO, we added the total number of HIV cases in all counties within the OPO’s donation service area.
Estimating the National HIV+ Deceased Donor Pool
To obtain a national estimate of the HIV+ deceased donor pool, we divided the number of exact HIV D+ referrals by the proportion of total HIV prevalent cases represented by those OPOs. In other words, if the OPOs that provided exact records represented 50% of the national prevalence of HIV and these OPOs reported 1,000 HIV+ referrals, we would estimate a potential of 2,000 HIV+ referrals nationally.
Statistical Analysis and Maps
All statistical analyses were performed using STATA 14 (StataCorp, College Station, TX). Maps were plotted using ArcGIS Pro 2.0 (Redlands, CA: Environmental Systems Research Institute). We calculated Spearman’s rank correlation coefficient to determine whether the number of exact HIV D+ referrals correlated geographically with prevalent HIV cases.
RESULTS
Survey Data
Of 58 OPOs, 55 (95%) completed the survey, with representation from all 11 United Network for Organ Sharing (UNOS) regions (Figure 1). For known HIV D+ referrals, 27 OPOs provided exact records; 21 provided estimates (17 accurate, 4 inaccurate) and 7 did not provide an estimate. For newly discovered HIV D+ cases, 28 OPOs provided exact records; 22 provided estimates (18 accurate, 4 inaccurate) and 5 did not provide an estimate. All 55 responding OPOs reported support for the HOPE Act and research related to HIV D+/R+ transplantation, and 45 (82%) of OPOs responded that they were willing to collaborate with Johns Hopkins University on HIV D+ research.
Figure 1.
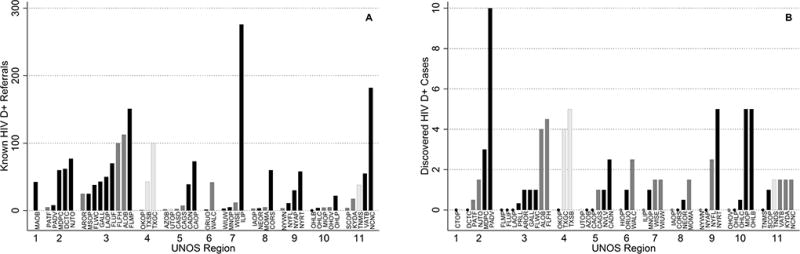
OPOs with Exact HIV D+ Referral Data
The 27 OPOs that provided exact records of known HIV D+ referrals in the prior year reported a total of 1,450 known HIV D+ referrals, ranging from 0 to 276 across OPOs (Figure 2A). Three OPOs reported more than 100 known HIV D+ referrals in the prior year: Carolina Donor Services (Greenville, North Carolina), Life Alliance Organ Recovery Agency (Miami, Florida), Gift of Hope Organ and Tissue Donor Network (Chicago, Illinois). The 28 OPOs that provided exact numbers of discovered HIV D+ cases reported a total of 39 discovered HIV D+ cases, ranging from 0 to 10 across OPOs in the prior year (Figure 1).
Figure 2.
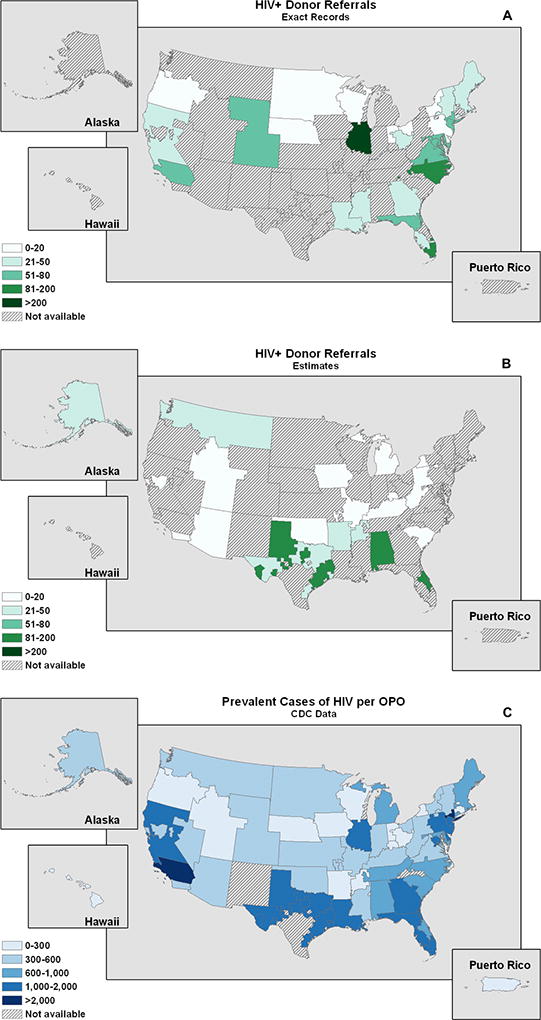
OPOs with Estimates of HIV D+ Referrals
The 17 OPOs that provided accurate estimates of known HIV D+ referrals reported a total of 352 annual HIV D+ referrals, ranging from 1 to 113 across OPOs in the prior year (Figure 2B). Two OPOs estimated more than 100 known HIV D+ referrals: TransLife (Winter Park, Florida) and Alabama Organ Center (Birmingham, Alabama). The 18 OPOs that provided accurate estimates of discovered HIV D+ referrals reported a total of 26 discovered HIV D+ referrals, ranging from 0 to 5 across OPOs in the prior year (Figure 1). The 4 OPOs that characterized their estimates of known HIV D+ referrals as potentially inaccurate estimated a total of 184 known HIV D+ referrals, ranging from 3 to 100 across OPOs in the prior year. One OPO estimated 100 known HIV D+ referrals: LifeGift (Houston, Texas). The 4 OPOs that characterized their estimates of discovered HIV D+ cases as potentially inaccurate reported a total of 11 discovered HIV D+ cases, ranging from 0 to 5 across OPOs in the prior year (Figure 1).
Estimate of National HIV D+ Pool
Based on 2015 CDC data, OPOs reporting exact referral data represented 67% of the prevalent cases of HIV in the US. OPOs reporting exact discovered HIV D+ data represented 59% of the prevalent cases of HIV in the US. Given their reported total of 1,450 known HIV D+ referrals and 39 discovered HIV D+ cases, we estimated 2,164 known HIV D+ referrals and 66 discovered HIV D+ cases nationally.
HIV D+ Referrals and Prevalent HIV Cases
Exact HIV D+ referrals per OPO donation service area were correlated geographically with the number of prevalent HIV cases per OPO donation service area (Spearman’s rank correlation coefficient = 0.66; p<0.001) (Figure 2).
DISCUSSION
In this national study of HIV D+ referrals, survey data was collected from 55 (95%) OPOs. Twenty-seven (47%) OPOs provided exact records of HIV D+ referrals, totaling 1,450 HIV D+ referrals in the prior year. Based on CDC HIV state prevalence data, these 27 OPOs represented 67% of the prevalent HIV cases in the US, yielding a national estimate of 2,164 potential HIV D+ referrals per year. In addition, 28 (51%) OPOs provided exact records of discovered HIV D+ cases, totaling 39 donors per year discovered to have a positive HIV screening test during donor evaluation. These OPOs represented 59% of the prevalent HIV cases in the US, yielding a national estimate of 66 discovered HIV+ cases annually.
Organs from HIV+ donors could benefit a substantial number of HIV+ individuals in need of transplantation, although the exact size of the HIV+ waitlist is unknown. Prior to HOPE Act implementation, candidate HIV status was not collected at the time of wait-listing. To estimate the potential pool of HIV+ transplant candidates, a recent study used pharmacy claims and data from the Scientific Registry of Transplant Recipients to identify 727 HIV+ kidney transplant candidates between 2009 and 201223. This was only a subset of HIV+ individuals who could benefit from transplantation, underscoring the substantial need for organs and the potential public health impact of HIV D+ organs for HIV+ individuals with end-stage kidney disease.
These OPO referral data indicate a large number of potential HIV D+ referrals nationwide, however, the prevalence of contraindications to donation among these HIV D+ referrals is unknown. Two prior studies provide estimates of medically-eligible HIV D+. The first was a national study which used data from two registries, the Nationwide Inpatient Survey and the HIV Research Network, and projected 534 and 494 potential HIV D+ per year, respectively.20 The second was a study of HIV D+ in Philadelphia by Richterman et al. which estimated 3–5 HIV D+ per year in Philadelphia, extrapolating this estimate to project 356 HIV D+ nationally.21 In these studies, up to 65% of HIV+ individuals who died were considered to be medically-ineligible for kidney or liver donation due to age, history of malignancy, and/or poorly-controlled HIV infection.
Our study projected the number of HIV D+ referrals, but cannot determine how many of these referrals would have proceeded to organ donation and recovery. We could not determine the proportion of HIV D+ referrals that were medically eligible as OPOs did not universally collect these data. If we assume 65% of the donors were medically ineligible, based on the findings of prior studies, our national estimate of eligible HIV D+ remains higher than both previous estimates. However, these prior reports may overestimate the number of medically eligible donors, due to limited clinical details in the registry and chart reviews. Furthermore, no studies to date have explored non-medical barriers to HIV+ donation, such as legal barriers at the state level, donor registration rates, and donation authorization rates.
Alternatively, previous reports may underestimate the medically-eligible HIV D+ pool for other reasons. Boyarsky et al. excluded 975/3798 (25%) potential HIV D+ due to missing CD4 count and HIV viral load and applied conservative donor eligibility criteria: more than 1,500 potential donors were excluded due to a plasma HIV RNA level >50 copies per milliliter and/or CD4 count <200 cells per microliter. However, the HOPE Safeguards and Research Criteria allow consideration of any HIV D+, regardless of the CD4 or viral load, provided there are no active opportunistic infections and a safe antiretroviral regimen is anticipated for the recipient.19 In addition, a recent study of 20 conventional brain death donors found that 55% of these HIV-uninfected donors had a CD4 <200.23 This indicates that in the setting of brain death, CD4 count may not be a reliable metric for infectious risk. In the study by Richterman et al., 23% of potential HIV D+ on mechanical ventilation were excluded due lack of documented brain death. Prior to HOPE Act research protocols, it is possible that hospitals did not routinely incorporate brain death testing for HIV+ patients on mechanical ventilation. Declaration of brain death for potential HIV D+ should become standard practice with increased awareness and targeted hospital education.
Several limitations of this study merit consideration. First, only 49% of responding OPOs provided exact records of HIV D+ referrals. However, this study represents the largest report of HIV D+ referrals to date in the US. Second, although hospitals are mandated to report all deaths to OPOs, several OPO respondents indicated that underreporting of HIV+ donor referrals was common due to the ban on HIV+ donation prior to the HOPE Act, meaning that these referrals might underestimate the true number. Finally for newly discovered HIV D+ cases, we could not distinguish between cases of pre-existing undiagnosed HIV, cases of acute HIV infection, or cases of donors with false positive HIV screening tests. Since the majority of OPOs utilize both HIV antibody and nucleic acid tests to screen donors, and these tests have false positive rates of 0.1–0.5%24 false positive tests are expected when screening large numbers of potential donors. In the past, organs from these false positive donors would have been discarded, but under the HOPE Act these organs can be utilized and represent another novel public health resource.
This national survey of US OPOs indicates that there are more than 1,450 HIV D+ referrals per year. Understanding the proportion of these donors that is medically ineligible or excluded for other reasons will be critical to successfully implement the HOPE Act. Based on these findings, support and resources can be targeted to OPOs with the highest numbers of HIV D+ referrals.
Acknowledgments
We thank and acknowledge the following OPOs for contributing to this research:
Alabama Organ Center; Arkansas Regional Organ Recovery Agency; Donor Network of Arizona;Donor Network West staff; Sierra Donor Services; OneLegacy (Tom Mone, CEO); LifeSharing; Donor Alliance; LifeChoice Donor Services; Washington Regional Transplant Community; TransLife; Life Alliance Organ Recovery Agency; LifeQuest; LifeLink of Florida; Lifelink of Georgia; Legacy of Life Hawaii; Iowa Donor Network; Gift of Hope Organ & Tissue Donor Network; Indiana Donor Network; Kentucky Organ Donor Affiliates; Louisiana Organ Procurement Agency; New England Organ Bank; The Living Legacy Foundation; Gift of Life Michigan (Richard Pietroski, CEO Emeritus); LifeSource Organ and Tissue Donation; Mid-America Transplant Services; Mississippi Organ Recovery Agency; Midwest Transplant Network; Carolina Donor Services; Nebraska Organ Recovery System; New Jersey Organ & Tissue Sharing Network; Nevada Donor Network; Center for Donation and Transplant; Finger Lakes Donor Recovery Network; LiveOnNY (Amy L. Friedman MD, Chief Medical Officer & Executive Vice President); Upstate New York Transplant Services; Lifebanc; Life Connection of Ohio; Lifeline of Ohio: LifeCenter Organ Donor Network: LifeShare of Oklahoma; Pacific Northwest Transplant Bank; Gift of Life (Rick Hasz, VP of Clinical Services and Sharon West, Data Analyst); Center for Organ Recovery & Education; LifeLink of Puerto Rico; We are Sharing Hope SC; Tennessee Donor Services; Mid-South Transplant Foundation; LifeGift; Southwest Transplant Alliance; Intermountain Donor Services; LifeNet Health; LifeCenter Northwest; Wisconsin Donor Network; University of Wisconsin Organ and Tissue Donation
FUNDING:
National Institutes of Health:
K23CA177321-01A1 (Durand), R34AI123023 (Durand), 1R01AI120938-01A1 (Tobian), K24DK101828 (Segev), F30DK116658-01 (Shaffer)
Johns Hopkins University Center for AIDS Research: 1P30AI094189 (Durand)
ABBREVIATIONS
- CDC
Centers for Disease Control and Prevention
- HIV
human immunodeficiency virus
- HIV+
HIV− infected
- HIV−
HIV− uninfected
- HOPE Act
HIV Organ Policy Equity Act
- HIV D+
HIV+ deceased donors
- HIV R+
HIV+ recipients
- OPO
Organ Procurement Organization
- UNOS
United Network for Organ Sharing
Footnotes
CONFLICTS OF INTEREST:
The authors declare no conflicts of interest.
AUTHORSHIP CONTRIBUTIONS
Ayla Cash M.P.H. wrote the manuscript, designed the survey, distributed the survey and performed statistical analyses. Xun Luo, M.D., M.P.H. performed statistical analyses. Eric K.H. Chow, M.Sc. performed geographical analyses and edited the manuscript. Mary Grace Bowring, M.P.H. performed geographical analyses and edited the manuscript. Ashton Shaffer, B.A. edited the manuscript. Brianna Doby, B.A. edited the manuscript. Corey E. Wickliffe, B.Sc. performed geographical analyses. Deborah McRann, R.N., B.S.N. designed the survey. Charles Alexander, R.N., M.S.N., M.B.A. designed the survey. Aaron A.R. Tobian. M.D., Ph.D. edited the manuscript. Dorry L. Segev M.D., Ph.D. distributed the survey, edited the manuscript and provided intellectual and operational oversight. Christine M. Durand M.D. wrote the manuscript, designed the survey, and provided intellectual and operational oversight.
References
- 1.Data Collection on Adverse Events of Anti HIVdSG. Smith C, Sabin CA, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24(10):1537–1548. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 2.Gupta SK, Eustace JA, Winston JA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40(11):1559–1585. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 3.Szczech LA, Grunfeld C, Scherzer R, et al. Microalbuminuria in HIV infection. AIDS. 2007;21(8):1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. Am J Kidney Dis. 2012;59(5):628–635. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucirka LM, Durand CM, Bae S, et al. Induction Immunosuppression and Clinical Outcomes in Kidney Transplant Recipients Infected With Human Immunodeficiency Virus. Am J Transplant. 2016;16(8):2368–2376. doi: 10.1111/ajt.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locke JE, Durand C, Reed RD, et al. Long-term Outcomes After Liver Transplantation Among Human Immunodeficiency Virus-Infected Recipients. Transplantation. 2016;100(1):141–146. doi: 10.1097/TP.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locke JE, Mehta S, Reed RD, et al. A National Study of Outcomes among HIV-Infected Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26(9):2222–2229. doi: 10.1681/ASN.2014070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363(21):2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terrault NA, Roland ME, Schiano T, et al. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl. 2012;18(6):716–726. doi: 10.1002/lt.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke JE, Reed RD, Mehta SG, et al. Center-Level Experience and Kidney Transplant Outcomes in HIV-Infected Recipients. Am J Transplant. 2015;15(8):2096–2104. doi: 10.1111/ajt.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roland ME, Barin B, Huprikar S, et al. Survival in HIV-positive transplant recipients compared with transplant candidates and with HIV-negative controls. AIDS. 2016;30(3):435–444. doi: 10.1097/QAD.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trullas JC, Cofan F, Barril G, et al. Outcome and prognostic factors in HIV-1-infected patients on dialysis in the cART era: a GESIDA/SEN cohort study. J Acquir Immune Defic Syndr. 2011;57(4):276–283. doi: 10.1097/QAI.0b013e318221fbda. [DOI] [PubMed] [Google Scholar]
- 13.Ragni MV, Eghtesad B, Schlesinger KW, Dvorchik I, Fung JJ. Pretransplant survival is shorter in HIV-positive than HIV-negative subjects with end-stage liver disease. Liver Transpl. 2005;11(11):1425–1430. doi: 10.1002/lt.20534. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian A, Sulkowski M, Barin B, et al. MELD score is an important predictor of pretransplantation mortality in HIV-infected liver transplant candidates. Gastroenterology. 2010;138(1):159–164. doi: 10.1053/j.gastro.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbott KC, Trespalacios FC, Agodoa LY, Ahuja TS. HIVAN and medication use in chronic dialysis patients in the United States: analysis of the USRDS DMMS Wave 2 study. BMC Nephrol. 2003;4:5. doi: 10.1186/1471-2369-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336–2337. doi: 10.1056/NEJMc0900837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller E, Barday Z, Mendelson M, Kahn D. HIV-positive-to-HIV-positive kidney transplantation--results at 3 to 5 years. N Engl J Med. 2015;372(7):613–620. doi: 10.1056/NEJMoa1408896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health Resources and Services Administration. Final Human Immunodeficiency Virus Organ Policy Equity (HOPE) Act safeguards and research criteria for transplantation of organs infected with HIV. Fed Register. 2015;80:34912–21. [Google Scholar]
- 19.Boyarsky BJ, Segev DL. From Bench to Bill: How a Transplant Nuance Became 1 of Only 57 Laws Passed in 2013. Ann Surg. 2016;263(3):430–433. doi: 10.1097/SLA.0000000000001352. [DOI] [PubMed] [Google Scholar]
- 20.Boyarsky BJ, Hall EC, Singer AL, Montgomery RA, Gebo KA, Segev DL. Estimating the potential pool of HIV-infected deceased organ donors in the United States. Am J Transplant. 2011;11(6):1209–1217. doi: 10.1111/j.1600-6143.2011.03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richterman A, Sawinski D, Reese PP, et al. An Assessment of HIV-Infected Patients Dying in Care for Deceased Organ Donation in a United States Urban Center. Am J Transplant. 2015;15(8):2105–2116. doi: 10.1111/ajt.13308. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. [Accessed January 1 2017];HIV Surveillance Report, 2015. 27 http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2016. [Google Scholar]
- 23.Serrano OK, Kerwin S, Payne WD, Pruett TL. CD4 Count in HIV− Brain-Dead Donors: Insight into Donor Risk Assessment for HIV+ Donors. Transplantation. 2017;101(4):831–835. doi: 10.1097/TP.0000000000001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seem DL, Lee I, Umscheid CA, Kuehnert MJ United States Public Health S. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep. 2013;128(4):247–343. doi: 10.1177/003335491312800403. [DOI] [PMC free article] [PubMed] [Google Scholar]


