Abstract
Male reproductive ageing has been mainly explained by a reduction in sperm quality with negative effects on offspring development and quality. In addition to sperm, males transfer seminal fluid proteins (Sfps) at mating; Sfps are important determinants of male reproductive success. Receipt of Sfps leads to female post-mating changes including physiological changes, and affects sperm competition dynamics. Using the fruit fly Drosophila melanogaster we studied ageing males’ ability to induce female post-mating responses and also determined the consequences of male ageing on their reproductive success. We aged males for up to 7 weeks and assayed their ability to: i) gain a mating, ii) induce egg-laying and produce offspring, iii) prevent females from remating and iv) transfer sperm and elicit storage after a single mating. We found that with increasing age, males were less able to induce post-mating responses in their mates; moreover ageing had negative consequences for male success in competitive situations. Our findings indicate that with advancing age male flies transferred less effective ejaculates and that Sfp composition might change over a male’s lifetime in quantity and/or quality, significantly affecting his reproductive success.
Keywords: fertility, sperm competition, ejaculate, sex peptide, seminal proteins, accessory gland
1. Introduction
Ageing is characterized by progressive declines in a number of bodily functions that accumulate in an increased risk of death. This decline is accompanied by reduced reproduction and fertility. Three phases of ageing can often be discerned in a number of model organisms, like Drosophila, whereby during the first, the ageing phase, performance decreases rapidly, while during the late-phase performance stabilizes at low levels to decline significantly ~2 weeks before death in a ‘death spiral’ (Shahrestani et al. 2012; Mueller et al. 2016). In general, a decrease in reproductive capacity in males is due to senescence of the soma, often expressed as lower mating success, and of the reproductive tissues, causing impaired fertility (reviewed in Johnson and Gemmell 2012). Explanations for decreased male fertility with age have focused mainly on diminished sperm quality (reviewed in Radwan 2003; Pizzari et al. 2007; Reinhardt et al. 2015). However, not only sperm and/or spermatogenic tissues age. We expect the entire ejaculate including its non-sperm components to show signs of senescence, contributing to poor reproductive success in older males. Here we focus on this aspect, investigating several consequences of ageing on male reproductive success in Drosophila melanogaster, a species where the link between male reproductive success and the non-sperm components of the ejaculate is well established.
Sperm of older males are predicted to have accumulated a larger number of mutations (Radwan 2003; Pizzari et al. 2007; Reinhardt et al. 2015) resulting in decreased offspring viability or quality. Data from a number of organisms are consistent with this prediction (Price and Hansen 1998; Jones et al. 2000; Jones and Elgar 2004; Karl and Fischer 2013), although there are exceptions (Schäfer and Uhl 2002; Fricke and Maklakov 2007; Avent et al. 2008; Krishna et al. 2012; Verspoor et al. 2015). It was further observed that older males sired fewer offspring when in competition over fertilization e.g. in the cellar spider Pholcus phalangioides (Schäfer and Uhl 2002), the hide beetle Dermestes maculatus (Jones et al. 2007) and the bulb mite Rhizoglyphus robini (Radwan et al. 2005). This reduced sperm competitive ability may be due to altered sperm quality or other characteristics, like the non-sperm component. For example, in the red junglefowl Gallus gallus older males achieve lower paternity share (McDonald et al. 2017). In this species sperm velocity can be influenced by the male through allocating a larger ejaculate (Cornwallis and O’Connor 2009). A recent study reported sperm velocity dependent variation in the red junglefowl seminal fluid proteome (Borziak et al. 2016) and, interestingly, a distinct seminal proteomic signature in older males. This suggests that the non-sperm components of the ejaculate might also change with age in ways that could affect male competitiveness. Since success in sperm competition is a key determinant of male fitness (Simmons 2001; for an example in D. melanogaster see Fricke et al. 2010) it is important to extend the study of male reproductive ageing to such non-sperm components of the ejaculate.
In D. melanogaster, non-sperm components of the ejaculate are vital for male fertilization success and play important roles in sperm competition (reviewed in Chapman 2001; Avila et al. 2011; Simmons and Fitzpatrick 2012). Along with sperm, a male transfers about 200 different seminal fluid proteins (Sfps) to a female (Findlay et al. 2008, 2009). Many Sfps are produced in the male’s accessory glands (AG) (reviewed in Avila et al. 2011). The AGs are two tube-like lobes connected to the ejaculatory duct. Their proteins are secreted by the glands’ ~1000 “main cells” and 40 “secondary cells”; the latter are located at the tip of the gland and contain large secretory vacuoles (Bertram et al. 1992). As males age, some AG secondary cells can be actively shed and even be transferred to females during mating (Leiblich et al. 2012). Here, we tested ageing D. melanogaster males’ ability to elicit known female post-mating responses to such non-sperm components of the ejaculate and tested whether this reduced ability contributes to diminished reproductive or competitive success of ageing D. melanogaster males.
The fact that Sfps in D. melanogaster play a crucial role in many post-copulatory traits (reviewed in Avila et al. 2011) prompted us to examine whether there were age dependent changes in their amount or action. Sfps for example, decrease the risk of sperm competition by decreasing female receptivity to remating (Chapman et al. 2003; Liu and Kubli 2003), affect the storage and retention of sperm (Harshman and Prout 1994; Neubaum and Wolfner 1999; Chapman et al. 2000; Prout and Clark 2000; Bloch Qazi and Wolfner 2003; Wong et al. 2008; Avila et al. 2010; Ravi Ram and Wolfner 2007) and boost egg production and laying by the female (Herndon and Wolfner 1995; Heifetz et al. 2000; Chapman et al. 2003; Liu and Kubli 2003) which increases the number of offspring a male can sire with a single mating (Fricke et al. 2009; Fricke and Chapman 2017). These female post-mating changes are accompanied by a remodelling of the female uterus into a mated conformation (Adams and Wolfner 2007; Kapelnikov et al. 2008a, b; Mattei et al. 2015) which is suggested to aid in sperm entry into storage (Adams and Wolfner 2007; Mattei et al. 2015). At least one seminal protein, Acp36DE, is necessary for these uterine conformational changes (Avila and Wolfner 2009). Since Sfps play such an important role in male reproductive success and post-mating competitiveness, we investigate whether they are affected by a male’s ageing. To that end, we measured whether males’ age affects their mates’ post-mating responses. We focused on responses elicited by the sex peptide (SP), which heightens a male’s reproductive success through a variety of means (Fricke et al. 2009; Fricke and Chapman 2017), including reducing female receptivity for up to ~4 days after mating, boosting egg-laying (Chen et al. 1988; Chapman et al. 2003; Liu and Kubli 2003) and regulating the efficient release of stored sperm (Avila et al. 2010). The other Sfp we examined was ovulin, which increases ovulation rate by mated females (Heifetz et al. 2000; Rubinstein and Wolfner 2013), but only exerts short-term effects on the female and has so far no known effect on sperm competition outcomes.
In this study, we examined the consequence of male ageing over a substantial part of male lifespan (from 4 days to 7 weeks post-eclosion) on the male’s ability to induce female post-mating responses. Upon noticing that there were age-dependent changes in the intensity of female post-mating responses induced by the male, we used an enzyme-linked immunosorbent assay (ELISA, Sirot et al. 2009) directly quantify the amounts of two Sfps (SP and ovulin) transferred in single-matings by males of different ages. We combined these measures with tests of age-dependent male reproductive success after a single mating as well as in competitive situations.
2. Materials and Methods
2.1 Fly stocks
2.1.1 Wild-type flies and Stubble (Sb) flies
The wild-type Dahomey stock has been maintained at large population size in cages with overlapping generations since it was collected in the 1970s in Dahomey, West Africa (now Benin). Hence we put no constraint on adult lifespan and allowed the flies to reproduce throughout their entire lifespan. The population was fed once a week by introducing three glass bottles into the population cage with 70 mL fresh standard sugar-yeast (SYA) food (Bass et al. 2007). Our Dahomey stock flies were kindly provided by Prof. Tracey Chapman (University of East Anglia, UK) and have been maintained in our laboratory in population cages for several generations prior to the experiments. Further we used flies with the Stubble (Sb) mutation, back-crossed into the wild-type Dahomey genetic background for four generations to increase genetic variability and have the mutation in a comparable genetic background. Sb, a dominant mutation that causes an easily-scored short-bristle phenotype, is recessive-lethal, hence the Sb males used in our assays were heterozygous for this mutation. Sb males were used in a direct competition assay as competitor males, enabling us to determine offspring paternity and collect fitness measures for our focal males from different age classes.
2.1.2 Sex peptide (SP) knockout flies
Following the protocol in Liu and Kubli (2003), flies that lacked SP (SP0) were heterozygous for SP0 (an allele with two mutations in SP, one a stop codon), and Δ130, a deletion that removes the SP gene; each SP allele was carried in stock over TM3,Sb,ry. Each line was backcrossed to Dahomey (3 generations for Δ130 TM3,Sb,ry; 4 for SP0/TM3,Sb,ry) to control for genetic background and increase vigor. The SP knockout flies were kindly provided by Prof. Tracey Chapman. SP0 males served as an age-matched baseline to compare to the strength of SP-elicited female post-mating responses by the wild-type males.
2.1.3 Fly culturing
Flies were maintained and all experiments were conducted in a constant climate room at 25°C and 60% humidity at a 12:12hr dark – light cycle. Mutant flies were kept in glass bottles containing 70mL of SYA food and regularly flipped onto fresh food.
To generate flies used in the experiments we allowed the parent generation to oviposit on grape-juice-agar plates [50 g agar, 600 mL red grape juice, 42.5 mL Nipagin (10% w/v solution) and 1.1 L water] supplemented with live-yeast paste. Following 24hrs of incubation, larvae were collected and 100 were transferred into a standard vial (diameter 2.5 cm, height 8.4 cm, containing 7 mL of SYA food) to develop under density controlled conditions. Throughout our experiments we added additional ad libitum live yeast granules or paste to our vials. To reduce variability in male age for our experiments we restricted wild-type Dahomey females to oviposit for 3–5 hrs. At eclosion, adults were collected as virgins and 20 individuals were kept in same sex groups in standard vials until the beginning of the experiment. If flies were kept for longer than 4 days prior to the experiment they were transferred to fresh food every 3–4 days.
2.2 Mating assays
We designed our experiments to use virgin rather than previously-mated males (see Jones and Elgar 2004 for such an approach), interpreting data from the latter comes with its own set of challenges: because D. melanogaster males transfer ~one third of their seminal proteins in each mating (Ravi Ram et al. 2005) and need at least four matings in short succession to largely-empty their Sfp stores (Hihara 1981), using a previously mated male would mean that his ejaculate would be a mix of old and fresh ejaculatory components. Moreover, the proportion of old vs. new components might vary among males from different male-age groups, if, for example old males invested more in a mating and/or replenished stores slower than young males. Moreover, the rate of sperm depletion (or replenishment) might not be the same as that for Sfps, resulting in further complication in interpretation of the ejaculates of older males. Hence for all of our assays we kept males as virgins and allowed them to age in male-only groups until being tested at the predetermined age and as such we are not able to distinguish male age from ejaculate age with our experimental set up.
All mating experiments were conducted in the morning after lights-on in a climate-controlled room at standard conditions. Males were placed individually in mating vials the day before and females were introduced the morning of the assay. We recorded the time at which pairs were placed together, and the times at which mating started and ended. This allowed us to calculate latency to mating (time between introduction and start of a mating) as well as copulation duration. Unless otherwise stated, flies were observed continuously.
2.2.1 Male reproductive ageing
To examine male reproductive ageing over much of the normal lifespan, we generated and tested males at 4 days, 2, 3, 4, 5, 6 and 7 weeks after eclosion. These seven male cohorts were set up over seven consecutive weeks and males were aged so that our assays could take place on the same day for all age groups. First, we measured the ability of males of each age to gain a first mating with a virgin female. Then, since we were particularly interested in the role of Sfps in reproductive ageing we measured female responses elicited by receipt of Sfps, particularly those induced by receipt of sex-peptide (SP). Specifically, we determined a female’s propensity to remate and the amount of eggs laid after a single mating. To assess the effect of SP we examined the responses by females mated to wild-type Dahomey males and, in parallel, age-matched SP0 males. We used 30 males per age group with the exception of the 7 week-old treatment, for which only 16 wild-type and 26 SP0 males had survived.
All females were 4 days old on the first day of the assay. After being placed together, pairs were observed continuously for 3 hrs and mating rate was recorded. Pairs that had not mated within this timeframe were excluded from further assays. After a mating was finished (a typical mating lasted ~20 min) the male was removed from the vial. The number of eggs laid by the female over the ensuing 24 hrs was measured. The next morning the female was transferred to a vial with a new wild-type virgin male (4 days old) and was given a 1.5 hr opportunity to remate. The number of females remating was recorded.
2.3 Long-term egg-to-adult survival after a single mating
Four day old virgin females were mated once to a male either 4 days, 2, 4 or 6 days post-eclosion (n = 35 per age group). After a successful mating females were transferred daily to fresh food and allowed to oviposit for nine days. Eggs laid on days 1–5, 7 and 9 were counted and vacated vials were incubated for another 11 days under standard conditions for offspring to eclose. Vials were then frozen and adult offspring were counted to assess egg to adult survival.
2.4 ELISA
To directly measure the amount of sex peptide (SP) and ovulin transferred in a single mating by males of different ages we used an enzyme-linked immunosorbent assay (ELISA) following the protocol described in Sirot et al. (2009). We mated females (4 days post eclosion) to either 4 day-, 2, 4 or 6 week-old males (n = 43–58 females per male age class), removed males after mating ended, and snap-froze the females in liquid nitrogen 30 mins after the start of a mating. In a few cases copulation was not completed after 30 mins, but previous studies indicate that by this time the whole ejaculate should have been fully transferred (Lung and Wolfner 1999; Gilchrist and Partridge 2000). From the frozen samples, 18 females for each male age group were used for the ELISA assay; the remaining females were used for sperm counts (see below). To quantify the amount of SP and ovulin transferred we dissected the lower reproductive tract (LRT) (consisting of the uterus, sperm storage organs, oviducts and parovaria) on ice, and processed it for ELISA quantification as described in Sirot et al. (2009), with the following minor modifications. Primary antibodies were used at 1:1000 (anti-SP) and 1:500 (anti-ovulin). For the SP-antibody the colorimetric reaction was stopped after 7 mins, while the ovulin-antibody was incubated for 30 mins as in Sirot et al. (2009). Each LRT sample was divided into four replicates. We processed two with anti-SP and two samples with anti-ovulin.
On every plate, a standard dilution curve of male AGs was included for each antibody, in addition to a blank sample (see Sirot et al. 2009) and a sample of a virgin female LRT as negative controls. The serial dilution for the standard curve for the SP-antibody was between 1/2 and 1/32 of a male AG. For the standard curve with anti-ovulin we diluted from 1/16 to 1/256 of an AG.
ELISA signals were quantified by measuring the absorption of the sample with a microplate reader at 450 nm (OD450), after the colorimetric reaction. We standardized the OD450 values to the blank sample, and subtracted the OD450 value for the virgin LRT from all other LRT samples’ values. The resulting OD450 values for one replicate of each duplicate sample was regressed against the OD450 value of the other replicate of the same sample. Duplicate samples with residuals greater than three standard deviations were considered to have low repeatability and were removed. The OD450 values of the remaining duplicate samples were averaged for each sample and subsequently converted to male AG equivalents using the AG standard curve.
2.5 Sperm counts and uterine conformation
The remaining females frozen 30 mins after mating were used to perform sperm counts and to determine the uterine conformation (Neubaum and Wolfner 1999; Adams and Wolfner 2007; Avila and Wolfner 2009). The LRT of 20 females per male age group were dissected and stained as in Neubaum and Wolfner (1999). Samples were coded with numbers for blind counts but care was taken that all age groups were represented each day sperm counts were done. We counted the number of sperm stored in the sperm storage organs with a focus on the seminal receptacle by carefully unrolling it using a dissecting needle. The spermathecae was omitted, as usually there are no sperm stored at this early time point (Manier et al. 2010). Orcein-stained seminal receptacle sperm were counted; each sample was counted twice by the same person. Samples with a repeatability of <93% were removed from the set (as in Avila et al. 2010), leaving us with a total of 12–17 samples per male age group.
Sperm storage is facilitated by conformational changes of the uterus that are induced by mating, and specifically by receipt of Sfps such as Acp36DE (Adams and Wolfner 2007; Avila and Wolfner 2009; Mattei et al. 2015). These changes serve to push the sperm mass towards the storage sites, and to open access to those sites. We also recorded uterine conformations in 28–30 preparations of tissues from females mated to males of a given age. Specifically, for each male age group we recorded as a present/ absent response whether the uterus conformation had proceeded to the rotund shape induced by mating and receipt of Acp36DE or whether it still looked virgin-like (see Adams and Wolfner 2007; Avila and Wolfner 2009).
2.6 Direct competition assay
To determine the combined effects of pre- and post-mating competitive success of males of different age on his reproductive success, we placed a focal wild-type virgin male who was 4 days, 2, 4 or 6 weeks post-eclosion into a vial together with a single Sb competitor male (4 days post-eclosion), and then added a single virgin wild-type female (4 days post-eclosion). We started with 42–44 replicate vials per male-age group. In this setting the two males directly competed for access to the female and for fertilizations. We allowed these trios to interact freely for five days and scored male mating success, as well as paternity success (via progeny counts). To estimate mating success for the two male types, on the first day of the experiment immediately after introducing the female we observed the vials continuously to score which male obtained the first mating for ~60 mins after the start of the experiment. We made sure all replicates had started their first mating before switching to conducting spot-checks every 20 min for the remainder of the 5 hr period for the first day. Then, we did spot-checks daily for the next four consecutive days, during the first 5 hrs after lights on. We marked competitor males by cutting off a small corner of their wings in order to easily distinguish them by eye from the focal males. Because we provided our focal males with a standardized background to compete against we could directly compare across the different age classes. Additionally every 24 hrs the flies were transferred to fresh vials, the vacated vials were incubated until offspring eclosion and then frozen for later counts of offspring by bristle phenotype, to assign paternity. Offspring vials from day 2 were lost for one of the age classes, therefore day 2 was omitted from the analysis for all age classes to ensure comparability between age classes. To quantify the progeny numbers for the two males - the competitor males were heterozygous for the Sb allele - we calculated their progeny number as double the number of Sb progeny; to determine the number of progeny of the non-Sb focal male, we subtracted the number of Sb progeny from the total number of non-Sb progeny; the remaining non-Sb progeny represented the number sired by the focal male.
2.7 Data analysis
The analyses were performed in R version 3.2.1 (R Core Team 2015) using RStudio version 7.6 (RStudio Team 2015). The following R packages were being applied: lmtest (Zeileis and Hothorn 2002), pgirmess (Giraudoux 2016), pscl (Jackman et al. 2015), lme4 (Bates et al. 2015), Hmisc (Harrell et al. 2017) and gplots (Warnes et al. 2016). Because data were not normally distributed, we applied generalized linear models (GLM, Nelder and Wedderburn 1972) with the appropriate data distribution and correction for over-dispersion via the quasi-extension if necessary. We included male age as a fixed factor in all models. To account for zero-inflation the sperm storage data were analyzed with the help of zero-altered negative binomial models (ZANB). Briefly, zero-altered negative binomial models are so-called two-part models consisting of a logistic part to model presence/absence of non-zero values and a count part to model non-zero count data with a zero-truncated negative binomial distribution (Zuur et al. 2009). The relative amount of transferred SP and ovulin measured via the ELISA was analysed using the Kruskal–Wallis test. Time measurements were analyzed using a Gamma data distribution which does not allow zeros. Since in the latency experiments a few replicates mated immediately (hence after 0 minutes), we added 1 minute to every measurement enabling us to use the appropriate data distribution. As we measured females repeatedly in our long-term test of egg-to-adult survival after a single mating we conducted generalized mixed effects models with the appropriate data distribution. We included male age, time after mating and their interaction as continuous variables and female identity as a random factor. If necessary we accounted for overdispersion by including an observation level random effect (Harrison 2014). All data were analyzed starting with the full model and throughout we applied a backward stepwise simplification approach excluding non-significant variables in order to arrive at the minimal adequate model (Crawley 2007), which we present throughout. P-values were assessed by analysis of deviance on nested models (likelihood ratio tests or F-ratio tests depending on the data distribution; likelihood ratio tests for mixed effects models; Crawley 2007), all tested terms (significant and non-significant) are reported in the results section.
3. Results
3.1 Male reproductive ageing
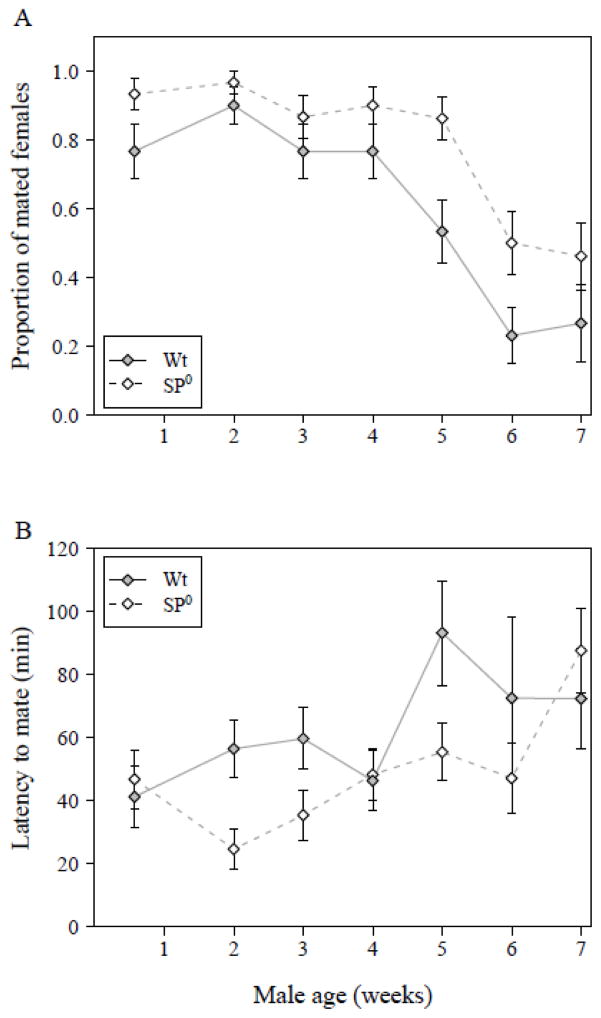
Mating rate decreased significantly with increasing male age (GLM with binomial error distribution, male age: N = 396, df = 6, Δ deviance = 87.42, χ2 = 87.42, P < 0.001). This general pattern of mating success across male age groups was the same for both the wild-type and the age-matched SP0 males (male age × genotype: N = 396, df = 6, Δ deviance = 1.54, χ2 = 1.54, P = 0.96). Whereas most 4 day old males achieved a mating, the proportion of successful matings dropped for older males, with a steep decrease occurring for males between ages of 5 and 6 weeks (Fig. 1A). Overall the proportion of males gaining a mating was significantly lower in wild-type males compared to SP0 males (male genotype: N = 396, df = 1, Δ deviance = 19.83, χ2 = 19.83, P < 0.001) over all age classes.
Figure 1.
(A) Mating rate during a 3-hr observation period. The bars show the proportion of 4-day old virgin females that mated to wild-type (filled symbol, solid line) or SP0 (open symbol, hatched line) males of the indicated ages (N = 396). (B). Latency to copulation. The Y-axis shows the minutes between introduction of a female into the mating vial and the start of mating (N = 286). Both figures show means ± SE.
Latency to copulation showed a similar pattern to that of mating rate. Changes in latency across male age groups were not significantly different between the two male genotypes (GLM with Gamma error distribution, male age × genotype: N = 286, Δ deviance = 10.54, F6, 272 = 1.70, P = 0.12). With increasing age, males not only showed a reduced mating rate, but they also had a significantly longer latency to copulation (male age: N = 286, Δ deviance = 15.83, F6, 278 = 2.52, P = 0.022) independent of male genotype (Fig. 1B). In general, SP0 males were significantly faster to mate than wild-type males (male genotype: N = 286, Δ deviance = 6.21, F1, 278 = 5.93, P = 0.016).
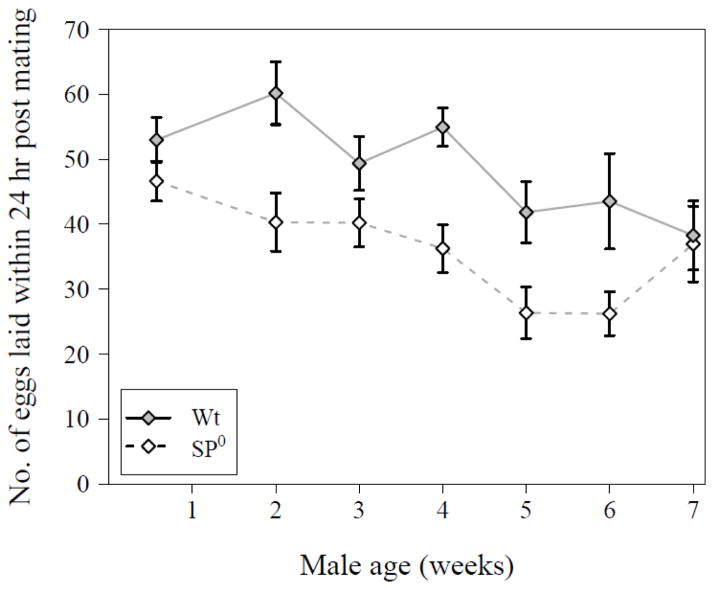
Older males were significantly poorer in inducing their mates to lay eggs within 24 hrs after a first mating (GLM with Poisson error distribution corrected for overdispersion, male age: N = 281, Δ deviance = 255.1, F6,273 = 3.76, P = 0.0013). These changes with male age were not significantly different between male genotypes (male age × genotype: N = 281, Δ deviance = 61.1, F6, 267 = 0.90, P = 0.50, see Fig. 2). As expected (Chapman et al. 2003; Liu and Kubli 2003), females mated to males producing no SP had an overall lower egg-laying rate than mates of wild-type males (male genotype: N = 281, Δ deviance = 282.8, F1, 273 = 25.00, P < 0.001).
Figure 2.
Number of eggs laid by females during the first 24hrs after mating with either a wild-type (filled symbol, solid line) or SP0 (open symbol, hatched line) male of the indicated ages (N = 281). Data are shown as means ± SE.
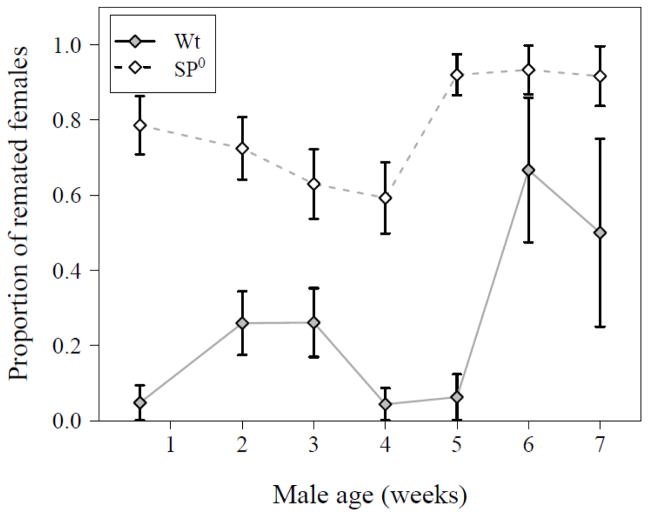
In contrast to the reproductive traits above, the willingness of a female to remate with a second male 24 hrs after a first mating was significantly affected by an interaction between male genotype and age (GLM with Binomial error distribution, N = 283, df = 6, Δ deviance = 13.37, χ2 = 13.37, P = 0.038). As expected (Chapman et al. 2003; Liu and Kubli 2003), females consistently remated more often when their first mate did not transfer SP. But the age-dependent decrease in male ability to suppress remating differed between control and SP0 males (Fig. 3). With increasing age, males were less efficient in suppressing female remating rate and this occurred earlier in the SP0 males (between 28 and 35 days post eclosion) than for the wild-type males (between 5 and 6 weeks).
Figure 3.
Proportion of females that remated with a second male 24 hrs after a first mating with either a wild-type (filled symbol, solid line) or SP0 (open symbol, hatched line) male of the indicated ages (N = 283, same mates of males as displayed in Fig. 1A). Data are shown as means ± SE.
3.2 Long-term egg-to-adult survival after a single mating
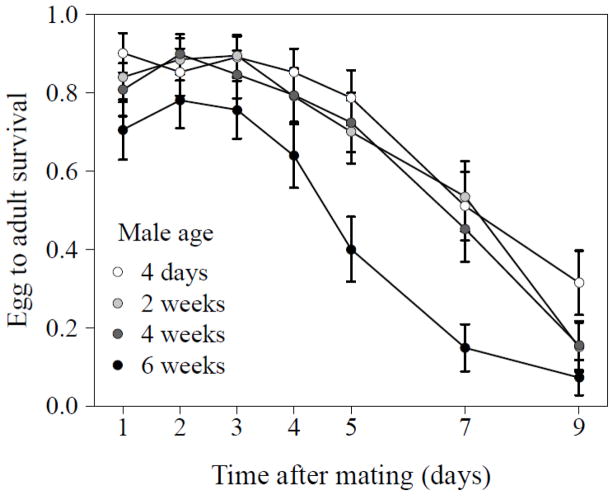
Egg-to-adult survival decreased significantly over the nine days after a single mating (GLMM with Binomial data distribution and observation level random effects, time: N = 140, df = 1, χ2 = 418.42, P <0.001, see Fig. 4) and varied significantly with male age (male age: N = 140, df = 3, χ2 = 20.73, P <0.001). In particular, progeny of the oldest males showed lower rates of egg-to-adult survival. However, the decline over time was similar across all male age groups (time x male age: N = 140, df = 3, χ2 = 2.80, P = 0.423). This difference in adult progeny numbers was not due to the production of fewer eggs by mates of older males, as there were no differences in egg-laying across male age classes (GLMM with negative binomial error distribution, male age: N = 140, df = 3, χ2 = 4.30, P = 0.23) and we only observed a significantly decreased in the number of eggs laid with time (N = 140, df = 1, χ2 = 138.16, P <0.001) and again this decline was similar across male age groups (time x male age: N = 140, df = 3, χ2 = 2.88, P = 0.41).
Figure 4.
Egg-to-adult survival of progeny from females mated once to a male of the indicated age over a nine day period. Data are presented as means ± SE of the raw data.
3.3 ELISA
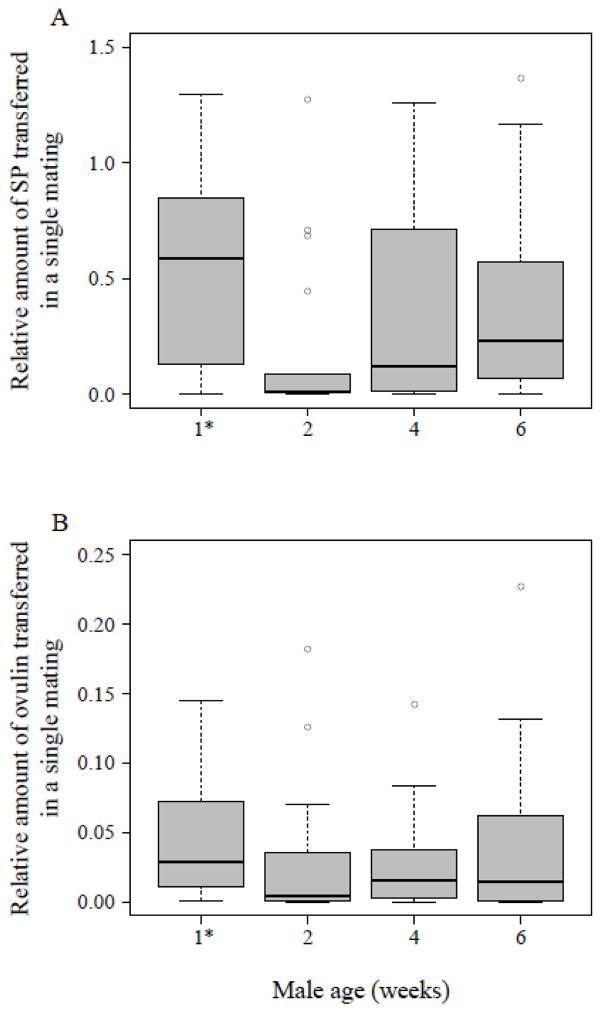
The relative amount of SP present in the female reproductive tract 30 mins after a single copulation significantly differed between male age classes (Kruskal-Wallis test, N = 68, df = 3, χ2 = 8.61, P = 0.035). Four day old males transferred a large amount of SP, while 2 week-old males transferred significantly less (Fig. 5A). Surprisingly the two older male age groups transferred quantities similar to those transferred by 4-day old males (thus, more than 2 week-old males transferred; see Fig. 5A).
Figure 5.
Relative amounts of (A) SP (N = 68) and (B) ovulin (N = 72) transferred to a female in a single mating by a wild-type male of the indicated age [1* males were four days old]. Females were flash frozen 30 mins after the beginning of the mating and the amount of SP or ovulin within the lower reproductive tract was measured via ELISA, as described in the text. Vertical lines represent the median, boxes the lower and upper quartile, whiskers extend to the extreme values within the 1.5 interquartile range and dots depict outliers outside the 1.5 interquartile range.
The relative amount of ovulin present in the female reproductive tract 30 mins after a single copulation did not differ significantly between male age classes (Kruskal-Wallis test, N = 72, df = 3, χ2 = 4.48, P = 0.21), However, median values for ovulin (Fig. 5B) show a similar pattern to median values for SP (Fig. 5A).
3.4. Sperm counts and uterine conformation
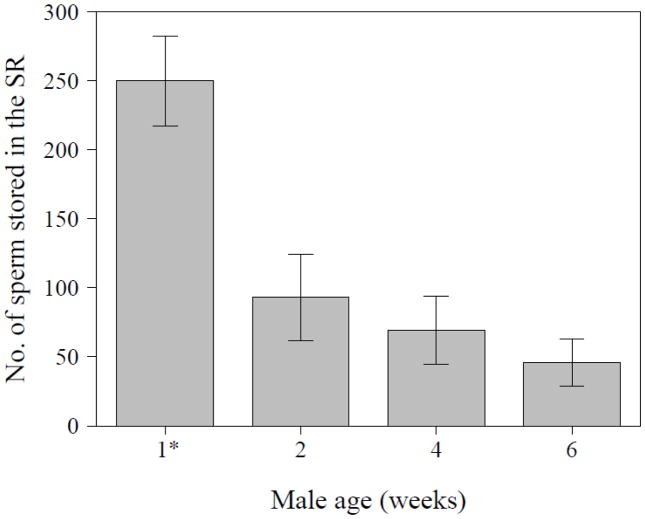
Since sperm begin to accumulate in the seminal receptacle (SR) by ~30 mins after the start of mating (Bloch Qazi and Wolfner 2003; Manier et al. 2010), we compared numbers of sperm in storage at this time in the mates of males of the four different age groups. The number of sperm in storage differed significantly between mates of males’ age groups, as did the probability of any sperm being stored at all at the time point examined (ZANB, N = 52, df = 3, count part: χ2 = 13.44, P = 0.004; logistic part: χ2 = 8.41, P = 0.038). In those females that had stored sperm, mean numbers of stored sperm in the SR decreased steadily with increasing age of their mate. Moreover, whereas 45% – 53% of females mated to older males (2, 4 and 6 weeks after eclosion) had no sperm at all in their SRs at 30 mins after mating, only 8% of females mated to 4-day old males had no sperm in their SRs. Together, these two effects caused a strong decrease of sperm numbers in the SR of females mated to older males (Fig. 6).
Figure 6.
Number of sperm stored in the female’s seminal receptacle (SR) at 30 mins after the start of mating with a wild-type male of the indicated age (N = 52) [1* males were four days old]. Data are presented as means ± SE.
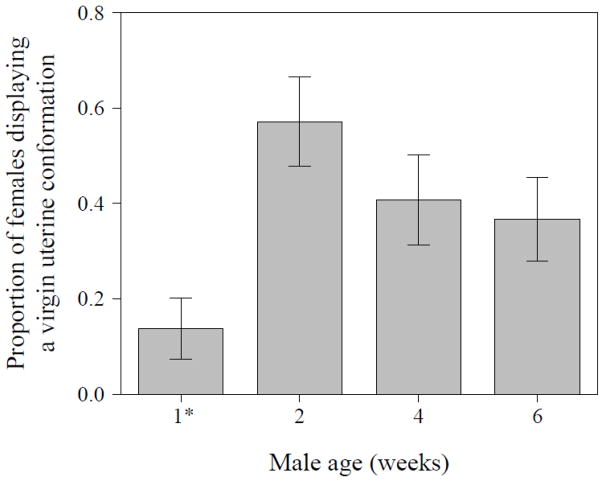
Given the lower amount of early sperm storage in mates of older males, we also examined whether male age affected the uterine conformational changes that facilitate sperm storage. The proportion of females showing a virgin-like uterus conformation 30 mins after mating was significantly different between mates of males from different age classes (GLM with Binomial error distribution, N = 114, df = 3, Δ deviance = 12.61, χ2 = 12.61, P = 0.006). It was lowest in females mated to young males (4 days after eclosion) and highest in females mated to 2 week- old males; older males showed an intermediate proportion of females with a virgin-like uterus conformation (Fig. 7).
Figure 7.
Proportion of females displaying a virgin-like uterine conformation at 30 mins after the start of mating with males of the indicated age (N = 114) [1* males were four days old]. Data are shown as means ± SE.
3.5 Direct competition assay
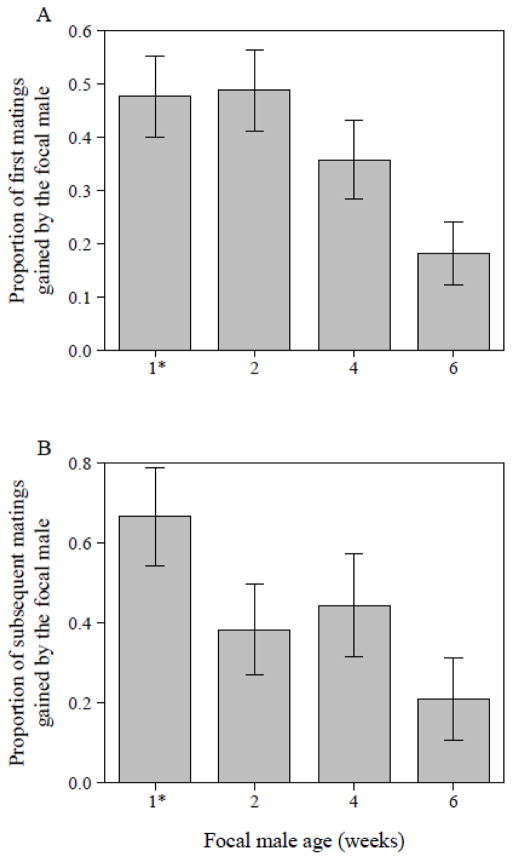
In the presence of a competitor, a focal male’s ability to secure the first mating significantly decreased with increasing focal-male age (GLM with Binomial error distribution, N = 171, df = 3, Δ deviance = 11.94, χ2 = 11.94, P = 0.008). The probability of a focal male gaining the first mating was similar to that of the competitor male when the focal male was 4 days to 2 weeks old. However, with older males the ratio skewed to favor the (younger) competitor male (Fig. 8A). Our daily checks revealed that this initial trend continued over the remaining five-day observation period: with increasing age, focal males gained a significantly lower proportion of the observed matings (GLM with Binomial data distribution, N = 64, df = 3, Δ deviance = 9.73, χ2 = 9.73, P = 0.021) and hence lost the pre-mating competition against younger competitor males (Fig. 8B).
Figure 8.
(A) Proportion of first matings gained by focal males of the indicated age [1* males were four days old], when in competition with 4-day-old Sb males (N = 171). Focal males were in direct competition with one Sb male as one female was held continuously with both types of males and the triplet was allowed to interact freely for five days. (B) Proportion of subsequent matings that focal males gained over a five day-period (N = 64, vials with additional matings observed via spot checks). Data are shown as means ± SE.
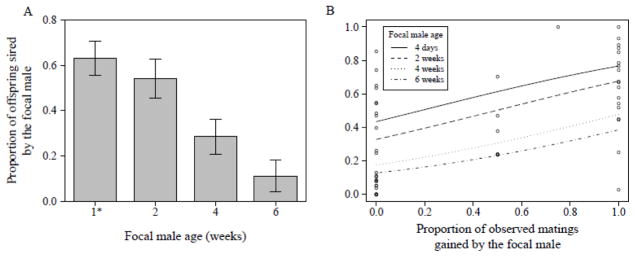
Similarly, the proportion of offspring sired by focal males over days 1 and 3 – 5 within this competitive setting was significantly influenced by the focal male’s age (GLM with Binomial data distribution corrected for overdispersion, N = 129, Δ deviance = 5789, F3,125 = 17.70, P < 0.001). There was a strong decline in male paternity share for males older than 2 weeks, and by 6 weeks focal male paternity share had declined to ~10% (Fig. 9A).
Figure 9.
(A) Overall proportion of offspring fathered over days 1 and 3–5 by focal males of the indicated age [1* males were four days old], relative to those sired by 4-day old competitor Sb males (N = 129). Focal males were in direct competition with one Sb male as one female was held continuously with both types of males and triplets were allowed to interact freely for five days. Data are presented as means ± SE. (B) Proportion of offspring fathered by focal males as a function of the proportion of observed matings gained by focal males (N = 49), when focal males were either 4 days (solid line), 2 weeks (dashed line), 4 weeks (dotted line) or 6 weeks (dashed and dotted line) old. Figure shows raw values (dots) and regression lines derived from the final statistical model.
This decline in paternity share in older males might be explained by diminishing success in pre-mating competition alone. But post-mating mechanisms might also play a role. To determine the relative extent to which pre-and post-mating success contributed to male reproductive success, we modelled the proportion of offspring sired by the focal male as a function of the proportion of observed matings he gained, including male age and the interaction of the two terms. The effect of proportional mating gain on offspring share was independent of male age (GLM with Binomial error distribution corrected for overdispersion, mating proportion × male age: N = 49, Δ deviance = 508.5, F3, 41 = 2.33, P = 0.09). Across all age classes males benefitted from gaining more matings by siring an increasing proportion of offspring (N = 49, Δ deviance = 1140.3, F1, 44 = 14.36, P < 0.001). Hence, success in direct male-male pre-mating competition also results in a higher fertilization success (Fig. 9B). However, male age significantly influenced paternity share (N = 49, Δ deviance = 1037.8, F3, 44 = 4.36, P = 0.009), showing diminishing returns with increasing male age (Fig. 9B). Older males are less successful in obtaining fertilizations even if they gain most of the observed matings, indicating the involvement of post-mating mechanisms.
4. Discussion
We have shown that, over a 6–7 week period, D. melanogaster males undergo reproductive senescence that strongly affects their reproductive success. Under laboratory conditions Dahomey wild-type males can live > 60 days, although only ~ 20 % survive past 50 days (HR, MK and CF unpublished data). Hence the timeframe we investigated spans a substantial part of a male’s lifespan. We found that reproductive ageing was accompanied by a decrease in a male’s mating probability and an increase in his latency-time to mating. D. melanogaster males have previously been found to undergo a 3 weeks aging phase in which the ability to mate and fertilise eggs diminishes rapidly, before in the late age phase mortality rates stabilize at low performance (Shahrestani et al. 2012). We also detected that with increasing age males were less efficient in inducing female post-mating changes. There was strong variation across traits for the male age at which we observed an age-dependent drop in male ability to induce female post-mating responses and also the strength of this response. For example after a single mating, males older than 5 weeks were less successful in preventing females from remating, whereas a males’ ability to trigger an egg laying boost had already weakened after 2 weeks of age. Similarly, 2 week- old males caused fewer sperm to storage shortly after mating (less than 50% compared to 4 day old males), and were about 3 times less efficient than younger males in inducing conformational changes in the female’s uterus. The combined effect of these age-dependent reductions strongly contributed to diminished male reproductive success in a competitive setting. As the post-mating traits we examined are mediated by male seminal fluid proteins (Avila et al. 2011), our data suggest that there is ageing of the male’s capacity to produce enough, or high-quality, Sfps. Producing a “well-composed” ejaculate (Perry et al. 2013) is key for male competitiveness and reproductive success. Our data indicate that Drosophila males are less able to do so as they age (see also Borziak et al. 2016 for an example in red junglefowl).
As males aged, we observed an age-dependent reduction in their ability to elicit critical post-mating responses by their mates. The responses we measured are caused by seminal fluid proteins transferred to females. For example, female propensity to remate and her level of egg production are regulated by receipt of her mate’s sex peptide (SP) (Chen et al. 1988; Chapman et al. 2003; Liu and Kubli 2003). Interestingly, in addition to these positive effects, repeated receipt of SP reduces female fitness and lifetime reproductive success and thus this Sfp possesses the potential to be a locus of sexual conflict (Wigby and Chapman 2005; Fricke et al. 2009; Isaac et al. 2010; Gioti et al. 2012; Smith et al. 2017). Because we found effects on SP-regulated traits in old vs. young males, we quantified directly the amount of SP transferred by these males. As expected, young males transferred the most SP, and 2 week old males transferred considerably (about 60 times) less. However, to our surprise, older males (4 weeks or older) transferred more SP than 2 week old males, with only 2.5 – 5 times less SP than seen for 4-day-old males, even though these oldest males were poor in suppressing female remating and in boosting female egg-laying. Even though for egg-laying effect sizes were modest (~10 eggs, see Fig. 2), effects were much strong for remating suppression (~50% more female remated in the two oldest age classes compared to mates from 4 day old males). Also the SP-lacking males showed a similar age-dependent increase in female remating rate (for males older than 5 weeks). Hence the ability of SP0 males to reduce female remating is additionally diminished in these males beyond their inability to transfer SP. These data suggest that factors other than the amount of SP transferred affect the male’s ability to induce post-mating responses as he ages.
One excellent candidate to explain the discord between amount of SP transferred and the reduced effectiveness in ageing wild-type males is sperm transfer. SP’s action beyond the first day requires its binding to, and release from, sperm (Peng et al. 2005; Apger-McGlaughon and Wolfner 2013). We found that the number of sperm initially stored in the females’ seminal receptacle decreased steadily with increasing male age. This strong effect was accompanied by a male-age-dependent decrease in the frequency in which any sperm were stored by the males’ mates at 30 mins after mating began, and by a male-age dependent decrease in ability to induce the uterine conformational changes that are thought to facilitate sperm storage (Adams and Wolfner 2007; Avila and Wolfner 2009; Mattei et al. 2015). Sperm storage depends on the number of sperm transferred by a male and on the efficiency of movement of those sperm into storage; Sfps, such as Acp36DE, are required for the latter (Bertram et al. 1996; Tram and Wolfner 1999; Chapman et al. 2000; Bloch Qazi and Wolfner 2003). We do not know whether male age negatively impacted the number of sperm transferred to females or the efficiency with which those sperm move into storage (or both). However, our finding that uterine conformational changes were ~3 times less well induced by older males suggests that at least this aspect of sperm storage is impaired as male age; this could reflect decreased production or quality of critical Sfps in older males. Furthermore, our data on the proportion of eggs that gave rise to adults over nine days of egg-laying are consistent with the idea that females mated to older (6-week-old) males have fewer sperm available for fertilizations. The proportion of eggs that gave rise to adults is a composite measure of fertilization success and larval/pupal survival to adulthood. Overall the proportion of eggs that gave rise to adults declined with time and this was similar for all male age groups, however 6-week- old males in general performed worse. Our data suggest that mates of older males had too few sperm in storage to sustain the production of progeny, however we cannot exclude that also egg-to-adult survival was reduced. This would be an interesting hypothesis to test in the future. Apart from reducing fertilization success the decreased sperm storage (as identified for the early time-point and assumed to persist after the process is completed (~6 hrs after mating, Manier et al. 2010)) by mates of older males would also mean that less SP would be retained by their mates. This would lead to smaller, and/or shorter-duration, effects of SP, despite the transfer of amounts of SP similar to those transferred by younger males.
Another possible explanation for the disconnect between similar amounts of SP transferred by old and young males, relative to the males’ different effects on their mates is that the SP transferred by older males could be of poorer quality than that from younger males. For example, some of the Sfps needed to modulate SP’s fate in the female (Ravi Ram and Wolfner 2009; Findlay et al. 2014) could be of lower quality or amount. Since we tested virgin males in our assays, our results measured the effects of a male’s first ejaculate. As this approach cannot tease apart the effects of male age vs. ejaculate age it could also be possible that the seminal fluid proteins degrade with time and become less effective. We know little of the effects of ageing on ejaculate quality and amount in D. melanogaster. However, in house mice (Mus domesticus) no signs of ejaculate ageing were found after two months of celibacy (Firman et al. 2015), whereas in guppies (Poecilia reticulata) ejaculate ageing was observed, characterized by reduced sperm velocity (Gasparini et al. 2010) and reduced sperm competitive success (Gasparini et al. 2017). Hence it would be worthwhile to disentangle the effect of organismal ageing and whether older males produce less well-composed ejaculates or whether the ejaculate itself ages faster in older males.
Interestingly, male age-dependent transfer of the two Sfps we examined showed different patterns. This might reflect the different cellular sources of these Sfps: ovulin is made in both cell types present in the AG (Monsma et al. 1990), whereas SP is only made in main cells. Main and secondary cells show different kinetics of accumulation of Sfps as a result of age at least in very young males (DiBenedetto et al. 1990; Monsma et al. 1990) and up to 10 days old males (Bertram et al. 1992), so it is possible that amounts of these proteins made and available in old males differ from those in young males and that these two Sfps have independent kinetics. Moreover, secondary cells in older males behave differently than in younger males, including delaminating and sometimes being transferred to females (Leiblich et al. 2012). An additional (but not mutually exclusive) explanation is that these same two Sfps show different allocation patterns as was observed in males in response to the presence of rivals (Wigby et al. 2009) or female mating status (Sirot et al. 2011). It may be that males maintain production of enough ovulin for longer and/or allocate it prudently and thus show no decline in amounts with age.
At the organismal level, we observed an age-dependent decrease in a male’s ability to gain a first mating, including an increase in latency time to copulation. The decrease in pre-copulatory performance as males’ age could have two possible explanations: First, due to a general physiological decline with age (e.g. Shahrestani et al. 2012), older males might not be able to court as much or as vigorously as young males; second old males might be less attractive to females, resulting in more vigorous resistance to these males by females. Work in D. melanogaster suggests a combination of both factors might be at work: Rezaei et al. (2015) reported that females showed more repelling behavior towards older males (52–53 days) than towards younger or intermediate-aged males, as well as that old males also showed less courtship behavior.
Overall in our assays as male age increased we saw a decline in males’ success in the individual pre- or post-mating traits measured. To assess the combined effects of these components on male reproductive success, we also tested males of different ages in a competitive setting, with a young rival continuously present. We found that in a competitive situation, with increasing age males became significantly less successful at both pre- and post-mating competition. Post-mating success was particularly important, as reproductive returns decreased steadily with advancing male age, even for males that gained a similar proportion of matings as four day old males (see Fig. 9B), even though all males increased their paternity share with each mating they gained. For example, while four day old males gained ~ 60 % of paternity when they gained 60 % of the matings, paternity dropped to ~ 45 % for 2 week- old males and was even lower for 4- and 6-week old males at the same value for proportional mating share (see Fig. 9B). The simplest explanation of our data is that ageing worsens a male’s ability to both i) gain a mating, and ii) induce several different Sfp-mediated post-mating traits. The cumulative effect of all of these individual declines is a significant reduction of male reproductive success with advancing age.
4.1 Conclusion
We found strong effects of male and/or ejaculate age on several pre- and post-copulatory traits in D. melanogaster. These included an age-dependent decline in a male’s ability to effectively induce post-mating changes in females that depend on the Sfps he transfers to her. This suggests an age dependent decline in male reproductive-gland function. It is possible that ageing males do not transfer sufficient amounts of Sfps and/or that the composition or quality of the Sfp set that they do transfer is no longer optimal. This may have been further facilitated by a parallel increase in ejaculate age. Our data support the idea that it is not just general senescence and decreased sperm quality/quantity that cause the lower reproductive success of older male D. melanogaster but also that senescence of non-sperm components of the ejaculate exerts a strong negative impact on a male’s post-mating competitiveness. In the future it will be interesting to determine whether and how the amount, blend, or quality of Sfps is impacted by ageing.
Highlights.
Ageing male flies gain fewer matings and induce lower female post-mating responses
These ageing induced declines reduce male reproductive success in competition
This suggests ejaculates of older males are less effective at inducing beneficial responses
We hypothesize that older male flies produce smaller or ill-composed ejaculates
Acknowledgments
We thank Tracey Chapman for sending the fly stocks used. Dolors Amorós-Moya and Sonja Schindler for help with the experiments, particularly with mating observations. We thank Norene Buehner, Frank Avila and Jessica Sitnik for instructions and advice on ELISA, sperm storage and uterine conformation assays. We thank the editor Thomas Johnson and two anonymous reviewers for their constructive suggestions. While at Cornell, HR was partly supported by the Fazit-Stiftung and also received support from the Münster Graduate School of Evolution. This work was supported by the German Science Foundation [FR 2973/1-1] to CF and partially by the National Institute of Health [R01-HD038921] to MFW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams EM, Wolfner MF. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J Insect Physiol. 2007;53:319–331. doi: 10.1016/j.jinsphys.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apger-McGlaughon J, Wolfner MF. Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. J Insect Physiol. 2013;59:1024–1030. doi: 10.1016/j.jinsphys.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avent TD, Price TAR, Wedell N. Age-based female preference in the fruit fly Drosophila pseudoobscura. Anim Behav. 2008;75:1413–1421. [Google Scholar]
- Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics. 2010;186:595–600. doi: 10.1534/genetics.110.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Wolfner MF. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci U S A. 2009;106:15796–15800. doi: 10.1073/pnas.0904029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MDW. Optimization of dietary restriction protocols in Drosophila. J Gerontol Biol Sci. 2007;62A:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015:67. [Google Scholar]
- Bertram MJ, Akerkar GA, Ard RL, Gonzalez C, Wolfner MF. Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mech Dev. 1992;38:33–40. doi: 10.1016/0925-4773(92)90036-j. [DOI] [PubMed] [Google Scholar]
- Bertram MJ, Neubaum DM, Wolfner MF. Localization of the Drosophila male accessory gland protein Acp36DE in the mated female suggests a role in sperm storage. Insect Biochem Mol Biol. 1996;26:971–980. doi: 10.1016/s0965-1748(96)00064-1. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi MC, Wolfner MF. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J Exp Biol. 2003;206:3521–3528. doi: 10.1242/jeb.00585. [DOI] [PubMed] [Google Scholar]
- Borziak K, Alvarez-Fernandez A, Karr TL, Pizzari T, Dorus S. The Seminal fluid proteome of the polyandrous Red junglefowl offers insights into the molecular basis of fertility, reproductive ageing and domestication. Sci Rep. 2016;6:35864. doi: 10.1038/srep35864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: Female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci U S A. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Neubaum DM, Wolfner MF, Partridge L. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc R Soc Lond Ser B-Biol Sci. 2000;267:1097–1105. doi: 10.1098/rspb.2000.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Stummzollinger E, Aigaki T, Balmer J, Bienz M, Bohlen P. A male accessory-gland peptide that regulates reproductive-behavior of female Drosophila melanogaster. Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Cornwallis CK, O’Connor EA. Sperm: seminal fluid interactions and the adjustment of sperm quality in relation to female attractiveness. Proc R Soc Lond B. 2009;276:3467–3475. doi: 10.1098/rspb.2009.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley MJ. The R Book. John Wiley & Sons; Chichester, UK: 2007. [Google Scholar]
- DiBenedetto AJ, Harada HA, Wolfner MF. Structure, cell-specific expression, and mating-induced regulation of a Drosophila melanogaster male accessory gland gene. Dev Biol. 1990;139:134–148. doi: 10.1016/0012-1606(90)90284-p. [DOI] [PubMed] [Google Scholar]
- Findlay GD, MacCoss MJ, Swanson WJ. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Res. 2009;19:886–896. doi: 10.1101/gr.089391.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Sitnik JL, Wang W, Aquadro CF, Clark NL, Wolfner MF. Evolutionary rate covariation identifies new members of a protein network required for Drosophila melanogaster female post-mating responses. PloS Genet. 2014;10:e1004108. doi: 10.1371/journal.pgen.1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Yi XH, MacCoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. Plos Biol. 2008;6:1417–1426. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firman RC, Young FJ, Rowe DC, Duong HT, Gasparini C. Sexual rest and post-meiotic sperm ageing in house mice. J Evol Biol. 2015;28:1373–1382. doi: 10.1111/jeb.12661. [DOI] [PubMed] [Google Scholar]
- Fricke C, Chapman T. Variation in the post-mating fitness landscape in fruit flies. J Evol Biol. 2017;30:1250–1261. doi: 10.1111/jeb.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke C, Maklakov AA. Male age does not affect female fitness in a polyandrous beetle, Callosobruchus maculatus. Anim Behav. 2007;74:541–548. [Google Scholar]
- Fricke C, Martin OY, Bretman A, Bussiere LF, Chapman T. Sperm competitive ability and indices of lifetime reproductive success. Evolution. 2010;64:2746–2757. doi: 10.1111/j.1558-5646.2010.01022.x. [DOI] [PubMed] [Google Scholar]
- Fricke C, Wigby S, Hobbs R, Chapman T. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster. J Evol Biol. 2009;22:275–286. doi: 10.1111/j.1420-9101.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- Gasparini C, Dosselli R, Evans JP. Sperm storage by males causes changes in sperm phenotype and influences the reproductive fitness of males and their sons. Evol Lett. 2017;1:16–25. doi: 10.1002/evl3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini C, I, Marino AM, Boschetto C, Pilastro A. Effect of male age on sperm traits and sperm competition success in the guppy (Poecilia reticulata) J Evol Biol. 2010;23:124–135. doi: 10.1111/j.1420-9101.2009.01889.x. [DOI] [PubMed] [Google Scholar]
- Gilchrist AS, Partridge L. Why it is difficult to model sperm displacement in Drosophila melanogaster: The relation between sperm transfer and copulation duration. Evolution. 2000;54:534–542. doi: 10.1111/j.0014-3820.2000.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Gioti A, Wigby S, Wertheim B, Schuster E, Martinez P, Pennington CJ, Partridge L, Chapman T. Sex peptide of Drosophila melanogaster males is a global regulator of reproductive processes in females. Proc R Soc B-Biol Sci. 2012;279:4423–4432. doi: 10.1098/rspb.2012.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudoux P. Package “pgirmess.” CRAN. 2016. [Google Scholar]
- Harrison XA. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ. 2014;2:e616. doi: 10.7717/peerj.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman LG, Prout T. Sperm Displacement Without Sperm Transfer In Drosophila-Melanogaster. Evolution. 1994;48:758–766. doi: 10.1111/j.1558-5646.1994.tb01359.x. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Lung O, Frongillo EA, Wolfner MF. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr Biol. 2000;10:99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Wolfner MF. A Drosophila Seminal Fluid Protein, Acp26aa, Stimulates Egg-Laying In Females For 1 Day After Mating. Proc Natl Acad Sci U S A. 1995;92:10114–10118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihara F. Effects of the male accessory gland secretion on oviposition and remating in females of Drosophila melanogaster. Zool Mag. 1981;90:307–316. [Google Scholar]
- Isaac RE, Li C, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc R Soc B-Biol Sci. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman S, Takh A, Zeileis A, Maimone C, Fearon J. Package “pscl.” CRAN. 2015. [Google Scholar]
- Johnson SL, Gemmell NJ. Are old males still good males and can females tell the difference? Bioessays. 2012;34:609–619. doi: 10.1002/bies.201100157. [DOI] [PubMed] [Google Scholar]
- Jones TM, Balmford A, Quinnell RJ. Adaptive female choice for middle-aged mates in a lekking sandfly. Proc R Soc B-Biol Sci. 2000;267:681–686. doi: 10.1098/rspb.2000.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TM, Elgar MA. The role of male age, sperm age and mating history on fecundity and fertilization success in the hide beetle. Proc R Soc Lond B. 2004;271:1311–1318. doi: 10.1098/rspb.2004.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TM, Featherston R, Paris DBBP, Elgar MA. Age-related sperm transfer and sperm competitive ability in the male hide beetle. Behav Ecol. 2007;18:251–258. [Google Scholar]
- Kapelnikov A, Rivlin PK, Hoy RR, Heifetz Y. Tissue remodeling: a mating-induced differentiation program for the Drosophila oviduct. BMC Dev Biol. 2008a;8:114. doi: 10.1186/1471-213X-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapelnikov A, Zelinger E, Gottlieb Y, Rhrissorrakrai K, Gunsalus KC, Heifetz Y. Mating induces an immune response and developmental switch in the Drosophila oviduct. Proc Natl Acad Sci U S A. 2008b;105:13912–13917. doi: 10.1073/pnas.0710997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl I, Fischer K. Old male mating advantage results from sexual conflict in a butterfly. Anim Behav. 2013;85:143–149. [Google Scholar]
- Krishna MS, Santhosh HT, Hegde SN. Offspring of older males are superior in Drosophila bipectinata. Zool Stud. 2012;51:72–84. [Google Scholar]
- Leiblich A, Marsden L, Grandy C, Corrigan L, Jenkins R, Hamdy F, Wilson C. Bone morphogenetic protein- and mating-dependent secretory cell growth and migration in the Drosophila accessory gland. Proc Natl Acad Sci U S A. 2012;109:19292–19297. doi: 10.1073/pnas.1214517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O, Wolfner MF. Drosophila seminal fluid proteins enter the circulatory system of the mated female fly by crossing the posterior vaginal wall. Insect Biochem Mol Biol. 1999;29:1043–1052. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. Resolving mechanisms of competitive fertilisation success in Drosophila melanogaster. Nature. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- Mattei AL, Riccio ML, Avila FW, Wolfner MF. Integrated 3D view of postmating responses by the Drosophila melanogaster female reproductive tract, obtained by microcomputed tomography scanning. Proc Natl Acad Sci U S A. 2015;112:8475–8480. doi: 10.1073/pnas.1505797112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald GC, Spurgin LG, Fairfield EA, Richardson DS, Pizzari T. Pre- and postcopulatory sexual selection favor aggressive, young males in polyandrous groups of red junglefowl. Evolution. 2017;71:1653–1669. doi: 10.1111/evo.13242. [DOI] [PubMed] [Google Scholar]
- Monsma SA, Harada HA, Wolfner MF. Synthesis of two Drosophila male accessory gland proteins and their fate after transfer to the female during mating. Dev Biol. 1990;142:465–475. doi: 10.1016/0012-1606(90)90368-s. [DOI] [PubMed] [Google Scholar]
- Mueller LD, Shahrestani P, Rauser CL, Rose MR. The death spiral: predicting death in Drosophila cohorts. Biogerontology. 2016;17:805–816. doi: 10.1007/s10522-016-9639-7. [DOI] [PubMed] [Google Scholar]
- Nelder JA, Wedderburn RWM. Generalized Linear Models. 1972;135(3):370–384. [Google Scholar]
- Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Chen S, Buesser S, Liu H, Honegger T, Kubli E. Gradual release of sprm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005;15:207–2013. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Perry JC, Sirot LK, Wigby S. The seminal symphony: how to compose an ejaculate. Trends Ecol Evol. 2013;28:414–422. doi: 10.1016/j.tree.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Dean R, Pacey A, Moore H, Bonsall MB. The evolutionary ecology of pre- and post-meiotic sperm senescence. Trends Ecol Evol. 2007;23:131–140. doi: 10.1016/j.tree.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Price DK, Hansen TF. How does offspring quality change with age in male Drosophila melanogaster? Behav. Genet. 1998;28:395–402. doi: 10.1023/a:1021677804038. [DOI] [PubMed] [Google Scholar]
- Prout T, Clark AG. Seminal fluid causes temporarily reduced egg hatch in previously mated females. Proc R Soc Lond Ser B-Biol Sci. 2000;267:201–203. doi: 10.1098/rspb.2000.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. 2015. [Google Scholar]
- Radwan J. Male age, germline mutations and the benefits of polyandry. Ecol Lett. 2003;6:581–586. [Google Scholar]
- Radwan J, Michalczyk L, Prokop Z. Age dependence of male mating ability and sperm competition success in the bulb mite. Anim Behav. 2005;69:1101–1105. [Google Scholar]
- Ravi Ram K, Ji S, Wolfner MF. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem Mol Biol. 2005;35:1059–1071. doi: 10.1016/j.ibmb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci U S A. 2009;106:15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. Plos Genet. 2007;3:2428–2438. doi: 10.1371/journal.pgen.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt K, Dobler R, Abbott J. An Ecology of Sperm: sperm diversification by natural selection. Annu Rev Ecol Evol Syst. 2015;46:435–459. [Google Scholar]
- Rezaei A, Siddaiah Krishna M, Santhosh HT. Male age affects female mate preference, quantity of accessory gland proteins, and sperm traits and female fitness in D. melanogaster. Zoolog Sci. 2015;32:16–24. doi: 10.2108/zs140121. [DOI] [PubMed] [Google Scholar]
- RStudio Team. RStudio: Integrated development for R. Inc; Boston: 2015. [Google Scholar]
- Rubinstein CD, Wolfner MF. Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc Natl Acad Sci U S A. 2013;110:17420–17425. doi: 10.1073/pnas.1220018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer MA, Uhl G. Determinants of paternity success in the spider Pholcus phalangioides (Pholcidae: Araneae): the role of male and female mating behaviour. Behav Ecol Sociobiol. 2002;51:368–377. [Google Scholar]
- Shahrestani P, Tran X, Mueller LD. Patterns of male fitness conform to predictions of evolutionary models of late life. J Evol Biol. 2012;25:1060–1065. doi: 10.1111/j.1420-9101.2012.02492.x. [DOI] [PubMed] [Google Scholar]
- Simmons LW. Sperm competition and its evolutionary consequences in the insects. Princeton University Press; Princeton: 2001. [Google Scholar]
- Simmons LW, Fitzpatrick JL. Sperm wars and the evolution of male fertility. Reproduction. 2012;144:519–534. doi: 10.1530/REP-12-0285. [DOI] [PubMed] [Google Scholar]
- Sirot LK, Buehner NA, Fiumera AC, Wolfner MF. Seminal fluid protein depletion and replenishment in the fruit fly, Drosophila melanogaster: an ELISA-based method for tracking individual ejaculates. Behav Ecol Sociobiol. 2009;63:1505–1513. doi: 10.1007/s00265-009-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Wolfner MF, Wigby S. Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2011;108:9922–9926. doi: 10.1073/pnas.1100905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DT, Clarke NVE, Boone JM, Fricke C, Chapman T. Sexual conflict over remating interval is modulated by the sex peptide pathway. Proc R Soc B-Biol Sci. 2017;284:20162394. doi: 10.1098/rspb.2016.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tram U, Wolfner MF. Male seminal fluid proteins are essential for sperm storage in Drosophila melanogaster. Genetics. 1999;153:837–844. doi: 10.1093/genetics/153.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspoor RL, Cuss M, Price TAR. Age-based mate choice in the monandrous fruit fly Drosophila subobscura. Anim Behav. 2015;102:199–207. [Google Scholar]
- Warnes GR, Bolker BM, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M, Venables B. Various R programming tools for plotting data. Gplots Package 2016 [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Wigby S, Sirot LK, Linklater JR, Buehner N, Calboli FC, Bretman A, Wolfner MF, Chapman T. Seminal fluid protein allocation and male reproductive success. Curr Biol. 2009;19:751–757. doi: 10.1016/j.cub.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Albright SN, Giebel JD, Ravi Ram K, Shuqing J, Fiumera AC, Wolfner MF. A role for Acp29AB, a predicted seminal fluid lectin, in female sperm storage in Drosophila melanogaster. Genetics. 2008;180:921–931. doi: 10.1534/genetics.108.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeileis A, Hothorn T. Diagnostic checking in regression relationships. R News. 2002;2:7–10. [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. Springer; New York: 2009. [Google Scholar]