Abstract
Objective
To investigate the association between weight change in older adults and mortality in a multiethnic population.
Methods
We performed a prospective analysis using data on weight change between the baseline (1993-1996) and the 10-year follow-up (2003-2007) surveys in relation to subsequent mortality among 63,040 participants in the Multiethnic Cohort Study in Hawaii and California. The participants were African American, Native Hawaiian, Japanese American, Latino, and white, aged 45-75 years at baseline, and did not report heart disease or cancer at either survey.
Results
During an average of 7.3 years of follow-up after the 10-year survey, 6,623 deaths were identified. Compared with individuals whose weight remained stable (±2.5 kg), those who lost weight and those with the highest weight gain (>10 kg) were at increased risk of all-cause mortality, with the risks greater for the weight-loss (hazard ratios [HR], 2.86; 95% confidence interval [95% CI], 2.62-3.11 for >10 kg) than the weight-gain group (HR, 1.25; 95% CI, 1.11-1.41 for >10 kg), thus resulting in a reverse J-shaped curve. Japanese Americans and Latinos had stronger associations of weight loss >10 kg with mortality than did African Americans, Native Hawaiians, and whites. The increase in risk with weight gain >10 kg was greater for older (≥55 years at baseline) than younger individuals while the increase in mortality associated with weight loss was greater for the normal weight (<25 kg/m2 at baseline) participants and never smokers, compared with overweight/obese persons and current smokers, respectively.
Conclusions
Our findings confirm the association between weight change and a higher mortality in a healthy, multiethnic population, with higher risks for weight loss than weight gain. Based on these observations, public health recommendation should focus on the prevention of weight loss, as well as weight stability within the non-obese range, for middle aged and older adults.
Introduction
Adult weight change, in addition to excess body weight,1-4 has frequently been examined in relation to longevity. In a meta-analysis of 26 prospective studies, unintentional weight loss was associated with a 22 to 39% higher risk of all-cause mortality.5 A recent meta-analysis of 26 prospective studies reported a 45% increased risk of all-cause mortality with weight loss, and a 7% increase in risk with weight gain in middle-aged populations.6 Among older adults (≥60 years), the increase in risk for all-cause mortality was 67% with weight loss and 21% with weight gain in another meta-analysis of 17 prospective studies.7 However, these previous studies were conducted mostly in white populations. Since racial/ethnic differences have been found in obesity status,8 body composition,9 and weight management behavior,10,11 weight change and its association with chronic diseases and longevity may vary across racial/ethnic groups.
The Multiethnic Cohort (MEC) that consists of participants primarily from five race/ethnicity groups – African Americans, Native Hawaiians, Japanese Americans, Latinos, and whites – provides a unique opportunity to investigate racial/ethnic differences in risk factors for chronic disease. In a previous analysis in the MEC, we reported a J-shaped association between baseline body mass index (BMI) and all-cause mortality in healthy never smokers.12 In the current study, using data from baseline and a 10-year follow-up survey, we investigated the association of weight change between the two surveys with subsequent mortality, and whether this relationship varied by race/ethnicity, age, baseline BMI, and smoking status among older adults in the MEC.
Materials and Methods
Study population
The MEC was established to investigate the association of lifestyle and genetic factors with cancer and other chronic diseases. The institutional review boards of the University of Hawaii and the University of Southern California approved the study protocol. In 1993-1996, more than 215,000 participants aged 45-75 years living in Hawaii and California (primarily Los Angeles county) entered the cohort by completing a 26-page mailed self-administered questionnaire and consenting to be in the study. As a result of targeted recruiting, participants were primarily African American, Native Hawaiian, Japanese American, Latino, or white living in Hawaii and California. Details of the study design and implementation are described elsewhere.13
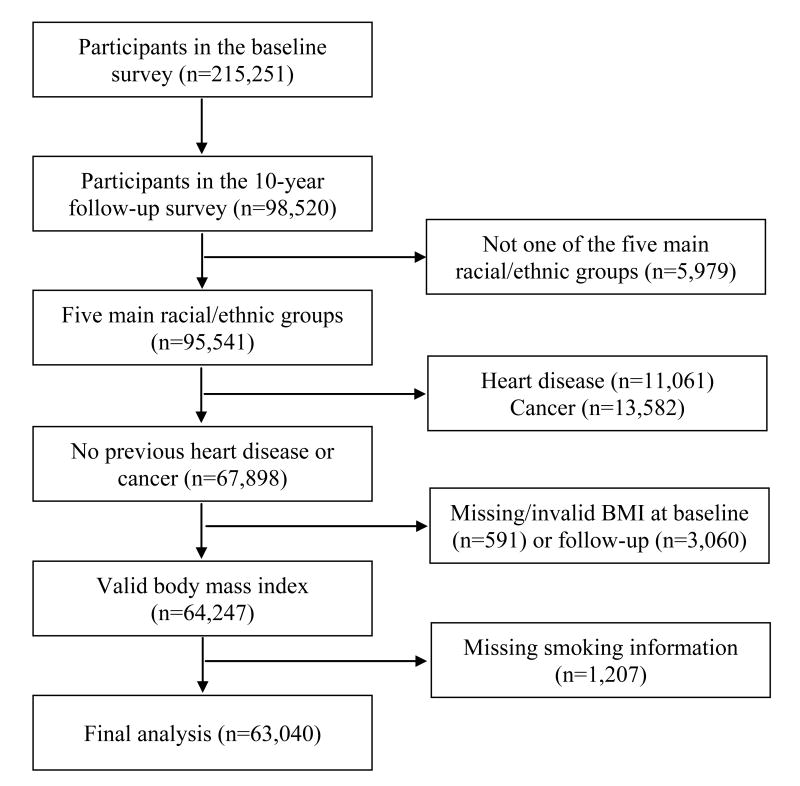
In 2003-2007, the 10-Year Follow-Up Questionnaire, which was very similar to the Baseline Questionnaire, was sent to all living participants (mean follow-up: 11.0 years). The approximately 98,500 participants who completed the 10-year follow-up questionnaire were the subjects of this study. For the current analyses, we successively excluded participants who were not of one of the five main racial/ethnic groups (n=5,979), who reported heart attack, angina at baseline or follow-up (n=11,061), who reported cancer at baseline or who had invasive cancer reported to tumor registries up to the date of the follow-up questionnaire (n=13,582), who had missing information on weight or height (n=442) or invalid BMI (<15 or >50 kg/m2, n=149) at baseline, who had missing weight (n=3,060), or missing smoking information at the 10-year follow-up (n=1,207). Therefore, a total of 63,040 participants remained for the analysis (Figure 1). Their mean age (SD) at the 10-year survey was 68.2 (8.3) years. Compared with those included in the analysis (n=63,040), participants who were excluded due to missing information on BMI and smoking (n=4,858) were more likely to be women (61.6% vs. 56.9%), older (71.3 vs. 68.2 years of mean age) and less educated (12.4 vs. 14.0 years of education). The entire MEC participants (∼215,000) were a somewhat better educated group compared to the general population in Hawaii and Los Angeles Country.13 In addition, participants who responded to the 10-year follow-up survey were more likely to be younger, never smokers, and more educated, compared with non-respondents. Therefore our findings, based on the analytic sample (∼63,000) who did not report a diagnosis of heart attack or cancer, reflect a younger, healthy, and better educated population.
Figure 1.
Flow diagram summarizing inclusion and exclusion criteria
Weight and covariates
BMI (as kg/m2) at baseline was calculated from self-reported weight and height from the Baseline Questionnaire. BMI at the 10-year follow-up was calculated from weight reported on the Ten-Year Follow-Up Questionnaire and height from the Baseline Questionnaire. Weight change between the two surveys was calculated by subtracting weight at baseline from weight at follow-up. On both questionnaires, cohort participants also provided information on diet, including alcohol consumption, cigarette smoking and history of medical conditions.
Outcome ascertainment
Deaths of cohort participants were identified by linkage to the death certificate files in Hawaii and California and to the U.S. National Death Index through December 31, 2012. During a mean 7.3 years of follow-up (525,762 person years) since the 10-year follow-up survey (2003-2007), we identified 6,623 deaths, 2,171 from cardiovascular disease and 1,967 from cancer.
Statistical analysis
We categorized weight change between the baseline and 10-year surveys into seven categories: weight loss (>10, >5–10, >2.5–5 kg), stable weight (within ±2.5 kg), and weight gain (>2.5–5, >5–10, >10 kg) based on previous studies14,15 and ensuring enough number of events in each weight change category. Cox proportional hazards regression with age as the time metric was used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of mortality related to weight change, using the stable weight group as the reference. The models were adjusted for race/ethnicity as a strata variable, and age, BMI at 10-year follow up and years between the baseline and 10-year surveys as covariates. BMI was selected for inclusion as it was related to subsequent mortality and to weight change. The interval between the two surveys was included to account for the autocorrelation of measures over time. For a majority (75.7%), the interval was between 8 and 12 years, since the goal had been 10 years. Smoking was an important confounding factor related to both BMI change and mortality. Thus some models were further adjusted for smoking at 10-year follow-up based on a comprehensive smoking model that was developed for lung cancer study in the MEC.16 The model explicitly included average number of cigarettes; average number of cigarettes squared; indicator variables for former and current smokers; number of years smoked (time-dependent); number of years since quitting (time-dependent); and interactions of race/ethnicity with the following variables: average number of cigarettes, average number of cigarettes squared, smoking status, and number of years smoked. We also considered education and alcohol consumption as covariates but did not include them in the final models because adjustment did not alter the associations. The proportionality assumption was tested by Schoenfeld residuals and was found to be valid. Since the associations between weight change and all-cause mortality were similar between men and women, some of these analyses were performed in men and women combined, with adjustment for sex as a strata variable.
We evaluated models for all-cause mortality and for cause-specific mortality (cardiovascular disease and cancer). We performed subgroup analyses to examine whether the associations between weight change and mortality varied by race/ethnicity, age group (45-54; 55-64; 65-75 years at baseline), baseline BMI (<25; 25–<30; ≥30 kg/m2), and smoking status (never, former, current). We also examined the associations by years between the baseline and follow-up surveys (<10, 10-12, >12 years), but did not present the results because there was little substantive difference. In sensitivity analyses, we excluded the 1,070 deaths that occurred within the first 2 years after the 10-year follow-up survey, and excluded the participants with baseline BMI <20 kg/m2 (n=3,591) who might have had an underlying illness. We also examined weight change per year (by dividing by the number of years between questionnaires) and percent weight change relative to the baseline weight. Tests for heterogeneity across subgroups were based on the Wald statistics for cross-product terms. Tests for heterogeneity between cardiovascular disease and cancer mortality were based on competing risk methodology.17 We also examined a nonlinear relation between weight change and mortality nonparametrically with restricted cubic splines.18 All analyses were conducted by using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Between the baseline and the 10-year follow-up surveys, mean (SD) weight change was 0.85 (7.2) kg in men and 1.3 (7.6) kg in women, but varied across the age groups. The mean (SD) weight change for those 45-54, 55-64 and 65-75 years of age at cohort entry was 2.4 (7.3), 0.42 (7.0), ‐1.5 (6.3) kg in men and 3.1 (7.9), 0.82 (7.2), ‐1.3 (6.8) kg in women, respectively. Overall, 4.2% of men and 4.5% of women lost more than 10 kg, while 6.8% of men and 8.2% of women gained more than 10 kg in weight (Table 1). In all, 41.4% of men and 39.2% of women maintained their weight within ±2.5 kg. Japanese Americans were more likely to report stable weight, while white men and women were more likely to gain weight. Compared to individuals in the weight loss groups, those in the weight gain groups were more likely to be younger and less likely to smoke at the 10-year follow-up.
Table 1. Characteristics of participants according to weight change at the follow-up survey (2003-2007).
| Weight change (kg) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Loss (>10) | Loss (>5–10) | Loss (>2.5–5) | Stable (±2.5) | Gain (>2.5–5) | Gain (>5–10) | Gain (>10) | |
| Men (n=27,181) | |||||||
| Participants, n | 1,146 | 2,450 | 3,033 | 11,242 | 3,838 | 3,626 | 1,846 |
| Age at baseline, year | 59.6 ± 8.5 | 59.8 ± 8.5 | 59.4 ± 8.3 | 57.6 ± 8.2 | 55.7 ± 7.7 | 54.2 ± 7.2 | 52.7 ± 6.6 |
| Age at follow-up, year | 70.7 ± 8.6 | 70.9 ± 8.5 | 70.3 ± 8.3 | 68.4 ± 8.2 | 66.7 ± 7.7 | 65.2 ± 7.2 | 64.0 ± 6.6 |
| Weight at baseline, kg | 94.3 ± 18.6 | 83.0 ± 14.6 | 78.6 ± 13.4 | 76.4 ± 12.6 | 78.1 ± 12.9 | 80.7 ± 13.7 | 87.8 ± 16.6 |
| Weight at follow-up, kg | 77.5 ± 18.5 | 75.8 ± 14.5 | 74.7 ± 13.4 | 76.5 ± 12.7 | 82.0 ± 13.0 | 88.0 ± 13.8 | 103.5 ± 18.5 |
| BMI at baseline, kg/m2 | 30.5 ± 5.3 | 27.6 ± 4.0 | 26.4 ± 3.6 | 25.7 ± 3.4 | 26.0 ± 3.5 | 26.5 ± 3.8 | 28.1 ± 4.8 |
| BMI at follow-up, kg/m2 | 25.0 ± 5.4 | 25.1 ± 4.0 | 25.1 ± 3.6 | 25.7 ± 3.4 | 27.3 ± 3.5 | 28.9 ± 3.9 | 33.2 ± 5.4 |
| Alcohol consumption, g/day | 8.5 ± 20.7 | 10.4 ± 21.3 | 10.1 ± 19.0 | 11.5 ± 20.1 | 11.9 ± 20.9 | 12.8 ± 23.4 | 12.8 ± 22.8 |
| Ethnicity | |||||||
| African American, % | 12.4 | 7.4 | 7.3 | 6.2 | 7.1 | 8.1 | 10.2 |
| Native Hawaiian, % | 15.1 | 8.7 | 7.0 | 6.0 | 6.5 | 8.5 | 12.3 |
| Japanese American, % | 23.2 | 37.0 | 42.8 | 42.6 | 36.9 | 29.2 | 14.9 |
| Latino, % | 20.2 | 18.6 | 18.4 | 18.5 | 20.2 | 19.0 | 20.2 |
| White, % | 29.1 | 28.2 | 24.5 | 26.6 | 29.2 | 35.2 | 42.4 |
| Smoking | |||||||
| Never, % | 35.3 | 35.8 | 38.2 | 39.5 | 40.3 | 36.7 | 34.2 |
| Former, % | 49.6 | 52.0 | 52.1 | 50.5 | 50.7 | 55.1 | 57.0 |
| Current, % | 15.1 | 12.2 | 9.7 | 10.0 | 9.0 | 8.2 | 8.8 |
| Women (n=35,859) | |||||||
| Participants, n | 1,599 | 3,062 | 3,794 | 14,062 | 5,391 | 5,007 | 2,944 |
| Age at baseline, year | 60.0 ± 8.7 | 60.5 ± 8.3 | 60.0 ± 8.4 | 58.1 ± 8.3 | 56.1 ± 7.7 | 54.4 ± 7.3 | 52.5 ± 6.8 |
| Age at follow-up, year | 71.3 ± 8.8 | 71.6 ± 8.3 | 71.0 ± 8.4 | 68.9 ± 8.3 | 67.0 ± 7.8 | 65.4 ± 7.4 | 63.8 ± 6.9 |
| Weight at baseline, kg | 83.7 ± 17.8 | 70.5 ± 15.4 | 65.3 ± 14.2 | 62.0 ± 13.1 | 63.6 ± 13.2 | 66.8 ± 13.7 | 73.1 ± 15.8 |
| Weight at follow-up, kg | 67.5 ± 16.7 | 63.2 ± 15.2 | 61.4 ± 14.1 | 62.1 ± 13.1 | 67.5 ± 13.3 | 74.1 ± 13.9 | 89.5 ± 18.5 |
| BMI at baseline, kg/m2 | 31.8 ± 6.3 | 27.5 ± 5.4 | 25.7 ± 4.9 | 24.5 ± 4.5 | 25.0 ± 4.6 | 25.8 ± 4.8 | 27.6 ± 5.6 |
| BMI at follow-up, kg/m2 | 25.6 ± 5.9 | 24.6 ± 5.3 | 24.2 ± 4.9 | 24.5 ± 4.5 | 26.5 ± 4.7 | 28.6 ± 4.9 | 33.8 ± 6.6 |
| Alcohol consumption, g/day | 2.9 ± 11.5 | 3.5 ± 10.1 | 3.9 ± 11.5 | 3.9 ± 9.8 | 3.9 ± 9.7 | 4.0 ± 9.9 | 3.9 ± 11.2 |
| Ethnicity | |||||||
| African American, % | 24.9 | 15.8 | 11.0 | 8.9 | 11.3 | 13.7 | 19.6 |
| Native Hawaiian, % | 13.2 | 7.1 | 6.0 | 6.5 | 7.4 | 9.1 | 12.5 |
| Japanese American, % | 15.0 | 31.5 | 38.9 | 42.7 | 36.9 | 24.6 | 11.5 |
| Latino, % | 17.7 | 18.4 | 16.8 | 14.9 | 17.6 | 19.6 | 18.5 |
| White, % | 29.2 | 27.2 | 27.3 | 27.1 | 26.8 | 33.0 | 38.0 |
| Smoking | |||||||
| Never, % | 59.5 | 63.2 | 64.8 | 66.2 | 63.8 | 57.2 | 50.8 |
| Former, % | 28.1 | 26.4 | 27.9 | 27.2 | 30.2 | 35.7 | 40.9 |
| Current, % | 12.3 | 10.4 | 7.3 | 6.6 | 6.0 | 7.1 | 8.3 |
Abbreviation: BMI, Body mass index. Data are presented as mean ± s.d. for continuous variables.
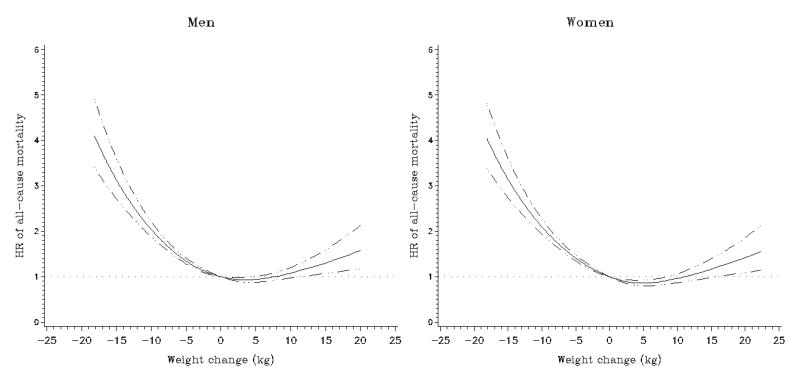
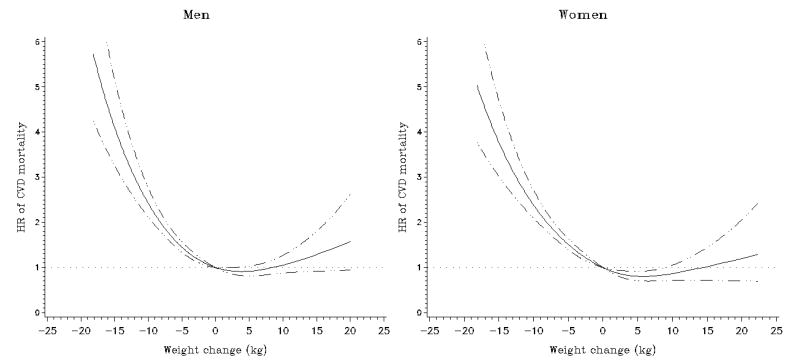
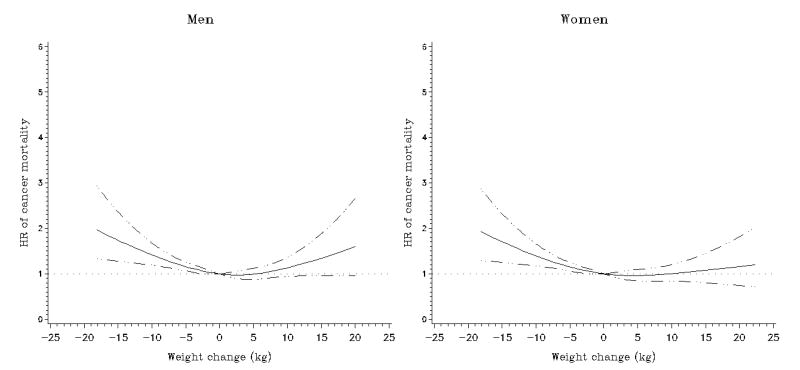
Compared to the stable weight group, all weight loss groups and the >10 kg weight gain group had higher all-cause mortality in each sex after adjustment for race/ethnicity, age, BMI, and interval between the two weight reports (Table 2 and Figure 2). Further adjustment for smoking slightly attenuated these associations: the relative risk estimates for the >10 kg loss and >10 kg gain groups were 2.85 (95% CI, 2.53 to 3.22) and 1.28 (95% CI, 1.08 to 1.52) in men, and 2.88 (95% CI, 2.55 to 3.25) and 1.22 (95% CI, 1.03 to 1.44) in women, respectively, resulting in a reverse J-shaped association. Similar associations were found for men and women (P for heterogeneity = 0.33). Interval (years) between the two weight reports was not significantly related to all-cause mortality. We observed comparable patterns for weight change per year (Supplementary Table 1) and percent weight changes (Supplementary Table 2). The increased risk associated with weight loss was greater for cardiovascular disease than for cancer mortality in both men and women (P's for heterogeneity < 0.001, Table 2 and Figures 3 and 4).
Table 2. Weight change between baseline (1993-1996) and follow-up (2003-2007) and subsequent mortality in the Multiethnic Cohort Study.
| Weight change (kg) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Causes of death | Loss (>10) | Loss (>5–10) | Loss (>2.5–5) | Stable (±2.5) | Gain (>2.5–5) | Gain (>5–10) | Gain (>10) |
| Men | |||||||
| All causes | |||||||
| No. of deaths | 360 | 530 | 478 | 1,228 | 354 | 306 | 189 |
| HR (95% CI)a | 2.99 (2.65-3.37) | 1.77 (1.59-1.96) | 1.28 (1.15-1.42) | 1.00 (ref) | 1.01 (0.90-1.14) | 1.05 (0.92-1.20) | 1.44 (1.21-1.70) |
| HR (95% CI)b | 2.85 (2.53-3.22) | 1.68 (1.52-1.87) | 1.28 (1.15-1.42) | 1.00 (ref) | 1.00 (0.89-1.13) | 1.00 (0.88-1.14) | 1.28 (1.08-1.52) |
| CVD | |||||||
| No. of deaths | 127 | 186 | 148 | 393 | 98 | 100 | 63 |
| HR (95% CI)a | 3.30 (2.69-4.05) | 1.93 (1.62-2.30) | 1.23 (1.02-1.49) | 1.00 (ref) | 0.85 (0.68-1.06) | 1.01 (0.80-1.26) | 1.19 (0.89-1.61) |
| HR (95% CI)b | 3.24 (2.64-3.98) | 1.88 (1.57-2.24) | 1.23 (1.02-1.49) | 1.00 (ref) | 0.84 (0.67-1.05) | 0.98 (0.78-1.23) | 1.13 (0.84-1.52) |
| Cancer | |||||||
| No. of deaths | 74 | 128 | 140 | 384 | 132 | 114 | 65 |
| HR (95% CI)a | 1.99 (1.55-2.57) | 1.42 (1.16-1.74) | 1.23 (1.02-1.50) | 1.00 (ref) | 1.17 (0.96-1.43) | 1.21 (0.98-1.51) | 1.62 (1.20-2.17) |
| HR (95% CI)b | 1.84 (1.43-2.37) | 1.33 (1.08-1.62) | 1.23 (1.01-1.49) | 1.00 (ref) | 1.16 (0.95-1.42) | 1.14 (0.92-1.42) | 1.38 (1.03-1.86) |
| Women | |||||||
| All causes | |||||||
| No. of deaths | 389 | 521 | 448 | 1,047 | 287 | 287 | 199 |
| HR (95% CI)a | 2.94 (2.61-3.32) | 1.91 (1.72-2.12) | 1.35 (1.21-1.51) | 1.00 (ref) | 0.86 (0.75-0.98) | 1.04 (0.91-1.19) | 1.41 (1.19-1.66) |
| HR (95% CI)b | 2.88 (2.55-3.25) | 1.87 (1.68-2.08) | 1.36 (1.22-1.52) | 1.00 (ref) | 0.84 (0.74-0.96) | 0.97 (0.85-1.11) | 1.22 (1.03-1.44) |
| CVD | |||||||
| No. of deaths | 165 | 193 | 160 | 321 | 86 | 82 | 49 |
| HR (95% CI)a | 3.82 (3.15-4.64) | 2.17 (1.81-2.61) | 1.51 (1.25-1.83) | 1.00 (ref) | 0.87 (0.68-1.10) | 1.05 (0.82-1.35) | 1.25 (0.90-1.73) |
| HR (95% CI)b | 3.76 (3.10-4.58) | 2.13 (1.78-2.56) | 1.53 (1.26-1.85) | 1.00 (ref) | 0.85 (0.67-1.08) | 0.99 (0.77-1.28) | 1.12 (0.80-1.55) |
| Cancer | |||||||
| No. of deaths | 63 | 119 | 113 | 341 | 112 | 112 | 70 |
| HR (95% CI)a | 1.56 (1.18-2.05) | 1.44 (1.17-1.78) | 1.11 (0.90-1.37) | 1.00 (ref) | 0.95 (0.76-1.18) | 1.10 (0.88-1.37) | 1.27 (0.95-1.69) |
| HR (95% CI)b | 1.49 (1.13-1.96) | 1.38 (1.12-1.71) | 1.11 (0.90-1.38) | 1.00 (ref) | 0.92 (0.74-1.14) | 1.00 (0.80-1.25) | 1.06 (0.79-1.42) |
| Men and women | |||||||
| All causes | |||||||
| No. of deaths | 749 | 1,051 | 926 | 2,275 | 641 | 593 | 388 |
| HR (95% CI)a | 2.96 (2.72-3.23) | 1.83 (1.70-1.98) | 1.31 (1.21-1.42) | 1.00 (ref) | 0.94 (0.86-1.02) | 1.05 (0.95-1.15) | 1.42 (1.26-1.60) |
| HR (95% CI)b | 2.86 (2.62-3.11) | 1.76 (1.64-1.90) | 1.31 (1.22-1.42) | 1.00 (ref) | 0.92 (0.84-1.01) | 0.99 (0.90-1.08) | 1.25 (1.11-1.41) |
| CVD | |||||||
| No. of deaths | 292 | 379 | 308 | 714 | 184 | 182 | 112 |
| HR (95% CI)a | 3.53 (3.07-4.06) | 2.03 (1.79-2.31) | 1.36 (1.19-1.55) | 1.00 (ref) | 0.86 (0.73-1.01) | 1.04 (0.88-1.23) | 1.25 (1.01-1.56) |
| HR (95% CI)b | 3.46 (3.01-3.98) | 1.99 (1.75-2.26) | 1.36 (1.19-1.56) | 1.00 (ref) | 0.85 (0.72-1.00) | 1.00 (0.84-1.18) | 1.14 (0.92-1.43) |
| Cancer | |||||||
| No. of deaths | 137 | 247 | 253 | 725 | 244 | 226 | 135 |
| HR (95% CI)a | 1.78 (1.48-2.15) | 1.44 (1.24-1.66) | 1.18 (1.02-1.36) | 1.00 (ref) | 1.06 (0.91-1.23) | 1.15 (0.99-1.35) | 1.42 (1.16-1.75) |
| HR (95% CI)b | 1.67 (1.38-2.01) | 1.35 (1.17-1.56) | 1.17 (1.02-1.36) | 1.00 (ref) | 1.04 (0.90-1.20) | 1.07 (0.92-1.25) | 1.21 (0.98-1.48) |
Abbreviations: BMI, Body mass index; CI, Confidence interval; CVD, cardiovascular disease; HR, Hazard ratio.
Adjusted by Cox regression for sex, race/ethnicity, age, BMI, and years between the baseline and follow-up surveys.
Further adjusted by Cox regression for smoking status, average number of cigarettes, squared average number of cigarettes, number of years smoked (time-dependent), number of years since quitting (time-dependent), and interactions between ethnicity and smoking status, average number of cigarettes, squared average number of cigarettes and number of years smoked.
Figure 2.
Multivariate association between weight change and subsequent all-cause mortality in the Multiethnic Cohort Study. Association estimated by Cox regression based on restricted cubic splines. Dashed lines represent 95% confidence intervals for adjusted estimates.
Figure 3.
Multivariate association between weight change and subsequent cardiovascular disease (CVD) mortality in the Multiethnic Cohort Study. Association estimated by Cox regression based on restricted cubic splines. Dashed lines represent 95% confidence intervals for adjusted estimates.
Figure 4.
Multivariate association between weight change and subsequent cancer mortality in the Multiethnic Cohort Study. Association estimated by Cox regression based on restricted cubic splines. Dashed lines represent 95% confidence intervals for adjusted estimates
Racial/ethnic-specific associations with all-cause mortality in men and women combined are presented in Table 3. HRs for weight loss >10 kg were higher in Japanese Americans and Latinos (P for heterogeneity between Japanese Americans and Latinos vs. the other three groups = 0.004 for weight loss >10 kg). Increased risk with weight gain >10 kg was found in African Americans, Japanese Americans, and Latinos, although the test for heterogeneity was not statistically significant. In analyses stratified by age at baseline (Table 3), the increases in risk of all-cause mortality associated with weight gain were greater in older groups (P for heterogeneity between ≥55 years vs. <55 years = 0.037 for weight gain >10 kg), while increases in risk with weight loss were similar across the three age groups. In analyses stratified by baseline BMI, increases in risk with weight loss were greater in the BMI <25 kg/m2 group, compared to the overweight (BMI 25–<30 kg/m2) and obese (BMI ≥30 kg/m2) groups, while increases in risk with weight gain >10 kg were similar across the three BMI groups (P for heterogeneity <0.001). In the joint analysis of baseline BMI (3 categories) and weight change (7 categories) comparing all 20 groups to a reference group with normal BMI (18.5–<25 kg/m2) and stable weight, all weight change categories in the obese group showed an increased risk (Supplementary Table 3). Never smokers showed a stronger association with weight loss and weight gain >10 kg than former or current smokers (P for heterogeneity = 0.011, Table 3).
Table 3. Weight change between baseline (1993-1996) and follow-up (2003-2007) and subsequent all-cause mortality in subgroups in the Multiethnic Cohort Study.
| Weight change (kg) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Loss (>10) | Loss (>5–10) | Loss (>2.5–5) | Stable (±2.5) | Gain (>2.5–5) | Gain (>5–10) | Gain (>10) | |
| Race/ethnicity | |||||||
| African American | |||||||
| No. of deaths | 161 | 156 | 123 | 259 | 72 | 91 | 81 |
| HR (95% CI)a | 2.46 (2.01-3.01) | 1.79 (1.47-2.19) | 1.30 (1.05-1.62) | 1.00 (ref) | 0.72 (0.55-0.94) | 0.93 (0.73-1.19) | 1.32 (1.00-1.74) |
| Native Hawaiian | |||||||
| No. of deaths | 98 | 78 | 40 | 137 | 52 | 43 | 51 |
| HR (95% CI)a | 2.55 (1.96-3.32) | 1.68 (1.27-2.23) | 0.84 (0.59-1.20) | 1.00 (ref) | 0.94 (0.68-1.30) | 0.71 (0.50-1.01) | 0.97 (0.68-1.39) |
| Japanese American | |||||||
| No. of deaths | 151 | 363 | 397 | 915 | 213 | 146 | 38 |
| HR (95% CI)a | 3.29 (2.76-3.92) | 1.86 (1.64-2.10) | 1.43 (1.27-1.61) | 1.00 (ref) | 0.97 (0.83-1.13) | 1.14 (0.95-1.37) | 1.40 (0.99-1.96) |
| Latino | |||||||
| No. of deaths | 136 | 172 | 146 | 361 | 127 | 119 | 82 |
| HR (95% CI)a | 3.28 (2.69-4.00) | 1.75 (1.45-2.10) | 1.29 (1.06-1.56) | 1.00 (ref) | 0.95 (0.77-1.16) | 1.01 (0.81-1.24) | 1.31 (1.01-1.70) |
| White | |||||||
| No. of deaths | 203 | 282 | 220 | 603 | 177 | 194 | 136 |
| HR (95% CI)a | 2.52 (2.15-2.96) | 1.60 (1.38-1.84) | 1.24 (1.06-1.44) | 1.00 (ref) | 0.94 (0.79-1.11) | 0.94 (0.80-1.11) | 1.07 (0.87-1.32) |
| P for heterogeneity | 0.22 | ||||||
| Age at baseline | |||||||
| 45-54 years | |||||||
| No. of deaths | 92 | 102 | 80 | 310 | 114 | 158 | 133 |
| HR (95% CI)a | 2.67 (2.10-3.39) | 1.78 (1.42-2.23) | 1.18 (0.92-1.50) | 1.00 (ref) | 0.80 (0.65-1.00) | 0.90 (0.74-1.09) | 0.89 (0.71-1.12) |
| 55-64 years | |||||||
| No. of deaths | 220 | 315 | 266 | 692 | 249 | 243 | 159 |
| HR (95% CI)a | 2.87 (2.46-3.35) | 1.96 (1.72-2.25) | 1.36 (1.18-1.57) | 1.00 (ref) | 0.99 (0.85-1.14) | 1.06 (0.91-1.24) | 1.42 (1.18-1.72) |
| 65-75 years | |||||||
| No. of deaths | 437 | 634 | 580 | 1,273 | 278 | 192 | 96 |
| HR (95% CI)a | 2.83 (2.52-3.17) | 1.65 (1.50-1.82) | 1.30 (1.18-1.44) | 1.00 (ref) | 0.94 (0.82-1.07) | 0.98 (0.84-1.14) | 1.41 (1.13-1.76) |
| P for heterogeneity | 0.51 | ||||||
| BMI at baseline | |||||||
| <25 kg/m2 | |||||||
| No. of deaths | 149 | 403 | 452 | 1,215 | 300 | 234 | 115 |
| HR (95% CI)a | 3.20 (2.64-3.87) | 1.78 (1.57-2.01) | 1.34 (1.19-1.49) | 1.00 (ref) | 0.99 (0.87-1.12) | 1.07 (0.92-1.25) | 1.65 (1.32-2.07) |
| 25–<30 kg/m2 | |||||||
| No. of deaths | 273 | 436 | 342 | 795 | 248 | 222 | 131 |
| HR (95% CI)a | 2.20 (1.84-2.64) | 1.54 (1.35-1.75) | 1.18 (1.04-1.35) | 1.00 (ref) | 1.03 (0.89-1.19) | 1.07 (0.90-1.26) | 1.39 (1.10-1.75) |
| ≥30 kg/m2 | |||||||
| No. of deaths | 327 | 212 | 132 | 265 | 93 | 137 | 142 |
| HR (95% CI)a | 2.58 (2.17-3.07) | 1.50 (1.24-1.80) | 1.18 (0.95-1.45) | 1.00 (ref) | 0.83 (0.66-1.06) | 1.03 (0.83-1.28) | 1.10 (0.87-1.38) |
| P for heterogeneity | <0.001 | ||||||
| Smoking status | |||||||
| Never | |||||||
| No. of deaths | 350 | 460 | 420 | 978 | 284 | 227 | 132 |
| HR (95% CI)a | 3.37 (2.97-3.83) | 1.92 (1.72-2.15) | 1.37 (1.22-1.53) | 1.00 (ref) | 0.99 (0.86-1.13) | 1.11 (0.95-1.28) | 1.42 (1.16-1.73) |
| Former | |||||||
| No. of deaths | 297 | 436 | 397 | 1,004 | 282 | 291 | 211 |
| HR (95% CI)a | 2.73 (2.39-3.11) | 1.66 (1.48-1.86) | 1.28 (1.14-1.43) | 1.00 (ref) | 0.87 (0.76-0.99) | 0.91 (0.79-1.04) | 1.19 (1.01-1.41) |
| Current | |||||||
| No. of deaths | 102 | 155 | 109 | 293 | 75 | 75 | 45 |
| HR (95% CI)a | 2.00 (1.59-2.53) | 1.64 (1.35-2.01) | 1.28 (1.02-1.60) | 1.00 (ref) | 0.86 (0.67-1.12) | 1.00 (0.76-1.30) | 1.05 (0.75-1.49) |
| P for heterogeneity | 0.011 | ||||||
Abbreviations: BMI, Body mass index; CI, Confidence interval; HR, Hazard ratio.
Adjusted by Cox regression for sex, race/ethnicity, age, BMI, years between the baseline and follow-up surveys, smoking status, average number of cigarettes, squared average number of cigarettes, number of years smoked (time-dependent), number of years since quitting (time-dependent), and interactions between ethnicity and smoking status, average number of cigarettes, squared average number of cigarettes and number of years smoked.
In sensitivity analyses, the results did not vary after excluding deaths that occurred within the first 2 years after the 10-year follow-up survey (Supplementary Table 4) or after excluding the participants with baseline BMI <20 kg/m2 (Supplementary Table 5).
Discussion
In this large multiethnic prospective cohort study of adults aged 45-75 at enrollment, a weight loss of more than 2.5 kg and a weight gain of more than 10 kg during the first eleven years of follow-up were associated with a higher risk of subsequent all-cause mortality in both men and women as compared to participants whose weight remained stable within ±2.5 kg. Increases in risk of all-cause mortality were greater with weight loss than with weight gain, resulting in a reverse J-shaped association. Similar patterns were found for cardiovascular disease and cancer mortality; however, increases in risk with weight loss were greater for cardiovascular disease than cancer mortality. Japanese Americans and Latinos showed stronger associations between weight loss and all-cause mortality than did African Americans, Native Hawaiians, and whites. Increases in risk with weight gain were higher in older than in younger participants, while increases in risk with weight loss were greater in the lower baseline BMI group and never smokers, compared with the higher baseline BMI group and current smokers, respectively. The reverse J-shaped association was observed in all racial/ethnic groups.
Past studies have examined weight loss during adulthood in relation to all-cause mortality. 19,20 A recent meta-analysis of 26 prospective studies reported that weight loss was related to an increased risk of all-cause mortality in middle-aged populations.6 An earlier meta-analysis of 26 prospective studies found that unintentional weight loss was associated with increased risk of all-cause mortality, whereas intentional weight loss had a small benefit for unhealthy adults but was associated with a marginally increased risk of death for healthy adults.5 Indeed, a meta-analysis of 12 randomized clinical trials (average ages: 43-69 years) reported a 15% lower all-cause mortality risk with weight loss interventions.21 Unfortunately, our analyses could not differentiate intentional versus unintentional weight loss. In our study, even a small weight loss (2.5-5 kg) was related to an increased risk of mortality in healthy older adults, which was generally preserved across subgroup analyses.
Since the relationship between body weight and mortality appears to vary by age,22 it is important to further investigate whether weight change associations with mortality persist in older populations.7 A recent meta-analysis of 17 prospective studies showed that weight loss (pooled RR=1.67, 95% CI, 1.51 to 1.85) and weight gain (pooled RR=1.21, 95% CI, 1.09 to 1.33) were associated with higher all-cause mortality among older adults (≥60 years).7 In this meta-analysis, the effect of weight gain appeared to be weaker than that of weight loss, which is consistent with our findings, and was also confirmed by another study in older adults.23 In the current study, large weight gain (>10 kg) was associated with an increased risk of mortality in the older age group (≥55 years at baseline) only, while weight loss was related to higher mortality in all age groups. Weight loss in the elderly is mainly related to muscle loss which leads to frailty and subsequently increases mortality.24
While most studies have been performed in white populations, 5,7 a study in Hong Kong, China found an increased risk of mortality with weight loss over 2 years among adults aged 70 years and older.25 Another prospective study in Japan also reported a reverse J-shaped association between weight change over 5 years and mortality among adults aged between 45 and 75 at baseline.14
In our previous study in the MEC among healthy never smokers, we found an increased risk of mortality with baseline BMI >27.5 kg/m2, compared with BMI 23-24.9 kg/m2, and a greater risk of mortality with BMI ≥35 kg/m2.12 In the current analyses, we detected no beneficial effect of a small or large weight loss on mortality among all participants, including those who were obese at baseline (BMI ≥30 kg/m2). Participants who were obese at baseline were at higher risk of mortality, compared with normal BMI participants, regardless of their weight change later. Therefore, both not being obese, as well as maintaining a stable weight, are important for longevity.
Weight change-mortality analyses are subject to confounding by an underlying condition, i.e., reverse causality. To minimize reverse causality, we excluded all participants who reported heart disease or cancer at the baseline or 10-year follow-up surveys. In sensitivity analyses, we removed deaths that occurred within the first two years of follow-up and obtained similar associations. Smoking is also a factor affecting both body weight and mortality. However, we found a more pronounced association in never smokers, which is consistent with a cohort study in Japan.14 Nevertheless, we cannot completely rule out the possibility of some effect of reverse causality and/or residual confounding.
The strengths of this study include a population-based prospective design, a large sample size, and participants with various racial/ethnic backgrounds. However, the sample size was limited for some subgroup analyses, and 7.3 years of follow-up may not yet be long enough to fully reflect the association between weight change and long-term mortality in our study. Because we used self-reported weight from both surveys, misclassification of weight may have occurred, although a pilot study with 60 female MEC participants found a high correlation between self-reported and measured weight (r=0.98).26 In older adults, body fat distribution changes to an increasing centralization of adiposity from the limbs to the trunk, even when total fat/weight remains constant.27,28 However, weight and BMI do not directly reflect body fat distribution. Nevertheless, adjustment for waist circumference, which was available from the follow-up survey only, did not substantially modify the results. The principal limitation of our study is the lack of information on the reasons for weight change, and thus we were not able to differentiate intentional versus unintentional weight loss. Intentional weight loss has been beneficial in interventional trials,21 whereas unintentional weight loss often represents underlying illness, which may increase mortality risk.7,29 Therefore, our finding for weight loss may not apply to obese patients who are advised to lose weight by health professionals.14 In addition, since no information on weight cycling was available, we were not able to consider weight change patterns and timing between the baseline and 10-year follow-up surveys. Although we excluded participants who reported heart disease or cancer either at baseline or follow-up surveys in all analyses and conducted a sensitivity analysis, which excluded all deaths occurring in the first 2 years or participants with baseline BMI <20 kg/m2 and led to similar results, we cannot completely rule out reverse causality. Also, despite a wide range of covariates available, we were not able to consider all potential covariates including socioeconomic status change. As discussed earlier, our findings from the study sample of ∼63,000 participants without heart disease or cancer may have limited generalizability. Mean weight at the 10-year follow-up survey in the entire MEC participants was lowered than in a national survey (1999-2002): 82.1 vs. 87.1 kg in men and 68.9 vs. 74.9 kg in women aged 60-74 years.30 Mean weight change was also lower than in national surveys (2003-2012), where a weight history questionnaire was asked and mean 10-year weight change was 4.5 kg gain in adults ≥36 years.31
In conclusions, our findings suggest that weight loss in mid to late adult life increases the risk of mortality both in men and women and across five different racial/ethnic groups. Excessive weight gain was also associated with increased risk of mortality. Based on these observations, public health recommendation should focus on the prevention of weight loss, as well as weight stability within the non-obese range, for middle aged and older adults.
Supplementary Material
Acknowledgments
This work was supported in part by the National Cancer Institute, National Institutes of Health (U01 CA164973).
Footnotes
Conflict of Interest: none declared.
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Solomon CG, Manson JE. Obesity and mortality: a review of the epidemiologic data. Am J Clin Nutr. 1997;66:1044S–1050S. doi: 10.1093/ajcn/66.4.1044S. [DOI] [PubMed] [Google Scholar]
- 2.McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev. 2009;22:93–108. doi: 10.1017/S0954422409990035. [DOI] [PubMed] [Google Scholar]
- 6.Karahalios A, English DR, Simpson JA. Change in body size and mortality: a systematic review and meta-analysis. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw246. [DOI] [PubMed] [Google Scholar]
- 7.Cheng FW, Gao X, Jensen GL. Weight change and all-cause mortality in older adults: a meta-analysis. J Nutr Gerontol Geriatr. 2015;34:343–368. doi: 10.1080/21551197.2015.1090362. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM., Jr Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016;17:262–275. doi: 10.1111/obr.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snook KR, Hansen AR, Duke CH, Finch KC, Hackney AA, Zhang J. Change in Percentages of Adults With Overweight or Obesity Trying to Lose Weight, 1988-2014. JAMA. 2017;317:971–973. doi: 10.1001/jama.2016.20036. [DOI] [PubMed] [Google Scholar]
- 11.Dorsey RR, Eberhardt MS, Ogden CL. Racial and ethnic differences in weight management behavior by weight perception status. Ethn Dis. 2010;20:244–250. [PubMed] [Google Scholar]
- 12.Park SY, Wilkens LR, Murphy SP, Monroe KR, Henderson BE, Kolonel LN. Body mass index and mortality in an ethnically diverse population: the Multiethnic Cohort Study. Eur J Epidemiol. 2012;27:489–497. doi: 10.1007/s10654-012-9695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanri A, Mizoue T, Takahashi Y, Noda M, Inoue M, Tsugane S. Weight change and all-cause, cancer and cardiovascular disease mortality in Japanese men and women: the Japan Public Health Center-Based Prospective Study. Int J Obes. 2010;34:348–356. doi: 10.1038/ijo.2009.234. [DOI] [PubMed] [Google Scholar]
- 15.de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol. 2014;179:1353–1365. doi: 10.1093/aje/kwu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 17.Therneau TM, Grambsh PM. Modeling survival data: extending the Cox model. Springer; New York: 2000. [Google Scholar]
- 18.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 19.Poobalan AS, Aucott LS, Smith WC, Avenell A, Jung R, Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev. 2007;8:503–513. doi: 10.1111/j.1467-789X.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 20.Simonsen MK, Hundrup YA, Obel EB, Gronbaek M, Heitmann BL. Intentional weight loss and mortality among initially healthy men and women. Nutr Rev. 2008;66:375–386. doi: 10.1111/j.1753-4887.2008.00047.x. [DOI] [PubMed] [Google Scholar]
- 21.Kritchevsky SB, Beavers KM, Miller ME, Shea MK, Houston DK, Kitzman DW, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One. 2015;10:e0121993. doi: 10.1371/journal.pone.0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 23.Karahalios A, Simpson JA, Baglietto L, MacInnis RJ, Hodge AM, Giles GG, et al. Change in body size and mortality: results from the Melbourne collaborative cohort study. PLoS One. 2014;9:e99672. doi: 10.1371/journal.pone.0099672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 25.Ho SC, Woo J, Sham A. Risk factor change in older persons, a perspective from Hong Kong: weight change and mortality. J Gerontol. 1994;49:M269–272. doi: 10.1093/geronj/49.6.m269. [DOI] [PubMed] [Google Scholar]
- 26.Lim U, Ernst T, Buchthal SD, Latch M, Albright CL, Wilkens LR, et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr Diabetes. 2011;1:e6. doi: 10.1038/nutd.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Hunter GR, Gower BA, Kane BL. Age Related Shift in Visceral Fat. Int J Body Compos Res. 2010;8:103–108. [PMC free article] [PubMed] [Google Scholar]
- 29.Han TS, Tajar A, Lean ME. Obesity and weight management in the elderly. Br Med Bull. 2011;97:169–196. doi: 10.1093/bmb/ldr002. [DOI] [PubMed] [Google Scholar]
- 30.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960-2002. Adv Data. 2004:1–17. [PubMed] [Google Scholar]
- 31.Veldheer S, Yingst J, Zhu J, Foulds J. Ten-year weight gain in smokers who quit, smokers who continued smoking and never smokers in the United States, NHANES 2003-2012. International journal of obesity (2005) 2015;39:1727–1732. doi: 10.1038/ijo.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.