Abstract
Objectives
Prenatal exposure to methamphetamine is associated with a range of neuropsychological, behavioural and cognitive deficits. A small number of imaging studies suggests that these may be mediated by neurostructural changes, including reduced volumes of specific brain regions. This study investigated potential volumetric changes in the brains of neonates with prenatal methamphetamine exposure. To our knowledge no previous studies have examined methamphetamine effects on regional brain volumes at this age.
Study Design
Mothers were recruited antenatally and interviewed regarding methamphetamine use during pregnancy. Mothers in the exposure group reported using methamphetamine ≥ twice/month during pregnancy; control infants had no exposure to methamphetamine or other drugs and minimal exposure to alcohol. MRI scans were performed in the first postnatal month, following which anatomical images were processed using FreeSurfer. Subcortical and cerebellar regions were manually segmented and their volumes determined using FreeView. Pearson correlations were used to analyse potential associations between methamphetamine exposure and regional volumes. The association between methamphetamine exposure and regional volumes were then examined adjusting for potential confounding variables.
Results
Methamphetamine exposure was associated with reduced left and right caudate and thalamus volumes. The association in the right caudate remained significant following adjustment for potential confounding variables.
Conclusions
Our findings showing reduced caudate and thalamus volumes in neonates with prenatal methamphetamine exposure are consistent with previous findings in older exposed children, and demonstrate that these changes are already detectable in neonates. Continuing research is warranted to examine whether reduced subcortical volumes are predictive of cognitive, behavioural and affective impairment in older children.
Keywords: Magnetic resonance imaging, prenatal methamphetamine exposure, neonate, caudate nucleus, thalamus, regional brain volumes
1. Introduction
Methamphetamine is one of the most widely used recreational drugs globally (Nguyen et al., 2010) and there is considerable evidence that exposure during pregnancy has marked effects on the infant (Carter et al., 2016; Good et al., 2010; Gorman et al., 2014; Ladhani et al., 2011; Smith et al., 2003). Given the dopaminergic activity of methamphetamine and its documented neurotoxicity in adult users (Chang et al., 2009; Sowell et al., 2010), it was expected that prenatal exposure would induce similar damaging effects on the infants concerned, considering the rapid development and growth of the central nervous system that occurs during this period (Salisbury et al., 2009). Neuropsychological and behavioural alterations have been observed in studies of children with prenatal methamphetamine exposure, including low arousal and lethargy (LaGasse et al., 2011; Paz et al., 2009), poor fine motor skills (Smith et al., 2011), poor psychomotor and emotional adjustment (Billing et al., 1988) and aggressive behaviour (Billing et al., 1994). Deficits in visuomotor integration (Chang et al., 2009), inhibitory control (Derauf et al., 2012a), and executive function and working memory (Piper et al., 2011) have also been observed in children with in utero methamphetamine exposure. In view of these findings, it is of interest to determine whether these deficits reflect structural alterations in the brain related to prenatal methamphetamine exposure, and whether these alterations can already be detected in the newborn.
Magnetic resonance spectroscopy, diffusion tensor imaging and functional MRI have shown a range of metabolic, microstructural and functional alterations as a consequence of prenatal methamphetamine exposure (Buchthal et al., 2009; Chang et al., 2016, 2009; Cloak et al., 2009; Colby et al., 2012; Lu et al., 2009; Roos et al., 2015; Roussotte et al., 2011; Smith et al., 2001). In children with prenatal methamphetamine and alcohol exposure, reductions in volume in the thalamus, striatum, and left parieto-occipital and right anterior prefrontal cortices, and increased volumes of several cortical regions, were observed compared to controls and to children with alcohol exposure only (Sowell et al., 2010). Similarly, reduced caudate volumes were observed in children aged 6-7 years with prenatal methamphetamine exposure (Derauf et al., 2012b). Putamen and globus pallidus volumes were reduced in methamphetamine exposed children, with a trend towards volume reductions of the caudate, thalamus, hippocampus and cerebellum (Chang et al., 2004). A number of these reductions were related to poorer cognitive function (Chang et al., 2004).
Prenatal methamphetamine exposure has been shown to have considerable, persistent effects on dopaminergic regions of the brain such as the fronto-striatal circuits, as well as certain non-dopaminergic regions. The neuroimaging literature, however, is sparse. To our knowledge, no volumetric studies have been conducted with neonates. Such an investigation in neonates is highly warranted, not least because of the sub-optimal postnatal environment into which these children are often born (Good et al., 2010; Nguyen et al., 2010; Piper et al., 2011; Smith et al., 2003). A more complete understanding of the structural abnormalities associated with prenatal methamphetamine exposure may assist in the development of more effective interventions for concomitant cognitive and behavioural deficits. The aim of this study was thus to investigate the volumetric changes in subcortical areas and cerebellum of the brains of neonates with prenatal methamphetamine exposure. We hypothesized that increased exposure to methamphetamine would be associated with reduced volume of the striatal structures.
2. Methods
2.1 Study sample
The cohort consisted of 39 infants born to women in a Cape Coloured (mixed ancestry) community of Cape Town, South Africa, who are participating in a larger prospective longitudinal study of prenatal alcohol and drug exposure on infant development (Carter et al., 2016; Jacobson et al., 2017; Taylor et al., 2015). The rate of methamphetamine (‘tik’) abuse in this community is the highest in South Africa (Peltzer et al., 2010), with 35-43% of drug treatment patients reporting it as their primary drug (Meade et al., 2015; Plüddemann et al., 2013). Rates of use among women in this community have been reported to be as high as 58% (Wechsberg et al., 2010). Methamphetamine use in this community has been strongly linked with the pervasive and unique gang culture of the area (Pasche and Myers, 2012), and is associated with increased interpersonal violence, mental health issues and sexual risk behaviours (Meade et al., 2012; Plüddemann et al., 2010; Wechsberg et al., 2008).
Pregnant mothers were recruited following initial antenatal care visits at midwife obstetric care units serving the community. The mothers were interviewed, once at recruitment and twice more before delivery, regarding their alcohol consumption using a time-line follow-back approach (Jacobson et al., 2008, 2002), and about the frequency of use (days per month) of methamphetamine, cigarettes and other drugs (marijuana (‘dagga’), methaqualone (‘mandrax’), and cocaine) during pregnancy. The present study consists of infants born to women in the cohort who reported using methamphetamine at least twice per month during pregnancy. The control group, recruited from the same community, comprises infants whose mothers abstained from alcohol and other drugs of abuse or who consumed no more than 2 drinks on 2 or fewer occasions during pregnancy. Exclusion criteria for pregnant women were as follows: < 18 years of age, HIV positive, treatment for medical conditions such as hypertension, epilepsy, diabetes or heart disease, and multiple pregnancy. Infant exclusion criteria were neural tube defects, major chromosomal anomalies, very low birth weight (<1200g), gestational age <30 weeks, and seizures (Carter et al., 2016).
Informed consent was obtained from each mother at recruitment and at the University of Cape Town (UCT) Child Development Research Laboratory and neuroimaging visits. Approval for this study was obtained from the ethics committees at Wayne State University and the Faculty of Health Sciences at UCT. Mothers were given breakfast and a snack during the morning at each visit. At the end of each visit, the mother received a small monetary compensation.
2.2 Interview Procedure
A staff driver and research staff nurse transported the mothers to our UCT Child Development Research Laboratory, where they were interviewed regarding their alcohol and drug use, and sociodemographic background information was obtained. They were also administered the 18-item Family Food Security interview (Bickel et al., 2000), which has been widely used to assess adequacy of food availability (e.g. (Health Canada, 2007)). Based on the mothers' responses, families were classified as food insecure if the mother reported moderate to severe hunger (Jacobson et al., 2017). Because no measure of weight prior to conception was available, we measured gestational weight gain (kg/wk) by subtracting the mother's weight at the first interview from her weight at the last one and dividing by number of weeks between the two interviews.
Urine samples were collected to examine the validity of maternal reports of drug use (Carter et al., 2016). Sample collection only began in the latter part of the larger cohort study (Jacobson et al., 2017). Urine samples from 105 women in the cohort were tested by the research nurse using the AccuTest™ 6+2 drugs of abuse panel (DTA Pty Ltd, Cape Town, South Africa), an immunochemical assay that detects metabolites of drugs commonly used in this community (amphetamines, cocaine, methaqualone, methamphetamine, opiates and marijuana (THC)), as well as pH and creatinine to test for sample adulteration. These samples were only available for 9 of the women in the current study. However, the urine assays from the larger cohort validated the maternal reports of drug use (Carter et al., in press). None of the women refused drug testing.
2.3 Scanning
Nonsedated infants were scanned between 1 and 4 weeks after birth, with the exception of two infants born prior to 34 weeks who were scanned at 7 and 9 weeks of age, respectively (Jacobson et al., 2017).
Infants and their mothers were transported to the Cape Universities Brain Imaging Centre (CUBIC) a minimum of 1 hour prior to scanning. During this period the infants were weighed and head circumference and crown-to-heel length were measured. Neonatal behavioural characteristics (Brazelton, 1984) were assessed by a trained developmental paediatrician (CDM). Following this, the infant was swaddled firmly and placed in a VacFix® vacuum cushion (S&S Par Scientific, Houston, TX) for later use in immobilising the infant's head in the coil, in accordance with an adapted protocol for neuroimaging assessment of nonsedated newborns (Laswad et al., 2009). Earplugs were used to protect the infant from the noise of the scanner. The infant was then fed by the mother and allowed to fall asleep.
MRI scanning was performed using a Siemens 3 T Allegra scanner. A custom-built circularly polarised birdcage coil, designed for use with neonates, was used for transmission and reception of the signal. Several sequences were acquired during the protocol. For this study a motion navigated multiecho gradient echo sequence was used, with the following protocol parameters: FOV 114 mm, 128 slices, TR 20 ms, TE 1.46/ 3.14/ 4.82/ 6.5/ 8.18/ 9.86/ 11.54/ 13.22 ms, 1 mm3 isotropic resolution. The sequence was acquired twice, with flip angles of 5° and 20°, respectively.
The sleeping infant was positioned in the scanner and immobilised by means of the VacFix® cushion. An oxygen saturation and pulse monitor probe was secured to the infant's foot, and this was monitored during the scanning period.
2.4 Data synthesis
The individual echoes from the two flip angle acquisitions were split, tissue parameters estimated and an image volume synthesised using FreeSurfer (Fischl et al., 2004). A flip angle of 24° was chosen as that which produced optimal contrast.
2.5 Manual Segmentation
Regions of interest (ROIs) were viewed and manually delineated using Freeview software (FreeSurfer image analysis suite http://surfer.nmr.mgh.harvard.edu/) run on a Lenovo ThinkPad X220 tablet. The following structures were traced bilaterally: caudate nucleus, nucleus accumbens, putamen, pallidum, thalamus, hippocampus, amygdala and cerebellar hemispheres, as well as the cerebellar vermis. Tracing protocols for each section followed the Infant Brain Segmentation Manual developed by the HST/MGH Athinoula A. Martinos Center for Biomedical Imaging (de Macedo Rodrigues et al., 2015), where training in the use of Freeview software and manual tracing of infant MRI scans was received. Tracings were rendered chiefly in the coronal plane, with guidance from the axial and sagittal planes where region boundaries were indistinct in a coronal view. The volumes of the traced regions were then calculated. Total brain volume was determined by extracting the brain from the anatomical T1-weighted volumes using an in-house script. Nine brains were independently retraced for inter-rater reliability purposes. Both tracers (FLW, NML) were blind to the exposure status of the infant brains. Dice overlap measures were determined for all ROIs using FreeSurfer.
2.6 Statistical Analysis
Statistical analysis was performed using SPSS (version 22; IBM, Armonk, NY). Eight variables were considered as potential confounders: maternal age at delivery, educational status (number of years), parity, maternal marijuana use (days per month during pregnancy), smoking (number of cigarettes per day during pregnancy), alcohol use (oz of absolute alcohol consumed per day during pregnancy), and infant sex and gestational age at scan (weeks). Total brain volume was not considered a potential confounder as it was unrelated to methamphetamine exposure.
T-tests or chi-square tests for categorical variables were used to compare sample characteristics between exposed and control groups. The methamphetamine use and smoking variables both contained outliers, which were recoded to 1 unit greater than the next highest value (see (Winer, 1971)). Prenatal alcohol use was skewed and was therefore logged for multivariable analyses.
Pearson correlation was used to analyse the relation between methamphetamine exposure and ROI volume in the methamphetamine exposed group. Following this, regions showing significant association with methamphetamine exposure were analysed by means of hierarchical multiple regression using a best estimate approach. Methamphetamine was entered first into the regression; the potential confounders described above which were at least weakly related to volume (p < 0.10) were then entered.
3. Results
3.1 Sample characteristics
We report data for 39 infants (18 methamphetamine exposed and 21 controls). Demographic and substance exposure characteristics of the infants are summarised in Table 1. Maternal education differed between groups, with controls achieving a higher level of education than the methamphetamine group. Food security and maternal weight gain during pregnancy did not differ between groups. The 24-hr dietary recall interviews for the 39 women showed no group difference for energy (calorie intake), carbohydrates, proteins, and fat (all p's > 0.20). The only differences found for this sample were that controls reported lower intake of cholesterol, fluoride and selenium in their diet.
Table 1. Sample characteristics (N = 39).
| Methamphetamine | Control | χ2 or t | p | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| (n =18) | (n =21) | |||||||
|
|
|
|||||||
| Mean | SD | Range | Mean | SD | Range | |||
| Maternal | ||||||||
| Age at delivery (years) | 27.0 | 4.2 | 19.8–34.0 | 26.7 | 5.9 | 18.8–36.4 | −0.19 | 0.850 |
| Education (years) | 9.3 | 1.4 | 7.0–12.0 | 10.5 | 1.4 | 7.0–12.0 | 2.60 | 0.013 |
| Parity | 3.2 | 1.6 | 1–6 | 2.4 | 1.3 | 1–5 | −1.70 | 0.098 |
| Socioeconomic statusa | 20.2 | 4.7 | 11.0–27.0 | 22.1 | 7.2 | 8.0–34.5 | 0.96 | 0.342 |
| Food security (%moderate-severe)b | 11.8 | 28.6 | 1.60 | 0.206 | ||||
| Gestational weight gain (kg/wk) | 0.4 | 0.3 | −0.2–1.1 | 0.4 | 0.3 | −0.3–0.8 | 0.26 | 0.794 |
| Dietary caloric intake (kj/day)c | 9601.06 | 3120.35 | 4278.80–15,863.10 | 9356.24 | 5881.96 | 3741.90–28,849.80 | −0.16 | 0.875 |
| Infant | ||||||||
| Sex (% male) | 33 | 57 | 2.21 | 0.137 | ||||
| Birth weight (g) | 2804.2 | 498.9 | 1370.0–3590.0 | 2915.7 | 541.7 | 1940.0–4200.0 | 0.67 | 0.510 |
| Head circumference (cm) | 32.9 | 1.9 | 27.0–35.0 | 33.3 | 2.0 | 31.0–38.0 | 0.67 | 0.509 |
| Crown-to-heel length (cm)d | 47.6 | 3.5 | 41.0–53.0 | 48.8 | 3.4 | 40.0–53.0 | 1.08 | 0.287 |
| Gestational age at birth (weeks) | 37.6 | 2.7 | 31.0–41.3 | 39.1 | 2.0 | 33.7–42.1 | 2.01 | 0.052 |
| Gestational age at scan (weeks) | 40.5 | 2.1 | 36.7–44.0 | 41.6 | 1.9 | 37.6–44.6 | 1.65 | 0.107 |
| Total intracranial volume (mm3) | 491,044 | 41,631 | 395,900–549,600 | 516,805 | 69,236 | 428,700–660,700 | 1.38 | 0.177 |
| Substance use | ||||||||
| Methamphetamine (days/month) | 6.1 | 3.8 | 0.7–12.0 | 0.0 | 0.0 | 0.0–0.0 | −7.34 | <0.001 |
| Smoking (number cigarettes/day) | 6.5e | 4.5 | 2.0–20.0 | 3.5f | 3.4 | 0.0–10.3 | −2.42 | 0.021 |
| Marijuana (days/month) | 5.8g | 8.4 | 0.0–30.5 | 0.0h | 0.1 | 0.0–0.2 | −3.18 | 0.003 |
| Alcohol (oz AA/day) | 0.2i | 0.4 | 0.0–1.4 | 0.0j | 0.0 | 0.0–0.1 | −2.15 | 0.038 |
Hollingshead (2011) Four Factor Index of Social Status Scale.
n = 20 for control.
Based on multiple pass 24-h dietary recall interviews, quantified using FoodFinder®.
n = 17 for methamphetamine group.
18/18 (100%) smoked.
13/21 (62%) smoked.
10/18 (56%) used marijuana.
2/21 (10%) used marijuana.
10/18 (56%) used alcohol.
1/21 (5%) used alcohol.
The sample consisted of 18 male and 21 female infants. Infants exposed to methamphetamine were born earlier, which is consistent with previous studies (Carter et al., 2016; Ladhani et al., 2011). Although the methamphetamine group weighed less at birth, there was no between-group difference in terms of gestational age at scan (calculated as gestational age at birth + age in weeks).
Mean methamphetamine, smoking, marijuana and alcohol use by mothers of the exposed group was higher than in the control group. Although control mothers did not use methamphetamine, 62% (n = 13) reported smoking, 9.5% (n = 2) used marijuana and 4.8% (n = 1) drank alcohol. The 1 control mother who reported alcohol use drank about 2 drinks/ occasion, which is considered light drinking and within the acceptable limits set for inclusion as a control. By contrast, all 18 of the mothers in the exposed group reported smoking, 55.6% (n = 10) reported using marijuana and 55.6% (n = 10) reported drinking alcohol during pregnancy.
Only 9 (23.1%) of the mothers in the sample had urine drug screens: 3 of the 9 were in the methamphetamine group, and all 3 admitted using methamphetamine; 2 admitted using marijuana, but all 3 denied using methaqualone. All 3 tested positive for methamphetamine, marijuana and methaqualone. The 6 control mothers denied using methamphetamine, marijuana and methaqualone, and all 6 tested negative for all drugs in the urine screen.
3.2 Inter-rater reliability of manual tracing
Volumes of traced regions and inter-rater reliability measures are reported in Table 2. Interrater reliabilities are reported as the median and range of the Dice coefficients calculated for 9 brains. The median Dice values of 10 ROIs (caudate, putamen, thalamus, and cerebellar hemispheres bilaterally, right and left hippocampus, and vermis) were greater or equal to 0.80. The remaining ROIs had Dice coefficients below 0.70. As Dice 0.80 is generally considered good interrater reliability, pallidum, nucleus accumbens, and amygdala bilaterally were discarded from further analyses.
Table 2. Volumes and interrater reliability measures.
| ROI | Volume (mm3) | Dice Coefficients | ||
|---|---|---|---|---|
|
| ||||
| Methamphetamine (n=18) Mean ± SD | Control (n=21) Mean ± SD | Median | Range | |
| Overall | - | - | 88.5 | 84.7 - 99.6 |
| Left caudate | 1241 ± 201 | 1300 ± 197 | 84.6 | 83.2 - 88.8 |
| Right caudate | 1175 ± 159 | 1264 ± 156 | 86.6 | 84.4 - 89.9 |
| Left putamen | 1679 ± 256 | 1806 ± 322 | 81.5 | 76.7 - 87.3 |
| Right putamen | 1720 ± 242 | 1798 ± 328 | 84.0 | 73.2 - 86.5 |
| Left pallidum | 650 ± 111 | 685 ± 123 | 66.8 | 37.7 - 76.1 |
| Right pallidum | 594 ± 91 | 626 ± 131 | 69.8 | 49.8 - 81.1 |
| Left nucleus accumbens | 150 ± 29 | 148 ± 41 | 38.3 | 18.0 - 63.4 |
| Right nucleus accumbens | 158 ± 41 | 147 ± 41 | 43.1 | 28.1 - 66.2 |
| Left thalamus | 4344 ± 352 | 4435 ± 713 | 90.3 | 85.3 - 92.1 |
| Right thalamus | 4264 ± 360 | 4470 ± 708 | 90.8 | 86.4 - 92.9 |
| Left hippocampus | 1187 ± 148 | 1149 ± 151 | 80.8 | 63.2 - 85.1 |
| Right hippocampus | 1173 ± 109 | 1144 ± 140 | 79.7 | 73.4 - 86.3 |
| Left amygdala | 383 ± 116 | 370 ± 74 | 64.2 | 44.6 - 75.6 |
| Right amygdala | 406 ± 124 | 374 ± 79 | 67.5 | 54.0 - 74.5 |
| Vermis | 1957 ± 383 | 1986 ± 386 | 84.7 | 71.4 - 89.7 |
| Left cerebellar cortex | 10207 ± 1500 | 10365 ± 1949 | 92.4 | 91.7 - 96.0 |
| Right cerebellar cortex | 10188 ± 1447 | 10501 ± 1820 | 93.3 | 88.6 - 95.8 |
3.3 Relation of methamphetamine exposure to subcortical regional volumes
Pearson correlation analyses investigated the associations between methamphetamine exposure and all subcortical regions which met the reliability threshold, in methamphetamine exposed infants (shown in Table 3). These analyses showed that heavier methamphetamine exposure was associated with reduced right caudate volume (Fig. 1), and reduced left caudate and bilateral thalamus volumes at a trend level. No association was observed between methamphetamine exposure and the other regions. Total intracranial volume was not significantly associated with methamphetamine exposure (r = -0.38, p = 0.122).
Table 3. Association between prenatal methamphetamine exposure and volume of subcortical structures (n = 18).
| Volume (ROI) | Methamphetamine exposure | |
|---|---|---|
|
| ||
| r | p | |
| Left caudate | -0.44 | 0.071 |
| Right caudate | -0.62 | 0.006 |
| Left putamen | 0.10 | 0.700 |
| Right putamen | -0.02 | 0. 946 |
| Left thalamus | -0.42 | 0.087 |
| Right thalamus | -0.46 | 0.054 |
| Left hippocampus | -0.38 | 0.116 |
| Right hippocampus | 0.02 | 0.946 |
| Vermis | -0.10 | 0.686 |
| Left cerebellum | -0.39 | 0.109 |
| Right cerebellum | -0.38 | 0.125 |
r is the Pearson correlation between methamphetamine exposure and volume in methamphetamine-exposed infants. Boldface denotes significant findings.
Figure 1.
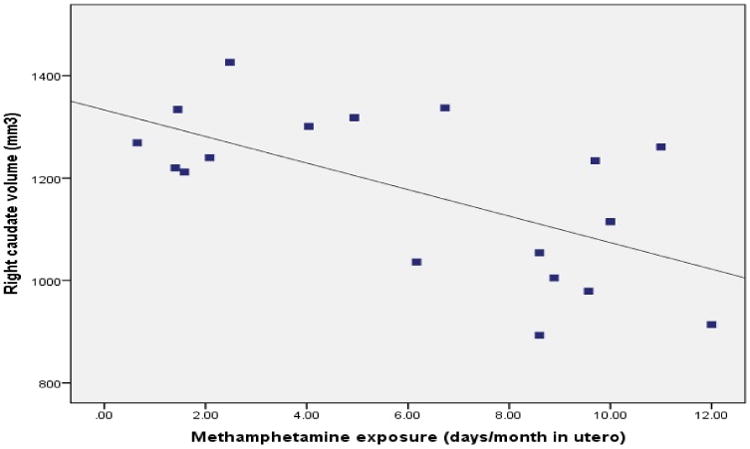
Relation of prenatal methamphetamine exposure across pregnancy to volume of the right caudate nucleus in methamphetamine exposed newborns (n = 18).
Potential confounding variables (see Table 4) were included in the subsequent regression analyses if they showed a correlation with ROI volume at p < 0.10. Cigarette smoking and alcohol exposure were associated with left and right caudate volume respectively, while gestational age at time of scan was associated with left and right thalamus volumes. Left and right caudate and thalamus volumes were then each regressed on methamphetamine, followed by the associated confounders for each ROI. Prenatal exposure to methamphetamine was associated with reduced volume in the right caudate after controlling for the potential confounder alcohol exposure (β = -0.55, p = 0.009). Methamphetamine exposure was associated with left caudate volume at a trend level after controlling for the potential confounder maternal smoking (β = -0.42; p = 0.065). After control for gestational age at scan, methamphetamine exposure was associated with left and right thalamus volumes at a trend level (left: β = -0.33, p = 0.094; right: β = -0.39, p = 0.063). Correlation and regression analyses which excluded control mothers who drank alcohol or used marijuana showed that their exclusion made essentially no change to the findings (results not shown). Similarly, exclusion of one methamphetamine-exposed infant who was born at 31 weeks produced no substantive changes (results not shown).
Table 4. Association of potential confounding variables with volumes of regions showing significant associations with methamphetamine (n = 18).
| Maternal age (years) | Education (years) | Parity | Marijuana (days/month) | Smoking (number of cigarettes/ day) | Alcohol (oz AA/day) | Infant sex | Gestation al age at scan (weeks) | |
|---|---|---|---|---|---|---|---|---|
| Left caudate | -0.02 (0.953) | 0.09 (0.733) | -0.23 (0.355) | 0.08 (0.749) | -0.42 (0.081) | 0.33 (0.180) | -0.26 (0.291) | 0.07 (0.796) |
| Right caudate | -0.17 (0.492) | -0.14 (0.594) | -0.25 (0.325) | 0.25 (0.326) | -0.14 (0.585) | 0.48 (0.046) | -0.32 (0.195) | 0.27 (0.281) |
| Left thalamus | -0.25 (0.325) | -0.31 (0.216) | -0.21 (0.400) | -0.14 (0.587) | -0.14 (0.594) | 0.24 (0.337) | -0.17 (0.506) | 0.63 (0.005) |
| Right thalamus | -0.35 (0.152) | -0.30 (0.226) | -0.279 (0.262) | -0.02 (0.930) | -0.07 (0.789) | 0.33 (0.182) | -0.06 (0.804) | 0.56 (0.016) |
Values are Pearson r (p) in methamphetamine-exposed infants. Results with a trend towards association (p < 0.10) are shown in boldface.
4. Discussion
This study is the first to investigate the effects of prenatal exposure to methamphetamine on regional brain volumes in neonates. As hypothesised, changes were observed in the left and right caudate nuclei, with reduced volume observed as a consequence of prenatal methamphetamine exposure. Similarly, the thalamus bilaterally showed a reduction in volume in association with prenatal methamphetamine exposure. Although the association in the left caudate and the thalamus remained at a trend level when confounding variables were included in the analysis, the effect of methamphetamine on the right caudate remained significant.
These results are consistent with those obtained in previous studies in which volumetric effects of prenatal methamphetamine exposure on subcortical structures were observed in older cohorts. In a study of children aged between 5 and 15 years, the striatum and thalamus were smaller in children exposed to both methamphetamine and alcohol prenatally, with the striatal volume reduction more severe than in children exposed to alcohol alone (Sowell et al., 2010). A study of 3- to 5-year-old children demonstrated significantly lower caudate volume in children with prenatal methamphetamine exposure than in controls, although no effect in the thalamus was observed (Derauf et al., 2012b). A trend towards reduction in volume was observed in the caudates of children aged 3-16 years with prenatal methamphetamine exposure (Chang et al., 2004). In an investigation of children aged 8-11 years with multiple substance exposure, including methamphetamine, trends towards reduced caudate and thalamus volume were observed, with significantly decreased total volume (Walhovd et al., 2007). By contrast, increased striatal volume was found in 6-year-olds with in utero methamphetamine exposure, findings which may reflect methodological differences, given that this was the only study that performed automated segmentation using FreeSurfer (Roos et al., 2014). In adult users, volumetric alterations have been observed in a number of studies. A reduction in caudate volume was noted in methamphetamine-dependent smokers (Morales et al., 2012). Other research, however, observed increased caudate volume in recently abstinent methamphetamine users (Chang et al., 2005; Jernigan et al., 2005).
The weaker effect of methamphetamine in the left caudate than the right was unexpected but may be due to chance confounding in this sample. The association of methamphetamine with volume was weaker in the left caudate than in the right before the addition of the confounding variables, while the association of cigarette smoking with volume was considerably stronger in the left caudate than in the right. Cigarette smoking has been shown to be associated with volume reductions in a variety of brain structures in adult smokers (Gallinat et al., 2006) and children with prenatal exposure (Ekblad et al., 2010).Given that the relative magnitudes of these effects might vary in different samples, it seems reasonable to suggest that both these exposures have bilateral impact, particularly in neonates. Findings in neonates are likely more “widespread” and may account for discrepancies in hemispheres, given that cognitive development is not refined and left brain dominance related to analytical learning develops later in life.
Previous studies of prenatal methamphetamine exposure reported volume reductions in additional regions, such as the hippocampus (Chang et al., 2004), as well as in a number of frontal cortical regions (Sowell et al., 2010). These alterations were not observed in the current study. It is worth noting that the strength of the association between methamphetamine and thalamus volume in this study might well have proved significant with a larger sample size; the same can be said of the association of methamphetamine exposure with cerebellar volumes. The strength of the association of methamphetamine with caudate volume in the current study, however, lends support to the hypothesis that prenatal methamphetamine specifically targets dopamine-rich regions. The thalamus relays outputs from the basal ganglia to the cortex (Haber, 2014) and has been shown to be profusely innervated with dopaminergic axons (García-Cabezas et al., 2007; Sanchez-Gonzalez, 2005), making it similarly vulnerable to dopamine-related insults (Salzwedel et al., 2016). This is in striking contrast to alcohol, which has been shown to be teratogenic to multiple regions of the brain (Astley et al., 2009; Coles et al., 2011; Meintjes et al., 2014; Sowell et al., 2008), and suggests that the mechanisms whereby methamphetamine and alcohol induce CNS damage are likely distinct from one another.
Reduced birth weight and lower gestational age at birth have previously been shown to be associated with prenatal methamphetamine exposure (Ladhani et al., 2011; Shah et al., 2012; Wouldes et al., 2014). This may be due to the vasoconstrictive effect of methamphetamine, causing reduced blood flow across the placenta (Stek et al., 1995) and consequent restriction of nutrient and oxygen supply to the fetus (Chang et al., 2004; Nguyen et al., 2010). Analysis of placental development in our cohort showed that methamphetamine use during pregnancy was associated with increased placental size, as well as increased placenta- to birth- weight ratio, lower gestational age at birth, and increased risk of intrauterine passing of meconium, which suggests that the exposed fetus may experience prolonged periods of hypoxia-ischemia (Carter et al., 2016).
The precise mechanisms whereby in utero methamphetamine exposure reduces the volumes of subcortical structures are not fully understood. Methamphetamine has been shown to be neurotoxic to dopaminergic and serotonergic axons and terminals in animal (Cappon et al., 2000; Castner et al., 2005; LaVoie et al., 2004; Marshall et al., 2007) and human studies (Lee et al., 2009; McCann et al., 1998), and in mature neurons appears to involve the production of reactive oxygen species (ROS), activation of P53 and subsequent apoptosis, and dysfunction of mitochondria (Quinton and Yamamoto, 2006). Alterations in dopamine and dopamine metabolite concentrations have been observed in the striatum of rats prenatally exposed to methamphetamine (Bubenikova-Valesova et al., 2009; Heller et al., 2001). Additionally, gene expression profiles are altered in rats with prenatal methamphetamine exposure, with substantial changes being observed in a number of genes coding for structural and guidance proteins which play vital roles in regulating normal neuronal development (Noailles et al., 2003). ROS production consequent to methamphetamine exposure in utero has been observed in animal studies (Wells et al., 2005; Wong et al., 2008), although the mechanisms by which this induces damage in the developing nervous system appear to be different from those in mature neurons (Wong et al., 2008). Methamphetamine exposure appears to have differential effects on dopamine neurons depending on the age at which exposure occurs (Morrow et al., 2011); in non-human primates in the second gestational trimester, neurons of the nigrostriatal pathway have been shown to be particularly vulnerable to methamphetamine-induced damage (Morrow et al., 2011). During this period primate dopamine neurons undergo natural cell death as part of the normal development and maturation of the neuronal population (Morrow et al., 2007), and it has been hypothesised that this might render them more highly susceptible to damage (Morrow et al., 2011).
To our knowledge, this is the first study in which reductions in volume of subcortical structures is demonstrated in neonates with prenatal methamphetamine exposure. Piper and colleagues have observed that children with prenatal methamphetamine exposure are very likely to be born into a poor postnatal environment (Piper et al., 2011). Primary caregiver psychological symptoms and poor quality home environment were shown to increase behavioural problems assessed on the Child Behavior Checklist, in studies of preschool age children with prenatal methamphetamine exposure (LaGasse et al., 2012; Twomey et al., 2013). Neonatal evaluation of the effects of methamphetamine exposure allows for assessments relatively unconfounded by the effects of postnatal environment. This may also be a consideration in understanding why effects were observed in a greater number of regions in studies of prenatal methamphetamine exposure which used older cohorts.
The women whose children were scanned in this study were recruited during pregnancy. This provided the advantage of allowing a more accurate recollection of methamphetamine use. This is a distinct advantage over studies of older children in which recall of precise drug use by the mother is less accurate, and it allows a more valid quantitative analysis of the effects of methamphetamine exposure. To our knowledge, this is the first study that shows an association between increasing methamphetamine exposure and reduced regional volumes rather than group differences as reported in previous research (Chang et al., 2004; Roos et al., 2014; Sowell et al., 2010; Walhovd et al., 2007).
An additional strength of this study is the use of manual tracing for segmentation of brain regions. Manual segmentation is regarded as the “gold standard” in volumetric analysis of brain structures (Pardoe et al., 2009; Rodionov et al., 2009; Sánchez-Benavides et al., 2010). Furthermore, automated segmentations of immature brains, such as those of infants and children, are likely to be inaccurate when the atlases on which these methods are based are derived from adult or child brains. Use of an age-appropriate atlas for automated segmentation produces more reliable results (Shi et al., 2011), but these require validation against full manual segmentation in a large infant sample. This is a particularly relevant issue when the subjects are neonates, as incomplete myelination of the neonatal brain (Rivkin, 2000) presents a challenge in correctly identifying regions of interest.
One limitation of this study is the potential confounding effects of polysubstance exposure, with the methamphetamine-exposed infants also having higher exposure to alcohol, cigarette smoking and marijuana. This is, unfortunately, almost inevitable in studies of prenatal drug exposure (Roos et al., 2015; Sowell et al., 2010). These other exposures were, however, controlled in the multivariable analyses in this study. Moreover, as mentioned above, recruitment during pregnancy allowed for a more accurate measure of substance use. Previous studies (Chang et al., 2004; Roos et al., 2014; Sowell et al., 2010) did not control for cigarette exposure, which was shown to be a significant confounding variable in the current study.
Another limitation is that the biological measures of exposure were available for only 9 of the 39 women in this sample. Additionally, drug use may not be detectable in urine if that use occurred several days before testing. However, the urine tests for the 9 showed that the maternal interviewing was successful in eliciting accurate responses regarding methamphetamine use. Of the 105 women with urine drug tests in our larger study, results were consistent with maternal reports for 96 (91.4%) for marijuana, cocaine, opiates and methamphetamine. None of the 3 women in the current sample who tested positive for methaqualone reported its use. In this community, methaqualone is commonly mixed with other drugs prior to being sold, often without the user's knowledge. Consistent with maternal reports, no urine tests were positive for cocaine or opiates.
It should be considered that some unexamined additional factor, such as malnutrition, may have played a role in these findings. However, both exposed and control groups were recruited from the same community, and socioeconomic status (Hollingshead, 2011) did not differ between groups. Moreover, the methamphetamine and control groups did not differ in food security, weight gain during pregnancy or calorie, carbohydrate, protein and fat intake, suggesting that nutritional status was similar in the two groups. Furthermore, neither birth weight nor crown-to-heel length, both of which might be considered measures of intrauterine nutritional status, was different between exposed and control children.
The sample size was small but comparable to other studies (Roos et al., 2014; Sowell et al., 2010) and sufficient to observe statistically significant effects. It is possible that with a larger cohort additional differences might have been significant. A potential concern, as in most such studies, is the issue of multiple comparisons. Eleven ROIs were included in the final analysis. However, the association of methamphetamine with right caudate volume remained significant, prior to the addition of confounders, even after controlling for multiple comparisons. Furthermore, as mentioned previously, the significant exposure effect was predicted a priori and found in the part of the striatum where it was expected, which provides additional support for its validity.
Given the rapid and heterochronous nature of early brain growth (Oishi et al., 2013), regional volumes obtained in the first weeks of life are particularly challenging to measure. Segmentation of the newborn brain is more time-consuming due to lower contrast-to-noise ratio (Gousias et al., 2012). Although the brain stem and posterior limbs of the internal capsule are myelinated in the newborn with white to gray matter contrast that is similar to the adult brain, other regions are unmyelinated with the gray/ white contrast inverted in T1-weighted images relative to adult contrast (Dubois et al., 2014; Xue et al., 2007). Despite these difficulties, we were able to demonstrate reductions in caudate and thalamus volumes in methamphetamine-exposed newborns, consistent with previously published research in older children. These reductions are thus already detectable in exposed infants in the first month postpartum. Future research is warranted to examine whether reduced subcortical volumes in methamphetamine-exposed neonates is predictive of the cognitive, behavioural and affective impairments observed in older children.
Highlights.
- Prenatal methamphetamine exposure is associated with reduced caudate volume in human newborns
- Prenatal methamphetamine exposure is associated with reduced thalamus volume in neonates
- Volume reductions previously observed in older children are already detectable in newborns
Acknowledgments
We thank A. Hess and A. Mareyam for their work in constructing the bird cage RF coil used in this study under the supervision of L. Wald, Director MRI Core, Martinos Center for Biomedical Imaging, Radiology, Massachusetts General Hospital; the Cape Universities Brain Imaging Centre radiographers N. Maroof and A. Siljeur; N. Dodge, our Wayne-State University-based data manager; and our University of Cape Town research staff M. September, B. Arendse, M. Raatz, and P. Solomon,. We greatly appreciate the participation of the Cape Town mothers and infants in the study.
Funding Sources: NIH grants R01-AA016781 (SJ), R21-AA020037 (SJ, EM, AvdK) and R00HD061485-03 (LZ), supplemental funding from the Lycaki/Young Fund, from the State of Michigan (SWJ and JLJ), and the South African Research Chairs Initiative (EM). FW is supported by a South African National Research Foundation Innovative Scholarship and the Duncan Baxter Scholarship from the University of Cape Town.
Abbreviations
- MRI
Magnetic resonance imaging
- ROI
Region of interest
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Contributors' Statement: FL Warton conceptualised the methamphetamine study, participated in subject scanning, conducted data analyses, prepared the manuscript and approved the final manuscript as submitted.
CMR Warton supervised and reviewed the segmentation data collection, critically reviewed the manuscript and approved the final manuscript as submitted.
SW Jacobson, JL Jacobson, A van der Kouwe, and EM Meintjes conceptualised and designed the infant scan study, supervised and reviewed data analysis, critically reviewed the manuscript and approved the final manuscript as submitted.
CD Molteno examined and prepared the infants for the neonatal scanning, monitored the infants during the scan, reviewed the manuscript and approved the final manuscript as submitted.
NM Lindinger conducted additional data analysis for interrater reliability purposes, reviewed the manuscript and approved the final manuscript as submitted.
RC Carter implemented and oversaw collection of the urine drug screening, trained and supervised collection of anthropometric measurements, reviewed the manuscript and approved the final manuscript as submitted.
L Zöllei designed data collection instruments, assisted in data analysis, reviewed the manuscript and approved the final manuscript as submitted.
P Wintermark designed the procedure for preparing the infants for the neonatal scanning without sedation and provided initial training and supervision in implementing these techniques.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1671–89. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security 2000 [Google Scholar]
- Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse Negl. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Steneroth G, Zetterström R. Predictive indicators for adjustment in 4-year-old children whose mothers used amphetamine during pregnancy. Child Abuse Negl. 1988;12:503–7. doi: 10.1016/0145-2134(88)90067-1. [DOI] [PubMed] [Google Scholar]
- Brazelton TB. Neonatal behavioral assessment scale. 2nd. J.B. Lippincott Co; 1984. [Google Scholar]
- Bubenikova-Valesova V, Kacer P, Syslova K, Rambousek L, Janovsky M, Schutova B, Hruba L, Slamberova R. Prenatal methamphetamine exposure affects the mesolimbic dopaminergic system and behavior in adult offspring. Int J Dev Neurosci. 2009;27:525–30. doi: 10.1016/j.ijdevneu.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Buchthal S, Ernst T, Anderson L, Cloak C, Kitamura R, Chang L. Proceedings 17th Scientific Meeting. International Society for Magnetic Resonance in Medicine; 2009. Brain Metabolite Changes in Infants Exposed in Utero to Methamphetamine and/or Nicotine; p. 1244. [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res. 2000;863:106–11. doi: 10.1016/S0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Carter RC, Senekal M, Dodge NC, Bechard L, Meintjes EM, Molteno CD, Duggan C, Jacobson JL, Jacobson SW. Maternal alcohol use and nutrition during pregnancy: diet and anthropometry. Alcohol Clin Exp Res. doi: 10.1111/acer.13504. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Wainwright H, Molteno CD, Georgieff MK, Dodge NC, Warton F, Meintjes EM, Jacobson JL, Jacobson SW. Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol Clin Exp Res. 2016;40:753–64. doi: 10.1111/acer.13022. [DOI] [PubMed] [Google Scholar]
- Castner SA, Vosler PS, Goldman-Rakic PS. Amphetamine sensitization impairs cognition and reduces dopamine turnover in primate prefrontal cortex. Biol Psychiatry. 2005;57:743–51. doi: 10.1016/j.biopsych.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: A possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Oishi K, Skranes J, Buchthal S, Cunningham E, Yamakawa R, Hayama S, Jiang CS, Alicata D, Hernandez A, Cloak C, Wright T, Ernst T. Sex-specific alterations of white matter developmental trajectories in infants with prenatal exposure to methamphetamine and tobacco. JAMA psychiatry. 2016;73:1217–1227. doi: 10.1001/jamapsychiatry.2016.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72:2068–75. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby JB, Smith L, O'Connor MJ, Bookheimer SY, Van Horn JD, Sowell ER. White matter microstructural alterations in children with prenatal methamphetamine/polydrug exposure. Psychiatry Res. 2012;204:140–8. doi: 10.1016/j.pscychresns.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Goldstein FC, Lynch ME, Chen X, Kable JA, Johnson KC, Hu X. Memory and brain volume in adults prenatally exposed to alcohol. Brain Cogn. 2011;75:67–77. doi: 10.1016/j.bandc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Macedo Rodrigues K, Ben-Avi E, Sliva DD, Choe MS, Drottar M, Wang R, Fischl B, Grant PE, Zöllei L. A FreeSurfer-compliant consistent manual segmentation of infant brains spanning the 0-2 year age range. Front Hum Neurosci. 2015;9:21. doi: 10.3389/fnhum.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, Lagasse LL, Smith LM, Newman E, Shah R, Neal CR, Arria AM, Huestis MA, Dellagrotta S, Dansereau LM, Lin H, Lester BM. Prenatal methamphetamine exposure and inhibitory control among young school-age children. J Pediatr. 2012a;161:452–9. doi: 10.1016/j.jpeds.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, Lester BM, Neyzi N, Kekatpure M, Gracia L, Davis J, Kallianpur K, Efird JT, Kosofsky B. Subcortical and cortical structural central nervous system changes and attention processing deficits in preschool-aged children with prenatal methamphetamine and tobacco exposure. Dev Neurosci. 2012b;34:327–41. doi: 10.1159/000341119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- Ekblad M, Korkeila J, Parkkola R, Lapinleimu H, Haataja L, Lehtonen L Group, P.S. Maternal smoking during pregnancy and regional brain volumes in preterm infants. J Pediatr. 2010;156:185–90.e1. doi: 10.1016/j.jpeds.2009.07.061. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–50. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- García-Cabezas MÁ, Rico B, Sánchez-González MÁ, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Good MM, Solt I, Acuna JG, Rotmensch S, Kim MJ. Methamphetamine use during pregnancy: maternal and neonatal implications. Obstet Gynecol. 2010;116:330–4. doi: 10.1097/AOG.0b013e3181e67094. [DOI] [PubMed] [Google Scholar]
- Gorman MC, Orme KS, Nguyen NT, Kent EJ, Caughey AB. Outcomes in pregnancies complicated by methamphetamine use. Am J Obstet Gynecol. 2014;211:429.e1–7. doi: 10.1016/j.ajog.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Gousias IS, Edwards AD, Rutherford MA, Counsell SJ, Hajnal JV, Rueckert D, Hammers A. Magnetic resonance imaging of the newborn brain: manual segmentation of labelled atlases in term-born and preterm infants. Neuroimage. 2012;62:1499–509. doi: 10.1016/j.neuroimage.2012.05.083. [DOI] [PubMed] [Google Scholar]
- Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282:248–57. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. Canadian Community Health Survey, Cycle 2.2, Nutrition (2004): Income-Related Household Food Security in Canada. Nutrition. 2007 doi:H164-42/2007E-PDF. [Google Scholar]
- Heller A, Bubula N, Freeney A, Won L. Elevation of fetal dopamine following exposure to methamphetamine in utero. Brain Res Dev Brain Res. 2001;130:139–42. doi: 10.1016/S0165-3806(01)00222-X. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale J Sociol. 2011;8:21–52. [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–25. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Molteno CD, Warton CMR, Wintermark P, Hoyme HE, De Jong G, Taylor P, Warton F, Lindinger NM, Carter RC, Dodge NC, Grant E, Warfield SK, Zöllei L, van der Kouwe AJW, Meintjes EM. Heavy prenatal alcohol exposure is related to smaller corpus callosum in newborn MRI scans. Alcohol Clin Exp Res. 2017;41:965–975. doi: 10.1111/acer.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–72. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Marcotte TL, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–72. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Ladhani NNN, Shah PS, Murphy KE Knowledge Synthesis Group on Determinants of Preterm/LBW Births. Prenatal amphetamine exposure and birth outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;205:219.e1–7. doi: 10.1016/j.ajog.2011.04.016. [DOI] [PubMed] [Google Scholar]
- LaGasse LL, Derauf C, Smith LM, Newman E, Shah R, Neal C, Arria A, Huestis MA, DellaGrotta S, Lin H, Dansereau LM, Lester BM. Prenatal methamphetamine exposure and childhood behavior problems at 3 and 5 years of age. Pediatrics. 2012;129:681–8. doi: 10.1542/peds.2011-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C, Huestis MA, Arria AM, Della Grotta S, Wilcox T, Lester BM. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicol Teratol. 2011;33:166–75. doi: 10.1016/j.ntt.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laswad T, Wintermark P, Alamo L, Moessinger A, Meuli R, Gudinchet F. Method for performing cerebral perfusion-weighted MRI in neonates. Pediatr Radiol. 2009;39:260–4. doi: 10.1007/s00247-008-1081-9. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Johnson A, O'Hare ED, Bookheimer SY, Smith LM, O'Connor MJ, Sowell ER. Effects of prenatal methamphetamine exposure on verbal memory revealed with functional magnetic resonance imaging. J Dev Behav Pediatr. 2009;30:185–92. doi: 10.1097/DBP.0b013e3181a7ee6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JF, Belcher AM, Feinstein EM, O'Dell SJ. Methamphetamine-induced neural and cognitive changes in rodents. Addiction. 2007;102(1):61–9. doi: 10.1111/j.1360-0443.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–22. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Towe SL, Watt MH, Lion RR, Myers B, Skinner D, Kimani S, Pieterse D. Addiction and treatment experiences among active methamphetamine users recruited from a township community in Cape Town, South Africa: A mixed-methods study. Drug Alcohol Depend. 2015;152:79–86. doi: 10.1016/j.drugalcdep.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Watt MH, Sikkema KJ, Deng LX, Ranby KW, Skinner D, Pieterse D, Kalichmann SC. Methamphetamine use is associated with childhood sexual abuse and HIV sexual risk behaviors among patrons of alcohol-serving venues in Cape Town, South Africa. Drug Alcohol Depend. 2012;126:232–9. doi: 10.1016/j.drugalcdep.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJW, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW. A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. NeuroImage Clin. 2014;5:152–60. doi: 10.1016/j.nicl.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Lee B, Hellemann G, O'Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125:230–8. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Redmond DE, Elsworth JD. Impact of methamphetamine on dopamine neurons in primates is dependent on age: implications for development of Parkinson's disease. Neuroscience. 2011;189:277–85. doi: 10.1016/j.neuroscience.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Redmond DE, Sladek JR, Elsworth JD. Apoptotic natural cell death in developing primate dopamine midbrain neurons occurs during a restricted period in the second trimester of gestation. Exp Neurol. 2007;204:802–7. doi: 10.1016/j.expneurol.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D, Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis MA, Haning W, Strauss A, Della Grotta S, Liu J, Lester BM. Intrauterine growth of infants exposed to prenatal methamphetamine: results from the infant development, environment, and lifestyle study. J Pediatr. 2010;157:337–9. doi: 10.1016/j.jpeds.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noailles PAH, Becker KG, Wood WH, Teichberg D, Cadet JL. Methamphetamine-induced gene expression profiles in the striatum of male rat pups exposed to the drug in utero. Brain Res Dev Brain Res. 2003;147:153–62. doi: 10.1016/j.devbrainres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria AV, Yoshida S, Chang L, Mori S. Quantitative evaluation of brain development using anatomical MRI and diffusion tensor imaging. Int J Dev Neurosci. 2013;31:512–24. doi: 10.1016/j.ijdevneu.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe HR, Pell GS, Abbott DF, Jackson GD. Hippocampal volume assessment in temporal lobe epilepsy: How good is automated segmentation? Epilepsia. 2009;50:2586–92. doi: 10.1111/j.1528-1167.2009.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasche S, Myers B. Substance misuse trends in South Africa. Hum Psychopharmacol. 2012;27:338–41. doi: 10.1002/hup.2228. [DOI] [PubMed] [Google Scholar]
- Paz MS, Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S, Liu J, Lester BM. Maternal depression and neurobehavior in newborns prenatally exposed to methamphetamine. Neurotoxicol Teratol. 2009;31:177–82. doi: 10.1016/j.ntt.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, Ramlagan S, Johnson BD, Phaswana-Mafuya N. Illicit drug use and treatment in South Africa: a review. Subst Use Misuse. 2010;45:2221–43. doi: 10.3109/10826084.2010.481594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Kolchugina GK, Butler RW, Corbett SM, Honeycutt EB, Craytor MJ, Raber J. Abnormalities in parentally rated executive function in methamphetamine/polysubstance exposed children. Pharmacol Biochem Behav. 2011;98:432–9. doi: 10.1016/j.pbb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plüddemann A, Dada S, Parry CDH, Kader R, Parker JS, Temmingh H, van Heerden S, de Clercq C, Lewis I. Monitoring the prevalence of methamphetamine-related presentations at psychiatric hospitals in Cape Town, South Africa. Afr J Psychiatry. 2013;16:45–9. doi: 10.4314/ajpsy.v16i1.8. doi: http://dx.doi.org/10.4314/ajpsy.v16i1.8. [DOI] [PubMed] [Google Scholar]
- Plüddemann A, Flisher AJ, McKetin R, Parry C, Lombard C. Methamphetamine use, aggressive behavior and other mental health issues among high-school students in Cape Town, South Africa. Drug Alcohol Depend. 2010;109:14–9. doi: 10.1016/j.drugalcdep.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8:E337–47. doi: 10.1208/aapsj080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin MJ. Developmental neuroimaging of children using magnetic resonance techniques. Ment Retard Dev Disabil Res Rev. 2000;6:68–80. doi: 10.1002/(SICI)1098-2779(2000)6:1<68∷AID-MRDD9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Rodionov R, Chupin M, Williams E, Hammers A, Kesavadas C, Lemieux L. Evaluation of atlas-based segmentation of hippocampi in healthy humans. Magn Reson Imaging. 2009;27:1104–9. doi: 10.1016/j.mri.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Roos A, Jones G, Howells FM, Stein DJ, Donald KA. Structural brain changes in prenatal methamphetamine-exposed children. Metab Brain Dis. 2014;29:341–9. doi: 10.1007/s11011-014-9500-0. [DOI] [PubMed] [Google Scholar]
- Roos A, Kwiatkowski MA, Fouche J, Narr KL, Thomas KGF, Stein DJ, Donald KA. White matter integrity and cognitive performance in children with prenatal methamphetamine exposure. Behav Brain Res. 2015;279:62–7. doi: 10.1016/j.bbr.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O'Connor MJ, Bookheimer SY, Sowell ER. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: the effects of methamphetamine, alcohol, and polydrug exposure. Neuroimage. 2011;54:3067–75. doi: 10.1016/j.neuroimage.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury AL, Ponder KL, Padbury JF, Lester BM. Fetal effects of psychoactive drugs. Clin Perinatol. 2009;36:595–619. doi: 10.1016/j.clp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel AP, Grewen KM, Goldman BD, Gao W. Thalamocortical functional connectivity and behavioral disruptions in neonates with prenatal cocaine exposure. Neurotoxicol Teratol. 2016;56:16–25. doi: 10.1016/j.ntt.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Benavides G, Gómez-Ansón B, Sainz A, Vives Y, Delfino M, Peña-Casanova J. Manual validation of FreeSurfer's automated hippocampal segmentation in normal aging, mild cognitive impairment, and Alzheimer Disease subjects. Psychiatry Res. 2010;181:219–25. doi: 10.1016/j.pscychresns.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA. The Primate Thalamus Is a Key Target for Brain Dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R, Diaz SD, Arria A, LaGasse LL, Derauf C, Newman E, Smith LM, Huestis MA, Haning W, Strauss A, Della Grotta S, Dansereau LM, Roberts MB, Neal C, Lester BM. Prenatal methamphetamine exposure and short-term maternal and infant medical outcomes. Am J Perinatol. 2012;29:391–400. doi: 10.1055/s-0032-1304818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, Shen D. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Pediatr. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–60. doi: 10.1212/WNL.57.2.255. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, Haning W, Arria A, Huestis M, Strauss A, Della Grotta S, Dansereau LM, Lin H, Lester BM. Motor and cognitive outcomes through three years of age in children exposed to prenatal methamphetamine. Neurotoxicol Teratol. 2011;33:176–84. doi: 10.1016/j.ntt.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Leow AD, Bookheimer SY, Smith LM, O'Connor MJ, Kan E, Rosso C, Houston S, Dinov ID, Thompson PM. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. J Neurosci. 2010;30:3876–85. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–44. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stek AM, Baker RS, Fisher BK, Lang U, Clark KE. Fetal responses to maternal and fetal methamphetamine administration in sheep. Am J Obstet Gynecol. 1995;173:1592–8. doi: 10.1016/0002-9378(95)90654-1. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Jacobson SW, van der Kouwe A, Molteno CD, Chen G, Wintermark P, Alhamud A, Jacobson JL, Meintjes EM. A DTI-based tractography study of effects on brain structure associated with prenatal alcohol exposure in newborns. Hum Brain Mapp. 2015;36:170–86. doi: 10.1002/hbm.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J, LaGasse L, Derauf C, Newman E, Shah R, Smith L, Arria A, Huestis M, DellaGrotta S, Roberts M, Dansereau L, Neal C, Lester B. Prenatal methamphetamine exposure, home environment, and primary caregiver risk factors predict child behavioral problems at 5 years. Am J Orthopsychiatry. 2013;83:64–72. doi: 10.1111/ajop.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Moe V, Slinning K, Due-Tønnessen P, Bjørnerud A, Dale AM, van der Kouwe A, Quinn BT, Kosofsky B, Greve D, Fischl B. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage. 2007;36:1331–44. doi: 10.1016/j.neuroimage.2007.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsberg WM, Jones HE, Zule WA, Myers BJ, Browne FA, Kaufman MR, Luseno W, Flisher AJ, Parry CDH. Methamphetamine (“tik”) use and its association with condom use among out-of-school females in Cape Town, South Africa. Am J Drug Alcohol Abuse. 2010;36:208–13. doi: 10.3109/00952990.2010.493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsberg WM, Luseno WK, Karg RS, Young S, Rodman N, Myers B, Parry CDH. Alcohol, cannabis, and methamphetamine use and other risk behaviours among Black and Coloured South African women: a small randomized trial in the Western Cape. Int J Drug Policy. 2008;19:130–9. doi: 10.1016/j.drugpo.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PG, Bhuller Y, Chen CS, Jeng W, Kasapinovic S, Kennedy JC, Kim PM, Laposa RR, McCallum GP, Nicol CJ, Parman T, Wiley MJ, Wong AW. Molecular and biochemical mechanisms in teratogenesis involving reactive oxygen species. Toxicol Appl Pharmacol. 2005;207:354–66. doi: 10.1016/j.taap.2005.01.061. [DOI] [PubMed] [Google Scholar]
- Winer B. Statistical principles in experimental design. 2nd. McGraw-Hill; 1971. [Google Scholar]
- Wong AW, McCallum GP, Jeng W, Wells PG. Oxoguanine glycosylase 1 protects against methamphetamine-enhanced fetal brain oxidative DNA damage and neurodevelopmental deficits. J Neurosci. 2008;28:9047–54. doi: 10.1523/JNEUROSCI.2557-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouldes TA, Lagasse LL, Huestis MA, Dellagrotta S, Dansereau LM, Lester BM. Prenatal methamphetamine exposure and neurodevelopmental outcomes in children from 1 to 3 years. Neurotoxicol Teratol. 2014;42:77–84. doi: 10.1016/j.ntt.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H, Srinivasan L, Jiang S, Rutherford M, Edwards AD, Rueckert D, Hajnal JV. Automatic segmentation and reconstruction of the cortex from neonatal MRI. Neuroimage. 2007;38:461–477. doi: 10.1016/j.neuroimage.2007.07.030. [DOI] [PubMed] [Google Scholar]


