Abstract
Background
Improved imaging methods are critical to assess neurodegeneration and remyelination in multiple sclerosis. Chronic hypointensities observed on T1-weighted brain MRI, “persistent black holes,” reflect severe focal tissue damage. Present measures consist of determining persistent black holes numbers and volumes, but do not quantitate severity of individual lesions.
Objective
Develop a method to differentiate black and gray holes and estimate the severity of individual multiple sclerosis lesions using standard magnetic resonance imaging.
Methods
38 multiple sclerosis patients contributed images. Intensities of lesions on T1-weighted scans were assessed relative to cerebrospinal fluid intensity using commercial software. Magnetization transfer imaging, diffusion tensor imaging and clinical testing were performed to assess associations with T1w intensity-based measures.
Results
Intensity-based assessments of T1w hypointensities were reproducible and achieved >90% concordance with expert rater determinations of “black” and “gray” holes. Intensity ratio values correlated with magnetization transfer ratios (R = 0.473) and diffusion tensor imaging metrics (R values ranging from .283 to −.531) that have been associated with demyelination and axon loss. Intensity ratio values incorporated into T1w hypointensity volumes correlated with clinical measures of cognition.
Conclusions
This method of determining the degree of hypointensity within multiple sclerosis lesions can add information to conventional imaging.
Keywords: Axonal loss, Multiple sclerosis, Quantitative MRI, Outcome measurement, MRI, T1w hypointensity
Introduction
Hypointense areas of white matter (WM) on T1-weighted (T1w) magnetic resonance images (MRIs) persisting for at least 12 months are markers of focal tissue injury in MS known as “persistent black holes” (PBH) 1. The pathologic correlate of a PBH is severe axon loss and matrix destruction 2–4. Some investigators label less hypointense BHs as “gray holes” (GHs) to reflect a lower degree of axonal loss. Acute contrast enhancing lesions (CELs) may also display decreased intensity on the concurrent non-contrasted T1w images. These “acute black holes” are due primarily to inflammation and edema, as most resolve within months of contrast resolution5.
PBHs have relevance to clinical outcomes and disease progression6. Several studies have shown that PBH numbers or volumes correlate with worse clinical test scores4, 6, 7. MS lesion “burden” has often been reported as the sum of lesion volumes, but the degree of tissue destruction may vary between lesions8, 9. A quantitative method to define the degree of hypointensity in individual MS lesions could improve patient monitoring, allow for better correlations to clinical measures, and may prove useful as an outcome measure in clinical trials of potential reparative therapies7.
Our goal was to develop a simple and objective method to identify and distinguish PBHs and PGHs and to estimate the severity of the underlying tissue damage in these lesions based on degree of T1w hypointensity.
Methods
Subject Population and Clinical Testing
Human Studies Committee approval was obtained. All 38 MS subjects contributing data gave informed consent. The following clinical tests, all validated for MS, were performed at times of imaging: Expanded disability status scale (EDSS), 25 foot timed walk (25FTW), nine-hole peg test (9HPT) and the 3- and 2-second versions of the Paced Auditory Serial Addition Test (PASAT).
Image Acquisition
Human Studies Committee approval was obtained and all subjects gave informed consent. Patients were imaged on a 3.0T Siemens Trio scanner (Siemens, Erlangen, Germany). T1w was acquired using the following parameters: Repetition Time (TR) = 600ms; Echo Time (TE) = 9ms; slice thickness = 2 mm; in-plane resolution = 1 × 1 mm3; total acquisition time = 4 min. T2w was acquired using the following parameters: TR = 7500ms; TE = 210ms; TI = 2500ms; slice thickness = 1 mm; in-plane resolution = 1 × 1 mm3; total acquisition time = 10 min. Magnetization Transfer (MT) images were acquired with the following parameters: TR = 38ms; TE = 11 ms; Flip Angle = 15 degrees; slice thickness = 3 mm; in-plane resolution = 1 × 1 mm3; total acquisition time = 8 min. Magnetization Transfer Ratio (MTR) maps were calculated pixel-by-pixel using the equation: MTR= (Soff−Son)/Soff ×100, where Son and Soff were signal intensities with and without saturation pulse. Axial Diffusion Weight images (DWI) covering the whole brain were acquired using a multi-b value diffusion weighting scheme (99 directions, maximum b-value 1500 s/mm2) and the following parameters: TR = 10,000 ms; TE = 120 ms; slice thickness = 2 mm; in-plane resolution = 2 × 2 mm3; total acquisition time = 16 min. Eddy current and motion artifacts of DWI were corrected, then susceptibility-induced off-resonance field was estimated and corrected. Whole brain voxel-wise DTI analyses were performed on DWI images by the in-house software developed using MATLAB10.
MS Lesion Classification
Areas of hypointensity on pre-gadolinium T1w images that met the definition of “persistent” by being present for at least 12 months were identified in the MS subjects. Amira 6.0.1 visualization and analysis software (FEI, Hillsboro, OR) was used to provide quantitative intensity values for each hypointense lesion on each scan, with lower values reflecting darker voxels. Lesion intensity assessment requires consideration of baseline intensity for each scan, to control for scan-to-scan variations. As cerebrospinal fluid (CSF) is not changed by MS pathology, CSF was used to provide a baseline for each individual scan.
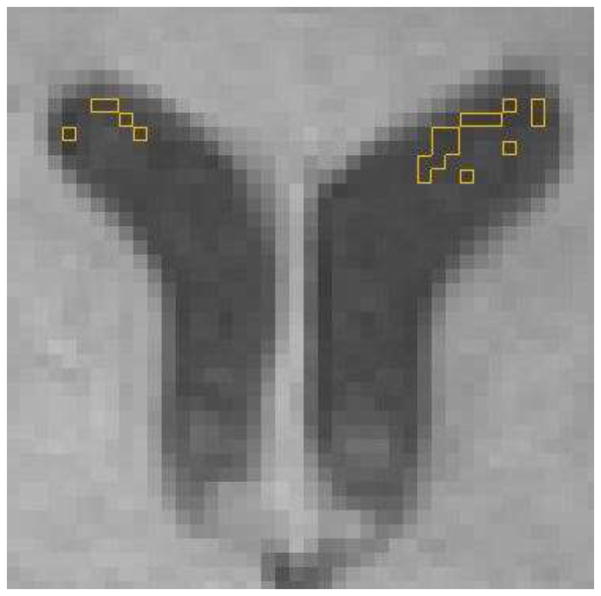
Selection of voxels representative of CSF intensity consisted of starting inferiorly and moving superiorly on axial slices, until the initial appearance of both anterior horns of the lateral ventricles. The axial slice 15mm superior to this was located (typically where the anterior horns of the lateral ventricles were widest), and was used to determine the median “Baseline Intensity” for CSF for that scan (Figure 1). After initially testing 1, 5, 20 and 40 voxels, using the median of 20 ventricular voxels was found to be representative of CSF and a feasible number to select, time-wise.
Figure 1. Illustration of determining “baseline intensity”.
The median value for the 20 voxels of lowest intensity (identified by yellow lines) within the lateral ventricles at an axial slice 15mm superior to the initial appearance of the lateral ventricles determined BI. Only voxels that were more than one voxel away from the ventricle edge were used.
To ensure exclusion of any voxels containing choroid plexus or partial volume effect from ventricle edge, the 20 lowest intensity, not necessarily contiguous, voxels within the CSF of the right and left anterior lateral ventricles at this level were selected. Voxels within one voxel distance from the ventricle edge were excluded to avoid voxels within periventricular lesions. In the rare situation of the baseline slice not containing 20 voxels unaffected by choroid plexus, the adjacent inferior axial image was also used to ensure a sample of 20 lowest intensity voxels within CSF.
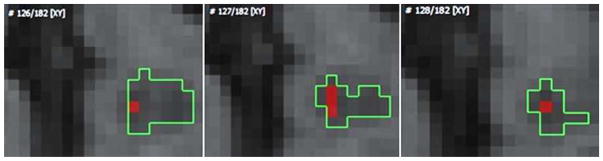
For each MS lesion, the “Lesion Intensity (LI)” was defined as the median of the 5 lowest intensity voxels on all T1w slices where the lesion was visible (Figure 2). This number of voxels was chosen to be representative of the lowest intensity voxels. It took less than 1 minute to identify the median of the 5 lowest intensity voxels. Moreover, small lesions of few voxels could usually be accommodated using 5 voxels.
Figure 2. Illustration of determining “lesion intensity”.
The five voxels of lowest intensity within a lesion were identified. The median intensity of these 5 voxels determined LI. Notice that voxels on three different slices were used for this particular lesion.
This value was divided by the BI for that scan to obtain an “Intensity Ratio (IR)” for the lesion relative to CSF:
Lesions were selected from throughout the brain. The only exclusion criterion for size was that a lesion have a minimum of 5 voxels, for intensity determinations. Lesion volumes were determined based on the voxel dimensions and the total number of voxels within each lesion ROI. Total WM volumes for each patient were generated using the SIENAX tool.
A single rater identified a region of interest (ROI) for a total of 181 chronic T1w hypointense MS lesions, 189 non-hypointense T2w hyperintense lesions and 113 normal appearing white matter (NAWM) areas, and determined IR for each hypointense lesion. Three of the 38 subjects had no PBH or PGH lesions.
Tests of Reproducibility
For intra-rater reproducibility determination, one examiner used the method on a single scan from each of 6 MS (2 RRMS, 2 SPMS, 2 PPMS) subjects. At two time-points one week apart, BI was determined and LI was determined for 20 lesions on the 6 scans.
For inter-rater reliability, another rater replicated the instruction protocol on 5 scans with 25 lesions from 5 subjects. We determined how potential differences in perceiving the initial appearance of the anterior lateral ventricles might affect the baseline slice chosen and the results. Thus, BI was determined on the 2 slices immediately adjacent to the baseline slice (1 inferior slice, 1 superior slice) for all 38 subjects in the study. Bland-Altman plots were used to compare the BI of the baseline slice to the BI of each of the adjacent slices11,12.
While at least 12 months was required to designate lesions as PBH, PGH or non-hole T2w lesions (NBH), some patients with multiple scans had intervals between consecutive scans of as few as 3 weeks, ranging to 54 weeks, with an average interval of 9.2 months. Reproducibility across these scans was assessed to determine whether scan-to-scan variations and/or time might affect the lesion classification method. For this, scans from 6 MS subjects (2 RRMS, 2 SPMS, 2 PPMS) with multiple MRI scans were used. Of the 6 subjects, three had scans from 2 time points, two had scans from 3 time points, and one had scans from 6 time points spanning 112 weeks. BI was determined on T1w non-contrasted scans from each different time point in each subject. 5 BH and 5 GH lesions across the 6 subjects were selected and LI was determined at each of the time points for every lesion. IRs across scans were compared for each lesion to evaluate reproducibility over time.
Correlations of Lesion Classification using Intensity-based metrics with Diffusion tensor imaging and magnetization transfer data
Alterations in DTI parameters and MTR have been associated with the pathological severity of MS lesions10. Thus, IR-based classifications of PBHs, PGHs, NBH and NAWM were compared with DTI-based parameters and MTR values.
IR values obtained for each of the 181 PBH or PGH lesions from standard T1w images were examined for associations with each of four DTI imaging parameters[Apparent Diffusion Coefficient (ADC), axial diffusivity (AD), radial diffusivity (RD), and fractional anisotropy (FA)] and with MTR values, all of which have been associated with tissue damage of various types 5, 13–18. Median values were used to represent the collective imaging parameters for each lesion ROI. Statistical modeling was used to account for intra-subject clustering of lesions.
Intensity-based Lesion Burden
IR of each hypointense lesion was incorporated into lesion burden by dividing each lesion volume by its IR; the result was termed the “Hypointense Lesion Weight”. The “Weighted Hypointense Lesion Burden” (WLB) was determined by summing all “Hypointense Lesion Weights” for an individual patient. Thus, greater weight was given to a BH than a GH. Given that WM volumes vary from subject to subject based on brain size, we also normalized WLB to WM volume. Total WM volumes for each patient were generated using “SIENAX” tool in “FSLView.”19 The volumes were normalized for subject head size. Each patient’s WLB was then divided by the total brain WM volume. This “Normalized Weighted Hypointense Lesion Burden” (NWLB) was analyzed for associations with clinical measures.
Statistical Analyses
Values of Gamma and Kendall’s tau-b were used for the assessment of concordance between subjective and IR classifications of lesion types. Bland-Altman plots were used to assess slice-to-slice reproducibility.
Multilevel linear repeated measures models were used to represent the association between IR and DTI parameters and MTR. AD, RD, ADC and MTR were approximately Gaussian on the original scale, whereas DTI FA was approximately Gaussian on a logarithmic scale. Pearson correlations and linear modeling were used to determine significance of associations between IR and each radiological marker. Clustering of lesions within patients was incorporated into the statistical models to account for dependency of multiple lesion data derived from the same individual. Correlation was assessed between the mean intensity ratio of each person and the mean of each radiological marker for the 38 patients to determine correlation coefficients.
WLB and NWLB were analyzed for associations with 25FTW, 9HPT and PASAT results using Spearman’s rho rank correlation and with EDSS using Kendall’s Tau b rank correlation.
Results
Subjects
Thirty-eight MS subjects (13 RRMS, 15 PPMS, 10 SPMS) contributed brain imaging data (Table 1), of whom 35 had hypointense lesions on T1w scans that persisted at least 12 months, establishing them as “persistent”.
Table 1.
Demographic profile of patients.
| Mean | SD | Range (Median) | |
|---|---|---|---|
| Age (years) | 51.5 | 10.6 | 25 – 72 (55) |
| Disease Duration (years) | 16.5 | 11.9 | 1.5 – 43.3 (13.7) |
| EDSS | 4.8 | 1.7 | 1.5 – 6.5 (6.0) |
| Gender | 26 females, 12 males | ||
| MS Subtypea | 13 RRMS, 15 PPMS, 10 SPMS | ||
RRMS = relapsing-remitting multiple sclerosis, PPMS = primary-progressive multiple sclerosis, SPMS = secondary-progressive multiple sclerosis
Intensity Ratio as a semi-quantitative measure of lesion severity
Two independent raters classified 25 lesions across 3 subjects (1 RRMS, 1SPMS, 1 PPMS) as “black holes” or “gray holes.” Based on this, provisional lesion type limits were set as:
Black Hole: 1.00 ≤ IR ≤ 1.70
Gray Hole: 1.71 ≤ IR ≤ 2.60
Non-Hole: IR > 2.60
An experienced rater then classified 103 lesions from 10 MS subjects as BH, GH or NBH, blinded to IR values. Subjective classifications were compared with IR-determined classifications (Table 2). Over 91 percent of the time the two methods were in accord. Values of Gamma and Kendall’s tau-b were 0.99 and 0.91, respectively, showing very strong concordance (Table 2).
Table 2.
Comparison of subjective lesion classification to classification based on Intensity Ratio.
| Intensity Ratio based categorizations | |||||
|---|---|---|---|---|---|
| BH | GH | NBH | total | ||
| Neurologist categorizations | BH | 27 | 3 | 0 | 30 |
| GH | 4 | 36 | 1 | 41 | |
| NBH | 0 | 1 | 31 | 32 | |
| Total | 31 | 40 | 32 | 103 | |
There were no cases of extreme discordance in which a BH by one method was classified as a NBH by the other method. For each method, one lesion was called a GH but was classified as NBH by the other method, both lesions were in IR range of 2.55 – 2.65. Seven discordantly classified lesions occurred for BH vs. GH. Each had IR in the 1.67 – 1.73 range, near the border between BHs and GHs. The kappa statistic was 0.87, indicating a strong agreement between methods, with no evidence for systematic discordance.
Time to apply method
After determination of the IR cutoffs for classification, the method was applied across 35 subjects to 181 T1w hypointense MS lesions. With practice, time to select the CSF baseline 20 voxels was 5 minutes. The time to select the 5 lowest intensity lesion voxels and determine median for a lesion was 1 minute.
Reproducibility
Intra-rater
One rater determined BI and LI in 20 MS lesions on 6 MR scans from 6 MS subjects at two time-points, one week apart. Exact concordance of BI and LI values was observed when the same rater re-examined the 20 MS lesions later.
Inter-rater
The two raters chose the same axial section for BI determination for three of the five subjects, with exact concordance in those cases. For the other two subjects’ scans, different but adjacent axial images were chosen for determination of BI. This resulted in BIs of 203.5 versus 201.5 in one subject, and 152 versus 150.2 in the other. No lesions changed in classification as a result of these BI differences. Both raters chose the exact same 5 lowest intensity voxels to determine median value in the lesions, obtaining identical LI values in all 25 lesions.
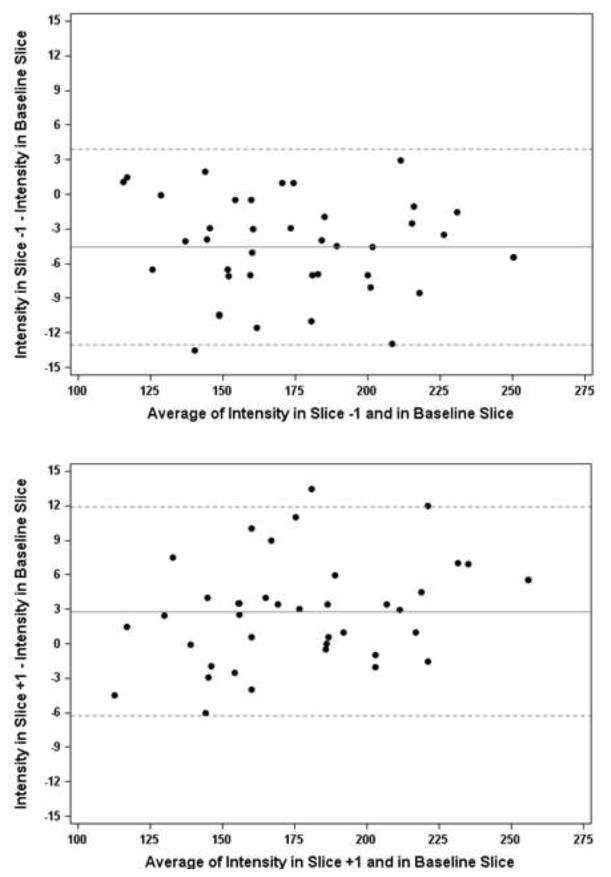
Bland-Altman plots were then used to assess differences when adjacent but different axial images were selected to determine BI (Figure 3a, 3b). Over 97% of points fell within the limits of agreement. The mean difference for the −1 scan was below 0, whereas the mean difference of baseline and +1mm scan was above 0, depicting a slight increase in intensity from −1 scan to the +1 scan.
Figure 3. Level of agreement between baseline intensity determinations for adjacent axial slices.
Bland-Altman plots were used to determine the limits of agreement (dotted lines represent ± 2 SD of the difference) when CSF intensity (BI) was assessed on adjacent axial images. 3a) compares BI obtained for the slice at 15mm above initial perception of lateral ventricles to the immediately inferior slice; 3b) compares BI obtained for the slice superior to and the slice at 15mm above initial perception of lateral ventricles. More than 97% of the points fell within the limits of agreement.
Reproducibility over time
Ten individual PBH or PGH lesions from 6 subjects were examined longitudinally over as long as 112 weeks. Some lesions were examined on as many as 6 different scans. The mean interval between scans was 9.2 months (range 3 weeks to 54 weeks). No lesion changed in classification when evaluated across scans. PGHs displayed more variability than PBHs. Standard deviations ranged from 1% to 4% of mean IR (Table 3).
Table 3.
Reproducibility of intensity ratios over time.
| Subject | MS Subtype | Lesion Type | Number of Scans | Time Intervala | Mean IR ± SD | IR Range/Valuesb | Mean Volume mm3 ± SD |
|---|---|---|---|---|---|---|---|
| 1 | RRMS | PGH | 3 | 2 months | 2.177 ± 0.043 | 2.127 – 2.203 | 32 ± 5 |
| 2 | RRMS | PBH | 6 | 26 months | 1.617 ± 0.018 | 1.594 – 1.636 | 108 ± 6 |
| PGH | 6 | 26 months | 2.151 ± 0.072 | 2.038 – 2.227 | 24 ± 5 | ||
| 3 | SPMS | PGH | 3 | 24 months | 1.811 ± 0.066 | 1.736 – 1.861 | 79 ± 10 |
| 4 | SPMS | PBH | 2 | 12 months | 1.418 ± 0.021 | 1.403, 1.433 | 140 ± 6 |
| PBH | 2 | 12 months | 1.630 ± 0.022 | 1.614, 1.646 | 233 ± 14 | ||
| 5 | PPMS | PBH | 2 | 11 months | 1.570 ± 0.006 | 1.566, 1.574 | 22 ± 0 |
| PBH | 2 | 11 months | 1.600 ± 0.005 | 1.592, 1.600 | 91 ± 2 | ||
| 6 | PPMS | PGH | 2 | 13 months | 1.738 ± 0.022 | 1.722, 1.753 | 21 ± 2 |
| PGH | 2 | 13 months | 2.398 ± 0.046 | 2.365, 2.430 | 17 ± 1 | ||
| Mean GH SD: 0.050 | |||||||
| Mean BH SD: 0.015 | |||||||
Time interval between the first scan and last scan
IR values were listed instead of range if the subject only had 2 scans
Comparisons of Intensity-Based Lesion Classification with MTR and Fractional anisotropy from T1w images
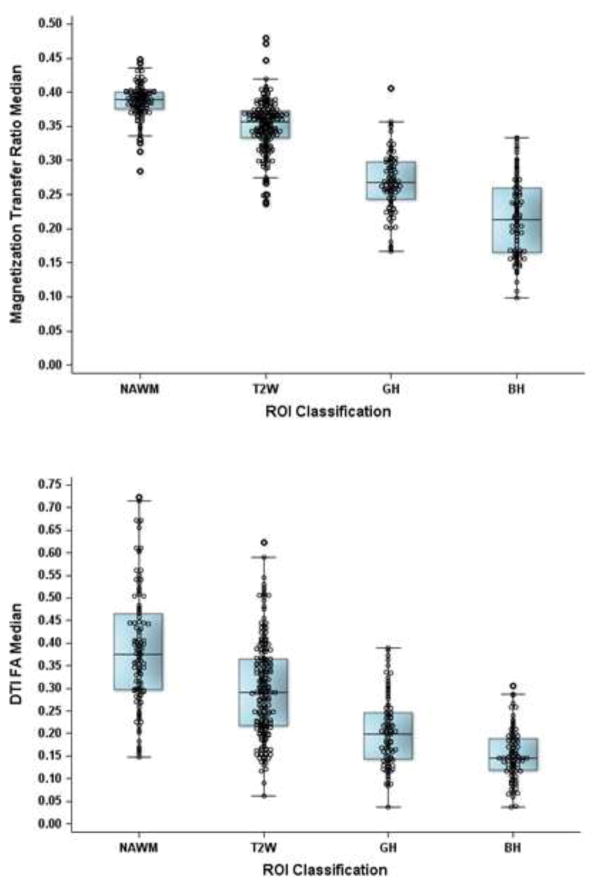
PBHs have reduced MTR and DTI-derived fractional anisotropy (FA) compared to mildly hypointense lesions, non-hypointense T2w lesions and NAWM5,20, 21. Thus, correlations of IR with MTR measures and DTI-derived FA of individual lesions were assessed. MTR and FA each progressively decreased as lesion classification moved from NAWM to PGH to PBH (p < 0.0001 for each pair of adjacent categories for both measures) (Figures 4a and 4b).
Figure 4. Associations of Magnetization Transfer Ratios and Fractional anisotropy values with IR-based classifications.
4a) IR-based lesion classifications in relation to MTR values and 4b) Fractional anisotropy (FA) values. Regions were classified as NAWM, or as non-black/gray hole, PGH and PBH using IR and showed decreasing trends from NAWM to PBH (p < 0.001, for each using model clustering lesions within patients).
Correlations of Intensity-Based Classification and Lesion Metrics with Diffusion Tensor Imaging Parameters from T1 images
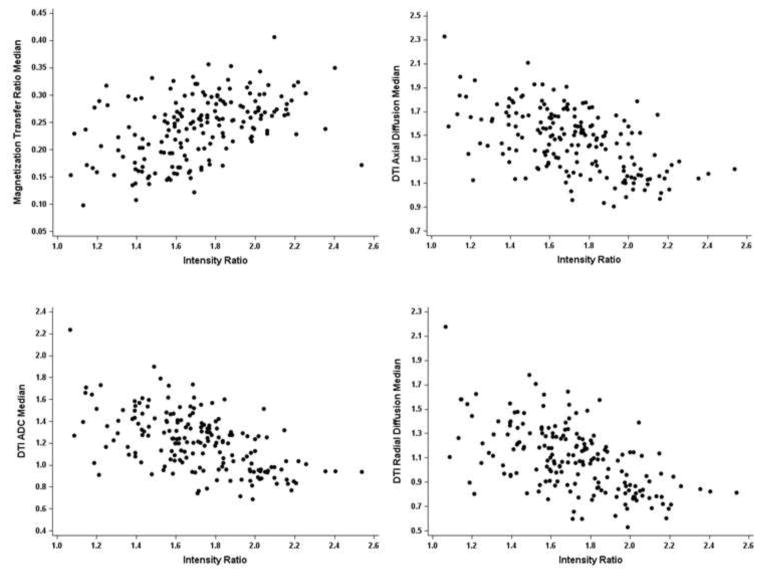
Lesion IRs values for the 181 PBH and PGH lesions from 35 MS patients were examined for correlations with MTR (Figure 5a), as well as DTI –derived parameters of AD, RD, and ADC (Figures 5b – 5d). MTR values directly correlated with IR values in the 181 PBHs and PGHs (Table 4; Figure 5a, p < 0.0001). DTI-derived AD, RD, and ADC were each inversely associated with IR (Table 4, p < 0.0001 for each). Log DTI FA values changed in the same direction associated with IR, increasing as IR increased, but associations did not reach statistical significance (p = 0.099; Table 4).
Figure 5. Associations of IR values with MTR and DTI-derived AD, RD, and ADC.
Associations of IR values of PGHs and PBHs with 5a) MTR (R= .473, p=.004), 5b) AD (R= −.515, p < .002), 5c) ADC (R= −.531, p=.001), and 5d) RD (R= −.530, p =.001) are shown. Pearson correlations and linear modeling were used to determine the strength of linear association between IR and each radiological marker. Correlation coefficients are based on the mean values per patient over all lesions to account for clustering.
Table 4.
Coefficients of correlation between IR and MTR and DTI parameters.
| Correlation Coefficienta | P-Valueb | |
|---|---|---|
| MTR | .473 | .0041 |
| DTI AD | −.515 | .0015 |
| DTI RD | −.530 | .0011 |
| DTI ADC | −.531 | .0010 |
| Log DTI FA | .283 | .0990 |
Correlation coefficients were weighted by the number of lesions per patient, to account for within-patient clustering.
Pearson correlations and linear modeling were used to determine significance of associations.
Normalized Weighted Lesion Burden
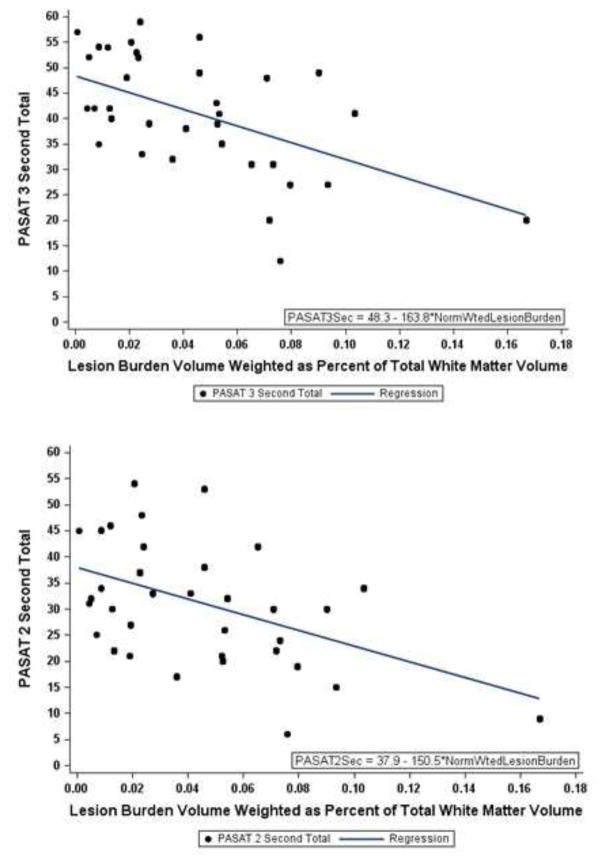
“Normalized WLB” correlated with cognition components of the MSFC, the 3 second PASAT (R = −0.572, p < 0.01) and 2 second PASAT (R = −0.428, p < 0.05)(Figures 6a and 6b).
Figure 6. Associations between IR and cognitive test scores.
6a) Normalized weighted lesion burden was inversely associated with 3 sec PASAT scores (Table 5: R= −.572, p < 0.01). 6b) Normalized weighted lesion burden was inversely associated with 2-sec PASAT scores (Table 5: R = − .428, p < 0.05).
Significant associations were not found for NWLB with motor tests such as 25 FTW, 9HPT or EDSS. However, associations of NWLB with 25FTW (R = 0.188, Spearman’s rho), 9HPT (0.071 and 0.107, Spearman’s rho) and EDSS (R=0.121, Kendall’s tau-b rank) were directionally valid (Table 5). Unweighted LB and non-normalized WLB also correlated significantly with 3 second (−.52 and −.54, respectively) and 2 second (−.39 and −.39, respectively) PASAT scores, but to a lesser extent than for NWLB.
Table 5.
Associations of Normalized Weighted Lesion Burden with clinical measures.
| Spearman’s rho | |
|---|---|
| PASAT 3 sec raw score | −0.572** |
| PASAT 2 sec raw score | −0.428* |
| 25 Foot Timed Walk – average of 2 trials | 0.188 |
| 9HPT Dominant Hand – average of 2 trials | 0.071 |
| 9HPT Non-dominant Hand – average of 2 trials | 0.107 |
| Kendall’s Tau b | |
| EDSS | 0.121 |
p < 0.01;
p < 0.05;
N = 38
Discussion
MRI plays an important role in diagnosis and management of MS. However, conventional T1w and T2w brain imaging techniques do not correlate well with MS disability22. Lesion-specific factors such as pathologic heterogeneity and different degrees of functional eloquence of varying CNS locations, coupled with suboptimal methods for measuring clinical disability contribute to a lack of strong correlations. Additionally, conventional T1w and T2w imaging contrasts are scan-specific and depend not only on the MR characteristics of tissue but also the pulse sequences and magnet strength, making them difficult to quantitate. MS lesions that are persistently hypointense on T1w images, so-called “persistent black holes,” contain more axon loss neuropathologically3. Numbers and volumes of T1w BHs have demonstrated better correlations with disability than T2w lesion measures6, 23–25. In a study that compared several imaging modalities performed at baseline in RRMS for correlations with worsening clinical disability over 10 years, PBH number and increasing PBH volumes performed best24. Comparisons of imaging and neuropathology in over 100 MS lesions found that degree of hypointensity was strongly associated with axonal density (r = 0.74, p < 0.0001).7
The development of anti-inflammatory therapies that reduce MS relapse rate has transformed the lives of many MS patients, and was aided greatly by the ability of gadolinium-enhancing lesions to serve as surrogates of relapses26. However, a comparable biomarker of neurodegeneration has not surfaced. Strong evidence supports the potential for using PBHs as a marker of neurodegeneration which might be exploited in individual patients and as an outcome measure in trials of potential neuroprotective agents.
Importantly, numbers and volumes of PBH change in concert with worsening in clinical disability24. A 2008 meeting of international experts in MS imaging established five criteria that imaging outcomes should fulfill to be used as surrogate markers for proof of concept neuroprotection studies in MS27, 28. These were pathological specificity, reproducibility, sensitivity to change, clinical relevance and response to treatment. PBH observed on T1w images were considered to be among the four most promising measures identified at that meeting (others were brain volumetrics, ocular coherence tomography, and MTR)28. The present study was performed because the value of PBHs as potential surrogate markers of neurodegeneration could be enhanced by more objective and quantitative measurements.
Another quantitative method of calculating BH intensity relative to NAWM and CSF intensities on the scan has been described29. This method depends on subjective identification of an area of NAWM, which may not be truly normal in an MS patients22,30. The reliability of this method has been questioned due to scan-to-scan variations in intensity and inclusion of a changing weight variable “k” in the intensity calculations31. Thus, we developed a simpler and more reproducible methodology to identify and assess level of hypointensity within MS lesions on standard T1w scans, using intensity of ventricular CSF as a benchmark on each scan. Metrics derived with the new method proposed herein were reproducible, stable in established lesions over time, and had face validity when compared to expert rater identifications of black and gray holes. While selecting the slice based upon the initial appearance of the anterior ventricles has some subjectivity and 40% of the time varied by an axial slice between two examiners, this did not change lesion categorizations. New T1w quantitative imaging techniques have been reported, and have improved clinical correlation compared to T2w lesion counts/volumes. 31,32. Notably, the hypointensity metrics we describe are standard imaging measures in clinical practice, and do not require extra sequences with longer scan times. Importantly, T1w hypointensity burdens that incorporated IR correlated with cognitive assessments.
In summary, we report upon an efficient method to estimate the degree of hypointensity within PBH that can be performed using standard T1w images and commercial imaging software. This method has potential for semi-automated integration into clinical trials and practice, as a marker of lesion severity and possibly lesion recovery. Next steps would include additional longitudinal studies with larger cohorts, different scanners, and multiple centers. This method may be especially useful for trials of therapeutics with potential to impact axonal degeneration.
Supplementary Material
Highlights.
A method to classify and estimate the severity of T1-weighted hypointensities (“persistent black and gray holes”) is presented
This severity quantification method correlated with diffusion tensor imaging and magnetization transfer ratios, methods that have been previously shown to associate with MS pathology
Incorporating lesion severity into calculations of T1-weighted hypointensity lesion burden improved correlations with tests of cognition
Acknowledgments
These studies were funded by a grant from the U.S. National Institutes of Health (PO1NS059560). Dr. Cross was supported by the Manny & Rosalyn Rosenthal – Dr. John L. Trotter MS Center Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation.
Footnotes
Conflict of Interest/Role of Funding source
Authors GA, KT, PS, SL, JV, and JW have no disclosures.
RTN has served as a consultant to Acorda, Alkermes, Bayer, Biogen, EMD Serono, Genentech, Genzyme, Novartis and Teva.
AHC has served as a consultant to AbbVie, Bayer, Biogen, EMD Serono, Genentech/Roche, Genzyme, Mallinckrodt, Novartis and Teva.
The funding sources had no role in the interpretation of data or the preparation/writing of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gautam Adusumilli, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri USA.
Kathryn Trinkaus, Department of Surgery, Washington University School of Medicine, St. Louis, Missouri USA.
Peng Sun, Department of Radiology, Washington University School of Medicine, St. Louis, Missouri USA.
Samantha Lancia, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri USA.
Jeffrey D. Viox, Department of Radiology, Washington University School of Medicine, St. Louis, Missouri USA
Jie Wen, Department of Radiology, Washington University School of Medicine, St. Louis, Missouri USA.
Robert T. Naismith, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri USA
Anne H Cross, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri USA.
References
- 1.Riva M, Ikonomidou VN, Ostuni JJ, et al. Tissue-specific imaging is a robust methodology to differentiate in vivo T1 black holes with advanced multiple sclerosis-induced damage. AJNR Am J Neuroradiol. 2009 Aug;30(7):1394–1401. doi: 10.3174/ajnr.A1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000 Jun;123(Pt 6):1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- 3.van Walderveen MA, Kamphorst W, Scheltens P, et al. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology. 1998 May;50(5):1282–1288. doi: 10.1212/wnl.50.5.1282. [DOI] [PubMed] [Google Scholar]
- 4.van Walderveen MA, Lycklama ANGJ, Ader HJ, et al. Hypointense lesions on T1-weighted spin-echo magnetic resonance imaging: relation to clinical characteristics in subgroups of patients with multiple sclerosis. Arch Neurol. 2001 Jan;58(1):76–81. doi: 10.1001/archneur.58.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Naismith RT, Xu J, Tutlam NT, et al. Increased diffusivity in acute multiple sclerosis lesions predicts risk of black hole. Neurology. 2010 May 25;74(21):1694–1701. doi: 10.1212/WNL.0b013e3181e042c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Truyen L, van Waesberghe JH, van Walderveen MA, et al. Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology. 1996 Dec;47(6):1469–1476. doi: 10.1212/wnl.47.6.1469. [DOI] [PubMed] [Google Scholar]
- 7.van Waesberghe JH, Kamphorst W, De Groot CJ, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999 Nov;46(5):747–754. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002 Jan 17;346(3):158–164. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 9.O’Riordan JI, Gawne Cain M, Coles A, et al. T1 hypointense lesion load in secondary progressive multiple sclerosis: a comparison of pre versus post contrast loads and of manual versus semi automated threshold techniques for lesion segmentation. Mult Scler. 1998 Oct;4(5):408–412. doi: 10.1177/135245859800400502. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Sun P, Wang Q, Trinkaus K, Schmidt RE, Naismith RT, Cross AH, Song SK. Differentiation and quantification of inflammation, demyelination, and axon injury or loss in multiple sclerosis. Brain. 2015 May;138:1223–1238. doi: 10.1093/brain/awv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. 1986;327:307–310. [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Measuring agreement in method comparison studies. Statistical Methods in Medical Research. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 13.DeBoy CA, Zhang J, Dike S, et al. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007 Aug;130(Pt 8):2199–2210. doi: 10.1093/brain/awm122. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Budde MD, Liang HF, et al. Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiol Dis. 2006 Mar;21(3):626–632. doi: 10.1016/j.nbd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Naismith RT, Xu J, Klawiter EC, et al. Spinal cord tract diffusion tensor imaging reveals disability substrate in demyelinating disease. Neurology. 2013 Jun 11;80(24):2201–2209. doi: 10.1212/WNL.0b013e318296e8f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002 Nov;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 17.Cercignani M, Iannucci G, Rocca MA, Comi G, Horsfield MA, Filippi M. Pathologic damage in MS assessed by diffusion-weighted and magnetization transfer MRI. Neurology. 2000 Mar 14;54(5):1139–1144. doi: 10.1212/wnl.54.5.1139. [DOI] [PubMed] [Google Scholar]
- 18.Enzinger C, Barkhof F, Ciccarelli O, et al. Nonconventional MRI and microstructural cerebral changes in multiple sclerosis. Nat Rev Neurol. 2015 Dec;11(12):676–686. doi: 10.1038/nrneurol.2015.194. [DOI] [PubMed] [Google Scholar]
- 19.Smith CD, Snowdon DA, Wang H, Markesbery WR. White matter volumes and periventricular white matter hypointensities in aging and dementia. Neurology. 2000;54(4):838–842. doi: 10.1212/wnl.54.4.838. [DOI] [PubMed] [Google Scholar]
- 20.Papanikolaou N, Papadaki E, Karampekios S, et al. T2 relaxation time analysis in patients with multiple sclerosis: correlation with magnetization transfer ratio. Eur Radiol. 2004 Jan;14(1):115–122. doi: 10.1007/s00330-003-1946-0. [DOI] [PubMed] [Google Scholar]
- 21.Vavasour IM, Laule C, Li DK, Traboulsee AL, MacKay AL. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? J Magn Reson Imaging. 2011 Mar;33(3):713–718. doi: 10.1002/jmri.22441. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulos K, Tozer DJ, Fisniku L, et al. TI-relaxation time changes over five years in relapsing-remitting multiple sclerosis. Mult Scler. 2010 Apr;16(4):427–433. doi: 10.1177/1352458509359924. [DOI] [PubMed] [Google Scholar]
- 23.Zivadinov R, Leist TP. Clinical-magnetic resonance imaging correlations in multiple sclerosis. J Neuroimaging. 2005;15(4 Suppl):10S–21S. doi: 10.1177/1051228405283291. [DOI] [PubMed] [Google Scholar]
- 24.Giorgio A, Stromillo ML, Bartolozzi ML, et al. Relevance of hypointense brain MRI lesions for long-term worsening of clinical disability in relapsing multiple sclerosis. Mult Scler. 2014 Feb;20(2):214–219. doi: 10.1177/1352458513494490. [DOI] [PubMed] [Google Scholar]
- 25.Barkhof F, Karas GB, van Walderveen MA. T1 hypointensities and axonal loss. Neuroimaging Clin N Am. 2000 Nov;10(4):739–752. [PubMed] [Google Scholar]
- 26.Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol. 2009 Mar;65(3):268–275. doi: 10.1002/ana.21606. [DOI] [PubMed] [Google Scholar]
- 27.Inglese M. MRI measures of neuroprotection and repair in multiple sclerosis. J Neurol Sci. 2011 Dec;311(Suppl 1):S16–23. doi: 10.1016/S0022-510X(11)70004-1. [DOI] [PubMed] [Google Scholar]
- 28.Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009 May;5(5):256–266. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 29.Tam RC, Traboulsee A, Riddehough A, Sheikhzadeh F, Li DK. The impact of intensity variations in T1-hypointense lesions on clinical correlations in multiple sclerosis. Mult Scler. 2011 Aug;17(8):949–957. doi: 10.1177/1352458511402113. [DOI] [PubMed] [Google Scholar]
- 30.Whittall KP, MacKay AL, Li DK, Vavasour IM, Jones CK, Paty DW. Normal-appearing white matter in multiple sclerosis has heterogeneous, diffusely prolonged T(2) Magn Reson Med. 2002 Feb;47(2):403–408. doi: 10.1002/mrm.10076. [DOI] [PubMed] [Google Scholar]
- 31.Thaler C, Faizy T, Sedlacik J, et al. T1- Thresholds in Black Holes Increase Clinical-Radiological Correlation in Multiple Sclerosis Patients. PLoS One. 2015;10(12):e0144693. doi: 10.1371/journal.pone.0144693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simoni S, Amaru F, Bonnier G, et al. MP2RAGE provides new clinically-compatible correlates of mild cognitive deficits in relapsing-remitting multiple sclerosis. J Neurol. 2014 Mar;216:1606–1613. doi: 10.1007/s00415-014-7398-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.