Abstract
Protein delivery is often used in tissue engineering applications to control differentiation processes, but is limited by protein instability and cost. An alternative approach is to control the cellular microenvironment through biomaterial-mediated sequestration of cell-secreted proteins important to differentiation. Thus, we utilized heparin-based microparticles to modulate cellular differentiation via protein sequestration in an in vitro model system of endochondral ossification. Heparin and poly(ethylene-glycol) (PEG; a low-binding material control)-based microparticles were incorporated into ATDC5 cell spheroids or incubated with ATDC5 cells in transwell culture. Reduced differentiation was observed in the heparin microparticle group as compared to PEG and no microparticle-containing groups. To determine if observed changes were due to sequestration of cell-secreted protein, the proteins sequestered by heparin microparticles were analyzed using SDS-PAGE and mass spectrometry. It was found that heparin microparticles bound insulin-like growth factor binding proteins (IGFBP)-3 and 5. When incubated with a small-molecule inhibitor of IGFBPs, NBI 31772, a similar delay in differentiation of ATDC5 cells was observed. These results indicate that heparin microparticles modulated chondrocytic differentiation in this system via sequestration of cell-secreted protein, a technique that could be beneficial in the future as a means to control cellular differentiation processes.
Keywords: heparin, microparticles, cell differentiation, protein sequestration, IGFBP
Graphical Abstract
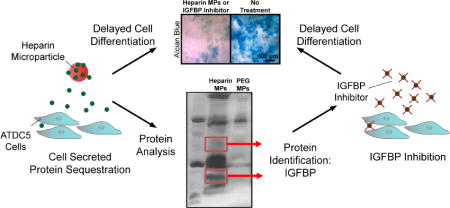
1. Introduction
Tissue engineering requires the precise regulation of cellular differentiation events [1]. Generally, this has been achieved through controlled delivery of recombinant protein, but this technique can be limited due to the short half-life and high expense of these molecules [2,3]. Additionally, this technique fails to directly modulate endogenous, cell-secreted proteins in the cellular microenvironment. Modulating local concentration of cell-secreted protein via biomaterial-based approaches could be particularly advantageous as it eliminates cost of recombinant proteins, may reduce off-target effects, and could potentially be effective over a longer period of time [2]. Recent examples demonstrate that biomaterial-mediated sequestration, or binding, of endogenously produced chemokines has successfully reduced local inflammation [4,5], increased function and survival of cardiac progenitor cells [6], and primed mesenchymal stem cells (MSCs) toward an osteogenic lineage [7]. While these results are encouraging, the ability of biomaterials to temporally modulate cell-secreted protein to affect cellular differentiation has not yet been fully explored.
Glycosaminoglycans (GAGs) have often been incorporated into biomaterials to enhance protein retention for controlled delivery applications and thus are an attractive material to employ for modulation of cell-secreted protein [8]. GAGs are polymers composed of highly sulfated disaccharide units, conferring a negative charge that allows binding, of positively-charged proteins [9]. In particular, because heparin/heparin sulfate has a strong negative charge and interactions between growth factors and heparin are specific and of high affinity but not covalent [10], materials have been modified with heparin and heparan sulfate to enhance protein loading, stability, and release in many controlled delivery vehicles [2,3,10–12]. Often, heparin is covalently modified and cross-linked into hydrogels to control delivery of proteins such as fibroblast growth factor (FGF)s, bone morphogenetic protein (BMP)s, vascular endothelial growth factor (VEGF)s, transforming growth factor (TGF)-βs, and other positively charged proteins to enhance tissue differentiation and healing [13–16]. In general, reduced burst released and lower total release is observed in hydrogels that contain heparin due to increased attraction of proteins to the biomaterial matrix [15–20]. Thus, incorporation of heparin into controlled delivery vehicles can affect the rate of protein release, and can be tuned for specific applications.
While GAGs have been employed for controlled protein delivery, they have not been fully investigated for protein sequestration, or binding, of proteins in the cellular microenvironment. Due to their protein binding ability, GAGs such as chondroitin sulfate, dermatan sulfate, and heparan sulfate are implicated in a variety of physiological processes, including metabolism, basement membrane organization, cell signaling, injury response and repair, and tissue development [21]. Several natural functions of GAGs are of particular interest when designing strategies to modulate differentiation via endogenous protein sequestration, including their ability to enhance protein activity at the cell-surface and to locally sequester protein, creating protein reservoirs of high concentration, in the extracellular matrix (ECM) [21]. For example, heparan sulfate is known to facilitate FGF signaling by acting as a co-receptor on the cell-surface [21]. In addition, chondroitin sulfate is known to bind or sequester Indian hedgehog (IHH) in the growth plate ECM, effectively creating IHH reservoirs and thus controlling its diffusion in growing limbs [22]. Finally, GAGs have been observed to inhibit development of kidney rudiments in organ culture, likely due to sequestration of cell-secreted protein away from cell receptors [23]. Taking inspiration from these functions of GAGs during development, in particular the ability of GAGs to sequester protein locally, we have developed a heparin-based microparticle (MP) [24] strategy to modulate cell-secreted proteins. Heparin-based microparticles are particularly advantageous in studying protein sequestration due to their high binding capacity [24] and their ability to be incorporated into 3D cell aggregates [25–28].
To investigate the ability of heparin-based MPs to temporally modulate cell differentiation, we employed an in vitro model system of endochondral ossification. Endochondral ossification is the process by which cartilage is converted to bone during long bone development [29]. This process occurs in the growth plate and involves maintenance of cells at specific stages of differentiation in a particular spatial organization, which is achieved by modulating local protein concentrations [29]. Many of these proteins are heparin-binding proteins, including BMPs, FGFs, IHH, insulin-like growth factors (IGFs), IGF binding proteins (IGFBPs), and VEGFs [29,30]. Thus, the differentiation process of endochondral ossification is an excellent model system in which to evaluate the ability of heparin-based materials to modulate differentiation through binding of endogenous protein.
As a model platform, we chose the ATDC5 cell line, a teratocarcinoma-derived murine cell line well-documented to produce heparin-binding protein as it undergoes endochondral ossification in vitro [31,32]. Unlike primary chondrocytes or mesenchymal stem cells, which are often used in models of endochondral ossification but can undergo dedifferentiation or early ossification [33], the ATDC5 cell line provided a robust and predictable model system upon which to thoroughly interrogate heparin-based approaches to modulate differentiation.
Previous work has shown that GAG-based materials have the potential to either delay or accelerate differentiation [6,23], likely depending upon the proximity of GAGs to cell surfaces and the binding affinity of sequestered proteins. As the heparin-based MPs used in this study have been shown to release little bound protein over time [24], we hypothesized that heparin-based MPs would sequester protein away from cell receptors and thus reduce cellular differentiation in our in vitro model system. Results from our studies indicated that heparin-based MPs were able to delay differentiation in both 3D (aggregate) and 2D (transwell) culture formats, without increasing cell number. In order to determine the mechanism behind the lower level of differentiation observed, SDS-PAGE and mass spectrometry were used to determine that heparin was sequestering cell-secreted IGFBPs. Finally, the addition of a small molecule inhibitor of IGFBPs produced similar results to those seen in cultures containing heparin MPs. Overall, these studies indicate that heparin MPs have the potential to modulate cellular differentiation through sequestration of endogenous protein, which is a novel strategy to direct cellular differentiation in future tissue engineering applications.
2. Materials and Methods
2.1 Material Synthesis
Heparin methacrylamide (MAm) was functionalized as previously described [7]. Briefly, 20 mg mL−1 heparin was reacted with 83 mM N-hydroxysulfosuccinimide sodium salt (Sigma-Aldrich), 100 mM N-(3-aminopropyl) methacrylamide hydrochloride (Polysciences), and 78 mM (N-3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) (Sigma-Alrich) in a pH 5 phosphate buffer for 2 hours on ice. Additional EDC was added, resulting in a final molarity of 156 mM. After 4 additional hours, the solution was dialyzed for 2–3 days and lyophilized.
Poly(ethylene-glycol) diacrylate (PEG-DA) (Sigma-Aldrich; 8 kDa for MPs in spheroid studies, 3.4 kDa for MPs in transwell studies) was functionalized according to previous methods [34]. Briefly, PEG (Sigma-Aldrich) was reacted with acryoloyl chloride (Sigma-Aldrich) at 100% molar excess in methylene chloride (Fisher Scientific). Triethylamine (Sigma-Aldrich) was added to the reaction to achieve a 1:1 molar ratio of triethylamine:PEG. This reacted under nitrogen purge overnight, at which point the aqueous and organic phases were separated and PEG was precipitated from the organic phase using diethyl ether (EDM Millipore) and dried using a solvent trap. All polymers were stored at −20°C prior to use. Polymers were characterized using NMR (See Supplementary Information).
2.2 Microparticle Fabrication and Characterization
Heparin MPs were formed via water-in-oil emulsion as previously described [24]. Briefly, an aqueous phase of 10% heparin MAm (wt%), 18 mM ammonium persulfate (Sigma-Alrich), and 18 mM N,N,N′,N′-Tetramethylethylenediamine (Sigma) was emulsified against corn oil with 1.67% (v/v) Tween-20 (polysorbate 20; BDH) at a 1:120 ratio aqueous:oil phase. MPs were cross-linked under nitrogen purge at 60°C for 30 minutes, then washed with acetone and water.
PEG MPs were formed via water-in-water (spheroid studies and BMP-2 pull-down studies) or water-in-oil (transwell and SDS-PAGE studies) emulsion techniques. For the water-in-water emulsion, a PEG-DA phase (150 mg mL−1 8 kDa PEG-DA, 2 mg mL−1 poly-L-lysine (PLL) (Sigma-Aldrich), 0.05% (w/v) Irgacure D2959 Photoinitiator (Ciba) in PBS) was emulsified against a dextran phase (50% (w/v) 70 kDa dextran (Sigma-Aldrich), 2 mg mL−1 PLL in PBS) at a 1:4 ratio by 30 seconds of vortexting and ultrasonication at 21 mW energy for 1 minute. For the water-in-oil emulsion, an aqueous phase (16 wt% 3.4 kDa PEG-DA, 0.05 wt% Irgacure D2959, and 2 mg mL−1 PLL) was homogenized against an oil phase (light mineral oil (white, Ameresco), 1.3% (v/v) Span-80 (sorbitan monooleate; TCI)) at a 1:16.7 ratio, then nitrogen purged for 1 minute. In both cases, MPs were then cross-linked under UV light (approximately 10.5 mW cm2–1) in a 35x10 mm petri dish for 10 minutes and washed in water.
MPs were sized using phase microscopy images and ImageJ. For cell studies, MPs were sterilized by incubating in 70% ethanol for 30 minutes followed by three 30 minute PBS washes. For BMP-2 binding study, 0.1 mg heparin and PEG MPs were incubated with 0.1 µg BMP-2 (murine, R&D Systems) overnight in 0.5 mL 0.1% bovine serum albumin (BSA) in PBS. MP solutions were then centrifuged and supernatant was removed. Concentration of BMP-2 in the supernatant was assessed by an enzyme-linked immunosorbent assay (ELISA) (R&D Systems) using kit standards, as per manufacturer’s instructions.
2.3 ATDC5 Cell Culture
ATDC5 cells were expanded in maintenance media (DMEM/F-12 with L-glutamine (Life Technologies), 5% fetal bovine serum (FBS; Atlanta biologics), 100 IU Penicillin (Mediatech), 100 ug mL−1 Streptomycin (Mediatech), 0.25ug mL−1 Amphotericin B1 (Mediatech), 30 nM Sodium Selenite (Sigma-Alrich), and 10 µg mL−1 transferrin (Life Technologies)) and experiments were conducted in mineralization media (maintenance media plus 10 µ g mL−1 insulin (Sigma-Aldrich), 10 mM sodium β-glycerophosphate pentahydrate (Alfa Aesar), and 50 µg mL−1 L-ascorbic acid-2-phosphate sequimmagnesium salt hydrate (Sigma-Aldrich)).
For spheroid studies, cells and MPs were combined at a 3:1 and 1:3 MP:cell ratio to form spheroids via forced aggregation into 400 µm agarose wells (Fig. 1a) [26]. After 18 hours, spheroids were transferred to mineralization media in low adherence dishes (6000 spheroids/plate; BD Biosciences, 10 cm diameter) on rotary culture at 65 RPM. Media was changed every 3 days. For transwell studies, ATDC5 cells were plated at a 6500 cells cm2–1 in 12-well plates in maintenance media until confluent (3–5 days). Once confluent, media was changed to 2.5 mL mineralization media and treatment groups were added. 2 mL of media was removed in each group (to account for 0.5 mL media remaining in transwell insert) and a fresh 2 mL of media was added each day. For MP groups, 3.28 mg MPs were added to each transwell insert.
Figure 1.
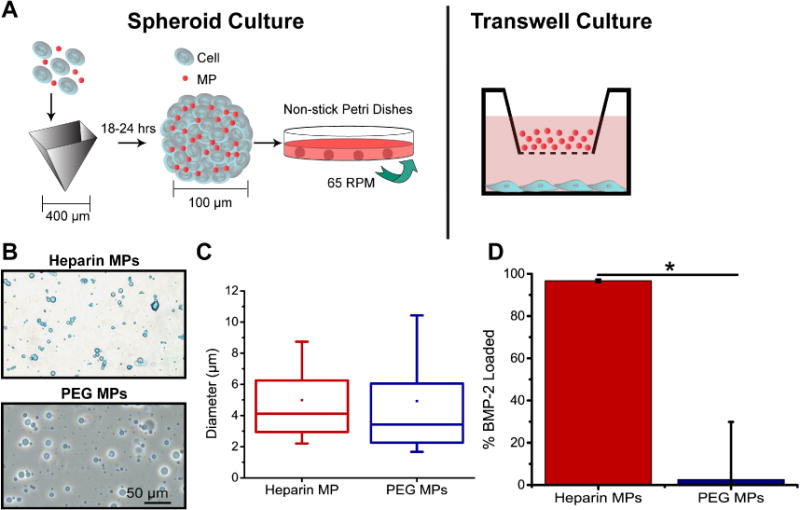
Similarly sized heparin and PEG MPs were fabricated and used in ATDC5 cell culture experiments. (A) MPs were incorporated in ATDC5 cell spheroids by aggregating MPs and single cells into agarose inserts. MPs were also cultured above ATDC5 cells in monolayer transwell culture. (B) Phase microscopy images of heparin (stained with alcian blue) and PEG MPs. (C) Average diameters for heparin and PEG MPs. (D) Percent BMP-2 loaded on heparin MPs vs. PEG MPs normalized to total BMP-2 in soluble control (Data are means +/− SD; *=significantly different than indicated group; p<0.05)
2.4 Histological Analysis
For histological analysis of ATDC5 spheroids, spheroids were imbedded in Histogel, sectioned at 10 µm sections and stained with Safranin-O according to standard protocols. Immunostaining for ECM deposition was performed using primary antibodies for collagen type II (Abcam). Antigen retrieval was performed by incubating in 20 µg mL−1 proteinase K (Sigma-Aldrich) for 10 minutes at 37°C, blocked with 1.5% goat serum (Fisher Scientific), and incubated with the primary antibodies at a 1:20 dilution overnight at 4°C. Secondary antibody binding with Alexa Fluor 488-conjugated goat polyclonal anti-mouse immunoglobulin G (IgG, Molecular Probes) was performed at room temperature for 1 hour. Samples were counterstained with Hoechst (Sigma-Aldrich) to visualize the nuclei.
For alcian blue and alizarin red staining in transwell culture, cell monolayers were rinsed in ddH2O and fixed in 95% ice cold methanol for 30 minutes. Cells were stained with either 1% alcian blue 8GX (Sigma-Aldrich) or 2% alizarin red (Sigma-Aldrich) pH 4.2 in 0.1 M HCl for 1 hour. Cells were then rinsed with ddH2O and imaged. Stain was extracted by incubating stained cells with solutions of 6 M guanidine-HCl (Sigma-Aldrich, alcian blue) or 5% SDS in 0.5M HCl (alizarin red) for 6 hours. Absorbance was measured at 630 and 405 nm for alcian blue and alizarin red extractions, respectively.
2.5 qPCR and DNA Analysis
Spheroids were rinsed in PBS and subjected to lysis buffer for an hour at 4°C before mRNA was extracted using a QIAshredder tissue homogenizer and RNeasy kit with DNase I digestion (Qiagen). For monolayer cells, cells were lifted with 0.05% trypsin and washed once in PBS, then incubated in lysis buffer for 15 minutes. cDNA was generated using SuperScript III Reverse Transcriptase (Invitrogen) with Oligo(dT)15 primers and dNTPs (Promega). Gene expression was analyzed using quantitative PCR amplification performed on a StepOnePlus Real-Time PCR System (Applied Biosystems) in the presence of SYBR Green/ROX master mix (Applied Biosystems). Custom-designed primer sequences for collagen II, aggrecan, collagen X, and RSP-18 (Invitrogen) can be found in Supplementary Table 1.
To analyze PCR amplification data, the raw fluorescence data were processed using LinRegPCR (v12.11; http://www.hartfaalcentrum.nl). The starting amplicon number (No) was calculated based on mean efficiencies (E) and cycle threshold (Ct) using the formula No = Nt/ECt, where Nt is the amplicon number at the cycle threshold. No for each target gene were normalized the starting amplicon numbers of the endogenous controls and then further normalized to no MP controls at each timepoint.
For DNA extraction, cells were trypsinized and then incubated in 1 mg mL−1 Collagenase 1A (Sigma-Aldrich) in Krebs-Ringer buffer (1.8 g L−1 D-glucose, 0.05 g L−1 magnesium chloride (anhydrous), 0.3 g L−1 potassium chloride, 7 g L−1 sodium chloride, 0.1 g L−1 sodium phosphate dibasic (anhydrous),0.2 g L−1 sodium phosphate monobasic (anhydrous), 1.3 g L−1 sodium bicarbonate, 0.03 g L−1 calcium, 10g L−1 BSA) overnight at 37°C in a 24 well plate on rotary. 1 mL 0.1% (v/v) Triton X-100 (Amresco) was added and solution was frozen at −80°C for 1 hour. Samples were then thawed, sonicated on ice for 30 minutes, frozen at −80°C for 1 hour and thawed. Samples were spun down and supernatant was used to assess DNA content with the CyQUANT Assay (ThermoFisher Scientific).
2.6 Microparticle SDS-PAGE
Heparin and PEG MPs that had been incubated with ATDC5 cells in a transwell insert for two days were analyzed via a combination of SDS-PAGE and non-specific-protein staining, a technique previously used to identify proteins of interest bound to microparticles and in nanoparticle protein coronas [35–37]. Heparin or PEG MPs were cultured in transwell with ATDC5 cells or media only for 2, 8, or 10 days. MPs were isolated and washed with PBS. MPs were combined with loading buffer (63 mM Tris-HCl pH 6.8, 350 mM SDS, 6.84 M glycerol, 0.75 mM Bromophenol Blue, 1.78 M β-mercaptoethanol) at a 4:1 MP:buffer ratio, then heated to 90°C for 5 minutes. MPs and a protein ladder (BioRad) were loaded onto a 12% polyacrylamide gel (BioRad) and ran at 200 V for 30–60 minutes. The SDS treatment and voltage difference promoted migration of proteins off the MPs while MPs remained in the wells due to size. The gel was stained with Silver Stain Plus (BioRad) or SYPRO Ruby (Thermo Fisher Scientific) according to the manufacturers’ protocol. The gel bands were excised and in-gel digestion was performed as previously described [38]. The peptides were analyzed with Nano-High Pressure Liquid Chromatography-Tandem Mass Spectrometry(nano-LC-MS/MS; see Supplementary Information for more details).
For IGFBP inhibitor studies, ATDC5 cells were cultured in maintenance media until confluent (3–5 days; see Section 2.3 for more details). Once confluent, media was changed to mineralization media, which included 70 µM NBI 31772 (IGFBP inhibitor; R&D Systems) in treatment groups. Cells were fed daily with fresh media containing the inhibitor.
2.7 Statistical Analysis
All results are depicted as mean ± standard deviation. ANOVA was used to identify significant factors and interactions, then Tukey’s post hoc test (significance level p ≤ 0.05) was used to generate pairwise comparisons between means of individual sample groups and determine statistically significant differences (Minitab 15 Statistical Software).
3. Results
3.1 Heparin and PEG MPs
The percent functionalization of heparin MAm, 3.4kDa PEG-DA and 8kDa PEG-DA was determined to be 15–27%, 58% and 71%, respectively (Supplementary Fig. 1). Heparin MAm MPs (referred to as heparin MPs) and PEG-DA MPs (referred to as PEG MPs and used as a low-binding material control) of similar size were formed (Fig. 1B,C). The average size of heparin and PEG MPs was 5.0±3.1 and 4.9±3.9 µm, respectively. When incubated in a solution of the protein BMP-2, (known to bind heparin [30], produced by ATDC5 cells [32], and implicated in endochondral ossification and chondrogenesis generally [29]), heparin MPs sequestered nearly 100% of the protein, similar to previous results [24], while PEG MPs bound very little (Fig. 1D).
Heparin and PEG MPs were incorporated into ATDC5 cell aggregates (spheroids) at high (3:1 MP:cells), medium (1:1 MP:cells), and low (1:3 MP:cells) ratios. Spheroid sections stained with Safranin-O appeared to contain more heparin MPs as the ratio of MPs:cells was increased (Supplementary Fig. 2A). Incorporation efficiency was similar for both heparin and PEG MPs, but decreased with increasing amounts of MPs so that the 3:1 MP:cell ratio effectively yielded a 2:1 MP:cell ratio (Supplementary Fig. 2B). The 3:1 (high) and 1:3 (low) MP:cell ratios were used for the remainder of the spheroid studies.
3.2 ATDC5 and Heparin MP Spheroid Studies
Heparin MPs were incorporated into ATDC5 cell spheroids and cultured on rotary culture for 18 days (Fig. 1A). All spheroids were observed to increase in size over time and developed a dense cell border (Supplementary Fig. 3). Heparin MPs stained a light purple with H&E staining, while PEG MPs were dehydrated and either appeared as small purple spheres or were lost due to due to sectioning and the solvents used in the staining process, leaving behind holes in the matrix. No differences in spheroid viability were observed in any of the groups at day 18 (Supplementary Fig. 4).
3.2.1 Gene Expression in Spheroid Studies
Markers of cellular differentiation, including gene expression and matrix molecule deposition, were assessed. Overall, heparin MPs reduced chondrocytic gene expression to a greater extent than PEG MPs (Fig. 2). For collagen II, high heparin and PEG MP-containing groups showed significantly lower gene expression than the no MP control at day 6, but the magnitude of the decrease was much greater for the heparin MP group (7.4±1.8- and 1.8±0.2-fold decreased for heparin and PEG, respectively). Collagen II expression in the high heparin-containing MP group was also significantly lower than the low heparin MP group on days 6 and 12, but higher on day 18. For aggrecan, gene expression decreased more in high heparin MP as compared to PEG MP-containing spheroids at day 6 (16.5±6.2- and 2.9±0.2-fold decrease for heparin and PEG, respectively). Furthermore, the significantly decreased expression for aggrecan persisted through day 18 for the high heparin MP-containing group, while increased gene expression was observed in PEG MP-containing groups at later time points. Lower heparin MPs groups appeared similar to no MP-containing control groups. No significant differences were observed for collagen X gene expression in heparin MP groups, while PEG MPs group demonstrated decreased collagen X expression at day 6 and increased collagen X gene expression at later timepoints compared to no MP controls.
Figure 2.
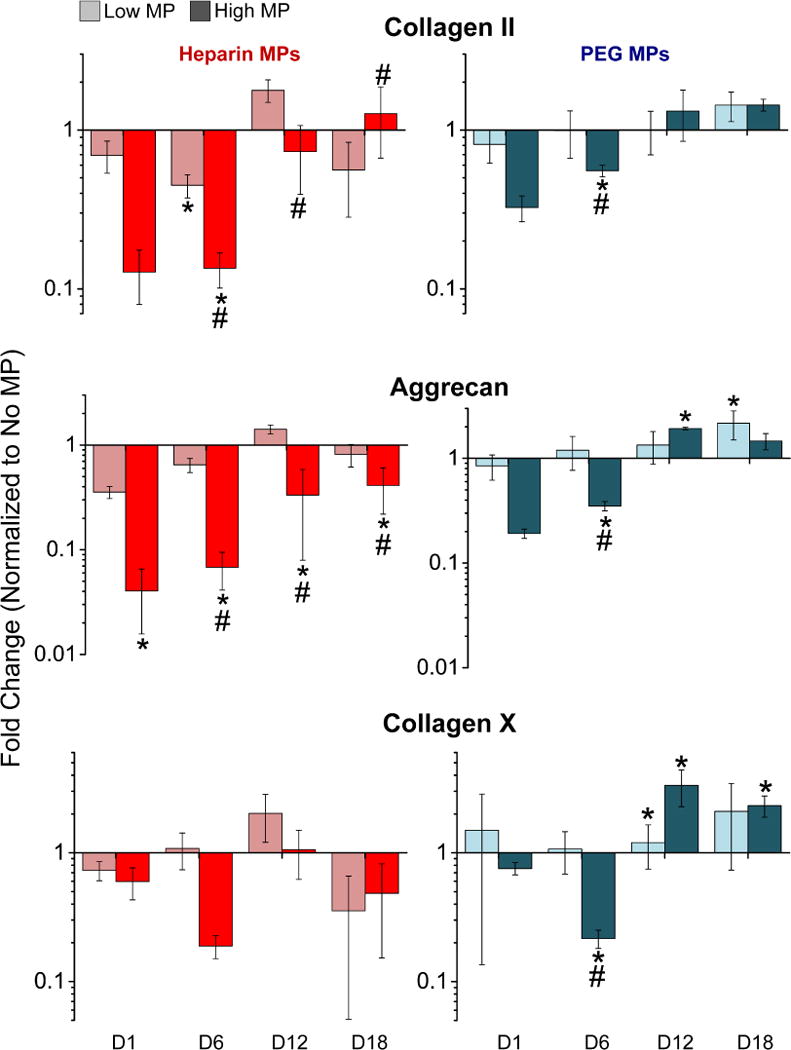
Gene expression of chondrocytic markers was decreased in ATDC5 cell spheroids with heparin MPs as compared to PEG and no MP groups. Fold change normalized to the no MP control is reported for collagen II, aggrecan, and collagen X (N=3 groups of 6000 spheroids; Data are means +/− SD; *=significantly different than no MP on same TP; #=significantly different than low MP on same TP; p<0.05).
3.2.2 Staining for Differentiation Markers in Spheroid Studies
Collagen II staining was not as evident in heparin MP groups at day 12 and in the high heparin MP group at day 18 as compared to no MP and PEG MP-containing controls (Fig. 3A). In addition, endogenous GAG staining was not as obvious in the high heparin MP group as compared to the no MP and PEG MP-containing groups at day 12 (Fig. 3B).
Figure 3.

Lower levels of staining for chondrocytic matrix molecules was observed in heparin MP containing spheroids. (A) Immunohistochemistry staining for collagen II (green) at days 12 and 18 for no MP, heparin MP, and PEG MP groups. Nuclei are shown in blue. (B) Safranin-O staining for GAG deposition (red) in heparin and PEG groups with low and high MPs. Heparin MPs appear dark red and PEG MPs appear as holes (removed in histological sectioning) or light purple. Inset boxes on day 18 are shown at high magnification below original image. Staining for GAG deposited by cells appears pericellularly (arrows) while GAG MPs appear round (arrowheads) (scale bars = 50 µm).
3.3 ATDC5 and Heparin MP Transwell Studies
3.3.1 Gene Expression in Transwell Studies
Heparin and PEG MPs were incubated in transwell culture with ATDC5 cells for 12 days at one MP dose (Fig. 1A). Gene expression of differentiation markers collagen II, aggrecan, and collagen X were downregulated in heparin MP groups (3.6±0.9, 3.0 ±1.2, and 4.0±1.4 fold down regulation, respectively) as compared to no MP controls at day 6, while no differences between PEG MP and no MP control groups were observed (1.2±0.2 fold upregulation, 1.0±0.2 fold upregulation, and 1.2±0.2 fold down-regulation, respectively; Fig. 4A). By day 12, no differences were observed in the high or low heparin or PEG MP groups as compared to no MP groups.
Figure 4.
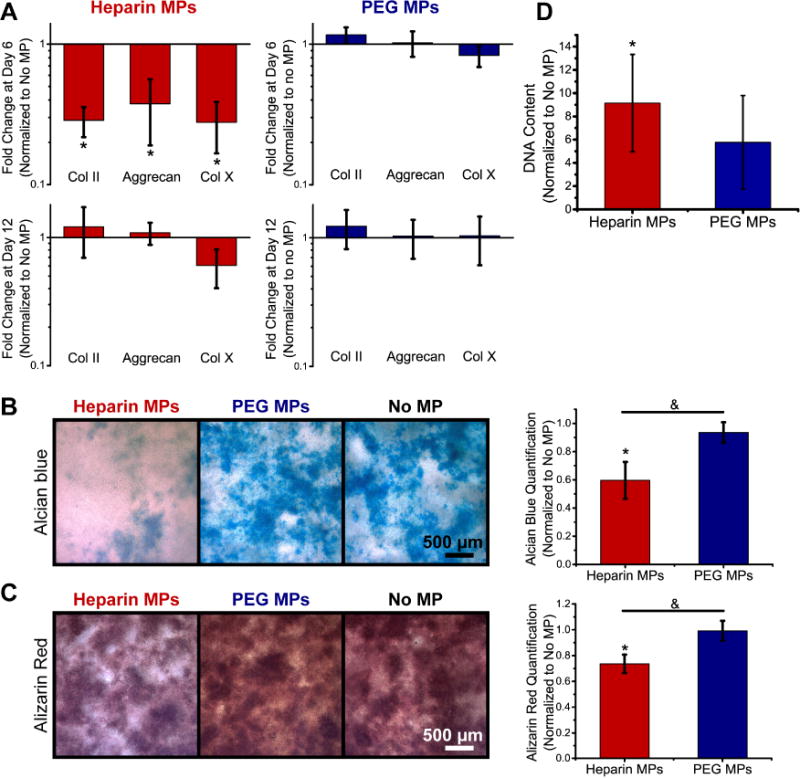
Differentiation markers were decreased in ATDC5 cells cultured in transwell with heparin MPs as compared to PEG and no MP groups. (A) Gene expression for differentiation markers collagen II, aggrecan, and collagen X at days 6 and 12 is reported as fold change normalized to the no MP group. (B) Representative images of GAG (alcian blue) and (C) mineral (alizarin red) deposition at day 12. Graphs show quantification of stain extraction, normalized to the no MP group. (D) Quantification of DNA content for heparin and PEG MP groups normalized to the no MP group (N=4; Data are means +/− SD; *=significantly different than no MP group, &=significantly different than indicated group, p<0.05).
3.3.2 Staining for Differentiation Markers in Transwell Studies
In 2D culture, GAG (alcian blue staining) and mineral deposition (alizarin red staining) was significantly decreased in heparin MP groups compared to no MP and PEG MP controls, as determined through a destaining assay (Fig. 4B,C). Additionally, DNA content was measured and found to increase in heparin MP groups compared to no MP controls (Fig. 4D).
3.4 Heparin MP Sequestered Protein Analysis
In SDS PAGE analysis, heparin MP groups showed protein profiles with multiple dark bands, while PEG MPs showed light bands, primarily in the MW range that would be expected for bovine serum albumin (BSA; ~65 kDa), a major component of the FBS used in cell culture (Fig. 5A). Heparin MPs incubated with cells (media + cell incubation) or in media alone (media incubation) for 2, 8, and 10 days were also analyzed using SDS-PAGE (Fig. 5B). (Note that while Silver Stain was used for Fig. 5A, Sypro Ruby stain was used for Fig. 5B as it is more compatible with mass spectrometry, a technique used to identify proteins. Silver stain can be more sensitive than Sypro Ruby, hence the relatively darker bands in Fig. 5A as compared to Fig. 5B.) Dark bands at 17 and 35 kDa were observed for heparin MPs incubated with cells, unlike the heparin MPs incubated with media alone. When analyzed with LC-MS/MS, a variety of bovine and murine proteins were identified, but most prominent were IGFBP-3 and 5, found in both the 17 and 35 kDa bands (Supplementary Fig. 5 and Tables 2–28).
Figure 5.
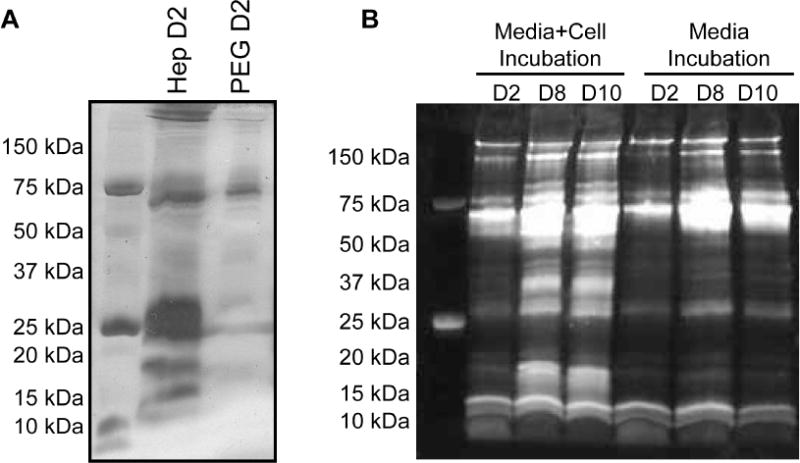
Heparin MPs were able to sequester cell-secreted proteins. (A) SDS-PAGE stained with silver stain for heparin and PEG MPs incubated in transwell with ATDC5 cells for 2 days. (B) SDS-PAGE stained with SYPRO Ruby for heparin MPs incubated overnight in transwell with cells (left, Media + Cell Incubation) or with media alone (right, Media Incubation) for 2, 8 and 10 days (N=3).
3.5 Effect of IGFBP Inhibitor on ATDC5 Cell Differentiation
When cultured with a small molecule inhibitor of IGFBP, less staining was observed in ATDC5 cells at day 12 for both GAG (alcian blue) and mineral (alizarin red) deposition, which was statistically significant when analyzed via a stain extraction assay (Fig. 6A). Additionally, the total cell number remained the same as indicated by no changes in DNA content in groups with and without the inhibitor (Fig. 6B).
Figure 6.
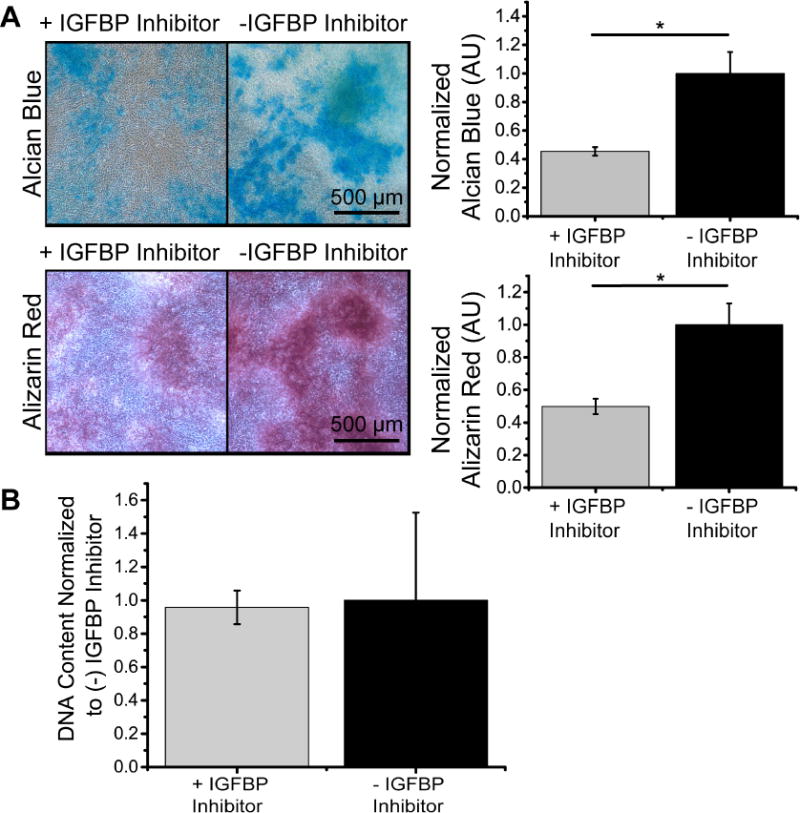
IGFBP inhibitor exhibited decreased cellular differentiation at day 12. (A) Representative images of GAG (alcian blue) and mineral (alizarin red) deposition for groups with and without an IGFBP inhibitor. Graphs show quantification of stain extraction, normalized to (−) IGFBP inhibitor group. (B) Quantification of DNA content for groups with and without IGFBP normalized to the (−) IGFBP group (N=4; Data are means +/− SD; *=significantly different than media only group, p<0.05).
4. Discussion
Overall, the objective of this study was to modulate cellular differentiation through heparin MP-mediated endogenous protein sequestration. ATDC5 cells in spheroid and monolayer culture were used as a model system upon which to test this technology, as these cells are known to undergo endchondral ossification and to secrete a variety of heparin-binding factors during differentiation, some of which include BMPs, FGFs, and IHH [32]. Heparin and PEG MPs (used as a low-binding material control; Fig. 1) were incorporated into ATDC5 spheroids to assess the influence of heparin MPs on differentiation. In high heparin MP spheroids, gene expression for differentiation markers collagen II and aggrecan was downregulated as compared to PEG MP and no MP-containing groups. Only subtle differences were observed for the low heparin MP-containing spheroids, suggesting that the observed changes in differentiation were dose-dependent. Interestingly, gene expression for the high heparin MP group was similar to that of the no MP-containing group by later time points, suggesting that the effect of heparin MPs was transient (Fig. 2). Decreased staining for collagen II and GAG was observed in the high heparin MP group as compared to PEG MP and no MP control groups, confirming trends observed in gene expression data (Fig. 3). Taken as a whole, these results indicate that heparin MPs transiently delayed or reduced endochondral ossification in a dose-dependent manner, possibly due to sequestration of cell-secreted soluble factors by the heparin MPs.
In order to further investigate the hypothesis that heparin-mediated soluble factor sequestration was responsible for changes in differentiation, a transwell culture system was employed, allowing for exchange of soluble factors but no physical contact between cells and MPs. Similar to what was observed in spheroid culture, heparin MPs reduced or delayed differentiation in ATDC5 cell transwell culture, which was not due to decreased cell number in the heparin MP groups (Fig. 4). In heparin MP groups, cell count appeared to be higher than in no MP groups, which could suggest that cells proliferated rather than differentiated (Fig. 4D), but more experiments would need to be performed to confirm this hypothesis. As was observed in spheroid culture, gene expression in heparin MP groups returned to baseline at later timepoints (Fig. 2 and 4A), suggesting that heparin MPs modulated differentiation in a temporally controlled fashion. Overall, as heparin MPs were able to effect these changes without physically contacting the cells, this set of experiments supports the hypothesis that the impediment of differentiation was due to sequestration of soluble factors.
To further confirm this hypothesis, proteins bound by heparin and PEG MPs from days 2 and 4 in transwell culture were run on SDS-PAGE gels, and observed protein profiles showed that PEG MPs bound fewer proteins than heparin MPs (Fig. 5A.), indicating that heparin MPs were able to bind protein from the culture environment. However, in these experiments, there were two possible sources for the proteins in the culture environment: cell-secreted proteins or serum proteins (from the culture media). A variety of serum proteins, including fibronectin, fibrinogen, kininogen, plasminogen, and lipoproteins [39–43], are known to bind heparin with strong affinity and some of these proteins have been shown to bind heparin MPs both in previous work and this study (Supplementary Tables 2–28) [37].
To investigate the source and identity of sequestered proteins, heparin MPs incubated with cells or in media alone were run on SDS-PAGE. Resulting protein profiles indicated that heparin MPs incubated with cells bound additional 17 and 35 kDa proteins in comparison to heparin MPs incubated in media alone (Fig. 5B), indicating binding of cell-secreted protein. In addition, the quantity of these proteins appeared to increase between days 2 and 8, but not between days 8 and 10. If protein bound to heparin MPs did increase over time, it is possible that heparin MPs became saturated with a particular set of proteins, which could be a result of the relative affinity between heparin and various proteins in the environment. This hypothesized mechanism could explain why heparin MP mediated delays in ATDC5 cell differentiation was dose-dependent and why a transient delay in differentiation was observed. Overall, these results provided further evidence that the heparin MPs likely sequestered cell-secreted protein in a manner that affected cell differentiation.
To further identify proteins sequestered by heparin MPs, mass spectrometry analysis of excised 17 and 35 kDa bands was performed. The most prominent proteins identified were IGFBP-3 and 5. IGFBPs are known to bind IGF-1 and 2 with high affinity and act as carrier proteins that increase the half-life of IGF and prevent IGF binding to its cell surface receptor, though some evidence indicates that they may facilitate receptor-ligand binding at times [44,45]. In order to release free IGF, proteases break IGFBPs into smaller fragments, ranging from 17–22 kDa [46]. It is likely that these fragments (in the case of IGFBP-3, 40–50 kDa) [47] or whole proteins (in the case of IGFBP-5, 28–35 kDa) [47] were observed in the SDS-PAGE protein profiles.
While other proteins were identified to be bound to heparin MPs in the mass spectrometry analysis (pleiotrophin, procollagen C-endopeptidase enhancer, histone, thrombospondin, collagens I, II, and III, etc.; Supplementary Tables 2–28), IGFBPs were very prominent (based on peptide spectra matches (PSM)) throughout all samples. Based on the role of IGFs in ATDC5 cell differentiation, IGFBPs were the sequestered protein hypothesized to be most likely to affect differentiation. In ATDC5 cells, IGF is known to enhance proliferation and differentiation [48]. This suggests that the binding of IGFBP by heparin may have reduced IGF signaling and downstream differentiation by 1) inhibiting IGFBP-IGF binding and reducing IGF half-life or 2) decreasing the effectiveness of IGF binding to its cell surface receptor. Alternatively, because there is evidence that lower concentrations of IGF are more potent at stimulating ATDC5 cell differentiation [48], it is possible that the binding of IGFBP by heparin may have released additional IGF and promoted proliferation, rather than differentiation.
In order to further confirm that heparin MP-mediated sequestration of IGFBPs caused the changes in differentiation observed, a small molecule known to inhibit IGFBP by displacing IGF (but not to interact with IGF receptors) [49], was added to ATDC5 cell culture media. Similar to the effect of heparin MPs on ATDC5 cells, a reduction in cellular differentiation was observed in groups containing the inhibitor (Fig. 6). While it is possible that the inhibitor could affect the cells independently of IGFBP blocking due to off-target effects, these experiments suggest that heparin MPs may bind and inhibit IGFBP in a manner similar to the small molecule inhibitor and support the hypothesis that heparin MPs inhibit cell differentiation through binding of cell-secreted protein. While the ability of heparin MPs to reduce cellular differentiation through sequestration of IGFBP may be specific to the ATDC5 cell line, these studies highlight the utility of heparin MPs for interrogating the role of secreted proteins in the cellular microenvironment.
This work shows the potential of heparin-based materials to modulate cellular differentiation through sequestration of cell-secreted protein, a novel tool to control differentiation. In previous studies, heparin or heparan sulfate have been speculated to bind proteins in the local microenvironment or shown to bind protein added exogenously to the culture system [4,7,50–56], but this work highlights the potential for heparin MPs to be used as a tool to control cell differentiation directly through sequestration of cell-secreted protein. In addition, heparin MPs are advantageous over soluble GAGs, as they are easier to localize within cellular systems. While other studies have used protein-specific antibody or peptide-based sequestration strategies to affect cellular differentiation [5,57–60], GAG-based sequestration systems may be particularly advantageous in therapeutic applications, as they can bind many (rather than one) endogenously-produced proteins implicated in evolving and time-dependent differentiation processes [30]. For example, heparin MPs could be employed to sequester osteogenic proteins to reduce early suture closure in the case of craniosynostosis [57,61] or to delay cellular differentiation in growth plate repair to prevent bone bridge formation [62,63]. Given the ability of heparin MPs to downregulate collagen X expression in this cell line (Fig. 2 and 4A), these particles may be further investigated for their ability to delay or restrict chondrocyte hypertrophy, which is a major challenge in cell therapies requiring chondrocytic differentiation [33]. Finally, as we have shown with our sequestered protein analysis techniques, GAG-based MPs could be used experimentally as a “first pass” to understand how cell-secreted protein sequestration affects a system of interest, and analysis of sequestered protein could inform design of future, protein-specific sequestration systems.
5. Conclusions
In summary, we have developed a technique by which cellular differentiation can be modulated solely through the introduction of a GAG-based biomaterial. Through sequestration of cell-secreted proteins, heparin MPs can be used to direct cell behavior without delivering exogenous protein, reducing cost while increasing efficiency in tissue regeneration applications. In addition, heparin may be therapeutically advantageous over other protein-specific affinity systems, as it can sequester multiple growth factors involved in differentiation processes. Experimentally, this technique could also potentially be used to identify proteins produced in the local microenvironment, illuminating new protein pathways to target in future applications requiring control of cellular differentiation for a wide range of regenerative medicine applications.
Supplementary Material
Statement of Significance.
In this work, we present a proof-of-principle set of experiments in which heparin-based microparticles are shown to modulate cellular differentiation through binding of cell-secreted protein. Unlike existing systems that rely on expensive protein with limited half-lives to elicit changes in cellular behavior, this technique focuses on temporal modulation of cell-generated proteins. This technique also provides a biomaterials-based method that can be used to further identify sequestered proteins of interest. Thus, this work indicates that glycosaminoglycan-based biomaterial approaches could be used as substitutes or additions to traditional methods for modulating and identifying the cell-secreted proteins involved in directing cellular behavior.
Acknowledgments
We wish to acknowledge the core facilities at the Parker H. Petit Institute for Bioengineering and Bioscience at the Georgia Institute of Technology for the use of their shared equipment, services, and expertise, as well as Liane Tellier for her assistance with polymer characterization.
Funding Sources
This study was supported with funding from the National Science Foundation (NSF) Graduate Research Fellowship (DGE-1148903) to TER, the Georgia Tech Petit Scholar Program to BDP, NSF (DMR 1207045), and National Institutes of Health Award Number R01AR062006. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindahl U, Li J. Int Rev Cell Mol Biol. 1st. Elsevier Inc; 2009. Chapter 3 Interactions Between Heparan Sulfate and Proteins— Design and Functional Implications; pp. 105–159. [DOI] [PubMed] [Google Scholar]
- 2.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 3.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–70. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 4.Lohmann N, Schirmer L, Atallah P, Wandel E, Ferrer RA, Werner C, Simon JC, Franz S, Freudenberg U. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci Transl Med. 2017;9:eaai9044. doi: 10.1126/scitranslmed.aai9044. [DOI] [PubMed] [Google Scholar]
- 5.Lin CC, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials. 2009;30:4907–14. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jha AK, Tharp KM, Ye J, Santiago-Ortiz JL, Jackson WM, Stahl A, Schaffer DV, Yeghiazarians Y, Healy KE. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials. 2015;47:1–12. doi: 10.1016/j.biomaterials.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seto SP, Casas ME, Temenoff JS. Differentiation of mesenchymal stem cells in heparin-containing hydrogels via coculture with osteoblasts. Cell Tissue Res. 2012;347:589–601. doi: 10.1007/s00441-011-1265-8. [DOI] [PubMed] [Google Scholar]
- 8.Freudenberg U, Liang Y, Kiick KL, Werner C. Glycosaminoglycan-Based Biohybrid Hydrogels: A Sweet and Smart Choice for Multifunctional Biomaterials. Adv Mater. 2016;28:8861–8891. doi: 10.1002/adma.201601908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller T, Goude MC, McDevitt TC, Temenoff JS. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014;10:1705–19. doi: 10.1016/j.actbio.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakiyama-Elbert SE. Incorporation of heparin into biomaterials. Acta Biomater. 2014;10:1581–7. doi: 10.1016/j.actbio.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58:1379–408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014;10:1588–1600. doi: 10.1016/j.actbio.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakiyama-Elbert SE. Incorporation of heparin into biomaterials. Acta Biomater. 2014;10:1581–7. doi: 10.1016/j.actbio.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai B, Nurcombe V, Cool SM. Heparan sulfate-based treatments for regenerative medicine. Crit Rev Eukaryot Gene Expr. 2011;21:1–12. doi: 10.1615/critreveukargeneexpr.v21.i1.10. http://www.ncbi.nlm.nih.gov/pubmed/21967329. [DOI] [PubMed] [Google Scholar]
- 15.Zieris A, Prokoph S, Levental KR, Welzel PB, Grimmer M, Freudenberg U, Werner C, Welzel PB, Grimmer M, Freudenberg U, Werner C. FGF-2 and VEGF functionalization of starPEG-heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials. 2010;31:7985–94. doi: 10.1016/j.biomaterials.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Watarai A, Schirmer L, Thönes S, Freudenberg U, Werner C, Simon JC, Anderegg U. TGFβ functionalized starPEG-heparin hydrogels modulate human dermal fibroblast growth and differentiation. Acta Biomater. 2015;25:65–75. doi: 10.1016/j.actbio.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Park JS, Woo DG, Yang HN, Lim HJ, Chung HM, Park KH. Heparin-bound transforming growth factor-beta3 enhances neocartilage formation by rabbit mesenchymal stem cells. Transplantation. 2008;85:589–596. doi: 10.1097/TP.0b013e3181639b3a. [DOI] [PubMed] [Google Scholar]
- 18.Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release. 2011;154:258–266. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhakta G, Rai B, Lim ZXH, Hui JH, Stein GS, van Wijnen AJ, Nurcombe V, Prestwich GD, Cool SM. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials. 2012;33:6113–6122. doi: 10.1016/j.biomaterials.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Il Choi W, Tae G, Kim YH, Kang SS, Kim SE, Kim S-H, Jung Y, Kim SH. Enhanced regeneration of the ligament–bone interface using a poly(l-lactide–co-ε-caprolactone) scaffold with local delivery of cells/BMP-2 using a heparin-based hydrogel. Acta Biomater. 2011;7:244–257. doi: 10.1016/j.actbio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–7. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 22.Cortes M, Baria AT, Schwartz NB. Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signaling in the developing growth plate. Development. 2009;136:1697–706. doi: 10.1242/dev.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sebinger DDR, Ofenbauer A, Gruber P, Malik S, Werner C. ECM modulated early kidney development in embryonic organ culture. Biomaterials. 2013;34:6670–82. doi: 10.1016/j.biomaterials.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE, McDevitt TC. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 2014;35:7228–7238. doi: 10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahoney MJ, Saltzman WM. Transplantation of brain cells assembled around a programmable synthetic microenvironment. Nat Biotechnol. 2001;19:934–9. doi: 10.1038/nbt1001-934. [DOI] [PubMed] [Google Scholar]
- 26.Bratt-Leal AM, Carpenedo RL, Ungrin MD, Zandstra PW, McDevitt TC. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32:48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goude MC, McDevitt TC, Temenoff JS. Chondroitin Sulfate Microparticles Modulate Transforming Growth Factor-β 1 -Induced Chondrogenesis of Human Mesenchymal Stem. Cells Tissues Organs. 2014:1–14. doi: 10.1159/000365966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann JA, Hettiaratchi MH, McDevitt TC. Enhanced Immunosuppression of T Cells by Sustained Presentation of Bioactive Interferon-γ Within Three-Dimensional Mesenchymal Stem Cell Constructs. Stem Cells Transl Med. 2017;6:223–237. doi: 10.5966/sctm.2016-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 30.Billings PC, Pacifici M. Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: mechanisms and mysteries, Connect. Tissue Res. 2015;56:272–280. doi: 10.3109/03008207.2015.1045066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atsumi T, Miwa Y, Kimata K, Ikawa Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev. 1990;30:109–16. doi: 10.1016/0922-3371(90)90079-c. http://www.ncbi.nlm.nih.gov/pubmed/2201423. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Fink T, Zhang X-Y, Ebbesen P, Zachar V. Quantitative transcriptional profiling of ATDC5 mouse progenitor cells during chondrogenesis. Differentiation. 2005;73:350–63. doi: 10.1111/j.1432-0436.2005.00038.x. [DOI] [PubMed] [Google Scholar]
- 33.Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic Differentiation of Mesenchymal Stem Cells: Challenges and Unfulfilled Expectations. Tissue Eng Part B Rev. 2014;20:596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn MS, Taite LJ, Moon JJ, Rowland MC, Ruffino KA, West JL. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 2006;27:2519–24. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 35.Belair DG, Murphy WL. Specific VEGF sequestering to biomaterials: influence of serum stability. Acta Biomater. 2013;9:8823–31. doi: 10.1016/j.actbio.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105:14265–70. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hettiaratchi MH, Chou C, Servies N, Smeekens JM, Cheng A, Esancy C, Wu R, McDevitt TC, Guldberg RE, Krishnan L. Competitive Protein Binding Influences Heparin-Based Modulation of Spatial Growth Factor Delivery for Bone Regeneration. Tissue Eng Part A. 2017 doi: 10.1089/ten.tea.2016.0507. ten.tea.2016.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2007;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 39.Zheng X, Baker H, Hancock WS, Fawaz F, McCaman M, Pungor E. Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnol Prog. 2006;22:1294–1300. doi: 10.1021/bp060121o. [DOI] [PubMed] [Google Scholar]
- 40.Fredenburgh JC, Leslie Ba, Stafford AR, Lim T, Chan HH, Weitz JI. Zn2+ Mediates High Affinity Binding of Heparin to the C Domain of Fibrinogen. J Biol Chem. 2013;288:29394–29402. doi: 10.1074/jbc.M113.469916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peysselon F, Ricard-Blum S. Heparin-protein interactions: from affinity and kinetics to biological roles. Application to an interaction network regulating angiogenesis. Matrix Biol. 2014;35:73–81. doi: 10.1016/j.matbio.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Andrade-Gordon P, Strickland S. Interaction of heparin with plasminogen activators and plasminogen: effects on the activation of plasminogen. Biochemistry. 1986;25:4033–4040. doi: 10.1074/jbc.M110.108720. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi M, Yamada KM. Divalent cation modulation of fibronectin binding to heparin and to DNA. J Biol Chem. 1982;257:5263–5267. [PubMed] [Google Scholar]
- 44.Beattie J, Phillips K, Shand JH, Szymanowska M, Flint DJ, Allan GJ. Molecular recognition characteristics in the insulin-like growth factor (IGF)-insulin-like growth factor binding protein -3/5 (IGFBP-3/5) heparin axis. J Mol Endocrinol. 2005;34:163–175. doi: 10.1677/jme.1.01656. [DOI] [PubMed] [Google Scholar]
- 45.Forbes BE, McCarthy P, Norton RS. Insulin-like growth factor binding proteins: A structural perspective. Front Endocrinol (Lausanne) 2012;3:1–13. doi: 10.3389/fendo.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem J. 2006;395:1–19. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R&DSystemsIncorporated, R&D Systems, (2016). https://www.rndsystems.com/ (accessed October 8, 2016).
- 48.Phornphutkul C, Wu KY, Yang X, Chen Q, Gruppuso PA. Insulin-like growth factor-I signaling is modified during chondrocyte differentiation. J Endocrinol. 2004;183:477–486. doi: 10.1677/joe.1.05873. [DOI] [PubMed] [Google Scholar]
- 49.Liu XJ, Xie Q, Zhu YF, Chen C, Ling N. Identification of a Nonpeptide Ligand That Releases Bioactive Insulin-like Growth Factor-I from Its Binding Protein Complex. J Biol Chem. 2001;276:32419–32422. doi: 10.1074/jbc.C100299200. [DOI] [PubMed] [Google Scholar]
- 50.Ayerst BI, Smith RAA, Nurcombe V, Day AJ, Merry CLR, Cool S. GDF5 Mediated Enhancement of Chondrocyte Phenotype is Inhibited by Heparin; Implication for the use of Heparin in the Clinic and in Tissue Engineering Applications. Tissue Eng Part A. 2016 doi: 10.1089/ten.TEA.2016.0364. ten.TEA.2016.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jha AK, Tharp KM, Ye J, Santiago-Ortiz JL, Jackson WM, Stahl A, Schaffer DV, Yeghiazarians Y, Healy KE. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials. 2015;47:1–12. doi: 10.1016/j.biomaterials.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin R, Moreira Teixeira LS, Dijkstra PJ, Van Blitterswijk CA, Karperien M, Feijen J. Chondrogenesis in injectable enzymatically crosslinked heparin/dextran hydrogels. J Control Release. 2011;152:186–195. doi: 10.1016/j.jconrel.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 53.Lei J, Trevino E, Temenoff J. Cell number and chondrogenesis in human mesenchymal stem cell aggregates is affected by the sulfation level of heparin used as a cell coating. J Biomed Mater Res Part A. 2016;104:1817–1829. doi: 10.1002/jbm.a.35713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson RA, McDonald MM, Nurcombe V, Little DG, Cool SM. The use of heparan sulfate to augment fracture repair in a rat fracture model. J Orthop Res. 2006;24:636–644. doi: 10.1002/jor.20103. [DOI] [PubMed] [Google Scholar]
- 55.Benoit DSW, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 56.Kim M, Kim SE, Kang SS, Kim YH, Tae G. The use of de-differentiated chondrocytes delivered by a heparin-based hydrogel to regenerate cartilage in partial-thickness defects. Biomaterials. 2011;32:7883–96. doi: 10.1016/j.biomaterials.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 57.Mooney MP, Losken HW, Moursi AM, Bradley J, Azari K, Acarturk TO, Cooper GM, Thompson B, Opperman La, Siegel M. Anti-TGF-beta2 antibody therapy inhibits postoperative resynostosis in craniosynostotic rabbits. Plast Reconstr Surg. 2007;119:1200-12-5. doi: 10.1097/01.prs.0000258403.49584.ec. [DOI] [PubMed] [Google Scholar]
- 58.Moursi AM, Winnard PL, Fryer D, Mooney MP. Delivery of transforming growth factor-beta2-perturbing antibody in a collagen vehicle inhibits cranial suture fusion in calvarial organ culture. Cleft Palate Craniofac J. 2003;40:225–32. doi: 10.1597/1545-1569(2003)040<0225:DOTGFA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 59.Impellitteri NA, Toepke MW, Lan Levengood SK, Murphy WL. Specific VEGF sequestering and release using peptide-functionalized hydrogel microspheres. Biomaterials. 2012;33:3475–84. doi: 10.1016/j.biomaterials.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin CC, Anseth KS. Controlling Affinity Binding with Peptide-Functionalized Poly(ethylene glycol) Hydrogels. Adv Funct Mater. 2009;19:2325. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen K, Krakora SM, Cunningham M, Singh M, Wang X, Hu FZ, Post JC, Ehrlich GD. Medical treatment of craniosynostosis: recombinant Noggin inhibits coronal suture closure in the rat craniosynostosis model. Orthod Craniofac Res. 2009;12:254–62. doi: 10.1111/j.1601-6343.2009.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarty RC, Xian CJ, Gronthos S, Zannettino ACW, Foster BK. Application of autologous bone marrow derived mesenchymal stem cells to an ovine model of growth plate cartilage injury. Open Orthop J. 2010;4:204–10. doi: 10.2174/1874325001004010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung R, Foster BK, Xian CJ. Preclinical studies on mesenchymal stem cell-based therapy for growth plate cartilage injury repair. Stem Cells Int. 2011;2011:570125. doi: 10.4061/2011/570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


