Abstract
Introduction
Past studies have shown that a large portion of individuals with Parkinson’s disease (PD) and mild cognitive impairment (MCI) will revert to a cognitively intact (CI) status in the future. Aging studies have shown that individuals who revert from MCI to CI are at increased risk for reconverting to MCI or dementia in the future. The current study examined if individuals who revert from PD-mild cognitive impairment (PD-MCI) to CI will be at increased risk for future PD-MCI and Parkinson’s disease dementia (PDD).
Method
The study utilized data from the Parkinson’s Progression Markers Initiative (PPMI). The sample included 364 newly diagnosed PD participants who were followed annually for up to 4 years. Based on the first and second assessments, we identified individuals who were CI at each assessment (CI-Stable) and individuals who were PDMCI at baseline but then reverted to CI (Reversion). Analyses examined if participants in the Reversion group were at greater risk, relative to the CI-Stable group, for cognitive impairment at future assessments.
Results
Participants in the Reversion group were at greater risk for future cognitive impairment (PD-MCI or PDD) at the 2nd, 3rd and 4th annual follow-up, relative to the CI-Stable group. The Reversion group continued to be at increased risk for future cognitive impairment when adjusting for age, gender, education, depressive symptoms, and motor severity.
Conclusion
A large proportion of individuals with PD-MCI will not show evidence of cognitive impairment within a year. However, these “reverters” continue to be at risk for future development of cognitive impairment.
INTRODUCTION
Parkinson’s disease (PD) is a common neurodegenerative disorder consisting of both motor and non-motor symptoms, including cognitive impairment. For over a decade, research has explored the concept of identifying non-demented PD patients who show evidence of mild cognitive impairment (PD-MCI) and are at risk for developing Parkinson’s disease dementia (PDD) in the future [1,2]. Although there have been relatively few longitudinal studies of PD-MCI, findings have generally shown that individuals with PD-MCI are at greater risk for developing PDD relative to cognitively intact (CI) PD patients [3,4]. Specifically, 20–60% of individuals with PD-MCI will develop PDD within a span 2 to 5 years post-PD-MCI diagnosis, with conversion rates being higher among older individuals with a greater duration of PD symptoms [1,5,6].
While the concept of MCI has been shown to be useful in identifying individuals at risk for future dementia, both in PD as well as other populations, MCI has been criticized for instability over time [7]. The longitudinal Norwegian ParkWest study showed that approximately 25% of individuals with PD-MCI no longer met criteria for PD-MCI after 1 year [5]. A similar pattern has been shown in aging studies. Population-based studies show that approximately 13–16% of non-PD individuals with MCI will revert to a cognitively intact status within a year [8–10]. These findings may suggest that the risk of transitioning from MCI to CI may be similar to the rate (2–31%) of transitioning from MCI to Alzheimer’s dementia (AD) [8].
Despite the fact that a large portion of individuals with MCI may revert to a CI status, these “reverters” may still be at an increased risk for developing dementia in the future. At least two longitudinal studies of non-PD elderly individuals have shown that reverters (individuals who met criteria for MCI at an initial visit, but were cognitively intact at a follow-up visit) were at increased risk (hazard ratios = 5.22 to 6.56) of reconverting to MCI or dementia at a future visit relative to those who never met criteria for MCI [9,10].
Given the evidence that MCI diagnoses may be unstable and individuals who revert from MCI to CI may be at risk for future cognitive decline, the aim of the current study was to examine if PD patients who revert from PD-MCI to CI are at increased risk for future PD-MCI and PDD relative to individuals who were CI and never met criteria for PD-MCI.
METHODS
Study Design
Data were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). Data was downloaded from the PPMI repository on April 11th, 2017. The PPMI is a longitudinal multi-site study of newly diagnosed (two years or less), untreated PD patients. Further details of the study have been published [11]. The study was approved by the institutional review board at each site and participants provided written informed consent.
The current sample included 364 individuals newly diagnosed with PD, who were followed for at least 2 years and up to 4 years (baseline, 1st, 2nd, 3rd and 4th annual follow-up). We excluded non-PD controls and PD patients who scored <22 on the Montreal Cognitive Assessment (MoCA) within the first two annual assessments.
Cognitive Status
Participants completed the MoCA and were classified as CI, PD-MCI, or PDD at each annual assessment. Cognitive status was determined based on the following criteria: CI if scored >26 on the MoCA, PD-MCI if scored between 26 and 23, and PDD if the MoCA was ≤22 [12,13]. It is important to note that PD-MCI was not determined based on the five neurocognitive tests administered as part of the PPMI. This decision was made a priori because 1) classifications based on one test per domain would reduce the ability to detect impairment, which severely increases the chance of committing type II error, and 2) measures of functional status were not administered until part way through the study, thus we would not be able to differentiate PD-MCI from PDD (as PDD requires evidence of functional impairment) without significantly reducing the sample size.
Based on participants’ cognitive status at baseline and year-1 follow-up, we identified 4 groups: individuals who were CI at the first two assessments (CI-Stable), those who were CI at baseline but converted to PD-MCI at the second assessment (Conversion), individuals who met criteria for PD-MCI at each assessment (PD-MCI-Stable), and individuals who met criteria for PD-MCI at baseline, but reverted to CI at the second assessment (Reversion).
Depression and Motor Severity
Symptoms of depression were assessed with the 15 item Geriatric Depression Scale (GDS). The GDS is a self-report questionnaire for older adults, where participants rate the presence or absence of depressive symptoms over the previous week. Total scores range from 0–15, with a score of ≥5 indicating clinically significant depression in PD [14].
Severity of motor symptoms were assessed with the Unified Parkinson’s Disease Rating Scale, part III (UPDRS-III).
Statistical Analyses
Ordinal regressions were computed to examine if individuals in the Conversion, PD-MCI-Stable group, or Reversion (relative to the CI-Stable group) were at increased risk for PD-MCI or PDD at future assessments. Specifically, in the unadjusted models, cognitive status at the 2-year follow-up (CI, PD-MCI or PDD) was entered as the dependent variable, and the four Conversion/Reversion/CI-Stable/MCI-Stable groups were entered as the independent variable. The CI-Stable group was set as the reference group, therefore risk of being classified as PD-MCI or PDD at a future assessment was relative to this group. Analyses were repeated with cognitive status at the 3-year and 4-year follow-up entered as the dependent variable.
In addition to the unadjusted models described above, we repeated the above analyses, but also included baseline age (years), gender, education (years), depressive symptoms (total GDS scores), and motor severity (total UPDRS-III scores) in the model.
RESULTS
Sample Characteristics
Sample characteristics are shown in Supplemental Table 1. At baseline, 257 participants (70.6%) were CI and 107 (29.4%) met criteria for PD-MCI. The distributions of baseline MoCA scores for each group are displayed in the Supplemental Figure. At the first annual follow-up, 184 (50.5%) participants were CI at both assessments (i.e. CI-Stable), 73 (20.1%) converted from CI to MCI (Conversion group), 65 (17.9%) met criteria for PD-MCI at both assessments (PD-MCI-Stable), and 42 (11.5%) participants met criteria for PD-MCI at baseline but then reverted to CI at the second assessment (Reversion group).
Regarding attrition between the 3rd and 4th annual follow-ups, of the 364 participants who were followed for the first 2 years (baseline, 1st and 2nd annual follow-ups), 302 (83.0%) returned for the 3rd annual assessment, and 262 (72.0%) returned for the 4th annual assessment. Participants who were not available at the 3rd annual assessment were more likely to have less severe motor symptoms (i.e., lower UPDRS-III scores) than individuals who were retained (t(362) = 3.38, p = 0.001), but otherwise did not differ in age, gender, education, GDS scores, or MoCA scores at baseline (all p values > 0.05). Participants who were not available at the 4th annual assessment were more likely to have more depressive symptoms (i.e., higher GDS scores; t(141) = 2.08, p = 0.039), but did not differ in age, gender, education, UPDRS-III scores, or MoCA scores at baseline (all p values > 0.05).
Unadjusted Odds of PD-MCI and PDD at Follow-Up
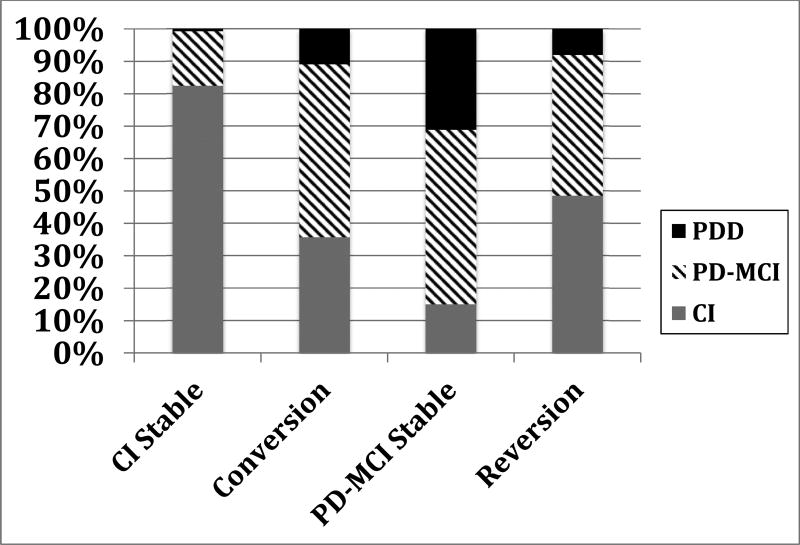
At the 2-year follow-up, 217 (59.6%) of the sample were CI, 122 (33.5%) met criteria for PD-MCI, and 25 (6.9%) met criteria for PDD. An ordinal regression revealed significant group differences in cognitive status at the 2-year follow-up (χ2 = 127.9, df = 3, p < 0.001; Figure 1). Specifically, participants in the PD-MCI-Stable (odds ratio; OR = 26.31, 95% CI = 13.3 – 51.9; p <0.001), Conversion (OR = 9.45, 95% CI = 5.1 – 17.4; p <0.001) or Reversion groups (OR = 7.27, 95% CI = 3.6 – 14.9; p <0.001) were more likely to be classified as PD-MCI or PDD at the 2-year follow-up relative to the CI-Stable group.
Figure 1.
Cognitive status at 2-year Follow-up by Group. CI = Cognitively Intact; PD-MCI = Parkinson’s Disease Mild Cognitive Impairment; PDD = Parkinson’s Disease Dementia.
The proportion of participants meeting criteria for CI, PD-MCI and PDD at years 3 and 4 are shown in Supplemental Table 2. Analyses of cognitive status at the 3rd and 4th year follow-up revealed similar findings (Table 1). The overall model significantly predicted cognitive status at year 3 (χ2= 80.2, df = 3, p < 0.001) and at year 4 (χ2= 64.5, df = 3, p < 0.001). Relative to participants who were CI the first two assessments, individuals in the PD-MCI-Stable, Conversion, or Reversion groups were more likely to be classified as PD-MCI or PDD at years 3 and 4.
Table 1.
Unadjusted Odds of Cognitive Status at Years 3 and 4.
| Year 3 | Year 4 | |||
|---|---|---|---|---|
| Odds Ratio |
p | Odds Ratio |
p | |
| Conversion* | 7.29 | <0.001 | 6.27 | <0.001 |
| PD-MCI-Stable* | 16.35 | <0.001 | 17.41 | <0.001 |
| Reversion* | 3.52 | 0.002 | 5.14 | <0.001 |
Odds relative to the CI-Stable group;
CI = Cognitively Intact; PD-MCI = Parkinson’s Disease Mild Cognitive Impairment
Adjusted Odds of PD-MCI and PDD at Follow-Up
Additional ordinal regression analyses were conducted, adjusting for age, gender, education, depressive symptoms, and motor severity at baseline. The overall adjusted model significantly predicted cognitive status at the 2-year follow-up (χ2 = 149.1, df = 7, p < 0.001; Table 2). Participants were more likely to be classified as PD-MCI or PDD at the 2-year follow-up if they were in the PD-MCI-Stable, Conversion, or Reversion groups relative to the CI-Stable group. Older age and more severe depressive symptoms were also risk-factors for being classified as PD-MCI or PDD at the 2-year follow up.
Table 2.
Adjusted Odds of Cognitive Status at 2-Year Follow-Up
| Odds Ratio |
p | 95% Confidence Interval |
|
|---|---|---|---|
| Age | 1.03 | 0.046 | 1.0 – 1.1 |
| Gender | 0.79 | 0.380 | 0.5 – 1.3 |
| Education | 0.93 | 0.100 | 0.9 – 1.0 |
| UPDRS-III | 1.01 | 0.410 | 0.9 – 1.1 |
| GDS | 1.18 | <0.001 | 1.1 – 1.3 |
| Conversion* | 8.41 | <0.001 | 4.5 – 15.9 |
| PD-MCI-Stable* | 23.33 | <0.001 | 11.4 – 47.7 |
| Reversion* | 7.70 | <0.001 | 3.6 – 16.3 |
Odds relative to the Cognitively Intact-Stable group;
PD-MCI = Parkinson’s Disease Mild Cognitive Impairment; UPDRS-III = Unified Parkinson’s Disease Rating Scale, part III; GDS= Geriatric Depression Scale.
Adjusted models of cognitive status were repeated at the 3rd and 4th year follow-up (Table 3). Findings were generally similar, such that individuals in the PD-MCI-Stable, Conversion, or Reversion groups were more likely to be classified as PD-MCI or PDD at years 3 and 4 relative to participants who were CI the first two assessments. Additionally, older age was a risk factor for being classified in a more severe cognitive status at Year 3 only, but not Year 4. Gender, years of education, depressive symptoms, and motor severity at baseline were not significantly predictive of cognitive status at years 3 or 4.
Table 3.
Adjusted Odds of Cognitive Status at Years 3 and 4.
| Year 3 | Year 4 | |||
|---|---|---|---|---|
| Odds Ratio |
p | Odds Ratio |
p | |
| Age | 1.06 | <0.001 | 1.02 | 0.161 |
| Gender | 0.60 | 0.093 | 0.72 | 0.362 |
| Education | 0.95 | 0.245 | 0.91 | 0.085 |
| UPDRS-III | 1.02 | 0.250 | 1.02 | 0.382 |
| GDS | 1.08 | 0.157 | 1.10 | 0.120 |
| Conversion* | 5.85 | <0.001 | 5.18 | <0.001 |
| PD-MCI-Stable* | 11.53 | <0.001 | 14.18 | <0.001 |
| Reversion* | 3.20 | 0.005 | 4.88 | <0.001 |
Odds relative to the Cognitively Intact-Stable group;
PD-MCI = Parkinson’s Disease Mild Cognitive Impairment; UPDRS-III = Unified Parkinson’s Disease Rating Scale, part III; GDS= Geriatric Depression Scale.
DISCUSSION
Previous research has shown that a large portion of individuals meeting criteria for PD-MCI will revert to CI status within a year [7–10]. This study provides evidence that individuals who revert from PD-MCI to CI continue to be at risk for future cognitive impairment. Specifically, individuals who reverted from PD-MCI to CI were 7 times more likely to re-develop evidence of cognitive impairment (either PD-MCI or PDD) within a year relative to PD participants who were consistently CI. Findings suggest that any diagnosis of PD-MCI may be clinically informative, even if subsequent evaluations fail to find evidence of cognitive impairment.
In the current study, participants who met criteria for PD-MCI at any point during the first year were at risk for future cognitive impairment, with participants who consistently met criteria for PD-MCI at the first two evaluations (i.e. PD-MCI-Stable) being at greatest risk for cognitive impairment at future evaluations. This is consistent with past studies showing that PD-MCI is a risk factor for PDD, and supports the utility of PD-MCI as a diagnostic category [3,4,15].
A unique aspect of the study was the ability to identify individuals who reverted from PD-MCI to CI. The finding that “reverters” continue to be at risk for future cognitive impairment is consistent with findings in non-PD aging samples. Two population based studies have shown that individuals who reverted from MCI to CI were more likely to either re-convert back to MCI or develop AD within 3 years relative to individuals without a history of MCI [9,10]. Individuals were more likely to revert from MCI to CI (as opposed to consistently meeting criteria for MCI) if they met criteria for non-amnestic, single domain MCI, or if they had lower severity of cognitive impairment as measured by the Mini Mental State Examination (MMSE) or low Clinical Dementia Ratings (CDR).
There has been at least one other study in PD that examined the risk of cognitive impairment among individuals who reverted from PD-MCI to CI [5]. A sample of 178 PD participants was followed for 5 years. Although only a small number (n = 10) of individuals reverted from PD-MCI at baseline to CI within a year, these “reverters” were at increased risk (unadjusted odds ratio = 10.7) for re-developing cognitive impairment within a year, relative to patients who were CI.
Past studies have speculated about explanations for reversion from MCI to a CI status. One explanation is that reversion may reflect an improvement in cognitive abilities due to interventions [5,10]. If an efficacious intervention, such as cognitive training, is implemented between evaluations, then it is expected that some individuals may no longer show evidence of cognitive impairment at subsequent assessments [16–18]. Alternatively, it has been suggested that cognitive performance fluctuates over time [9]. In PD, acute neuropsychiatric fluctuations (including fluctuations in subjective cognitive abilities), similar to motor fluctuations or cognitive fluctuations in Lewy body disease, have been reported to be relatively common.[19] It is possible that PD-MCI reversion may be partially related to these acute cognitive fluctuations. Although the current study did control for depression, future studies may benefit by accounting for fluctuations in other neuropsychiatric symptoms, such as apathy, as past studies have shown apathy to have a stronger relationship with cognition and overall disease severity.[20–22]
Perhaps more likely, reversion of PD-MCI may represent normal, non-pathological fluctuations in cognitive abilities or may reflect measurement error (including practice effects between the initial and subsequent assessments). Past longitudinal studies have investigated the stability of the MoCA. Two studies of healthy older adults or individuals with PD have found that MoCA scores were generally stable when tested 2–5 months apart, with a test-retest intra-class correlation of 0.81 in healthy controls and 0.79 in individuals with PD.[23,24] Both studies found that MoCA scores increased by approximately half a point on average when retested, with a standard error of measurement of 1.5 (reported among elderly controls only). While these studies support the MoCA as a valid and stable assessment of cognition, mild improvements in performance are common. Therefore, reversion from PD-MCI to CI may not reflect a “true” improvement in cognitive status or the disease process, but rather measurement error, particularly among individuals who’s scores are just below the cut-off. Despite the reasons for reversion, over time as the underlying neurodegenerative process continues to progress, these individuals (i.e. reverters) may be at increased risk for re-converting to MCI and eventually dementia.
Limitations of the current study include the classification of PD-MCI and PDD status based on a short cognitive screener (the MoCA). The Movement Disorder Society (MDS) previously published diagnostic criteria for PD-MCI and PDD [25,26]. The recommended evaluation consisted of two tests per cognitive domain (attention/working memory, executive functioning, language, memory and visuospatial functioning), which were not administered as part of the larger PPMI study. Although it would be possible to classify PD-MCI using only one test per domain, this would likely result in a high rate of type II error due to the fact that it would be impossible to detect individuals who meet criteria for PD-MCI, single domain (which is approximately 67% of individuals with PD-MCI). [27, 28] Furthermore, assessment of functional abilities is required to differentiate PD-MCI from PDD. PPMI implemented a categorization of functional status after study initiation, and utilization of this categorization would have significantly reduced the sample size.[29] However, the MoCA is a recommended screener for PD-MCI based on MDS Level I guidelines, and the proportion of participants with MCI at baseline (29.4%) is relatively consistent with a past MDS review of PD-MCI (19% – 38%) [1]. Additionally, a past study showed the MoCA had good psychometrics, with a sensitivity and specificity of 95 and 87, respectively, for identifying PDD, and a sensitivity and specificity of 90 and 75 for identifying PD-MCI [12]. It is important to point out that although the PD-MCI Reversion group’s mean MoCA score at baseline (mean = 25.2) is close to the 26/27 cut-off point, and the PD-MCI-Stable group’s mean MoCA score at baseline (mean = 25.0) is similarly close to the 26/27 cut-off. Therefore, it is unlikely that the difference between these groups (i.e. why do some individuals revert, while others remain PD-MCI stable) is not due to one group performing closer to the cut-off. The current study was unable to examine different MCI profiles (e.g., amnestic versus non-amnestic, or single domain versus multi-domain). Future studies may benefit from examining whether risks for reversion or future cognitive decline differ as a function of MCI profiles. Furthermore, the sample consisted of participants newly diagnosed with PD. As such, the sample may be atypical and non-representative of the community/general PD population, which may limit the generalizability of findings. Also, there were only a small proportion of participants who developed PDD (<7%) during the course of the study. Due to the small proportion of participants with PDD, cognitive status was treated as an ordinal variable (i.e. CI<PD-MCI<PDD), which limited us from analyzing PDD as a separate dichotomous outcome (i.e. PDD versus not PDD). Future studies may additionally benefit from examining the role of starting/stopping medications (particularly cholinergic medications or other medications that may affect cognitive functioning) on PD-MCI reversion/stability.
Overall, findings from the current study suggest that even though a large proportion of individuals with PD-MCI will not show evidence of cognitive impairment within a year, these “reverters” continue to be at risk for the future development of cognitive impairment. Clinically, a diagnosis of PD-MCI may serve as a harbinger of future cognitive decline, and reversion from PD-MCI to CI may only be temporary for a large portion of patients.
Supplementary Material
Highlights.
Mild cognitive impairment is a risk factor for future cognitive decline.
A large portion of individuals “revert” from PD-MCI to a cognitively intact status.
Reverters continue to be at risk for future cognitive impairment within 2–4 years.
Acknowledgments
Acknowledgements/ Study Funding
Jacob Jones, Ph.D. is supported by a NIMH T32 Training Grant (MH19535).
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Avid
Radiopharmaceuticals, Biogen Idec, BioLegend, Bristol-Meyers Squibb, GE Healthcare, Genentech, GlaxoSmithKline, Eli Lilly and Company, Lundbeck, Merck, Meso Scale Discovery, Pfizer Inc., Piramal Imaging, Roche group, Sanofi-Genzyme, Servier, Takeda, TEVA, and UCB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Tröster AI, Weintraub D. MDS task force on mild cognitive impairment in Parkinson's disease: Critical review of PD-MCI. Mov Disord. 2011;26(10):1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez HH, Crucian GP, Okun MS, Price CC, Bowers D. Mild cognitive impairment in Parkinson’s disease: the challenge and the promise. Neuropsychiatr Dis Treat. 2005;1(1):37–50. doi: 10.2147/nedt.1.1.37.52295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord. 2006;21(9):1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Burn D, Barone P, Pagonabarraga J, Allcock L, Santangelo G. Mild cognitive impairment in Parkinson disease A multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen KF, Larsen JP, Tysnes O, Alves G. Natural course of mild cognitive impairment in Parkinson disease A 5-year population-based study. Neurology. 2017;88(8):767–774. doi: 10.1212/WNL.0000000000003634. [DOI] [PubMed] [Google Scholar]
- 6.Wood KL, Myall DJ, Livingston L, Melzer TR, Pitcher TL, MacAskill MR, Geurtsen GJ, Anderson TJ, Dalrymple-Alford JC. Different PD-MCI criteria and risk of dementia in Parkinson's disease: 4-year longitudinal study. NPJ Parkinsons Dis. 2016;2:15027. doi: 10.1038/npjparkd.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 8.Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr. 2004;16(2):129–140. doi: 10.1017/s1041610204000092. [DOI] [PubMed] [Google Scholar]
- 9.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition Risk factors and prognosis. Neurology. 2012;79(15):1591–1598. doi: 10.1212/WNL.0b013e31826e26b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz VS, Christianson T, Geda YE, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82(4):317–325. doi: 10.1212/WNL.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, Coffey C, Kieburtz K, Flagg E, Chowdhury S, Poewe W. The parkinson progression marker initiative (PPMI) Prog. Neurobiol. 2011;95(4):629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, Melzer TR, Kirwan J, Keenan R, Wells S, Porter RJ. The MoCA well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 13.Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weintraub D, Oehlberg KA, Katz IR, Stern MB. Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry. 2006;14(2):169–175. doi: 10.1097/01.JGP.0000192488.66049.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 16.Linda C, Woods RT. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer's disease: A review. Neuropsychol Rehabil. 2004;14(4):385–401. [Google Scholar]
- 17.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30(1):1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PloS One. 2012;7(7):e40588. doi: 10.1371/journal.pone.0040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Cherif AA. Nonmotor fluctuations in Parkinson’s disease Frequent and disabling. Neurology. 2002;59(3):408–413. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 20.Jones JD, Marsiske M, Okun MS, Bowers D. Latent Growth-Curve Analysis Reveals that Worsening Parkinson’s Disease Quality of Life is Driven by Depression. Neuropsychology. 2015;29(4):603–609. doi: 10.1037/neu0000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones JD, Mangal P, Lafo J, Okun MS, Bowers D. Mood Differences among Parkinson’s Disease Datients with Mild Cognitive Impairment. The Journal of Neuropsychiatry and Clinical Neurosciences. 2016;28(3):211–216. doi: 10.1176/appi.neuropsych.15090221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butterfield LC, Cimino CR, Oelke LE, Hauser RA, Sanchez-Ramos J. The Independent Influence of Apathy and Depression on Cognitive Functioning in Parkinson's Disease. Neuropsychology. 2010;24(6):721. doi: 10.1037/a0019650. [DOI] [PubMed] [Google Scholar]
- 23.Feeney J, Savva GM, O’Regan C, King-Kallimanis B, Cronin H, Kenny RA. Measurement Error, Reliability, and Minimum Detectable Change in the Mini-Mental State Examination, Montreal Cognitive Assessment, and Color Trails Test among Community Living Middle-Aged and Older Adults. Journal of Alzheimer's Disease. 2016;53(3):1107–1114. doi: 10.3233/JAD-160248. [DOI] [PubMed] [Google Scholar]
- 24.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord. 2008;23(7):1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 25.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 26.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, Evidente V, Shill HA, Adler CH. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007;22(9):1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 28.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord. 2006;21(9):1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 29.Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Siderowf A, Aarsland D, Barone P, Burn D, Chahine LM, Eberling J. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Mov Disord. 2015;30(7):919–927. doi: 10.1002/mds.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.