Abstract
The Gulf Coast tick Amblyomma maculatum Koch is increasingly relevant to medical and veterinary communities as human infection rates of Rickettsia parkeri rise, the risk of introduction of Ehrlichia ruminantium increases, and the range of this tick expands into the densely populated Mid-Atlantic region of the United States. We report on the results of five years of field surveillance to better describe the ecology of A. maculatum in newly established populations in southeastern Virginia. We document habitat preferences, host preferences, and the phenology of the adult human-biting life stage. We discuss key ecological factors needed for A. maculatum establishment and the influence of the successional process and anthropogenic activities on the persistence of A. maculatum populations in Virginia.
Keywords: Amblyomma maculatum, invasions, range expansions, succession, ticks
INTRODUCTION
The Gulf Coast tick, Amblyomma maculatum, is an aggressive, human-biting tick of increasing medical and veterinary significance (Paddock and Goddard, 2015). It is a known vector of several emerging pathogens including Rickettsia parkeri, the agent of Tidewater spotted fever (Paddock et al., 2004). Human infection with R. parkeri results in an eschar-associated febrile illness that is milder than the more familiar Rocky Mountain spotted fever caused by the related Rickettsia rickettsii (Paddock et al., 2008). Veterinary pathogens transmitted naturally or experimentally by this tick include Hepatozoon americanum, the principal agent of American canine hepatozoosis (Baneth, 2011), Rickettsia felis, the agent of feline rickettsiosis, Leptospira pomona, causal agent of leptospirosis in livestock, and Ehrlichia ruminantium, the agent of heartwater in ruminants (Stromdahl and Hickling, 2012). In addition, A. maculatum is often infected with spotted fever group rickettsiae of unknown pathogenicity, including “Candidatus Rickettsia andeanae” and other newly described organisms (Paddock et al., 2010; Jiang et al., 2012). In addition to carrying various microbes, A. maculatum is a relatively large tick, and bites can cause inflammation, edema, abscesses, and predisposition to myiasis, anemia, and secondary infections, especially in livestock (Paddock et al., 2010; Teel et al., 2010; Jiang et al., 2012). As the effects of these pathogens on human and animal health have become better understood over the last decade, there has been a renewed interest in understanding the ecology A. maculatum in order to prevent disease (Paddock and Goddard, 2015).
The historical range of A. maculatum throughout the Caribbean, South and Central America is extensive, including records from Mexico, Jamaica, Belize, the West Indies, Columbia, Venezuela, and Peru (Teel et al., 2010; Paddock and Goddard, 2015). The southern extent of the range of A. maculatum is in question because of the morphological similarity of A. maculatum to the closely related A. tigrinum and A. triste (Estrada-Peña et al., 2005). In the early 1900s, A. maculatum was common along the Gulf Coast of the U.S. from Louisiana to Texas, invading into the coastal Atlantic region up through South Carolina in the mid-1900s (Teel et al., 2010). After the 1950s, the distribution of A. maculatum drastically changed, as the cattle industry facilitated the establishment of this tick in Kansas and Oklahoma in the 1950s-1980s (Teel et al., 2010; Paddock and Goddard, 2015). Researchers in Arkansas collected over 200 A. maculatum between 2006 and 2009, and now consider A. maculatum to be established in that state (Trout et al., 2010; McAllister et al. 2016). Between 2009 and 2011, A. maculatum populations reached sufficient levels to be considered established in North Carolina, having previously been known only from sporadic records (Harrison et al., 2010; Varela-Stokes et al., 2011). Newly established A. maculatum populations have also recently been discovered in southeastern and northern Virginia (Fornadel et al., 2011; Wright et al., 2011), Delaware (Florin et al., 2014), and Kentucky (Pagac et al., 2014). Incidental collections of individual ticks reported nearly everywhere further north are generally along migratory bird flyways; migrating birds and an expanding white-tailed deer population have been suggested as hosts that may be implicated in the continued expansion of this tick (Paddock and Goddard, 2015).
Human infections with R. parkeri are reported everywhere this tick is found (Sumner et al., 2007; Jiang et al., 2012; Paddock and Goddard, 2015). However, newly established populations of this tick, especially in Mid-Atlantic states, have tended to exhibit higher infection prevalence than populations found in areas where these ticks have long been established (Paddock et al., 2010; Sumner et al., 2007; Cohen et al., 2009; Jiang et al., 2012; Trout et al., 2010; Wright et al., 2011; Fornadel et al., 2011; Varela-Stokes et al., 2011; Ferrari et al., 2012). Some of the first cases of R. parkeri infection were described in Virginia, which has only had established A. maculatum populations for a short while, indicating this tick can start spreading disease as soon as it arrives (Paddock et al., 2004; Whitman et al., 2007). Recent evidence suggests that spillover of R. parkeri to other common tick species, including the lone star tick (Amblyomma americanum), may occur in areas invaded by A. maculatum (Gaines et al., 2014; Henning et al., 2014; Wright et al., 2015). As this tick continues to expand its range, understanding the ecological mechanisms by which new populations arise and persist is critical to protecting human and animal health. Here, we describe the results of five years of field surveillance on newly established populations of A. maculatum in southeastern Virginia, and discuss implications for the continued expansion of A. maculatum into the Mid-Atlantic.
MATERIALS AND METHODS
Collection of questing ticks
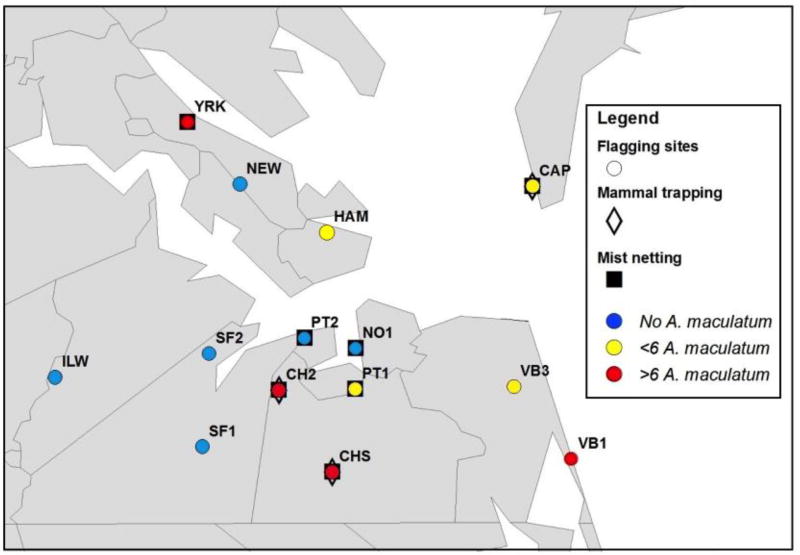
Questing A. maculatum were collected from public and privately owned lands throughout southeastern Virginia from for five years from 2010 to 2014. Fifteen sites representing a variety of woodland, grassland, successional, and anthropogenically disturbed habitats were sampled weekly during the summer months and at least monthly throughout the rest of the year (Fig. 1). All transects were marked with surveyor’s flags so that the same transect was walked during each sampling event. In order to determine the area sampled at each site, GPS coordinates were taken along each transect. ArcGIS 9.3 (www.esri.com) was used to draw a line connecting the GPS points from each transect and to draw a 2m buffer around the line.
Figure 1.
Map of Amblyomma maculatum collection sites, 2010–2014. Map shows southeastern Virginia, U.S. counties and independent cities, with circles marking sites that were flagged weekly or bi-weekly from 2010 to 2014. For years of sampling and brief habitat descriptions of these sites, see Table 1. Sites where mist netting also took place are surrounded by a square, and sites where small mammal trapping also took place are marked with a diamond. Fifteen sampling sites in southeastern Virginia were sampled, eight of which yielded at least one A. maculatum in five years of sampling from 2010–2014. Blue sites yielded no A. maculatum, yellow sites indicate where fewer than six A. maculatum were collected, and red sites indicate established populations of more than six A. maculatum collected over five years.
Ticks were collected using 1m2 white denim flags attached to wooden dowel rods swept over the ground and through low vegetation. All ticks found clinging to the flag material were removed from the flag with forceps and placed in containers labeled with the time and date of collection, as well as the sampling location, temperature, and other weather data. Ticks were frozen at −20 C until morphologically identified (Keirans and Durden, 1998) ; they were then stored frozen at −80 C. Only sites where more than six adult ticks or multiple life stages were collected within a single collection period were considered to represent established populations (Fish and Howard, 1999).
Collection of ticks from hosts
Host-targeted sampling techniques were employed from 2010 to 2014, including collection of ticks from large mammals at hunt check locations during the fall of each year, collaboration with veterinarians to collect ticks from companion animals, serendipitous sampling of road-killed mammals, small mammal live-trapping, avian sampling using mist-nets, and opportunistic hand-capture of reptiles at flagging sites (ODU IACUC # 11-012, 13-018, 13-008). In addition, ticks found crawling on or attached to humans throughout southeastern Virginia were submitted periodically to the Old Dominion University Tick Research Team between 2011 and 2014 as a result of local outreach and educational programs.
Large wild mammals were sampled for ticks through collaborations with local hunters, game managers, and state biologists, and through checking road-killed animals for ticks. Any ticks found on dead mammals were removed with forceps, places in vials, and returned to the laboratory where they were frozen at −20 C until processing. Domesticated animals were sampled through collaborations with veterinarians with practices in southeastern Virginia. If any ticks were found on an animal patient, ticks were removed, labeled, and frozen until they could be forwarded to the Old Dominion University Tick Research Team for identification and processing.
Small mammal communities were sampled at two primary flagging sites with established populations of A. maculatum, CHS and CH2. These sites represented mid to late successional habitats adjacent to the Great Dismal Swamp National Wildlife Refuge in the City of Chesapeake, Virginia, and were dominated by grasses and forbs with mixed coniferous and deciduous trees that were 5–15 years old. Transects or grids of modified Fitch live traps (Rose, 1994) were laid at each site. Trapping was conducted monthly or quarterly from 2010 to 2014. Traps were baited with birdseed and sunflower seeds, set in the evening, and checked for three consecutive mornings. Trapped mammals were examined for ticks, and any ticks were removed using forceps, placed in vials, and returned to the laboratory to be frozen until identification and processing. All mammals were handled according to the guidelines set forth by the American Society of Mammalogists (Sikes and Gannon, 2011).
Avian hosts were sampled from 2013 to 2014 at a subset of the flagging sites (Fig. 1) through weekly or bi-weekly mist netting, conducted as previously described (Heller et al., 2016). Starting in 2013, local reptiles were sampled at the weekly flagging transect sites, with an emphasis on collecting ticks from native lizards, snakes, and turtles. Reptiles were captured using standard collection techniques, including the implementation of artificial cover objects and visual encounter surveys, and examined for ticks before being released (McDiarmid et al., 2012). Ticks were removed from reptiles with forceps and placed in vials, before being returned to the laboratory and frozen until identification and processing.
Molecular methods and identification
All ticks collected from flags and from hosts were identified to species using morphological characters (Clifford et al., 1961; Keirans and Durden, 1998). If morphological identification was impossible because of uncertain morphological characters or damage to the specimen, a fragment of the 16S mitochondrial rRNA gene was sequenced using previously established methods (Nadolny et al., 2015) to determine tick species. While adult A. maculatum are morphologically distinct from other U.S. ticks in the genus Amblyomma, immatures can be difficult to tell apart from other Amblyomma and Dermacentor species, especially when engorged. Unengorged immatures from flags and engorged immature ticks from hosts were identified via restriction fragment length polymorphism (RFLP) assay and/or sequencing of the 16S mitochondrial gene (Fornadel et al., 2011).
RESULTS
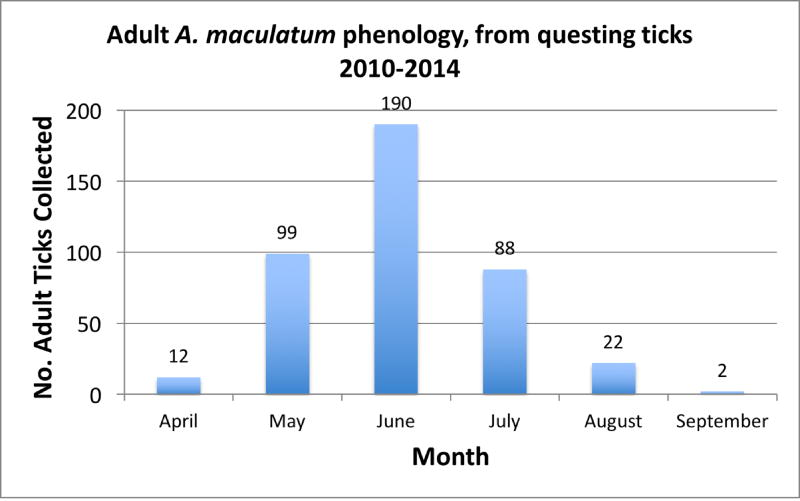
Questing adult A. maculatum ticks were collected from eight of 14 sites sampled from 2010–2014 (Fig. 1, Table 1). Of those sites where A. maculatum was collected, four yielded sufficient numbers of adults within a single sampling period to be considered populations (Fig. 1). Adults were collected only during the warmer months, from April through September, with peak activity in June (Fig. 2). While immature A. maculatum were infrequently collected on flags, a small number were collected over the five years (Table 2). Amblyomma maculatum immatures were flagged almost exclusively immediately after grassy fields had been mowed at sites where large numbers of adults were regularly collected. Larvae and nymphs were sporadically collected from spring through late summer, however, insufficient numbers of immatures were collected to establish a clear phenology of these life stages in Virginia.
Table 1.
Amblyomma maculatum (AM) adults collected by flagging, 2010–2014. All sites were in southeastern Virginia. Site codes correspond to those mapped in Figure 1, and habitat types and A. maculatum density are included for each site. NS indicates that that site was not sampled in those years. General habitat types were determined by observing vegetation communities along transects. Observed successional events are qualitative changes to each site from natural or anthropogenic factors observed during the study period.
| County | Site | AM 2010 |
AM 2011 |
AM 2012 |
AM 2013 |
AM 2014 |
Total AM |
AM/ 100 m2 |
Habitat Type | Observed Successional Events |
|---|---|---|---|---|---|---|---|---|---|---|
| Chesapeake | CHS | 54 | 104 | 88 | 17 | 7 | 270 | 2.66 | Lowland mid-successional old field with drainage ditches | Grass/perennials to shrubs/young trees |
| Chesapeake | CH2 | NS | NS | NS | 6 | 18 | 24 | 4.44 | Early and mid-successional old field, bordering pine-mixed hardwood forests | Grass/perennials to shrubs |
|
|
||||||||||
| Hampton | HAM | NS | 2 | 2 | 0 | 5 | 9 | 2.16 | Old field and mixed hardwood forest edge; Early successional old field | Annual mowing to maintain grass/perennials at old field |
|
|
||||||||||
| Isle of Wight | ILW | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Loblolly-longleaf pine savannah; Edge of bald-cypress-tupelo swamp bordering loblolly pine-oak forest | Longleaf pine savannah maintained through prescribed burns |
|
|
||||||||||
| Newport News | NEW | NS | NS | NS | NS | 0 | 0 | 0 | Pine-mixed hardwood forest edge | None |
|
|
||||||||||
| Norfolk | NO1 | NS | 0 | 0 | 0 | 0 | 0 | 0 | Pine-mixed hardwood forest | None |
|
|
||||||||||
| Northampton | CAP | 0 | 0 | 1 | 0 | 0 | 1 | 0.57 | Pine-mixed hardwood forest; Rear dune shrub community bordering parking lot; Early successional old field | Frequent mowing to maintain grass in old field |
|
|
||||||||||
| Portsmouth | PT1 | 3 | 0 | 0 | 0 | 0 | 3 | 0.31 | Pine-mixed hardwood forest, some bordering a salt marsh | Clear cut portion of forest to rebuild wetlands |
| Portsmouth | PT2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Pine-mixed hardwood forest, some bordering a salt marsh | None |
| Suffolk | SF1 | NS | NS | 0 | 0 | NS | 0 | 0 | Early successional old field bordering pine-mixed hardwood forest | Closed landfill, fields maintained through frequent mowing |
| Suffolk | SF2 | NS | NS | NS | NS | 0 | 0 | 0 | Pine-mixed hardwood forest | None |
| Virginia Beach | VB1 | 9 | 6 | 27 | 31 | 18 | 91 | 8.55 | Maritime live oak forest edge; Early successional old field | Biennial mowing to maintain grass/perennials at old field |
|
|
||||||||||
| Virginia Beach | VB3 | 0 | 1 | 0 | 0 | 0 | 1 | 0.13 | Pine-mixed hardwood forest; Loblolly pine-oak forest; Maritime dune grassland | None |
|
|
||||||||||
| York | YRK | 0 | 1 | 13 | 0 | 0 | 14 | 2.14 | Pine-mixed hardwood forest bordering river and bald cypress-tupelo swamp; Early successional old field; Mature loblolly pine-oak forest bordering managed pine forest | Biennial mowing to maintain grass/perennials at old field |
Figure 2.
Phenology of adult Amblyomma maculatum collected in Virginia, U.S. over five years. Numbers shown are total adult A. maculatum collected at all sites and in all years through equal weekly flagging effort.
Table 2.
Amblyomma maculatum immatures collected by flagging, 2010–2014. All A. maculatum (AM) nymphs and larvae were collected in southeastern Virginia. Site codes correspond to those mapped in Figure 1.
| Month | AM nymphs | AM larvae | Site |
|---|---|---|---|
| March | 9 | VB1 | |
| April | 1 | 5 | CH2 (n), YRK (l) |
| May | 1 | VB1 | |
| August | 6 | VB1 | |
| September | 2 | VB1 |
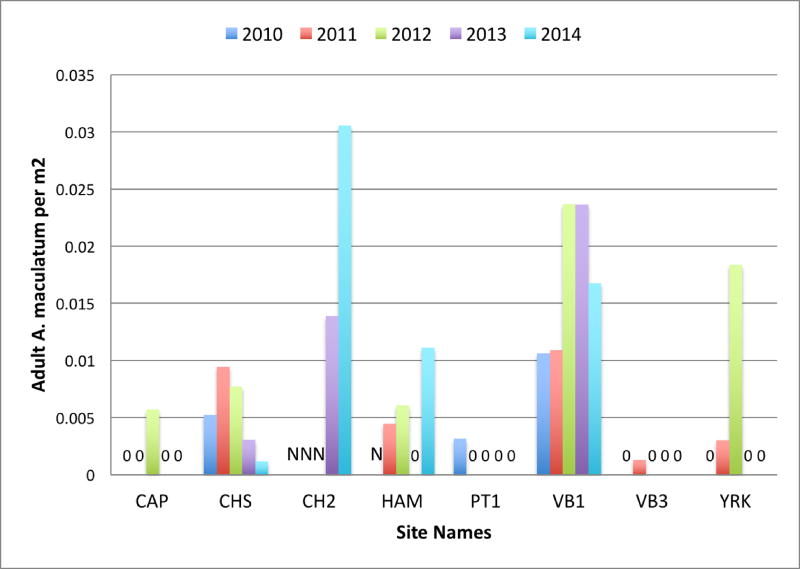
Amblyomma maculatum adults were collected from a variety of habitat types over the five years (Table 1), but all of the most productive sites where A. maculatum could be reliably collected were characterized by open habitat, including mowed fields (YRK, HAM, and parts of VB1) and old fields undergoing ecological succession to forest (CHS and CH2). Sites with large swaths of open habitat had the highest densities of A. maculatum per m2 (Fig. 3), and maintained those populations of A. maculatum over multiple years (Table 1).
Figure 3.
Amblyomma maculatum adults collected per m2 at each site, 2010–2014, Virginia, U.S. The numbers of ticks collected per m2 sampled are low because all sampling events, including during times when A. maculatum were not active, are included in the denominator.
Sites where populations of A. maculatum were established did not maintain static numbers of ticks from year to year (Table 1, Fig. 3). In particular, the largest population of A. maculatum at the CHS site reached peak abundance in 2011, with decreasing numbers each subsequent year, indicating the decline of this population. This decline corresponded with the progression of ecological succession, and the closure of the canopy at that site. Another site, CH2, yielded increasing numbers of A. maculatum in the two years it was sampled, indicating that this population may be undergoing an increase in A. maculatum numbers, similar to the first few years of sampling VB1 and CHS. This site is earlier in the successional process, and will not undergo canopy closure for several more years. While numbers of A. maculatum were fewer at VB1 (Table 1), A. maculatum were the most densely populated tick at this site (Fig. 3). The VB1 site is a nature preserve that comprises grassy areas that are anthropogenically maintained through annual mowing as well as preserved dune and maritime forest communities.
At each site where A. maculatum was collected, other common species of ticks were also found, including Dermacentor variabilis, Amblyomma americanum, Ixodes affinis, and Ixodes scapularis (Table 3). At almost every site, A. americanum was by far the most abundant tick species, with the exception of D. variabilis being most abundant at two sites, PT1 and CH2. Amblyomma maculatum was the second most abundant tick species at only one site, VB1.
Table 3.
Adult tick species composition of sites where A. maculatum was collected, 2010–2014. Site codes correspond to those mapped in Figure 1. Combined 2010–2014 collections of questing adult Amblyomma maculatum (AM), Dermacentor variabilis (DV), Amblyomma americanum (AA), Ixodes affinis (IA), and Ixodes scapularis (IS) are included for each site.
| County | Site | DV | AA | IS | AM | IA | Total adults | % AM |
|---|---|---|---|---|---|---|---|---|
| Chesapeake | CHS | 879 | 1360 | 50 | 270 | 130 | 2689 | 10.04% |
| Chesapeake | CH2 | 234 | 202 | 1 | 24 | 25 | 486 | 4.94% |
| Hampton | HAM | 112 | 586 | 41 | 9 | 17 | 765 | 1.18% |
| Northampton | CAP | 65 | 448 | 17 | 1 | 42 | 573 | 0.17% |
| Portsmouth | PT1 | 135 | 25 | 2 | 3 | 0 | 165 | 1.82% |
| Virginia Beach | VB1 | 83 | 298 | 9 | 91 | 3 | 484 | 18.80% |
| Virginia Beach | VB3 | 25 | 857 | 77 | 1 | 321 | 1281 | 0.08% |
| York | YRK | 27 | 1423 | 54 | 14 | 57 | 1575 | 0.89% |
|
| ||||||||
| Total | 1560 | 5199 | 251 | 413 | 595 | 8018 | 5.15% | |
Of 370 individual large animal hosts, from 15 species sampled, and 3,293 ticks removed, only seven were adult A. maculatum. These seven ticks were collected from six individual animals, including a cat (Felis catus), a dog (Canis lupus familiaris), three white-tailed deer (Odocoileus virginianus), and a feral hog (Sus scrofa) between 2010 and 2014 (Table 4). Most A. maculatum were sharing hosts with other common ticks at the time of collection, including Dermacentor albipictus, D. variabilis, A. americanum, and I. scapularis (Table 4).
Table 4.
Hosts infested with Amblyomma maculatum in southeastern Virginia. Hosts were infested with one or more A. maculatum (AM) adult (A), nymph (N), or larva (L).
| Host | Site | Month | AM (L) |
AM (N) |
AM (A) |
DV (A) |
AA (L) |
AA (N) |
AA (A) |
IS (A) |
DA (N) |
Total ticks |
Monthly host IR |
Annual host IR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Felis catus (Domesticated Cat)** | ||||||||||||||
|
| ||||||||||||||
| HAM | Jul | 1 | 1 | 33.33% | 4.35% | |||||||||
|
| ||||||||||||||
| Odocoileus virginianus (White-tailed Deer) | ||||||||||||||
|
| ||||||||||||||
| VB1 | Oct | 2 | 1 | 1 | 2 | 2 | 8 | 1.85% | 2.50% | |||||
| CHS | Dec | 1 | 8 | 9 | 10.00% | 2.50% | ||||||||
| Tappahannock | Jun | 1 | 6 | 7 | 14 | 12.50% | 2.50% | |||||||
|
| ||||||||||||||
| Canis lupus familiaris (Domesticated Dog) | ||||||||||||||
|
| ||||||||||||||
| NEW | May | 1 | 1 | 1.72% | 0.81% | |||||||||
|
| ||||||||||||||
| Sus scrofa (Feral Swine) | ||||||||||||||
|
| ||||||||||||||
| VB1 | Jul | 1 | 15 | 2 | 11 | 29 | 100.00% | 5.26% | ||||||
|
| ||||||||||||||
| Microtus pennsylvanicus (Meadow Vole) | ||||||||||||||
|
| ||||||||||||||
| CHS | Feb | 1* | 1 | NA | NA | |||||||||
| CHS | Apr | 1 | 1 | NA | NA | |||||||||
|
| ||||||||||||||
| Sigmodon hispidus (Hispid cotton rat) | ||||||||||||||
|
| ||||||||||||||
| CHS | Aug | 2 | 2 | NA | NA | |||||||||
|
| ||||||||||||||
| Oryzomys palustris (Marsh Rice Rat) | ||||||||||||||
|
| ||||||||||||||
| CHS | July | 2 | 2 | NA | NA | |||||||||
| CHS | Dec | 1 | 1 | NA | NA | |||||||||
Immatures were identified using a RFLP assay, with * indicating sequence confirmation. Site and month where the host was collected are included for each host. For mammals hosting adults, co-feeding ticks collected from the same host are reported, including Dermacentor variabilis (DV), Amblyomma americanum (AA), Ixodes scapularis (IS), and Dermacentor albipictus (DA). The monthly and annual infestation rate (IR), or percentage of hosts checked infested with AM, are included for each host species hosting adults. Co-feeding ticks and IR are not reported for rodent hosts, because of challenges associated with identifying immature engorged ticks.
The actual number of ticks from this cat is unknown; this A. maculatum tick may have been co-feeding with A. americanum larvae or I. scapularis adult ticks that were received by the ODU Tick Research Team from one veterinary clinic on the same day
Immature A. maculatum were more difficult to sample than adults. Of 643 small mammals captured and 1,087 individual ticks collected from these small mammals, only seven engorged immature A. maculatum from five rodents could be conclusively identified using molecular techniques (Table 4). Of 944 ticks collected and identified using molecular techniques from 1,888 birds sampled year-round from 2012–2014, not a single A. maculatum immature was collected (Heller et al., 2016; Walters laboratory, unpublished data). Similarly, 11 larval ticks collected from 81 native reptiles were determined, via sequencing, to be I. scapularis. Of 544 ticks found crawling on or attached to humans that were submitted to the Old Dominion University tick laboratory between 2011 and 2014, four adult A. maculatum were identified (<1%).
DISCUSSION
Phenology
The seasonal phenology of A. maculatum shifts depending on geographical distribution (Teel et al., 2010). The three life stages minimally overlap in peaks of activity; however, A. maculatum from inland populations in Oklahoma and Kansas become active five months earlier than ticks from historic populations in Texas (Williams and Hair, 1976; Johnson, 1990; Teel et al., 1998; Teel et al., 2010). In Texas, adults feed in September while in Kansas and Oklahoma, adults feed from April through early June (Teel et al., 2010). The phenology of A. maculatum adults in Virginia falls between those described for Texas and Oklahoma (Teel et al., 2010), with peak adult collections in the month of June. It is most similar to populations described from Mississippi, where adults were collected from March through November with peaks in July and August (Goddard and Paddock, 2005). Survival and duration in each life stage is dependent on environmental variables, including habitat, temperature, and humidity (Teel et al., 2010). A period of fall and winter quiescence of adults is standard in all areas in the U.S. where this tick species has been collected.
Virginia A. maculatum nymphs were collected from March through September and a few larvae were collected on flags in April, but these small numbers were insufficient to establish immature phenology. In Texas, peak larval and nymphal feeding seasons are in January and February, respectively, while in Kansas and Oklahoma, larvae peak in June and nymphs in July (Teel et al., 2010). Mississippi A. maculatum nymphs have been collected from February through August, and larvae have been collected from June though November (Paddock and Goddard, 2005; Portugal and Goddard, 2015). If the trends from these populations hold true for Virginia A. maculatum, peak larval abundance would be expected several months after the adult peak, followed closely by nymphs, probably in late summer and early fall. Immature A. maculatum are notoriously difficult to collect (Goddard, 2007), and additional work with novel collection methods such as swabbing rodent burrows should increase our understanding of immature phenology in this tick species (Portugal and Goddard, 2015).
Predictors of A. maculatum presence and absence
Established populations of A. maculatum were discovered at only four of twelve sites sampled continuously over multiple years, indicating that high sampling frequency does not guarantee discovery of this tick. Unlike A. americanum, this tick is not universally distributed throughout the landscape of southeastern Virginia. A specific set of abiotic and biotic factors may be necessary to support a population; if these factors are in place, large numbers of A. maculatum may emerge in just a few years. The life cycle of A. maculatum is generally completed in 1–2 years (Teel et al., 2010). Engorged females lay an average of 8,000 and up to 15,000 eggs (Drummond and Whetstone, 1970; Wright, 1971), demonstrating the ability of this tick species to undergo rapid population expansion after arrival in a suitable new habitat.
In southeastern Virginia and throughout its range, A. maculatum has been found sharing habitats and hosts with other ticks. One observed trend of where A. maculatum would be found was the presence of D. variabilis, as the habitat and host preferences of these ticks seem to strongly overlap (Table 3). Because of this overlap, as well as superficial morphological similarities between immature stages and females of these two tick species (Paddock and Goddard, 2015), recognizing when A. maculatum has invaded may require careful surveillance by experienced acarologists.
In addition to populations of D. variabilis at sites where many A. maculatum were found, these sites had other commonalities, most notably particular habitat characteristics. No A. maculatum were found in forested habitats including bald cypress-tupelo swamps, pine savannahs, and mixed hardwood forests. All A. maculatum sites comprised open successional old field habitat with little shade, dominated by grasses and shrubs (Table 1). These xeric habitats are similar to other areas where A. maculatum have been found in other regions; along the Gulf Coast and in the inland Midwestern U.S., A. maculatum is common in grass-dominated habitats such as prairies, scrublands, oak savannahs, and mesquite (Scifres et al., 1988; Teel et al., 2010). Other newly identified populations of A. maculatum in the Mid-Atlantic have been found in disturbed grassy habitats, including a large population at a landfill in northern Virginia (Fornadel et al., 2011) and small numbers in a disturbed secondary growth habitat (Florin et al., 2014). In general, A. maculatum habitat has been described as southern coastal habitat with high rainfall, temperature and humidity, with open, non-shaded habitats where A. maculatum can quest for hosts in the heat of the day (Paddock and Goddard, 2015).
Most open habitats in Virginia are anthropogenically influenced through prescribed burning, tree clearing, or mowing to create fields or other open spaces for human use. All observed successional-related events at field sites are noted in Table 1. Once these open habitats are created, ecological succession is inevitable unless they are anthropogenically maintained, because of the continual fire suppression along the east coast (for a review of fire and is effects on A. maculatum, see Paddock and Goddard, 2015). Amblyomma maculatum populations appear in secondary successional habitats in Virginia, when there is tall grass with shrubs, but populations seem to disappear as the canopy closes and field becomes forest, as observed at our CHS site (Table 1). The landscape of the U.S. east coast is extremely developed, so patches of open habitat occur mainly by human design, such as when an agricultural field is left fallow, or an area is cleared for construction. These sites are often near areas with large human populations, including patches in urban or suburban developments. By creating and maintaining these open habitats, especially in areas with significant human habitation, humans are inviting A. maculatum to establish and generating new human health risks.
Regulation of A. maculatum populations
If open areas are not maintained by natural fire or anthropogenic influence, A. maculatum populations seem to be unable to last more than a few years in southeastern Virginia habitats. This ephemeral nature may be unique to A. maculatum populations in the southeast, as open habitats and rangelands persist along the Gulf Coast and in the central U.S. where A. maculatum are well established. As A. maculatum ticks move northward into the Mid-Atlantic, more ephemeral populations can be expected, with short-lived open habitats used as stepping stones to continue the process of expansion into otherwise unsuitable habitat. Anthropogenically disturbed habitat islands seem to be the habitat best able to facilitate the establishment and spread of A. maculatum populations in the Mid-Atlantic region. Inland populations of A. maculatum have also been described as “ephemeral” because of periodic expansions and contractions associated with patterns of drought and rainfall (Teel et al., 2010). It is possible that precipitation patterns in the central U.S. may regulate those populations as anthropogenic activity regulates coastal populations, which receive more regular precipitation. Alternatively, peripheral populations at expansion fronts may be inherently more fragile and prone to periodic extirpation than more established populations in the historic range of A. maculatum.
Pathways for dispersal and spread
Because Mid-Atlantic populations of A. maculatum generally exist in habitat islands, they are unlikely to be founded by contiguous populations of ticks (Fig. 4). In order for a population to arise, ticks must be translocated from an existing population to a suitable habitat with an appropriate population of hosts. Amblyomma maculatum has been collected from over 71 species of birds and mammals, including humans, in the United States alone (Teel et al., 2010). Rodents and birds are most often hosts of larvae and nymphs, while adult ticks generally parasitize larger mammals. There are no reports of A. maculatum collected from reptile hosts. The wide host range utilized by this tick, combined with its ability to withstand xeric habitats, has likely contributed to the successful spread of A. maculatum over the last few decades (Paddock and Goddard, 2015).
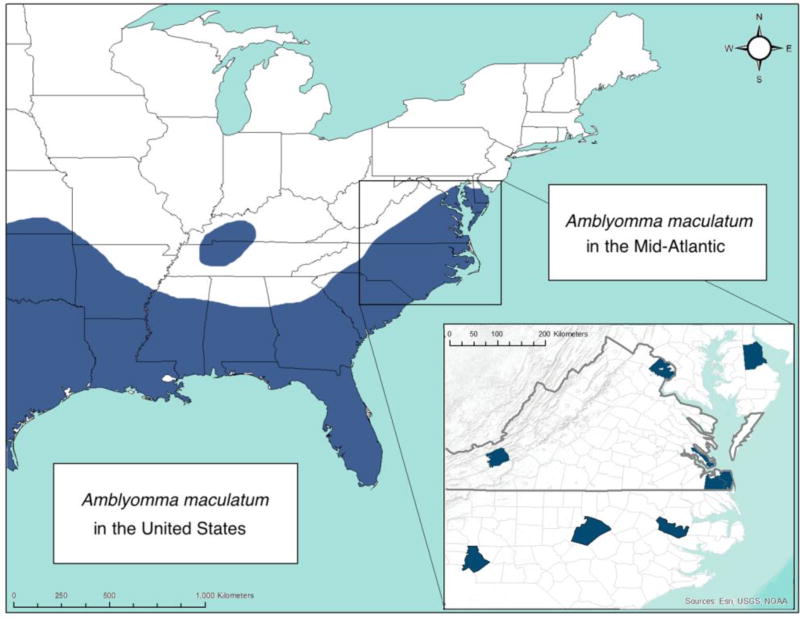
Figure 4.
Range map of Amblyomma maculatum in the United States. Dark blue indicates the known range of A. maculatum in the eastern U.S. Virginia is demarcated in bold grey. Inset map shows counties with reported populations of >6 adult or multiple life stages of A. maculatum collected in North Carolina, Virginia, and Delaware. The data included in the main map are from Paddock and Goddard 2015, data for the inset map are from David Gaines, personal communication (western Virginia population), Florin et al. 2014 (Delaware), Nadolny et al. 2014, Wright et al. 2011, this study (Virginia), and Varela-Stokes et al. 2011 (North Carolina).
Because A. maculatum feeds on a large variety of avian and mammalian hosts with varying dispersal capabilities, there are many possible pathways for arrival at a new site. Amblyomma maculatum was not documented parasitizing any novel hosts in Virginia (Table 4), but it is possible to reconstruct potential pathways to colonization in the Mid-Atlantic using previously reported hosts of A. maculatum. Cattle have been implicated in invasions of A. maculatum in the central US, and previous studies have suggested that migratory birds might be transporting immature A. maculatum, as incidental collections of this tick have historically been along flyways (Teel et al., 2010). The expansion of feral swine across the southeastern and central U. S., and the growing population of white-tailed deer have also been suggested as facilitators of the A. maculatum expansion (Paddock and Goddard, 2015). There is no significant cattle industry in southeastern Virginia, so discussion of possible invasion routes will focus on wild hosts that are present in the Mid-Atlantic. To closely examine these possibilities and determine which hosts could be most important in range expansion and new population establishment, a table of relevant ecological and life history characteristics of each of the hosts of A. maculatum in the U. S. was compiled (Table S1). Tick dispersal ability depends on the behaviors of these hosts during the months when their life stages are questing.
Small bodied, non-migratory hosts with small home ranges have limited dispersal ability for immature A. maculatum ticks. This includes rodents and small non-migratory birds with home ranges generally <1 ha, that generally disperse <2 km from their natal area (Table S1). Rodent hosts can become extremely abundant in suitable habitat, with some species reaching over 100 animals per ha in prime ecological conditions (Table S1). Although these hosts probably do not play much of a role in tick movement, it is likely that high rodent densities are important at sites where A. maculatum arrive on more wide-ranging hosts. Large rodent populations may be necessary for a population of A. maculatum to establish in a new area, because rodents provide a critical food source for immature ticks. If the phenology curves are as expected (Paddock and Goddard, 2005; Portugal and Goddard, 2015; Teel et al. 2010), immature A. maculatum in Virginia should quest in the late summer and early fall, just as rodent populations reach seasonal peaks after reproducing during the spring and summer. Timing the arrival of A. maculatum to years in the successional process when field rodents have had time to establish robust populations but before the canopy closes may be critical to enable population establishment.
Small-bodied animals with home ranges <50 ha that disperse up to 200 km, including lagomorphs, sciurids, and birds that are partial migrants in Virginia have moderate dispersal potential for immature A. maculatum (Table S1). Partial migrants do not breed in Canada or winter in the neotropics, but will sometimes move up to a few hundred kilometers seasonally. While members of this group have the ability to disperse over long distances, they often do not move more than a few km from their natal area. Populations of these hosts are generally lower than those of small-bodied hosts with small home ranges, but the capacity of these longer-ranging hosts to transport immatures over distances is greater. Most movement of animals in this group occurs during the late summer and fall, when immature A. maculatum may be questing.
Medium and large mammals with home ranges over 50 ha that disperse up to and beyond 200 km are likely to be the most important long distance dispersers of adult ticks (Table S1). Adult A. maculatum quest in the summer months, which corresponds to the timing when first year offspring of these large mammals are dispersing from their place of birth. The natal dispersal timing of juvenile striped skunks (Mephitis mephitis), white-tailed deer, black bears (Ursus americanus), and bobcats (Lynx rufus) aligns particularly well with A. maculatum phenology. Adult A. maculatum were collected into August and September, so it is possible that dispersing juvenile coyotes (Canis latrans), grey foxes (Urocyon cinereoargenteus), and raccoons (Procyon lotor) which generally move in fall would also have an opportunity to facilitate movement of these ticks from habitat patch to habitat patch. Male A. maculatum attach to hosts and then release a pheromone that aggregates females to mate, increasing the tick load on these large-bodied hosts and the likelihood that hosts will distribute multiple ticks across a landscape (Gladney, 1971; Gladney et al., 1974). Because A. maculatum mate on-host, engorged females can lay eggs directly after detaching in a suitable habitat. Because these mammals can host many adult ticks and move them over long distances in short amounts of time, larger mammals translocate the ticks that are most able to found new populations. If engorged females drop off in areas of suitable habitat with high densities of small mammal and bird hosts, their larvae stand a good chance of survival.
Adult ticks are not the only life stage that can be moved long distances. Migratory birds are known hosts for immature A. maculatum, and have been documented moving engorged larval and nymphal ticks thousands of kilometers during spring and fall migrations from the ancestral range of A. maculatum in the neotropics north to Canada (Ogden et al., 2008; Teel et al., 2010; Scott et al., 2012; Florin et al., 2014). These birds may transport A. maculatum immatures southward during the fall, and northward during the spring. Immature A. maculatum were collected during the spring, summer, and fall in Virginia. These ticks may move northward during the spring, continuing the invasion into the Mid-Atlantic, or southward in the fall back to sites in areas where A. maculatum is already established. Once engorged immatures drop off birds in an appropriate habitat, they must survive to molt, mate, and reproduce in order to seed a new population. Because of challenges associated with survival in the environment, including predation and desiccation (Needham and Teel, 1991), models of tick dispersal have shown faster population establishment with mammalian dispersers (Leighton et al., 2012).
Genetic evidence indicates that long distance dispersal must be taking place in order to facilitate gene flow between remote populations, and that geographically proximate populations of A. maculatum do not have higher levels of gene flow than distant ones (Nadolny et al., 2015). This recent study suggests that a combination of mammalian and avian hosts are likely responsible for dispersal, but that dispersal of immatures by migratory birds is necessary to facilitate the observed gene flow between far-flung populations. Combining genetic evidence of gene flow between populations with our understanding of preferred hosts that are abundant in Virginia (Table S1), the most likely pathway for a new population of these ticks to arise in the Mid-Atlantic seems to be through the introduction of multiple genotypes to an anthropogenically disturbed isolated habitat patch by migratory birds and long-distance dispersing mammals. The habitat patch must be at an appropriate stage in ecological succession to be dominated by large populations of rodent hosts in order to provide sufficient blood meals for immature ticks. Using abundant local hosts, a population can grow to large numbers in a short period of time. Once established, the A. maculatum population may be more likely to persist only until the canopy closes and the community of small, high-density hosts such as field rodents declines (Langley and Shure, 1980). Ticks may then disperse to other patches, either geographically proximate or distant.
Adult A. maculatum were found feeding on domestic dogs and cats in Virginia, and anthropogenic movement of these ticks may be an important factor in this tick’s range expansion. Amblyomma maculatum adults have been removed from dogs and cats with no travel history in Ontario, Canada, where these ticks are not established (Scott et al., 2001). Willingness to feed on domesticated animals may enable A. maculatum to invade human-dominated areas faster than otherwise predicted.
In conclusion, tick ranges are not fixed in time or space and may change rapidly; the geographic range of A. maculatum is much more extensive today than it was 70 years ago (Teel et al., 2010; Paddock and Goddard, 2015). This tick species is one of the top four tick species parasitizing humans in the southeastern US, and human infections with R. parkeri seem to arise concurrently with the discovery of newly established A. maculatum populations (Paddock and Goddard, 2015). Because of the morphological similarities between A. maculatum and D. varabilis at all but one life stage, it may be difficult to tell when A. maculatum have arrived, so vigilance is required. Enhanced surveillance is recommended in anthropogenically disturbed open habitats during the summer months in Mid-Atlantic states to detect new populations of this invader. Once detected, medical and veterinary personnel should be alerted to the increased threat of pathogens vectored by this tick species.
Supplementary Material
Acknowledgments
Thanks to the entire ODU Tick Research Team for their assistance in collecting ticks. We also acknowledge the Nature Conservancy, Weyanoke Bird and Butterfly Sanctuary, Back Bay National Wildlife Refuge, Kiptopeke State Park, York River State Park, First Landing State Park, Hoffler Creek Wildlife Refuge, and the Elizabeth River Project for permission to use their land. The project was supported in part by grant no. K25AI067791 (to H.D.G.) from the National Institute of Allergy and Infectious Diseases. R. Nadolny was supported by the Science, Mathematics and Research for Transformation (SMART) scholarship from the Department of Defense and the American Society for Engineering Education. This project was also supported by the Jayne Koskinas Ted Giovanis Foundation for Health and Policy, a private foundation based in Highland, Maryland dedicated to effecting change in health care for the greater public good. The opinions, findings, and conclusions or recommendations expressed in this material are those of the author and not necessarily those of the Jayne Koskinas Ted Giovanis Foundation for Health and Policy, its directors, officers, or staff.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Ammon ME. In: Lincoln’s sparrow (Melospiza lincolnii), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 1995. Retrieved from the Birds of North America Online: https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Baneth G. Perspectives on canine and feline hepatozoonosis. Vet. Parasitol. 2011;181:3–11. doi: 10.1016/j.vetpar.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Bekoff M. Canis latrans. Mamm. Species. 1977:1–9. [Google Scholar]
- Bendel PR, Gates JE. Home range and microhabitat partitioning of the southern flying squirrel (Glaucomys volans) J. Mammal. 1987;68:243–255. [Google Scholar]
- Best LB. Nestling biology of the Field Sparrow. The Auk. 1977:308–319. [Google Scholar]
- Blair WF. The Florida marsh rabbit. J. Mammal. 1936;17:197–207. [Google Scholar]
- Brennan LA, Hernandez F, Williford D. In: Northern bobwhite (Colinus virginianus), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2014. Retrieved from the Birds of North America, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Cameron GN, Kincaid WB. Species removal effects on movements of Sigmodon hispidus and Reithrodontomys fulvescens. Am. Midl. Nat. 1982;1:60–67. [Google Scholar]
- Cameron GN, Spencer SR. Sigmodon hispidus. Mamm. Species. 1981;158:1–9. [Google Scholar]
- Carey M, Carey M, Burhans DE, Nelson DA. In: Field sparrow (Spizella pusilla), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2008. Retrieved from the Birds of North America, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Cavitt JF, Haas CA. In: Brown thrasher (Toxostoma rufum), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2014. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Chapman JA, Hockman JG, Ojeda MM. Sylvilagus floridanus. Mamm. Species. 1980;136:1–8. [Google Scholar]
- Chapman JA, Willner GR. Sylvilagus palustris. Mamm. Species. 1981;153:1–3. [Google Scholar]
- Clifford CM, Anastos G, Elbl A. The larval ixodid ticks of the eastern United States (Acarina-Ixodidae) Misc. Publ. Entomol. Soc. Am. 1961;2:213–237. [Google Scholar]
- Cohen SB, Yabsley MJ, Garrison LE, Freye JD, Dunlap BG, Dunn JR, Mead DG, Jones TF, Moncayo AC. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA. Emerg. Infect. Dis. 2009;15:1471–1473. doi: 10.3201/eid1509.090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DE, Emlen JT. The placental scar as a measure of fertility in rats. J. Wildlife Manage. 1948;12:162–166. [Google Scholar]
- Dolan PG, Carter DC. Glaucomys volans. Mamm. Species. 1977;78:1–6. [Google Scholar]
- Drummond RO, Whetstone TM. Oviposition of the Gulf Coast Tick. J. Econ. Entomol. 1970;63:1547–1551. [Google Scholar]
- Estrada-Peña A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone A. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst. Parasitol. 2005;60:99–112. doi: 10.1007/s11230-004-1382-9. [DOI] [PubMed] [Google Scholar]
- Evans M, Gow E, Roth RR, Johnson MS, Underwood TJ. In: Wood thrush (Hylocichla mustelina), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2011. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Ferrari FA, Goddard J, Paddock CD, Varela-Stokes AS. Rickettsia parkeri and Candidatus Rickettsia andeanae in Gulf Coast ticks, Mississippi, USA. Emerg. Infect. Dis. 2012;18:1705–1707. doi: 10.3201/eid1810.120250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish D, Howard C. Methods used for creating a national Lyme disease risk map. Mor Mortal Wkly Rep CDC Surveill. Summ. 1999;48:21–24. [Google Scholar]
- Florin DA, Brinkerhoff RJ, Gaff H, Jiang J, Robbins RG, Eickmeyer W, Butler J, Nielsen D, Wright C, White A, Gimpel ME, Richards AL. Additional U.S. collections of the Gulf Coast tick, Amblyomma maculatum (Acari: Ixodidae), from the State of Delaware, the first reported field collections of adult specimens from the State of Maryland, and data regarding this tick from surveillance. Syst. Appl. Acarol. 2014;19:257–262. [Google Scholar]
- Fornadel CM, Zhang X, Smith JD, Paddock CD, Arias JR, Norris DE. High rates of Rickettsia parkeri infection in Gulf Coast ticks (Amblyomma maculatum) and identification of “Candidatus Rickettsia Andeanae” from Fairfax County, Virginia. Vector-Borne Zoonotic Dis. 2011;11:1535–1539. doi: 10.1089/vbz.2011.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzell EK, Haroldson KJ. Urocyon cinereoargenteus. Mamm. Species. 1982:1–8. [Google Scholar]
- Gaines DN, Operario DJ, Stroup S, Stromdahl E, Wright C, Gaff H, Broyhill J, Smith J, Norris DE, Henning T, Lucas A, Houpt E. Ehrlichia and Spotted Fever Group rickettsiae surveillance in Amblyomma americanum in Virginia through use of a novel six-plex real-time PCR Assay. Vector-Borne Zoonotic Dis. 2014;14:307–316. doi: 10.1089/vbz.2013.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladney WJ. Mate-seeking by female Amblyomma maculatum (Acarina: Ixodidae) on a bovine. Nature. 1971;232:401–402. doi: 10.1038/232401a0. [DOI] [PubMed] [Google Scholar]
- Gladney WJ, Grabbe RR, Ernst SE, Oehler DD. The Gulf Coast tick: evidence of a pheromone produced by males. J. Med. Entomol. 1974;11:303–306. doi: 10.1093/jmedent/11.3.303. [DOI] [PubMed] [Google Scholar]
- Goddard J. Seasonal activity of Amblyomma spp. in Mississippi. J. Vector Ecol. 2007;32:157–158. doi: 10.3376/1081-1710(2007)32[157:saoasi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Goddard J, Paddock CD. Observations on distribution and seasonal activity of the Gulf Coast tick in Mississippi. J. Med. Entomol. 2005;42:176–179. doi: 10.1093/jmedent/42.2.176. [DOI] [PubMed] [Google Scholar]
- Gowaty PA, Plissner JH. In: Eastern bluebird (Sialia sialis), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2015. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved : 19 August 2017. [Google Scholar]
- Greenlaw JS. In: Eastern towhee (Pipilo erythrophthalmus), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2015. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Guzy MJ, Ritchison G. In: Common yellowthroat (Geothlypis trichas), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 1999. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Haggerty TM, Morton ES. In: Carolina wren (Thryothorus ludovicianus), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2014. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Halkin SL, Linville SU. In: Northern cardinal (Cardinalis cardinalis), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 1999. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Harrison BA, Rayburn WH, Toliver M, Powell EE, Engber BR, Durden La, Robbins RG, Prendergast BF, Whitt PB. Recent discovery of widespread Ixodes affinis (Acari: Ixodidae) distribution in North Carolina with implications for Lyme disease studies. J. Vector Ecol. 2010;35:174–179. doi: 10.1111/j.1948-7134.2010.00044.x. [DOI] [PubMed] [Google Scholar]
- Heller EL, Wright CL, Nadolny RM, Hynes WL, Gaff HD, Walters EL. New Records of Ixodes affinis (Acari: Ixodidae) parasitizing avian hosts in southeastern Virginia. J. Med. Entomol. 2016;53:441–445. doi: 10.1093/jme/tjv175. [DOI] [PubMed] [Google Scholar]
- Henning TC, Orr JM, Smith JD, Arias JR, Norris DE. Spotted fever group Rickettsiae in multiple hard tick species from Fairfax County, Virginia. Vector-Borne Zoonotic Dis. 2014;14:482–485. doi: 10.1089/vbz.2013.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkert JR, Kroodsma DE, Gibbs JP. In: Sedge wren (Cistothorus platensis), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2001. Retrieved from the Birds of North America Online, https://birdsna.org. [Google Scholar]
- Jackson BJ, Jackson JA. In: Killdeer (Charadrius vociferus), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2000. Retrieved from the Birds of North America Online, https://birdsna.org. [Google Scholar]
- Jaster LA, Jensen WE, Lanyon WE. In: Eastern meadowlark (Sturnella magna), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2012. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Jiang J, Stromdahl EY, Richards AL. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from humans in the United States. Vector-Borne Zoonotic Dis. 2012;12:175–182. doi: 10.1089/vbz.2011.0614. [DOI] [PubMed] [Google Scholar]
- Johnson BJ. M.S. Thesis. Oklahoma State University; Stillwater: 1990. Seasonal distribution and population dynamics of Amblyomma maculatum Koch: Ixodidae in Oklahoma. [Google Scholar]
- Johnson LS. In: House wren (Troglodytes aedon), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2014. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Keirans JE, Durden LA. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 1998;35:489–495. doi: 10.1093/jmedent/35.4.489. [DOI] [PubMed] [Google Scholar]
- Kershner EL, Walk JW, Warner RE. Postfledging movements and survival of juvenile eastern meadowlarks (Sturnella magna) in Illinois. Auk. 2004;121:1146–1154. [Google Scholar]
- Koprowski JL. Sciurus carolinensis. Mamm. Species. 1994;480:1–9. [Google Scholar]
- Krohne DT, Dubbs BA, Baccus R. An analysis of dispersal in an unmanipulated population of Peromyscus leucopus. Am. Midl. Nat. 1984;112:146–156. [Google Scholar]
- Lackey JA, Huckaby DG, Ormiston BG. Peromyscus leucopus. Mamm. Species. 1985;247:1–10. [Google Scholar]
- Langley AK, Jr, Shure DJ. The effects of loblolly pine plantations on small mammal populations. Am. Mid. Nat. 1980;103:59–65. [Google Scholar]
- Larivière S. Ursus americanus. Mamm. Species. 2001;647:1–11. [Google Scholar]
- Larivière S, Walton LR. Lynx rufus. Mamm. Species. 1997:1–8. [Google Scholar]
- Leighton Pa, Koffi JK, Pelcat Y, Lindsay LR, Ogden NH. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 2012;49:457–464. [Google Scholar]
- Lotze JH, Anderson S. Procyon lotor. Mamm. Species. 1979:1–8. [Google Scholar]
- Lowther PE. In: Brown-headed cowbird (Molothrus ater), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 1993. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- McAllister CM, Durden LA, Robison HW. The ticks (Arachnida: Acari: Ixodida) of Arkansas. Journal of the Arkansas Academy of Science. 2016;70:141–154. [Google Scholar]
- McDiarmid RW, Foster MS, Guyer C, Gibbons JW, Chernoff N. Reptile biodiversity: standard methods for inventory and monitoring. University of California Press; 2011. [Google Scholar]
- McGuire B, Pizzuto T, Bemis WE, Getz LL. General ecology of a rural population of Norway rats (Rattus norvegicus) based on intensive live trapping. Am. Midl. Nat. 2006;155:221–236. [Google Scholar]
- Mowbray TB. In: Swamp sparrow (Melospiza georgiana), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 1997. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Nadolny R, Gaff H, Carlsson J, Gauthier D. Comparative population genetics of two invading ticks: evidence of the ecological mechanisms underlying tick range expansions. Infect. Genet. Evol. 2015;35:153–162. doi: 10.1016/j.meegid.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham GR, Teel PD. Off-host physiological ecology of ixodid ticks. Annual Rev. Entomol. 1991;36:659–681. doi: 10.1146/annurev.en.36.010191.003303. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Hanincová K, Barker IK, Bigras-Poulin M, Charron DF, Heagy A, Francis CM, O’Callaghan CJ, Schwartz I, Thompson RA. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008;74:1780–1790. doi: 10.1128/AEM.01982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SLF, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME. Rickettsia parkeri Rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin. Infect. Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Fournier PE, Sumner JW, Goddard J, Elshenawy Y, Metcalfe MG, Loftis AD, Varela-Stokes A. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl. Environ. Microbiol. 2010;76:2689–2696. doi: 10.1128/AEM.02737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Goddard J. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae) J. Med. Entomol. 2015;52:230–252. doi: 10.1093/jme/tju022. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SLF, Tamminga CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Pagac BB, Miller MK, Mazzei MC, Nielsen DH, Jiang J, Richards AL. Rickettsia parkeri and Rickettsia montanensis, Kentucky and Tennessee, USA. Emerg. Infect. Dis. 2014;20:1750–1752. doi: 10.3201/eid2010.140175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardieck KL, Ziolkowski DJ, Jr, Hudson MAR. North American Breeding Bird Survey Dataset 1966 – 2014, version 2014. U.S. Geological Survey, Patuxent Wildlife Research Center. 2015 < www.pwrc.usgs.gov/BBS/RawData/>. Date retrieved: 19 August 2017.
- Payne NF. Range extension of the marsh rabbit in Virginia. Chesapeake Science. 1975;16:77–78. [Google Scholar]
- Peer BD, Bollinger EK. In: Common grackle (Quiscalus quiscula), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 1997. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Portugal JS, III, Goddard J. Collections of immature Amblyomma maculatum Koch (Acari: Ixodidae) from Mississippi, USA. Syst. Appl. Acarol. 2015;20:20–24. [Google Scholar]
- Recht MA. The biology of domestic rats: telemetry yields insight for pest control. Proceedings of the 13th Vertebrate Pest Conference. 1988;21:98–100. [Google Scholar]
- Rose RK. Instructions for building two live traps for small mammals. Va. J. Sci. 1994;45:151–158. [Google Scholar]
- Scifres CJ, Oldham TW, Teel PD, Drawe DL. Gulf Coast tick (Amblyomma maculatum) populations and responses to burning of coastal prairie habitats. southwest. Assoc. Nat. 1988;33:55–64. [Google Scholar]
- Scott JD, Anderson JF, Durden LA. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J. Parasitol. 2012;98:49–59. doi: 10.1645/GE-2874.1. [DOI] [PubMed] [Google Scholar]
- Scott JD, Fernando K, Banerjee SN, Durden LA, Byrne SK, Banerjee M, Mann RB, Morshed MG. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J. Med. Ent. 2001;38:493–500. doi: 10.1603/0022-2585-38.4.493. [DOI] [PubMed] [Google Scholar]
- Sikes RS, Gannon WL. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011;92:235–253. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KG, Tarvin KA, Woolfenden GE. In: Blue jay (Cyanocitta cristata), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2013. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Smith Robert J, Hatch Margret I, Cimprich David A, Moore Frank R. In: Gray Catbird (Dumetella carolinensis), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2011. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Smith WP. Odocoileus virginianus. Mamm. Species. 1991;388:1–13. [Google Scholar]
- Stalling DT. Reithrodontomys humulis. Mamm. Species. 1997;565:1–6. [Google Scholar]
- Stromdahl EY, Hickling GJ. Beyond Lyme: Aetiology of tick-borne human diseases with emphasis on the southeastern United States. Zoonoses Public Hlth. 2012;59:48–64. doi: 10.1111/j.1863-2378.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Paddock CD. Gulf coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg. Infect. Dis. 2007;13:751–753. doi: 10.3201/eid1305.061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GD, Harestad AS, Price K, Lertzman KP. Scaling of natal dispersal distances in terrestrial birds and mammals. Conserv. Ecol. 2000;4:16. [Google Scholar]
- Teel PD, Hopkins SW, Donahue WA, Strey OF. Population dynamics of immature Amblyomma maculatum (Acari: Ixodidae) and other ectoparasites on meadowlarks and northern bobwhite quail resident to the coastal prairie of Texas. J. Med. Entomol. 1998;35:483–488. doi: 10.1093/jmedent/35.4.483. [DOI] [PubMed] [Google Scholar]
- Teel PD, Ketchum HR, Mock DE, Wright RE, Strey OF. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 2010;47:707–722. doi: 10.1603/me10029. [DOI] [PubMed] [Google Scholar]
- Trout RT, Steelman CD, Szalanski AL, Loftin K. Establishment of Amblyomma maculatum (Gulf Coast Tick) in Arkansas, U.S.A. Florida Entomol. 2010;93:120–122. [Google Scholar]
- Vanderhoff N, Sallabanks R, James FC. In: American robin (Turdus migratorius), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2014. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Varela-Stokes AS, Paddock CD, Engber B, Toliver M. Rickettsia parkeri in Amblyomma maculatum ticks, North Carolina, USA, 2009–2010. Emerg. Infect. Dis. 2011;17:2350–2353. doi: 10.3201/eid1712.110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery PD. In: Grasshopper Sparrow (Ammodramus savannarum), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 1996. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Wade-Smith J, Verts BJ. Mephitis mephitis. Mamm. Species. 1982;173:1–7. [Google Scholar]
- Whisson DA, Quinn JH, Collins KC. Home range and movements of roof rats (Rattus rattus) in an old-growth riparian forest, California. J. Mammal. 2007;88:589–594. [Google Scholar]
- Whitman TJ, Richards AL, Paddock CD, Tamminga CL, Sniezek PJ, Jiang J, Byers DK, Sanders JW. Rickettsia parkeri infection after tick bite, Virginia. Emerg. Infect. Dis. 2007;13:334–336. doi: 10.3201/eid1302.061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RW. Neotoma floridana. Mamm. Species. 1980;139:1–7. [Google Scholar]
- Williams RE, Hair JA. Influence of Gulf Coast ticks on blood composition and weights of eastern meadowlarks in Oklahoma. Ann. Entomol. Soc. Am. 1976;69:403–404. [Google Scholar]
- Wilson WH., Jr . In: Palm warbler (Setophaga palmarum), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 2013. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
- Wolfe JL. Oryzomys palustris. Mamm. Species. 1982;176:1–5. [Google Scholar]
- Wood Gene W, Barrett RH. Status of wild pigs in the United States. Wildlife Soc. B. 1979;7:237–246. [Google Scholar]
- Wright CL, Nadolny RM, Jiang J, Richards AL, Sonenshine DE, Gaff HD, Hynes WL. Rickettsia parkeri in Gulf Coast ticks, southeastern Virginia, USA. Emerg. Infect. Dis. 2011;17:896–898. doi: 10.3201/eid1705.101836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Sonenshine DE, Gaff HD, Hynes WL. Rickettsia parkeri transmission to Amblyomma americanum by cofeeding with Amblyomma maculatum (Acari: Ixodidae) and potential for spillover. J. Med. Entomol. 2015;52:1090–1095. doi: 10.1093/jme/tjv086. [DOI] [PubMed] [Google Scholar]
- Wright JE. Response of ovipositing Amblyomma maculatum Koch (Acarina: Ixodidae) to photoperiod. J. Med. Entomol. 1971;8:529–531. doi: 10.1093/jmedent/8.5.529. [DOI] [PubMed] [Google Scholar]
- Yasukawa K, Searcy WA. In: Red-winged blackbird (Agelaius phoeniceus), The Birds of North America Online. Poole A, editor. Ithaca: Cornell Lab of Ornithology; 1995. Retrieved from the Birds of North America Online, https://birdsna.org. Date retrieved: 19 August 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.