Abstract
Prescription drug monitoring programs (PDMPs) are a response to the prescription opioid epidemic, but their impacts on prescribing and health outcomes remain unclear, with conflicting reports. We sought to determine if prescriber use of Oregon’s prescription drug monitoring program (PDMP) led to fewer high-risk opioid prescriptions or overdose events. We conducted a retrospective cohort study from October, 2011 through October, 2014, using statewide PDMP data, hospitalization registry, and vital records. Early PDMP registrants (n=927) were matched with clinicians who never registered during the study period, using baseline prescribing metrics in a propensity score. Generalized estimating equations were used to examine prescribing trends following PDMP registration, using 2-month intervals. We found a statewide decline in measures of per capita opioid prescribing. However, compared with non-registrants, PDMP registrants did not subsequently have significantly fewer patients receiving high-dose prescriptions; overlapping opioid and benzodiazepine prescriptions, inappropriate prescriptions, prescriptions from multiple prescribers, or overdose events. At baseline, frequent PDMP users wrote fewer high-risk opioid prescriptions than infrequent users; this persisted during follow-up with few significant group differences in trend. Thus, although opioid prescribing declined statewide after implementing the PDMP, registrants did not demonstrate greater declines than non-registrants.
Keywords: Prescription Drug Monitoring Program, opioids, risky prescribing, health policy, cohort study
INTRODUCTION
Prescription opioid use in the United States increased over much of the past two decades, with parallel increases in opioid-related overdoses, mortality, hospitalization, and addiction treatment.24,25,28 One response has been creation of electronic internet-based prescription drug monitoring programs (PDMPs) in nearly every state, tracking outpatient prescriptions for controlled substances. By allowing prescribers and pharmacists to view all prescriptions for controlled substances from all sources, these programs were intended to facilitate judicious prescribing and to illuminate the activities of prescribers, pharmacies, and patients. Creating and using these systems became a central recommendation of the White House Office of National Drug Control Policy13 and a part of recent opioid prescribing recommendations from the Centers for Disease Control (CDC).10
Despite growing use, the impact of PDMPs on opioid prescribing and health outcomes remains unclear.12 Some studies suggested important changes in prescribing and overdose risk,2,27 while others reported little or no impact.4,21,22,29 Only a few studies quantified effects on overdose hospitalizations or mortality, with conflicting results.21,22,27,29 Most PDMP evaluations compared states with and without such programs, or prescribing patterns before and after implementation. Such comparisons may be confounded by other influences on prescribing (e.g., new medical literature, clinical guidelines, insurance coverage policies, media reports, and other changes in public policy). State-to-state variations in PDMP design and policies are substantial, and may also influence comparisons.26
Like most states, Oregon experienced increases in opioid prescribing, misuse, and overdoses after 2000.25 The state implemented a PDMP that became available to clinicians in September, 2011, with voluntary registration and use.8 Seeking to minimize some confounding risks in previous studies, we evaluated Oregon’s PDMP by comparing prescribing patterns and patient outcomes of clinicians with similar baseline prescribing patterns who did or did not register to use the system.
The analyses had 3 aims:
Describe statewide trends in opioid prescribing and health impact of opioids (opioid-related deaths and hospitalizations) following initiation of the PDMP.
Compare changes in opioid prescribing between early PDMP registrants and non-registrants. Among PDMP registrants, we also examined changes in prescribing according to frequency of PDMP use.
Assess the likelihood of opioid-related mortality or hospitalization among patients of clinicians who were early registrants versus those who did not register for the PDMP.
We hypothesized that opioid prescribing and related hospitalization and death declined statewide following initiation of the PDMP (Aim 1). We further hypothesized that clinicians who registered for the PDMP, compared to those who did not, would decrease the proportion of patients with high opioid doses, the proportion with risky co-prescriptions, the number with opioid prescriptions from multiple prescribers, and the number of inappropriate prescriptions. Similarly, we hypothesized that among registrants, those who used the PDMP frequently would demonstrate greater changes in these measures than those who used the PDMP infrequently (Aim 2). Finally, we hypothesized that, compared with non-registrants, registrants would have fewer patients who were hospitalized or died from opioid overdoses (Aim 3).
METHODS
Overview
This project was approved by Institutional Review Boards at Oregon Health & Science University and the Public Health Division of the Oregon Health Authority, where the PDMP is housed. Data were obtained from the Oregon PDMP, Oregon vital records, and a statewide hospital discharge registry. We first examined statewide trends in prescribing and health outcomes following initiation of the PDMP. To better assess the degree to which those trends might be related to PDMP use, we then compared changes in opioid prescribing patterns between clinicians who registered for the PDMP during 3 early months of operation and those who never registered. We focused on early registrants because they were more likely than later registrants to write frequent and high-dose opioid prescriptions, and others have reported that high-risk prescribers are disproportionately responsive to state policices.6 Because of substantial differences in opioid prescribing during the pre-registration period between those who did or did not register for the PDMP, we identified pairs of early-registered and nonregistered clinicians with similar pre-registration prescribing patterns. Finally, we compared opioid prescribing between registrants who accessed the PDMP frequently, and those who accessed it infrequently.
The Oregon PDMP
Licensed prescribers are encouraged to register online, and pay a small annual fee for use of the PDMP. The program is accessible online with a password at all times, but is not integrated with electronic medical records. Oregon neither requires registration nor mandates use of the PDMP system in any specific circumstances. Prescribers can voluntarily access an online “dashboard” that identifies patients with unusual prescription patterns, but there is no proactive alert system that identifies patients with alarming doses, numbers of prescribers, or drug combinations.
Preparation of PDMP data
Our de-identified analytic file included PDMP data from October 1, 2011 through October 31, 2014. Patient age was categorized by a public health analyst into 10-year intervals to maximize anonymity. Gender, ethnicity, race, other demographic data, and payment source were not collected during study years.
Oregon’s PDMP is maintained by a commercial vendor. Because the vendor uses a largely deterministic, proprietary algorithm for matching prescription fills for a single patient, it may not always uniquely identify patients in the face of nicknames, misspellings, transposed digits or characters, name changes, or changes in residence. A public health analyst therefore used probabilistic linking software (The Link King v7.1.21)3,5 to match individuals within and between data sets, based on name, birthdate, and ZIP Code.
Inclusion of prescriptions
We identified opioid preparations using Food and Drug Administration (FDA) National Drug Codes. Tramadol was not included in the PDMP during study years, and we excluded buprenorphine-naloxone combinations. Conversion reference tables from CDC were used to calculate “morphine milligram equivalents” (MME) for each prescription fill. A clinical pharmacist (NO) assigned a conversion factor if one was not available, as well as designation as long- or short-acting, based on information from the drug name and drugs with equivalent features.23
Inclusion of Clinicians
The primary analysis included “early registrants” who registered for the PDMP in December, 2011 through February, 2012, the “registration interval”. This provided 2 months (October and November, 2011) of PDMP data prior to registration for all clinicians (the “baseline interval”). Non-registrants were clinicians who had not registered for the PDMP as of October, 2014.
Clinicians who never wrote a prescription for opioids in our data set were excluded. We also excluded clinicians who registered for the PDMP before December 1, 2011, because we could not obtain 2 full months of baseline prescribing data.
Measures of PDMP Querying Behavior
To compare registrants who frequently accessed the PDMP with those who did not, we examined the ratio of database queries to opioid prescriptions filled over the full length of our database. We defined high and low use based on the median ratio, approximately 1 query for every 6 opioid prescriptions.
Outcome measures
The primary prescribing outcomes were 4 metrics associated with an increased risk of opioid overdose: high doses (≥ 90 MME/day), overlapping opioid and benzodiazepine prescriptions, opioid prescriptions from multiple prescribers (≥3 in any 2-month interval), and inappropriate opioid prescriptions.9,10,14,30 The definition of “high dose” corresponded to a recommendation in recent CDC guidelines that discouraged prescriptions exceeding this dose.10 Although the Oregon PDMP did not record a days’ supply during the study years, we defined an opioid prescription as overlapping a benzodiazepine prescription if it occurred within 30 days before or after the benzodiazepine prescription. The definition of an “inappropriate opioid prescription” was adapted from a previous study,9 and represented a new prescription for the same opioid following a prescription of at least 30 tablets, occurring within 7 days, from a different prescriber. These were chosen as primary outcomes because of the previously demonstrated associations with outcome risks.
Secondary Measures included 10 additional measures of opioid prescribing designed to capture numbers of prescriptions, doses per prescription and related factors (Table 1). Trends were examined by calculating prescribing metrics for each 2-month interval from October, 2011 through September, 2014.
Table 1.
Baseline opioid prescribing of PDMP early registrants and non-registrants, before and after propensity score matchinga
| Before matching | After propensity score matching | |||||
| Prescribing metric, per prescriber during the 2-month baseline time period, mean (SD) | Non-Registered n = 12,005 |
Registered n = 964 |
SMDb | Non-Registered n = 927 |
Registered n = 927 |
SMDb |
| Primary prescribing outcomes | ||||||
| Opioid patients with an average daily MME ≥90 over the time period (2 months)c | 0.79 (3.9) | 6.06 (17.0) | 0.428 | 3.9 (9.9) | 4.5 (11.4) | 0.063 |
| % of opioid prescriptions that overlap a sedative-hypnotic prescription within 30 daysd | 5.8 (14.5) | 9.6 (10.6) | 0.296 | 9.5 (13.3) | 9.2 (10.5) | 0.024 |
| Patients with ≥ 3 opioid prescribers in a 2 mo. interval | 1.3 (3.2) | 4.8 (6.2) | 0.717 | 4.5 (6.4) | 4.5 (5.7) | <0.001 |
| Inappropriate opioid prescriptionse | 0.23 (0.9) | 0.59 (1.1) | 0.354 | 0.60 (1.4) | 0.56 (1.1) | 0.030 |
| Secondary prescribing outcomes | ||||||
| Opioid prescriptions | 18.5 (44.2) | 88.0 (125) | 0.743 | 72.9 (88.2) | 76.3 (103) | 0.036 |
| Benzodiazepine prescriptions | 6.0 (20.5) | 29.7 (48.0) | 0.643 | 24.8 (43.5) | 25.8 (39.3) | 0.025 |
| Units per opioid prescription (most often pills) | 26.7 (40.4) | 49.0 (36.3) | 0.583 | 47.6 (37.4) | 47.5 (35.6) | 0.002 |
| Patients who filled at least one opioid prescription | 12.0 (24.8) | 51.1 (56.6) | 0.897 | 44.8 (47.4) | 46.0 (49.3) | 0.026 |
| % of patients with overlapping long-acting and short-acting opioid prescriptions within 30 d | 2.8 (10.0) | 6.0 (9.2) | 0.329 | 5.4 (10.3) | 5.5 (8.5) | 0.017 |
| Opioid prescriptions per patient with at least one opioid prescription | 1.4 (0.60) | 1.6 (0.51) | 0.292 | 1.6 (0.93) | 1.5 (0.49) | 0.054 |
| Total MME per prescriptionc | 273 (606) | 616 (692) | 0.529 | 568 (754) | 578 (649) | 0.014 |
| Total MME per patient with at least one opioid prescriptionc | 694.6 (1,851.6) | 1,454.0 (1,825.7) | 0.413 | 1,298.0 (1,713.7) | 1,345.6 (1,653.7) | 0.028 |
| Patients with ≥ 3 opioid-dispensing pharmacies in 2 mo. | 0.91 (3.1) | 4.5 (8.9) | 0.538 | 3.4 (6.1) | 3.7 (6.4) | 0.056 |
| Prescribers with at least 1 opioid prescription in baseline period, n (%) | 7,089 (59.1) | 878 (91.1) | 0.797 | 846 (91.3) | 841 (90.7) | 0.019 |
| Geographic Location | ||||||
| Prescribers in an urban location, n (%) | 9,538 (80) | 707 (73) | 0.144 | 629 (68) | 672 (73) | 0.102 |
Matching based on all covariates and a caliper of .05 SD of the propensity score using “baselline” data from October – November, 2011. Registrants are those who registered in December 2011 – February 2012, and non-registrants were those who never registered before November, 2014.
SMD = Standardized Mean Difference
Metrics that use MME exclude prescriptions that did not have complete conversion factor, quantity, or strength information (0.2% of opioid prescriptions). MME calculations exclude buprenorphine and pentazocine.
“Sedative-hypnotics” included benzodiazepines, non-benzodiazepine sedative-hypnotics, and carisoprodol.
Inappropriate opioid prescriptions are those where a second prescription with the same drug name, from a different prescriber, was filled within 7 days of a first prescription that contained ≥30 pills.
We identified opioid-related deaths from Oregon vital records and opioid-related hospitalizations from the hospital discharge registry. Heroin-related deaths and hospitalizations were excluded, as were events if no opioid prescription was filled in the prior 5-months. A death or hospitalization was linked to the most recent prescriber of an opioid, sedative-hypnotic, or carisoprodol for that patient.
International Classification of Diseases, Version 9 Clinical Modification (ICD-9-CM) diagnosis codes for relevant hospitalizations were described earlier.7
Propensity Matching of PDMP Registrants and Non-registrants
Because PDMP registrants and non-registrants differed substantially in baseline opioid prescribing (Table 1) we undertook a propensity score matching process, using baseline prescribing measures. We performed one-to-one matching of registered and non-registered providers based on a propensity score derived from a multivariable logistic model that included all 14 prescribing metrics listed in Table 1 and an indicator for urban or rural ZIP code. We required a caliper of 0.05 standard deviation of the propensity score.
Statewide Population-based prescribing
We used quarterly data for measures of statewide prescribing and health outcomes. State population estimates for denominators were updated annually, using estimates from Portland State University’s Population Research Center. For context, the state population is approximately 4 million. We focused on 7 measures that gave a broad view of statewide prescribing patterns in terms of overall volume of opioid prescribing, use of multiple prescribers and pharmacies, potentially inappropriate prescribing, and health outcomes (hospitalization and death).
Examination of later PDMP registrants
Because early PDMP registrants differed from later registrants in their pre-registration prescribing patterns, we conducted an additional analysis comparing clinicians who registered a year after initiation of the PDMP with those who had not registered at all prior to October 2014. As with the early registrants, we used propensity score matching to identify pairs of clinicians with similar pre-registration prescribing patterns. We included a 6-month enrollment window (October 1, 2012 through March 31, 2013) because enrollment was slower at this point than early in the PDMP’s existence.
Analysis
The propensity score matching was tested by examining standardized mean differences (SMD) in the prescribing metrics before and after matching. An SMD of <0.1 is considered a negligible imbalance of covariate means between groups.1
The primary comparison between matched PDMP registrants and non-registrants was an assessment of prescribing trends over time, aggregating prescription data into 2-month intervals. For these analyses we used generalized estimating equations (GEE) regression modeling with robust sandwich variance estimators (GEE Poisson model for count data and GEE Gaussian regression for percentage data). The GEE models included an indicator for PDMP registration status, categorical time, and their interaction, as well as any factors that were imbalanced after propensity score matching. We clustered all models by matched provider and used a first-order autoregressive covariance structure to account for within-provider temporal correlation. We tested changes in prescribing outcomes over time between groups through an omnibus test of the two-way interaction between the indicator for PDMP registration and categorical time (termed parallel lines test).
To facilitate an understanding of actual numbers of prescriptions, patients, or doses, we also tabulated cumulative means of the prescribing metrics in the short term (6 months following the registration period) and the longer term (30 months following the registration period). Differences in rates were tested using a GEE Poisson model accounting for clustering of matched pairs assuming an exchangeable correlation structure and were adjusted for rural or urban physician location.
Comparisons between registrants who queried the PDMP frequently or infrequently were similarly performed using GEE Poisson models. Because these groups had substantial baseline prescribing differences, we adjusted the prescribing outcomes using Poisson regression, with the baseline prescribing metric and urban or rural location as covariates.
Deaths and hospitalizations were tabulated in the 6-month and 30-month intervals, then compared using a two-sample exact rate ratio test.11 Analysis was conducted using SAS v.9.4 (SAS Institute, Inc.) and R version 3.3.2; statistical significance was set at p-value<0.05.
RESULTS
Statewide trends
Statewide, there was a decrease in the number of opioid units (overwhelmingly pills, but including liquid doses, suppositories, patches, and injections) dispensed per capita beginning in the first quarter of PDMP operation and continuing through 3 years of operation (from 16.9 to 15.0 units per capita per quarter, Figure 1a). Similarly, there was a gradual downward trend in the total number of daily morphine equivalents dispensed per capita (from 2.80 per quarter to 2.41, Figure 1b).
Figure 1.
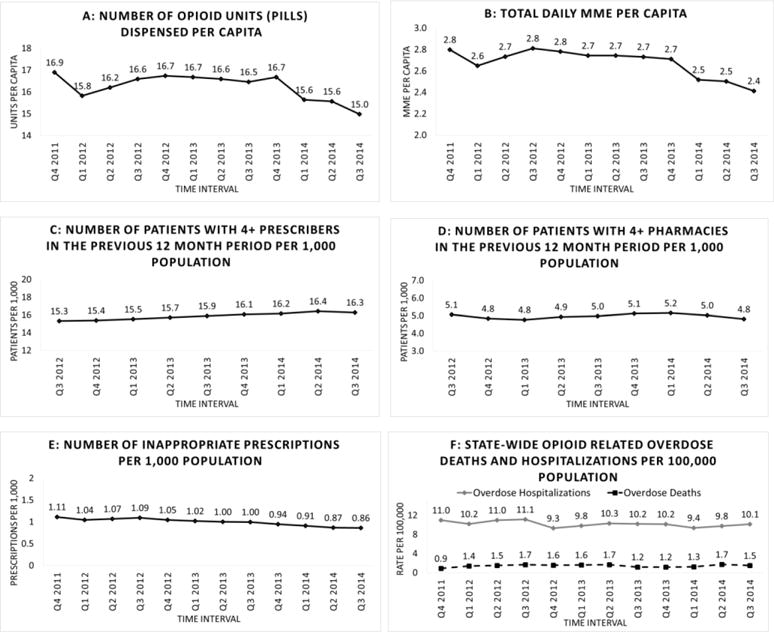
Statewide Oregon trends in selected measures of opioid prescribing and impact, October 2011–September, 2014
In contrast, there was no decrease in the number of patients (per 1,000 population) with ≥ 4 prescribers or using ≥ 4 pharmacies in the previous 12 month period (Figures 1 c and d). The number of inappropriate prescriptions per 1,000 population showed a slight gradual decline (From 1.11 to 0.86, Figure 1e).
The statewide number of opioid-related hospitalizations and overdose deaths per 1,000 population remained relatively constant over 3 years following initiation of the PDMP (Figure 1f).
Prescribers and overall prescriptions
There were 17,734 clinicians who wrote at least one opioid prescription between September 1, 2011 and October 31, 2014. Of these, 964 registered for the PDMP during our early registration interval. There were 12,005 who had not registered by October 31, 2014 (Figure 2). Baseline prescribing characteristics of registrants and non-registrants were substantially different, with non-registrants prescribing fewer opioids by every measure (Table 1). The propensity matching process successfully matched 927 early registrants with an equal number of non-registrants (Table 1), with SMDs less than 0.1 for all prescribing metrics. After matching, only urban or rural location of the clinician had an SMD >0.1, so this was used as a covariate in GEE models.
Figure 2.
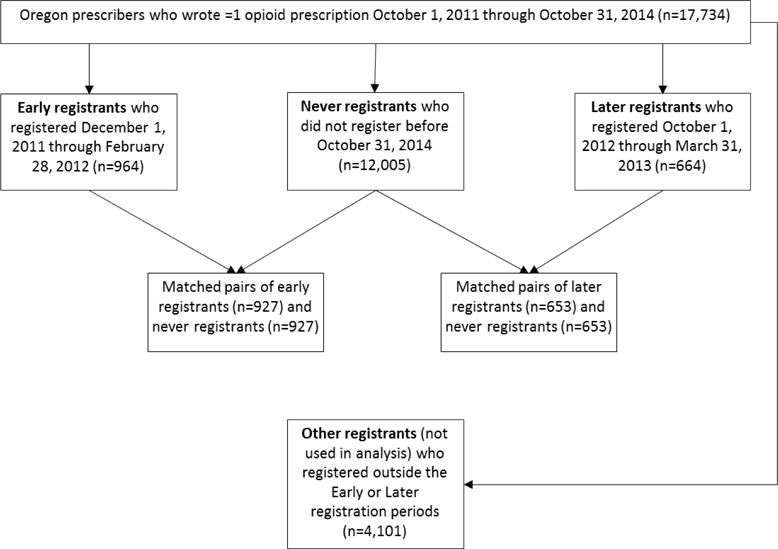
Derivation of prescriber samples for analysis.
The matched (registered and non-registered) clinicians combined wrote a mean of 75 opioid prescriptions during the 2-month baseline period, provided to a mean of 45 individual patients. The most common prescriptions were for short-acting opioids combined with acetaminophen, including hydrocodone, oxycodone or codeine (n = 93,498, 68% of prescriptions).
Comparing Registrants and Non-Registrants
The numbers of patients with high dose prescriptions, multiple prescribers, or inappropriate prescriptions fell gradually over time, in both registered and non-registered groups (Figure 3). However, contrary to our hypothesis, registered clinicians did not show greater or faster declines than non-registrants, either in the short-term or the long-term following PDMP registration (Figure 3 and Table 2). In fact, except for inappropriate prescriptions, registrants wrote slightly more of these prescriptions, though differences were small. The proportion of opioid prescriptions that overlapped with benzodiazepine prescriptions remained nearly flat in both groups, but with significantly more among registrants than non-registrants (Figure 3). In cumulative analyses over 30 months, registrants wrote significantly more high-dose prescriptions and overlaps, though non-registrants wrote more inappropriate prescriptions (Table 2).
Figure 3.
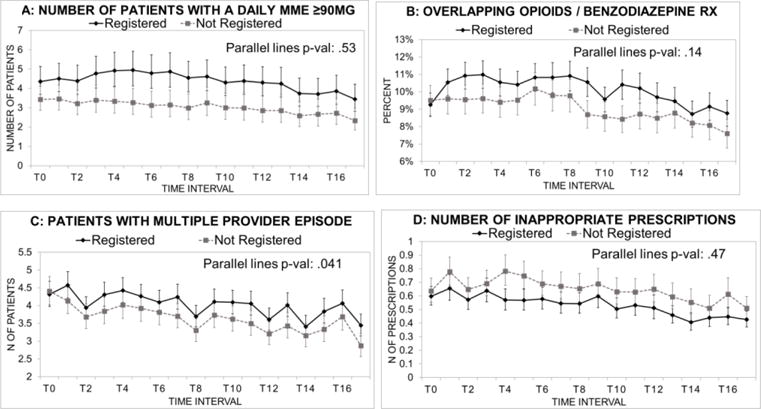
Trends in primary prescribing outcomes, comparing early PDMP registrants and matched non-registrants. Data are means per prescriber.a
a Data are based on GEE models adjusted for urban or rural location of the prescriber. Error bars are 95% confidence intervals. T0 through T16 represent 2-month time intervals beginning with October and November, 2011. Inappropriate opioid prescriptions are those where a second prescription with the same drug name, from a different prescriber, was filled within 7 days of a first prescription that contained ≥30 pills. A “multiple provider episode” refers to a patient with ≥ 3 opioid prescribers in the 2 month interval
Table 2.
Prescribing and health outcomes for matched registered and non-registered prescribers in the months following the “registration interval”.
| Prescribing in the 6 month interval March 2012-August, 2012 | Prescribing in the 30 month interval March 2012-August, 2014 | |||||
|---|---|---|---|---|---|---|
| Prescribing metric, per prescriber, adjusted mean (95% CI)a (deaths and hospitalizations are group totals) | Non- Registered n = 927 |
Registered n = 927 |
p- value | Non- Registered n =927 |
Registered n =927 |
p-value |
| Primary prescribing outcomes | ||||||
| Opioid patients with an average daily MME ≥90 in the intervalb | 4.46 (3.77, 5.26) | 6.04 (5.08, 7.17) | .012 | 6.08 (5.25, 7.05) | 7.98 (6.91, 9.22) | .012 |
| % of opioid prescriptions that overlap a sedative-hypnotic prescription within 30 daysc | 11.0% (10.2, 11.9) | 12.2% (11.4, 12.9) | .043 | 12.2% (11.3, 13.1) | 13.3% (12.5, 14.1) | .050 |
| Patients with ≥ 3 opioid prescribers in any 2 mo. interval | 5.76 (5.21, 6.36) | 5.99 (5.50, 6.53) | .540 | 31.7 (28.9, 34.6) | 35.1 (32.5, 38.0) | .088 |
| Inappropriate opioid prescriptionsd | 1.78 (1.58, 2.01) | 1.48 (1.31, 1.67) | .035 | 8.30 (7.38, 9.32) | 6.58 (5.89, 7.34) | .006 |
| Secondary prescribing outcomes | ||||||
| Opioid prescriptions | 207 (189, 226) | 245 (224, 267) | .006 | 939 (855, 1031) | 1162 (1067, 1267) | .001 |
| Benzodiazepine prescriptions | 72.6 (64.6, 81.7) | 87.6 (79.3, 96.7) | .014 | 326 (290, 366) | 422 (383, 465) | .001 |
| Units per opioid prescription (most often pills) | 48.8 (46.2, 51.5) | 51.3 (49.1, 53.6) | .146 | 50.7 (48.4, 53.1) | 53.2 (51.0, 55.5) | .122 |
| Patients who filled at least one opioid prescription | 93.4 (87.0, 100.3) | 105 (98.5, 112) | .016 | 321 (297, 347) | 352 (329, 377) | .079 |
| % of patients with overlapping long-acting and short-acting opioid prescriptions within 30 d | 5.18% (4.59, 5.77) | 6.00% (5.42, 6.57) | .057 | 5.59% (5.00, 6.19) | 6.20% (5.60, 6.80) | .159 |
| Opioid prescriptions per patient with at least one prescription | 1.87 (1.78, 1.95) | 2.14 (2.05, 2.23) | <.001 | 3.08 (2.85, 3.33) | 3.61 (3.38, 3.85) | .002 |
| Total MME per prescriptionb | 569 (518, 625) | 646 (600, 696) | .041 | 596 (553, 644) | 656 (614, 701) | .061 |
| Total MME per patient with at least one opioid prescriptionb | 2,580 (2344, 2841) | 2,974 (2721,3252) | .031 | 7,697 (7043, 8411) | 8,934 (8155, 9787) | .017 |
| Patients with ≥ 3 pharmacies in a 2 month interval | 2.43 (2.20, 2.68) | 2.84 (2.59, 3.12) | .016 | 14.7 (13.4, 16.2) | 17.7 (16.3, 19.2) | .003 |
| Percent of prescribers with at least 1 opioid prescription | 91.3% (89.1, 93.0) | 97.9% (96.7, 98.7) | <.001 | 97.4% (96.0, 98.2) | 99.6% (98.9, 99.9) | .001 |
| Health-related outcomese | ||||||
| Opioid related hospitalization | 158 | 199 | 0.034 | 777 | 1087 | <0.001 |
| Opioid overdose death | 12 | 11 | 1.00 | 19 | 44 | 0.002 |
Mean adjusted rates from Poisson GEE models are presented with upper and lower confidence intervals.
MME calculations exclude prescriptions with incomplete conversion factor, quantity, or strength information (0.2% of opioid prescriptions). MME calculations exclude buprenorphine and pentazocine.
“Sedative-hypnotics” included benzodiazepines, non-benzodiazepine sedative-hypnotics, and carisoprodol
Inappropriate opioid prescriptions are those where a second prescription with the same drug name, from a different prescriber, was filled within 7 days of a first prescription that contained ≥30 pills.
Each event is attributed to the prescriber of the most recent opioid, benzodiazepine, or non-benzodiazepine sedative hypnotic prescription filled in the 5 months prior to the event. Some patients are counted multiple times, once for every hospitalization event. Heroin related events are excluded.
Among secondary prescribing outcomes, all showed gradual declines among both registered and non-registered prescribers. Where significant group differences occurred, the non-registrants generally demonstrated greater decreases (Table 2, online supplemental Figure 1).
Cumulative prescription opioid-related hospitalizations and deaths were greater among patients of registrants than among those of non-registrants, in both 6-month and 30-month follow-up intervals (Table 2). Except for short term mortality, differences were statistically significant.
Frequent PDMP Users versus Infrequent Users
When comparing registrants who frequently queried the PDMP with those who infrequently queried, we found differences in prescribing patterns, even at baseline. Those who became frequent PDMP users demonstrated significantly more cautious opioid prescribing by several metrics both at baseline and throughout the follow-up period (Figure 4, Supplemental Figure 2, and Table 3). Differences between frequent and infrequent PDMP users were small, and most comparisons were not statistically significant.
Figure 4.
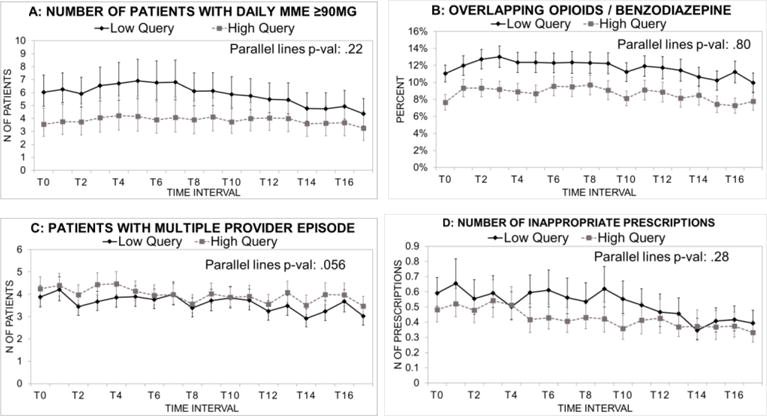
Trends in primary prescribing outcomes among registrants, comparing frequent users (>1 query per 6 prescriptions) with infrequent users.a Data are means per prescriber.
a Data are based on GEE models adjusted for urban or rural location of the prescriber. Error bars are 95% confidence intervals. T0 through T16 represent 2-month time intervals beginning with October and November, 2011. Inappropriate opioid prescriptions are those where a second prescription with the same drug name, from a different prescriber, was filled within 7 days of a first prescription that contained ≥30 pills. A “multiple provider episode” refers to a patient with ≥ 3 opioid prescribers in the 2 month interval.
Table 3.
Prescribing among PDMP-registrants who queried the PDMP frequently (>1 query per 6 opioid prescriptions) or infrequently.
| Prescribing in the 6 month interval March 2012-August, 2012 | Prescribing in the 30 month interval March 2012-August, 2014 | |||||
|---|---|---|---|---|---|---|
| Prescribing metric, per prescriber, adjusted mean (95% CI)a (deaths and hospitalizations are group totals) | Prescribers with infrequent queries n = 464 |
Prescribers with frequent queries n = 463 |
p-value | Prescribers with infrequent queries n =464 |
Prescribers with frequent queries n =463 |
p-value |
| Primary Prescribing Outcomes | ||||||
| Opioid patients with an average daily MME ≥90 in the intervalb | 5.09 (4.27, 6.07) | 3.56 (2.86, 4.43) | .015 | 6.73 (5.67, 7.98) | 5.74 (4.62, 7.14) | .310 |
| % of opioid prescriptions that overlap a sedative-hypnotic prescription within 30 daysc | 12.9% (12.0, 13.9) | 11.1% (10.2, 12.1) | .016 | 13.7% (12.8, 14.6) | 12.6% (11.5, 13.7) | .122 |
| Patients with ≥ 3 opioid prescribers in any 2 mo. interval | 5.75 (5.15, 6.42) | 6.01 (5.43, 6.65) | .544 | 32.4 (29.2, 36.0) | 35.8 (32.8, 38.9) | .123 |
| Inappropriate opioid prescriptionsd | 1.50 (1.31, 1.72) | 1.37 (1.15, 1.64) | .430 | 7.02 (6.19, 7.96) | 5.93 (5.19, 6.78) | .081 |
| Secondary prescribing outcomes | ||||||
| Opioid prescriptions | 226 (209, 245) | 200 (182, 220) | .034 | 1066 (987, 1153) | 977 (885, 1079) | .135 |
| Benzodiazepine prescriptions | 70.6 (64.5, 77.2) | 67.0 (59.7, 75.2) | .452 | 348 (315, 383) | 336 (293, 385) | .694 |
| Units per opioid prescription (most often pills) | 47.1 (44.4, 50.0) | 44.8 (42.4, 47.4) | .297 | 48.3 (45.7, 51.0) | 48.5 (45.6, 51.6) | .923 |
| Patients who filled at least one opioid prescription | 90.7 (84.2, 97.7) | 100 (93.7, 108) | .053 | 296 (271, 322) | 377 (347, 410) | <.001 |
| % of patients with overlapping long-acting and short-acting opioid prescriptions within 30 d | 6.19% (5.70, 6.68) | 6.16% (5.46, 6.85) | .944 | 6.21% (5.71, 6.71) | 6.47% (5.72, 7.22) | .588 |
| Opioid prescriptions per patient with at least one opioid prescription | 1.98 (1.90, 2.06) | 1.89 (1.80, 1.99) | .132 | 3.24 (3.04, 3.44) | 2.91 (2.69, 3.15) | .027 |
| Total MME per prescriptionb | 580 (530, 634) | 502 (460, 548) | .013 | 580 (537, 626) | 529 (488, 574) | .084 |
| Total MME per patient with at least one opioid prescriptionb | 2,654 (2467, 2856) | 2,436 (2137, 2776) | .272 | 7,909 (7215, 8669) | 7,580 (6687, 8593) | .589 |
| Patients with ≥ 3 pharmacies in a 2 month interval | 2.54 (2.27, 2.84) | 3.14 (2.82, 3.49) | .004 | 15.6 (14.0, 17.5) | 20.5 (18.7, 22.6) | <.001 |
| Percent of prescribers with at least 1 opioid prescription | 99.7% (98.4, 99.96) | 99.5% (98.5, 99.9) | .305 | 99.78%e | 99.57%e | – |
| Health-Related Outcomes (n)f | ||||||
| Opioid related hospitalization | 116 | 83 | 0.024 | 612 | 475 | <0.001 |
| Opioid overdose death | 6 | 5 | 1.00 | 26 | 18 | 0.295 |
Mean adjusted rates from Poisson GEE models are presented with upper and lower confidence intervals.
Metrics that use MME exclude prescriptions that did not have complete conversion factor, quantity, or strength information (0.2% of opioid prescriptions). MME calculations exclude buprenorphine and pentazocine.
“Sedative-hypnotics” included benzodiazepines, non-benzodiazepine sedative-hypnotics, and carisoprodol
Inappropriate opioid prescriptions are those where a second prescription with the same drug name, from a different prescriber, was filled within 7 days of a first prescription that contained ≥30 pills.
Unadjusted percentages. Statistical model did not converge because values are too close to 100% for both groups.
Each event is attributed to the prescriber of the most recent opioid, benzodiazepine, or non-benzodiazepine sedative hypnotic prescription filled in the 5 months prior to the event. Some patients are counted multiple times, once for every hospitalization event. Heroin related events are excluded.
Patients of registrants who queried frequently experienced significantly fewer opioid-related hospitalizations than those of registrants who queried infrequently. Cumulative overdose deaths were similar between groups (Table 3).
Analysis of Later Registrants
In our additional analysis, we compared later (1 year later) PDMP registrants with matched non-registrants. The propensity matching process successfully matched 653 later registrants with an equal number of non-registrants (Supplemental Table 2), with SMDs less than 0.1 for all but one prescribing metric. As with early registrants, subsequent opioid prescribing metrics were not significantly lower among later registrants than among non-registrants (online supplement Table 3).
DISCUSSION
Following implementation of Oregon’s PDMP, there were statewide declines in per capita numbers of inappropriate opioid prescriptions, MMEs dispensed, and pills dispensed. Despite these changes, opioid-related deaths and hospitalizations remained stable. Contrary to our hypothesis, prescribers who registered for the PDMP did not demonstrate greater declines than non-registrants with regard to subsequent numbers of high-dose opioid prescriptions, overlaps with benzodiazepines, patients with multiple prescribers, inappropriate prescriptions, patients with at least one opioid prescription, mean dose per prescription, mean dose per patient, or numbers of patients using multiple pharmacies. In fact, for some measures, registrants demonstrated more high-risk prescribing than matched non-registrants. Furthermore, there were more opioid-related hospitalizations and deaths among patients of registrants than of matched non-registrants.
Registrants who used the PDMP frequently showed less opioid prescribing and fewer opioid-related hospitalizations than infrequent PDMP users, but the prescribing pattern was apparent even at baseline. More cautious prescribers were apparently more likely to make frequent use of the PDMP, rather than frequent use making prescribers more cautious. More generally, it seems unlikely that use of the PDMP caused greater opioid prescribing, hospitalizations, or deaths among registrants than among non-registrants. Rather, it appears that in spite of matching, prescribers (and corresponding patient mix) were self-selected into user or non-user categories with somewhat differing average prescribing patterns.
Among prescribers who did not register for the PDMP, there were decreases in the number who wrote at least one opioid prescription per 2-month interval, mean pills per prescription, opioid dose per prescription, and total morphine equivalents per patient per month. Thus, it appears that non-registered prescribers, who outnumbered registered prescribers, accounted for some of the statewide trends.
Among nonregistered prescribers, the number of clinicians who wrote any opioid prescription fell more than among clinicians who registered to use the PDMP, though both declined (supplemental Figure 1J). This raises the possibility of patient migration from non-registered clinicians to those who were registered and who were perhaps more likely to prescribe opioids. In part, this could result from clinicians dismissing problematic patients from their practices.17,20 Such migration might account for smaller changes in some prescribing metrics among registrants than among non-registrants.
We can speculate that some of the statewide decline in opioid prescribing may have resulted from an “observer effect” in which clinicians perceived that prescribing patterns were being more closely scrutinized. Clinician registration and use of the PDMP did not appear to explain observed changes in prescribing. However, registrants may have shared experiences using the PDMP with non-registrants, potentially influencing their prescribing behaviors. Other factors in the environment were likely important, such as greater reporting of opioid prescribing and related mortality in professional publications, greater media coverage of these trends, new clinical guidelines, and new reimbursement restrictions. For example, in the spring of 2012, the Oregon Health Authority implemented a Medicaid prior authorization program for opioid doses exceeding 120 MME/day. This was followed by a substantial and rapid decline in high-dose Medicaid opioid pharmacy claims.15
Though the apparent lack of impact of the PDMP is disappointing, this remains a relatively new innovation in clinical care. More experience, system refinements, and related policy changes may be needed before reaching conclusions about efficacy. In surveys and interviews, clinicians generally welcome the information from PDMPs, but have noted barriers such as cumbersome access and navigation requirements, time demands, difficulty integrating complex information, and uncertainty how to respond to the information.16–18, 20,31,33,34
PDMP Refinements might include the use of prescriber “dashboards” of higher risk patients, proactive alerts, mandatory registration, mandatory querying for certain prescriptions, improved online interfaces, and integration into electronic medical records. Some states have incorporated some of these approaches, and there is modest evidence to support their use,26 but their application has been inconsistent. There is some evidence to suggest that useful policies outside the PDMP might include prior authorization requirements for high-dose prescriptions or for long-acting opioids and “pill mill” laws such as those implemented in Florida.19
Further, greater PDMP impact may require better training of clinicians in use of this relatively new innovation. Such training may be important because previous studies have suggested that clinicians vary widely regarding frequency of PDMP use, responses to the data, and approaches to discussion with patients.16–18,20
Our study has some important strengths. We are unaware of other studies that have compared prescribing behavior, hospitalization, and mortality among PDMP system registrants and non-registrants. We also have used a particularly broad range of outcome measures, and a novel measure of querying frequency (the ratio of a clinician’s PDMP queries to opioid prescriptions filled). We believe such methods, as well as improved conceptual models,12 may be useful for further studying the impacts of PDMP system differences and system improvements.
Our study has important limitations, as well. We have no prescriber demographic or clinical specialty information to compare registrants and non-registrants or to use for adjustment in statistical models. We also have no data regarding patient diagnoses. Thus, differences in patient diagnostic mix between PDMP registrants and non-registrants could be important, though we matched carefully on prescribing patterns. Changes of established prescribing habits in response to implementing the PDMP may require more time and longer periods of observation. We could not directly track migration of patients from one clinician to another. Propensity score matching reduces baseline group differences but is not as effective as random allocation in producing equivalent study groups. However, random allocation to PDMP registration or frequency of use is infeasible. We had no way to account for prescribers who left the state or stopped practicing after being included in the study. The PDMP does not capture inpatient prescriptions, those filled at federal facilities, or those filled out of state. The apparent lack of change in opioid-related mortality or hospitalization with overall declines in prescribing could partly reflect greater use of illicit drug sources in the face of decreasing dosages. States with differing PDMP content, requirements for use, prescriber alerts, and other PDMP features might have different results.26
In conclusion, although there was a statewide decline in several measures of per capita opioid prescribing, PDMP registrants did not demonstrate greater declines than non-registrants. Refinements in the PDMP program and related policies may be necessary to improve their impact. Future studies may profitably focus on implementing and rigorously evaluating such refinements, including development of “best practices” for clinicians in using and responding to PDMP data.
Supplementary Material
Highlights.
We asked if a prescription drug monitoring program (PDMP) changed opioid risks.
We matched early PDMP registrants with similarly prescribing non-registrants.
Prescribing risk generally decreased among both registrants and non-registrants.
Frequent PDMP users showed similar trends to infrequent users.
Factors other than PDMP appeared to have greater influence on prescribing trends.
Perspective.
Factors other than PDMP use may have had greater influence on prescribing trends. Refinements in the PDMP program and related policies may be necessary to increase PDMP impact.
Acknowledgments
Supported by grant number R01 DA031208 from the National Institute on Drug Abuse, and by Grant number UL 1RR024140, from the National Center for Advancing Translational Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Richard A. Deyo receives royalties from UpToDate for authoring topics on low back pain and previously received honoraria for board membership at the nonprofit Informed Medical Decisions Foundation. His salary at Oregon Health & Science University is supported in part by an endowment from Kaiser Permanente. He received a financial award from NuVasive, as part of a lifetime achievement award from the International Society for Study of the Lumbar Spine. Dennis McCarty has research awards from the National Institute on Drug Abuse. All other authors declare no conflicts of interest.
References
- 1.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27:2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 2.Bao Y, Pan Y, Taylor A, Radakrishnan S, Luo F, Pincus HA, Shackman BR. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Affairs. 2016;35:1045–1051. doi: 10.1377/hlthaff.2015.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beil H, Preisser JS, Rozier RG. Accuracy of record linkage software in merging dental administrative data sets. Journal of Public Health Dentistry. 2013;73:89–93. doi: 10.1111/j.1752-7325.2012.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady JE, Wunsch H, DiMaggio C, Lang BH, Giglio J, Li G. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep. 2014;129:139–47. doi: 10.1177/003335491412900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell KM, Deck D, Krupski A. Record linkage software in the public domain: a comparison of Link Plus, The Link King, and a ‘basic’ deterministic algorithm. Health Informatics J. 2008;14:5–15. doi: 10.1177/1460458208088855. [DOI] [PubMed] [Google Scholar]
- 6.Chang HY, Lyapustina T, Rutkow L, Daubresse M, Richey M, Faul M, Stuart EA, Alexander GC. Impact of prescription drug monitoring programs and pill mill laws on high-risk opioid prescribers: a comparative interrupted time series analysis. Drug Alcohol Depend. 2016;165:1–8. doi: 10.1016/j.drugalcdep.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deyo RA, Hallvik SE, Hildebran C, Marino M, Dexter E, Irvine JM, O’Kane N, Van Otterloo J, Wright DA, Leichtling G, Millet LM. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naïve patients: a statewide retrospective cohort study. J Gen Intern Med. 2017;32:21–27. doi: 10.1007/s11606-016-3810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyo RA, Irvine JM, Hallvik SE, Hildebran C, Beran T, Millet LM, Marino M. Leading a horse to water: facilitating registration and use of a prescription drug monitoring program. Clin J Pain. 2015;31:782–787. doi: 10.1097/AJP.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dormuth CR, Miller TA, Huang A, Mamdani MM, Juurlink DN. Effect of a centralized prescription network on inappropriate prescriptions for opioid analgesics and benzodiazepines. Can Med Assoc J. 2012;184:E852–E856. doi: 10.1503/cmaj.120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fay MP. Two-sided exact tests and matching confidence intervals for discrete data. R Journal. 2010;2:53–58. [Google Scholar]
- 12.Finley EP, Garcia A, Rosen K, McGeary D, Pugh MJ, Potter JS. Evaluating the impact of prescription drug monitoring program implementation: a scoping review. BMC Health Serv Res. 2017;17:420. doi: 10.1186/s12913-017-2354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gugelmann HM, Perrone J. Can prescription drug monitoring programs help limit opioid abuse? JAMA. 2011;306:2258–2259. doi: 10.1001/jama.2011.1712. [DOI] [PubMed] [Google Scholar]
- 14.Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174:796–801. doi: 10.1001/jamainternmed.2013.12711. [DOI] [PubMed] [Google Scholar]
- 15.Hartung D, Kim H, Ahmed S, Middleton L, Keast S, Deyo R, McConnell KJ. Impact of a high dose opioid policy on prescription opioid use, misuse, and overdose outcomes. Presented at Annual Research Meeting; Academy Health, Boston. June 26, 2016. [Google Scholar]
- 16.Hildebran C, Cohen DJ, Irvine JM, Foley C, O’Kane N, Beran T, Deyo RA. How clinicians use prescription drug monitoring programs: a qualitative inquiry. Pain Med. 2014;15:1179–86. doi: 10.1111/pme.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildebran C, Leichtling G, Irvine JM, Cohen DJ, Hallvik SE, Deyo RA. Clinical Styles and Practice Policies: Influence on Communication with Patients Regarding Worrisome Prescription Drug Monitoring Program Data. Pain Med. 2016;17:2061–2066. doi: 10.1093/pm/pnw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irvine JM, Hallvik SE, Hildebran C, Marino M, Beran T, Deyo RA. Who Uses a Prescription Drug Monitoring Program and How? Insights from a Statewide Survey of Oregon Clinicians. J Pain. 2014;15:747–55. doi: 10.1016/j.jpain.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy-Hendricks A, Richey M, McGinty EE, Stuart EA, Barry CL, Webster DW. Opioid overdose deaths and Florida’s crackdown on pill mills. Am J Public Health. 2016;106:291–297. doi: 10.2105/AJPH.2015.302953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leichtling GJ, Irvine JM, Hildebran C, Cohen DJ, Hallvik SE, Deyo RA. Clinicians’ use of prescription drug monitoring programs in clinical practice and decision-making. Pain Med. 2017;18:1063–1069. doi: 10.1093/pm/pnw251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meara E, Horwitz JR, Powell W, McClelland L, Zhou W, O’Malley J, Morden NE. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med. 2016;375:44–53. doi: 10.1056/NEJMsa1514387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nam YH, Shea DG, Shi Y, Moran JR. State prescription drug monitoring programs and fatal drug overdoses. Am J Manag Care. 2017;23:297–303. [PubMed] [Google Scholar]
- 23.O’Kane N, Hallvik SE, Marino M, Van Otterloo J, Hildebran C, Leichtling G, Deyo RA. Preparing a prescription drug monitoring program data set for research purposes. Pharmacoepidemiology and Drug Safety. 2016;25:993–997. doi: 10.1002/pds.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 25.Oregon Health Authority, Center for Prevention & Health Promotion, Injury & Violence Prevention Section. Drug overdose deaths, hospitalizations, abuse & dependency among Oregonians. 2014 Available at: https://public.health.oregon.gov/DiseasesConditions/InjuryFatalityData/Documents/oregon-drug-overdose-report.pdf, accessed August 22, 2017.
- 26.Pardo B. Do more robust prescription drug monitoring programs reduce prescription opioid overdose? Addiction. 2016 Dec 23; doi: 10.1111/add.13741. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Affairs. 2016;35:1324–1332. doi: 10.1377/hlthaff.2015.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulozzi LJ, Jones CM, Mack KA, Rudd RA. Vital signs: overdoses of prescription opioid pain relievers, United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- 29.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12:747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 30.Paulozzi LJ, Kilbourne EM, Shah NG, Nolte KB, Desai HA, Landen MG, Harvey W, Loring LD. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13:87–95. doi: 10.1111/j.1526-4637.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- 31.Perrone J, DeRoos FJ, Nelson LS. Prescribing practices, knowledge, and use of prescription drug monitoring programs (PDMP) by a national sample of medical toxicologists, 2012. J Med Toxicol. 2012;8:341–352. doi: 10.1007/s13181-012-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths – United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–82. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 33.Rutkow L, Turner L, Lucas E, Hwang C, Alexander GC. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Affairs. 2015;34:484–492. doi: 10.1377/hlthaff.2014.1085. [DOI] [PubMed] [Google Scholar]
- 34.Young HW, Tyndall JA, Cottler LB. The current utilization and perceptions of prescription drug monitoring programs among emergency medicine providers in Florida. Int J Emerg Med. 2017;10:16. doi: 10.1186/s12245-017-0140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


