Abstract
Prune belly syndrome (PBS), also known as Eagle-Barrett syndrome, is a rare congenital disorder characterized by absence or hypoplasia of the abdominal wall musculature, urinary tract anomalies, and cryptorchidism in males. The etiology of PBS is largely unresolved, but genetic factors are implicated given its recurrence in families. We examined cases of PBS to identify novel pathogenic copy number variants (CNVs). A total of 34 cases (30 males and 4 females) with PBS identified from all live births in New York State (1998–2005) were genotyped using Illumina HumanOmni2.5 microarrays. CNVs were prioritized if they were absent from in-house controls, encompassed ≥10 consecutive probes, were ≥20 Kb in size, had ≤20% overlap with common variants in population reference controls, and had ≤20% overlap with any variant previously detected in other birth defect phenotypes screened in our laboratory. We identified 17 candidate autosomal CNVs; 10 cases each had one CNV and four cases each had two CNVs. The CNVs included a 158 Kb duplication at 4q22 that overlaps the BMPR1B gene; duplications of different sizes carried by two cases in the intron of STIM1 gene; a 67 Kb duplication 202 Kb downstream of the NOG gene, and a 1.34 Mb deletion including the MYOCD gene. The identified rare CNVs spanned genes involved in mesodermal, muscle, and urinary tract development and differentiation, which might help in elucidating the genetic contribution to PBS. We did not have parental DNA and cannot identify whether these CNVs were de novo or inherited. Further research on these CNVs, particularly BMP signaling is warranted to elucidate the pathogenesis of PBS.
Keywords: Prune belly syndrome, Eagle-Barrett syndrome, copy number variant, abdominal wall musculature
INTRODUCTION
Prune belly syndrome (PBS) is a rare congenital disorder characterized by absence or hypoplasia of the abdominal wall musculature, urinary tract anomalies, and cryptorchidism in males (Lloyd et al., 2013; Seidel et al., 2015). A wide variability in disease severity exists with some patients also experiencing other associated defects including pulmonary hypoplasia, renal hypoplasia, cardiac defects, imperforate anus, and intestinal malrotation (Jennings, 2000). PBS profoundly affects a child’s physical, emotional, social, and school functioning (Arlen et al., 2016). The estimated prevalence of PBS in the United States is 3.8 per 100,000 live-births (Routh et al., 2010) with a higher occurrence in males than in females (5:1 ratio) (Druschel, 1995).
The etiology of PBS remains largely unresolved. Mesenchymal developmental defects have been suggested as the underlying defect in PBS (Stephens and Gupta, 1994; Straub and Spranger, 1981). Although PBS often presents as a sporadic condition, familial cases of PBS (Balaji et al., 2000; Ramasamy et al., 2005), as well as occurrence with chromosomal defects (Amacker et al., 1986; Fryns et al., 1991), suggest a genetic contribution. Specifically, PBS has been associated with chromosomal anomalies including trisomy 21 (Amacker et al., 1986) and large deletions in the long arm of chromosome 6 (Fryns et al., 1991). Additionally, there have been several reports showing that PBS occurs in both twin and non-twin siblings, as well as in successive generations (Balaji et al., 2000; Ramasamy et al., 2005). A study from a national database also noted a twofold higher proportion of PBS among blacks compared to the general population (Routh et al., 2010). Previous studies have also reported a gene deletion of the hepatocyte nuclear factor-1β gene in PBS cases (Haeri et al., 2010; Murray et al., 2008).
Studies examining PBS gene defects have been limited. To our knowledge, no study has systematically screened the genome of PBS cases for copy number variants (CNVs). We aimed to identify CNVs in PBS patients.
MATERIALS AND METHODS
Cases
The New York State (NYS) Congenital Malformations Registry (CMR) mandates reporting of major structural birth defects identified within the first two years of life. Hospitals enter birth defect descriptions as text fields which are then coded using the expanded British Pediatric Association (BPA) coding system. To identify PBS cases, we conducted a population-based review searching for a BPA code corresponding to congenital PBS (BPA code 756.720) in the NYS CMR database. We also searched the text field for ‘prune belly syndrome’ and ‘Eagle-Barrett syndrome’ to ensure maximum case capture. In total, we identified 38 PBS cases with archived newborn screening dried blood spots from among 2,023,083 live-births occurring in NYS from January 1, 1998 through December 31, 2005. We excluded those with a known genetic syndrome (Turner syndrome (n=1) and Beckwith-Wiedemann syndrome (n=1)) and those where the BPA code and the clinical narrative were inconsistent (n=2). A total of 34 cases were studied; 18 with isolated PBS and 16 with PBS and other associated defects. We classified as isolated PBS cases those with other genitourinary defects that could have occurred secondarily to the primary PBS defects, such as renal dysplasia, hydronephrosis, ureteropelvic junction obstruction, renal pelvic obstruction, ureteral dilation/obstruction, and cryptorchism. The non-isolated PBS cases had other major birth defects such as gastrointestinal and heart defects. Demographic and clinical characteristics of mothers and cases were extracted from NYS vital records and compared with a random sample of NYS live-births (n=7,683). Statistical analyses were performed using t-tests or Fisher’s exact tests, where applicable with a p-value<0.05 used for statistical significance. All cases were de-identified by removing any personally identifying data and assigning a random identification number prior to genotyping and analysis. This study was approved by the NYS Department of Health Institutional Review Board (NYS IRB #07-007) and the National Institutes of Health Office of Human Subjects Research Protection (OHSRP#3687).
Genotyping
DNA was extracted from two 3-mm dried bloodspot punches using a lab-developed method (Saavedra-Matiz et al., 2013). Genotyping was performed at the University of Minnesota using IlluminaHumanOmni2.5-8_v1 bead arrays and the Infinium HD assay protocol. Data were analyzed with Illumina GenomeStudio v2011.1 with a genotype no-call threshold set at <0.15. In total, 34 PBS samples were genotyped concurrently with 140 cases with other unrelated phenotypes, three technical controls, and one HapMap control (in duplicate). Genotype clustering was based on the data generated in this project. Clusters were reviewed and cleaned based on Illumina’s Infinium Genotyping Data Analysis Technical Note (Illumina, 2014). A total of 2,278,660 autosomal probes, 55,207 probes on chromosome X, and 2,560 probes on chromosome Y were included in the CNV analysis. For autosomal SNPs, the average PBS sample SNP call rate ± SD (range) was 99.6% ± 0.7% (98.2–99.9%) and the mean log R ratio deviation was 0.133 ± 0.041 (0.096–0.287). After cleaning, SNP genotype reproducibility (based on two duplicates included among the 174 samples genotyped) was 100%.
CNV Calling and Annotation
Autosomal CNVs were called using PennCNV v2011/05/03 (Wang et al., 2007) and Illumina’s cnvPartition algorithm v3.1.6. For both algorithms, data were GC-wave adjusted, and the minimum number of probes required for a CNV call was three. The confidence threshold for CNV calling was set to the default value of 10 for PennCNV and 35 for cnvPartition. Sex chromosome CNVs were called using PennCNV after recomputing Log R ratio (LRR) and B allele frequency (BAF) values using sex-specific centroids. Median values for R and theta were computed for each marker on the X and Y chromosomes in males and females separately and then applied using in-house software that implement the standard formulas (Peiffer et al., 2006) to generate new LRR and BAF values. These new values were then fed into PennCNV as “autosomal” probes using custom sex-specific population frequency of B allele (.pfb) and GC content (.gcmodel) files. The PennCNV function clean_cnv.pl was run with default parameters to merge adjacent CNV calls. Autosomal CNV call files were annotated using custom C++ programs as previously (Rigler et al., 2015) to compare concordance between CNV calling algorithms, count the number of cases and controls carrying overlapping CNVs in the current study, determine overlap with an in-house database of CNVs generated from cases and controls of other unrelated defects, determine overlap with the Database of Genomic Variants archive (DGV2), and identify intersecting transcripts and genes (Iafrate et al., 2004). Transcripts included full-length coding transcripts and full-length non-coding transcripts with a well characterized biotype downloaded from GENCODE (version 19, accessed via UCSC genome browser May 2014) (Harrow et al., 2012). Genes were defined as those included in the Consensus Coding Sequence project (CCDS; release 15, accessed via UCSC genome browser June 2014) (Pruitt et al., 2009). Each sex chromosome CNV call was manually reviewed and annotated.
CNV Selection and Prioritization
We prioritized CNVs that were not detected in our in-house controls (i.e. PBS CNVs of the same type and with the same predicted breakpoints), encompassed a minimum of 10 consecutive single nucleotide polymorphism (SNP) probes, were at least 20 Kb in size, had ≤20% overlap with common variants in HapMap (Altshuler et al., 2010) and Children’s Hospital of Philadelphia (CHOP) (Shaikh et al., 2009) CNV datasets, and had ≤20% overlap with any variant previously detected in other birth defect phenotypes screened in our laboratory. We uploaded CNVs meeting these requirements to the DGV2 genome browser (release data 2014-10–16 version), using build37/hg19 coordinates, and examined them for overlap with known CNVs.
CNV Validation
Seventeen autosomal CNVs were selected for validation studies using two to four quantitative real-time PCR (qPCR) TaqMan assays (Applied Biosystems, Carlsbad, CA) per CNV region. Genomic DNA was extracted from one 3-mm dried blood spot, diluted 1:10 in water, and amplified using TaqMan Environmental Master Mix (ABI) in 5µl reaction volumes. A fragment of the RNaseP H1 RNA gene was co-amplified and used as an internal control (TaqMan Copy Number Reference Assay, ABI). Assays were run in quadruplicate on either an ABI 7900HT or an ABI QuantStudio. CopyCaller software v2.0 (ABI) was used to analyze the real-time data using relative quantitation (2−ΔΔCt method). The manual Ct threshold was set to 0.2 with the automatic baseline on. CopyCaller software parameters were as follows: the median ΔCt for each experiment was used as the calibrator, wells with an RNaseP Ct > 38 were excluded and the zero copy ΔCt threshold was set to 6. The average copy number and a software-generated confidence value were calculated for each subject. Samples with confidence values ≥0.95 were considered valid; samples with confidence values <0.95 were rerun in quadruplicate. Since multiple assays targeted each CNV, in all cases, no single sample contained all low confidence calls throughout a CNV region. One probe (Hs06815519_cn) was excluded due to discordant results obtained when retesting multiple samples with low confidence calls (Supplemental Table S1). All assays were initially tested in each of the 34 cases and 24 control subjects. We subsequently screened all validated CNVs against an additional 174 control samples from unaffected NYS births using at least one assay targeting the area of interest. Therefore, a total of 198 unaffected controls were screened using at least one assay in the candidate CNV region.
RESULTS
We identified a total of 34 PBS cases (30 males and 4 females) in the NYS CMR from a population of 2,023,083 live-births (1,036,842 males) resulting in a birth prevalence of 1.68 in 100,000 live-births or 2.89 in 100,000 male live-births. Patients with PBS were significantly more likely than the random sample of control infants from NYS (n=7,683) to be male (88% vs 51%), and to be born early (36.5 vs 39.2 weeks’ gestation) with a lower birthweight (2994 vs 3330 grams) (p<0.001) but there were no differences in small for gestational age (SGA) status. The proportion of African-American infants born with PBS was greater than that of the general population (35% vs 18%; p-value borderline significant=0.077). The two groups did not differ with respect to maternal age and education at delivery, parity, smoking status, or body mass index (Table I).
TABLE I.
Demographic characteristics of patients with prune belly syndrome as compared to a random sample of New York State live-births
| PBS Cases (n=34) |
NY Birth Sample (n=7,683) |
p-value | |
|---|---|---|---|
| Maternal characteristics | |||
|
| |||
| Age years, mean (SD) | 27.7 (6.5) | 28.8 (6.2) | 0.31 |
| Age years, n (%) | 0.87 | ||
| <20 | 2 (5.9) | 612 (8.0) | |
| 20–34 | 27 (79.4) | 5637 (73.4) | |
| ≥35 | 5 (14.7) | 1434 (18.7) | |
| Race/ethnicity, n (%) | 0.077 | ||
| Non-Hispanic White | 15 (44.1) | 4339 (56.6) | |
| Non-Hispanic African American | 12 (35.3) | 1369 (17.9) | |
| Hispanic | 4 (11.8) | 1359 (17.7) | |
| Other | 3 (8.8) | 600 (7.8) | |
| Education years, n (%) | 0.33 | ||
| <12 | 7 (20.6) | 1335 (17.6) | |
| 12 | 13 (38.2) | 2221 (29.2) | |
| >12 | 14 (41.2) | 4045 (53.2) | |
| Nulliparous, n (%) | 14 (41.2) | 3165 (41.2) | 1.0 |
| Smoking, n (%) | 5 (14.7) | 760 (9.9) | 0.38 |
| Prepregnancy BMI kg/m2, n (%) | 0.13 | ||
| ≤24.9 | 7 (36.8) | 2368 (56.4) | |
| 25–29.9 | 8 (42.1) | 992 (23.6) | |
| ≥30 | 4 (21.1) | 837 (19.9) | |
|
| |||
| Newborn characteristics | |||
|
| |||
| Male, n (%) | 30 (88.2) | 3916 (51.0) | <0.001 |
| Gestational age weeks, mean (SD) | 36.7 (3.4) | 38.8 (2.3) | <0.001 |
| Birth weight g, mean (SD) | 2994 (790) | 3330 (577) | <0.001 |
| Low birth weight, n (%) | 7 (20.6) | 500 (6.5) | 0.006 |
| SGA, n (%) | 5 (16.1) | 883 (12.1) | 0.42 |
BMI: body mass index, SGA: small for gestational age.
Data missing for PBS patients on: BMI: 15, gestational age: 3, SGA: 3; for NY State Births on: maternal smoking: 12, race/ethnicity: 16, education: 82, birth weight: 1, BMI: 3486, gestational age: 377, SGA: 378. Among the 381 missing SGA, 4 infants born <22 weeks’ gestation were not coded since the Kramer (Kramer et al. 2001) cut-off points start at 22 weeks’ gestation.
The microarray analysis of all 34 PBS patients resulted in a total of 3,109 autosomal PennCNV calls, 72 PennCNV calls on chromosome X, zero calls on chromosome Y, and 1,296 autosomal cnvPartition calls. Only two CNVs on chromosome X met our inclusion criteria; both were in the same patient, but neither was followed up for validation (Supplemental Table SII). We selected 18 candidate PBS-associated autosomal CNVs of interest in 14 different cases for follow-up (7 with isolated PBS and 7 with PBS and associated defects, Supplemental Table SIII). Ten cases each carried one candidate PBS-associated CNV while four cases each had two candidate CNVs. Two cases carried the same CNV resulting in 17 CNVs for follow-up.
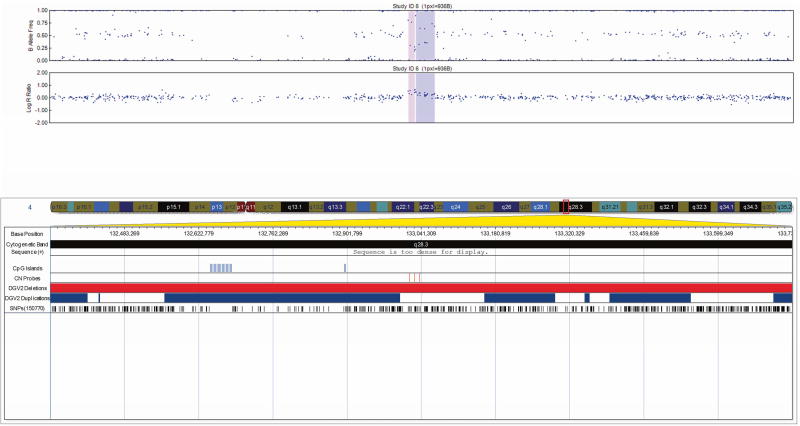
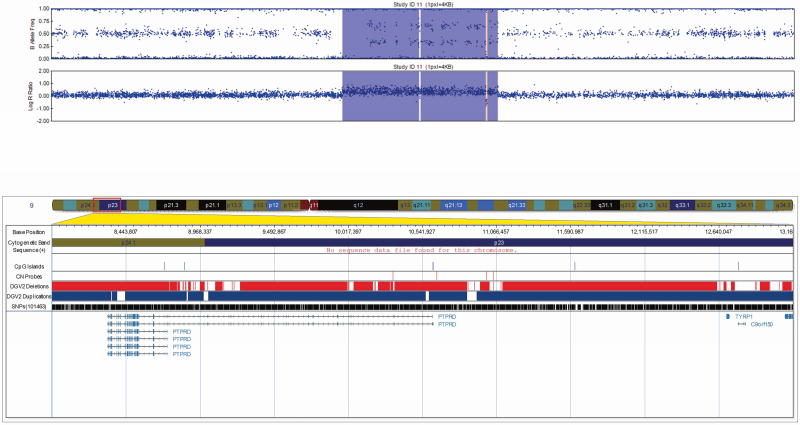
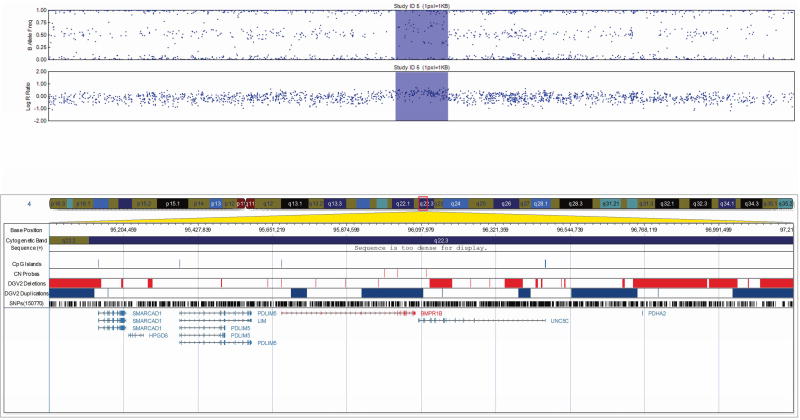
All 17 CNVs were validated by qPCR (Table II) and ranged in size from 20 Kb to 1.3 Mb. The CNVs consisted of four heterozygous deletions and eleven duplications, each intersecting at least one gene/transcript. One CNV predicted as a triplication followed by duplication was determined to be two triplications with an intervening duplication by qPCR (Fig. 1). Two additional duplications were intergenic. One complex CNV was confirmed to be a large duplication with an intervening deletion by qPCR (Fig. 2).
TABLE II.
Rare CNVs identified and validated in 14 patients with prune belly syndrome
| Locus | Phenotype | Genomic Coordinates | Size (bps) | Type | Study Subject ID |
Gene(s)/Transcript(s) |
|---|---|---|---|---|---|---|
| 1p34.2 | Multiple | 43,013,801..43,034,105 | 20,305 | Dupl | 1 | CCDC30 |
| 1q25.3 | Multiple | 183,918,161..184,193,929 | 275,769 | Dupl | 2 | GLT25D2; TSEN15;Y_RN A |
| 2p12 | Isolated | 81,185,831..81,340,758 | 154,928 | Dupl | 3 | NA |
| 3q26.3 2 | Multiple | 176,966,522..176,993,657 | 27,136 | Dupl | 4 | NA |
| 4q22.3 | Multiple | 96,020,228..96,178,679 | 158,452 | Dupl | 5 | BMPR1B; UNC5C |
| 4q24 | Multiple | 104,795,495..105,078,507 | 283,013 | Dupl | 5 | RP11-703G6.1; U6 |
| 4q28.3 | Isolated | 133,016,451..133,066,208 | 49,758 | Dupl* | 6 | RP11-789C2.1 |
| 5p15.2 | Isolated | 11,864,077..11,888,474 | 24,398 | Dupl | 6 | CTNND2 |
| 6p24.3 | Multiple | 10,503,758..10,692,232 | 188,475 | Het Del | 7 | C6orf52; GCNT2; GCNT6; RP11360O19.4; Y_RNA |
| 6p12.3 | Multiple | 49,778,621..49,806,196 | 27,576 | Dupl | 8 | CRISP1; RP11719J20.1; RP3417L20.3; RP3-417L20.4 |
| 6q12 | Isolated | 66,861,468..67,383,327 | 521,860 | Het Del | 9 | AC002485.1 |
| 7p12.2 | Isolated | 49,217,143..49,317,234 | 100,092 | Dupl | 10 | AC010971.1 |
| 9p23 | Isolated | 9,973,935..11,265,271 | 1,101,337 | Complex** | 11 | PTPRD; RP1187N24.2; RP11-87N24.3 |
| 11p15.4 | Isolated | 3,923,570..3,964,500 | 40,931 | Dupl | 12 | STIM1 |
| Isolated | 3,938,197..3,956,049 | 17,853 | Dupl | 13 | ||
| 13q14.3 | Multiple | 54,223,562..54,495,998 | 272,437 | Het Del | 14 | RP11-474J1.1 |
| 17p12 | Multiple | 11,354,433..12,697,336 | 1,342,904 | Het Del | 4 | AC005358.1; AC005358.3; ARHGAP44; DNAH9; MAP2K4; MIR744; MYOCD; RP11-678P16.1; SHISA6; U11; ZNF18 |
| 17q22 | Multiple | 54,886,671..54,953,327 | 66,657 | Dupl | 14 | NOG^; COIL^; C17orf67; DGKE; RP11-670E13.2; U6 |
Dupl: Duplication, Het Del: Heterozygous deletion, NA: not applicable.
Bolded genes are highlighted in the figures and/or discussion section.
Coordinates (hg19) predicted using PennCNV.
CNV predicted as duplication was determined to be a triplication with an intervening duplication via qPCR.
CNV predicted to be complex by both algorithms was determined to be a large region of copy-number 3 with a region in the middle of copy-number 1 via qPCR.
Gene located downstream of CNV.
A total of 15 of the 18 candidate CNVs were called by both the PennCNV and cnvPartition algorithms (overall, cnvPartition calls had >72% overlap with PennCNV calls). One CNV called by cnvPartition had 27.3% overlap with PennCNV, one CNV was identified by cnvPartition only, and another was identified by PennCNV only.
FIG 1.
Visualization of the 49 Kb triplication/duplication determined to be two triplications with an intervening duplication by qPCR in patient 6 at 4q28, exported from the Illumina Genome Viewer. The top panel depicts B-allele frequency (ratio of minor to major alleles) and the bottom panel depicts the logR ratio data (signal intensity). Tracks provided by Illumina show cytobands, CpG islands and the location of SNPs on the array. Custom tracks were created to display the location targeted by copy number assays used to validate CNVs (“CN probes”), and copy number losses (“DGV Deletions,” shown in red) and gains (“DGV Duplications,” shown in blue), both of which were downloaded from the DGV2 database (2014-10-16 version). CNV calls made using the pennCNV algorithm are highlighted (heterozygous deletions in orange and duplications are in blue). hg19 coordinates shown.
[Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552-4833.]
FIG 2.
Visualization of the 1.1 Mb complex CNV determined to be a large duplication with an intervening deletion by qPCR in patient 11 at 9p23, exported from the Illumina Genome Viewer. The top panel depicts B-allele frequency (ratio of minor to major alleles) and the bottom panel depicts the logR ratio data (signal intensity). Tracks provided by Illumina show cytobands, CpG islands and the location of SNPs on the array. Custom tracks were created to display the location targeted by copy number assays used to validate CNVs (“CN probes”), and copy number losses (“DGV Deletions,” shown in red) and gains (“DGV Duplications,” shown in blue), both of which were downloaded from the DGV2 database (2014-10-16 version). A subset of genes/transcripts overlapping CNVs are listed below the panels. CNV calls made using the pennCNV algorithm are highlighted (heterozygous deletions in orange and duplications are in blue). hg19 coordinates shown.
[Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552-4833.]
We prioritized CNVs as PBS candidates based on the genes involved and their function. Findings of interest included a 158 Kb duplication at 4q22 overlapping the 3’ end of bone morphogenetic protein receptor type 1B (BMPR1B) (Fig. 3); two intronic Stromal Interaction molecule 1 (STIM1) gene duplications of different sizes; a 67 Kb duplication 202 Kb downstream of the Noggin (NOG) gene; and a 1.34 Mb deletion encompassing the Myocardin (MYOCD) gene.
FIG. 3.
Visualization of the 158 Kb duplication in patient 5 at 4q22 overlapping the 3’ end of bone morphogenetic protein receptor type 1B (BMPR1B), exported from the Illumina Genome Viewer. The top panel depicts B-allele frequency (ratio of minor to major alleles) and the bottom panel depicts the logR ratio data (signal intensity). Tracks provided by Illumina show cytobands, CpG islands and the location of SNPs on the array. Custom tracks were created to display the location targeted by copy number assays used to validate CNVs (“CN probes”), and copy number losses (“DGV Deletions,” shown in red) and gains (“DGV Duplications,” shown in blue), both of which were downloaded from the DGV2 database (2014-10-16 version). A subset of genes/transcripts overlapping CNVs are listed below the panels. Genes mentioned in the tables/text are in red. CNV calls made using the pennCNV algorithm are highlighted (heterozygous deletions in orange and duplications are in blue). hg19 coordinates shown.
[Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552-4833.]
DISCUSSION
Our study identified several CNVs that encompassed genes involved in mesodermal, muscle, and urinary tract development and differentiation. During the embryonic period, the intermediate and lateral plate mesoderm are the origins of the abdominal wall musculature. The intermediate mesoderm also forms the urogenital ridge which is the origin of the kidneys and the urinary tract (Sadler, 1994). Defective mesodermal development could potentially explain the multisystem defects of the abdominal wall musculature and urinary tract that characterize PBS (Moerman et al., 1984; Straub and Spranger, 1981).
A defining feature of PBS is abdominal muscle deficiency. Muscle development is a multifactorial and highly regulated process. The BMP signaling pathway is a member of the transforming growth factor-beta superfamily and is suggested to tightly regulate the formation of lateral mesoderm which later develops the hypaxial musculature (Dietrich et al., 1998). Hypaxial musculature is the origin of the body wall musculature including rectus abdominis muscle (Dietrich et al., 1998). BMPs function through receptors and activate gene transcription through signaling pathways such as Smad (Zhang and Li, 2005). BMPs regulate development by precisely controlling differentiation of stem cells in various organ systems via the specific combinations of type II receptors with one of type Ia or 1b receptors (Zhang and Li, 2005).
We identified four CNVs that overlap or are in close proximity to genes, including BMPR1B, STIM1, MYOCD, and NOG, which are linked to embryonic muscle development. The BMPR1B and NOG genes are involved in the BMP signaling pathway. Knocking out BMP signaling in mice resulted in multiple malformations and early embryonal death (Zhao, 2003). In addition to its association with osteoblastic differentiation (Shi et al., 2016), BMPR1B is expressed locally in somites (Danesh et al., 2009). Since BMPR1B is a receptor of the BMP signaling pathway (Zhang and Li, 2005), it is possible that defects at BMPR1B expression at these cells result in ineffective receptor combinations. That the distribution and combination of type I and type II BMP receptors defines the effect that BMP molecules have on each cell (Costantini, 2012) suggest that defective BMPR1B could lead to malfunctioning receptor combination during embryogenesis, which could cause insufficient BMP pathway molecule-BMP receptor interaction. As a result, muscle-determining genes during embryogenesis might not be effectively expressed, leading to improper hypaxial musculature formation. This might explain rectus abdominis muscle weakness and atrophy seen in PBS.
We also identified a 67 Kb duplication 202 Kb downstream of NOG, a BMP antagonist that acts by directly binding on BMPs, preventing interaction with their receptors (Furthauer et al., 1999). NOG acts as a regulator of BMP signaling to control and maintain the balance between active and inactive BMPs (Furthauer et al., 1999). The role of regulated BMP antagonism is well established (Costamagna et al., 2016; Mine et al., 2008; Stafford et al., 2011). Dysregulation of BMP antagonism by a malfunction in NOG expression could lead to a disruption of that balance. Ineffective NOG activity has been associated with lower musculature differentiation potential in vivo (Costamagna et al., 2016) and could explain rectus abdominis defects in PBS.
BMP signaling has been shown to have a defining role in urogenital development and ureter morphogenesis, key features of PBS (Cain et al., 2008; Manson et al., 2015; Raatikainen-Ahokas et al., 2000; Wang et al., 2009). BMPR1B is expressed in the branching ureter, the ureter epithelium, and the Wolffian duct (Miyazaki et al., 2000). BMPs are highly expressed in the mesenchyme in a specific pattern through embryonic life and they have a vital role in urogenital development (Cain et al., 2008; Costantini, 2012; Dunn et al., 1997; Manson et al., 2015; Tsujimura et al., 2016). BMP4 expression defects have been associated with enlarged kidneys in mouse studies (Dunn et al., 1997). Since a differential distribution of receptors for BMP molecules has been suggested to contribute to the pattern of action of those factors (Costantini, 2012), defective BMPR1B expression in ureter epithelium could complicate ureter formation. Furthermore, ineffective activation or disruption of the BMP signaling due to a defective BMPR1B receptor, as well as dysregulation of BMP molecule balance due to impaired NOG antagonism, could both result in defective ureter formation potentially leading to urinary tract defects such as the blockage and hydronephrotic phenotype seen in PBS.
Two cases had duplications of STIM1 intron 1–2 Intron 1–2 includes enhancers, promoter flank regions and transcription factor binding sites which are regulatory elements for the expression of STIM1. STIM1 encodes a transmembrane protein that acts as a calcium sensor at the endoplasmic reticulum and mediates internal calcium store repletion after depletion through the store-operated calcium-entry (SOCE) mechanism (Kiviluoto et al., 2011). SOCE has a role in muscle development, because defective STIM1 signaling in mice results in ineffective muscle differentiation (Stiber et al., 2008). The fact that BMPR1B, NOG and STIM1 all have a clear role in embryonic muscle development supports the contention that a genetic component could contribute to the abdominal muscle defects in PBS.
In one additional case we identified a 1.34 Mb deletion encompassing the MYOCD gene. MYOCD regulates smooth muscle cell differentiation in the ureter (Caubit et al., 2008; Martin et al., 2013), which might suggest a connection with defective urinary tract development. Furthermore, the BMP signaling pathway is a downstream regulator of MYOCD expression (Caubit et al., 2008). This emphasizes the complexity of urinary tract development regulation by genetic factors and lends further support to urinary tract defects in PBS potentially having an underlying genetic basis.
Our study has several strengths. To our knowledge, this is the first population-based genome-wide search for PBS CNVs. We found evidence of expression with different intensities for BMPR1B, STIM1, and NOG genes in the embryonal kidney (Harding et al., 2011; McMahon et al., 2008) and for MYOCD in the embryonal ureter (Martin et al., 2013). We observed shorter gestational age and lower birth weight in PBS cases compared to a random sample of NYS births. Our estimated birth prevalence of 2.9 per 100,000 male live-births is lower than has been previously reported of 3.6 per 100,000 male live-births (Lloyd et al., 2013) possibly due to the exclusion of syndromic cases. Similar to another study we reported a twofold higher proportion of PBS among African-Americans in comparison to the general population (Routh et al., 2010). One limitation of our study is that we did not have parental DNA and were unable to identify whether these CNVs were de novo or inherited. We reported on two interesting complex CNVs, one with a triplication and a duplication and another with a large duplication and an intervening deletion, we do not know the significance of these CNVs as they intersected transcripts of unknown function. Some PBS cases may not have been reported however; PBS is an obvious defect to medical professionals (Lloyd et al., 2013). Additionally, estimates available from the NYS CMR suggest that the registry identifies a large proportion of children born with congenital malformations in NYS (Sekhobo and Druschel, 2001). Lastly, bias in genomic databases reduce our ability to confidently rule out the presence of CNVs in the normal population and assess the clinical significance of the identified CNVs (Duclos et al., 2011).
In conclusion, we identified several CNVs that included genes involved in mesodermal, muscle and urinary tract development and differentiation, all systems that are affected in PBS. Our findings support a genetic contribution to PBS etiology. The BMP signaling pathway is a well-known contributor to mesodermal differentiation and formation (Mine et al., 2008; Zhao, 2003). Thus, identifying CNVs in BMPR1B and NOG gene areas, both regulators of the BMP pathway, strengthens the hypothesis that abnormal mesoderm development leads to PBS phenotypes. Further research on the genetic factors identified, particularly of BMP signaling and genes associated with mesodermal development, is warranted.
Supplementary Material
Acknowledgments
We thank Natalie Weir at the Minnesota Core Laboratories and the staff at the Biomedical Genomics Center Facility at the University of Minnesota for microarray genotyping; Zoë L. Edmunds for assistance with qPCR assays and April J. Atkins, Emily C. McGrath, and Adam C. Gearhart for laboratory and technical assistance at the Wadsworth Center, New York State Department of Health; Sandra D. Richardson at the Congenital Malformations Registry, New York State Department of Health for data management; and Dr. Karl G. Hill with the Social Development Research Group (5R01DA024411) at the University of Washington for generously sharing population B allele frequency and GC content files for PennCNV software. This study makes use of data generated by the DECIPHER Consortium. A full list of centers who contributed to the generation of the data is available from http://decipher.sanger.ac.uk and via email from decipher@sanger.ac.uk. Funding for the DECIPHER project was provided by the Wellcome Trust. Those who carried out the original analysis and collection of the data bear no responsibility for the further analysis or interpretation of it. Some data used for comparison in this manuscript were obtained from the ISCA Consortium database (www.iscaconsortium.org), which generates this information using NCBI’s database of genomic structural variation (dbVar, www.ncbi.nlm.nih.gov/dbvar/), study nstd37. Samples and associated phenotype data were provided by ISCA Consortium member laboratories.
Grant sponsor: Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development; Contract numbers: HHSN275201100001I, HHSN27500005; Grant sponsor: NICHD; Contract number: N01-DK-73431; Grant sponsor: National Human Genome Research Institute.
Abbreviations
- BAF
B allele frequency
- BPA
British Pediatric Association
- CMR
Congenital Malformations Registry
- CNV
copy number variant
- DGV
Database of Genomic Variants
- LRR
Log R ratio
- NYS
New York State
- PBS
prune belly syndrome
- qPCR
quantitative realtime PCR
- SGA
small for gestational age
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None.
References
- Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, Peltonen L, Dermitzakis E, Bonnen PE, Altshuler DM, Gibbs RA, de Bakker PI, Deloukas P, Gabriel SB, Gwilliam R, Hunt S, Inouye M, Jia X, Palotie A, Parkin M, Whittaker P, Yu F, Chang K, Hawes A, Lewis LR, Ren Y, Wheeler D, Gibbs RA, Muzny DM, Barnes C, Darvishi K, Hurles M, Korn JM, Kristiansson K, Lee C, McCarrol SA, Nemesh J, Dermitzakis E, Keinan A, Montgomery SB, Pollack S, Price AL, Soranzo N, Bonnen PE, Gibbs RA, Gonzaga-Jauregui C, Keinan A, Price AL, Yu F, Anttila V, Brodeur W, Daly MJ, Leslie S, McVean G, Moutsianas L, Nguyen H, Schaffner SF, Zhang Q, Ghori MJ, McGinnis R, McLaren W, Pollack S, Price AL, Schaffner SF, Takeuchi F, Grossman SR, Shlyakhter I, Hostetter EB, Sabeti PC, Adebamowo CA, Foster MW, Gordon DR, Licinio J, Manca MC, Marshall PA, Matsuda I, Ngare D, Wang VO, Reddy D, Rotimi CN, Royal CD, Sharp RR, Zeng C, Brooks LD, McEwen JE. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amacker EA, Grass FS, Hickey DE, Hisley JC. An association of prune belly anomaly with trisomy 21. Am J Med Genet. 1986;23:919–923. doi: 10.1002/ajmg.1320230406. [DOI] [PubMed] [Google Scholar]
- Arlen AM, Kirsch SS, Seidel NE, Garcia-Roig M, Smith EA, Kirsch AJ. Health-related quality of life in children with prune-belly syndrome and their caregivers. Urology. 2016;87:224–227. doi: 10.1016/j.urology.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Balaji KC, Patil A, Townes PL, Primack W, Skare J, Hopkins T. Concordant prune belly syndrome in monozygotic twins. Urology. 2000;55:949. doi: 10.1016/s0090-4295(00)00452-0. [DOI] [PubMed] [Google Scholar]
- Cain JE, Hartwig S, Bertram JF, Rosenblum ND. Bone morphogenetic protein signaling in the developing kidney: present and future. Differentiation. 2008;76:831–842. doi: 10.1111/j.1432-0436.2008.00265.x. [DOI] [PubMed] [Google Scholar]
- Caubit X, Lye CM, Martin E, Core N, Long DA, Vola C, Jenkins D, Garratt AN, Skaer H, Woolf AS, Fasano L. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development. 2008;135:3301–3310. doi: 10.1242/dev.022442. [DOI] [PubMed] [Google Scholar]
- Costamagna D, Mommaerts H, Sampaolesi M, Tylzanowski P. Noggin inactivation affects the number and differentiation potential of muscle progenitor cells in vivo. Sci Rep. 2016;6:31949. doi: 10.1038/srep31949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F. Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip Rev Dev Biol. 2012;1:693–713. doi: 10.1002/wdev.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh SM, Villasenor A, Chong D, Soukup C, Cleaver O. BMP and BMP receptor expression during murine organogenesis. Gene Expr Patterns. 2009;9:255–265. doi: 10.1016/j.gep.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Healy C, Sharpe PT, Lumsden A. Specification of the hypaxial musculature. Development. 1998;125:2235–2249. doi: 10.1242/dev.125.12.2235. [DOI] [PubMed] [Google Scholar]
- Druschel CM. A descriptive study of prune belly in New York State, 1983 to 1989. Arch Pediatr Adolesc Med. 1995;149:70–76. doi: 10.1001/archpedi.1995.02170130072017. [DOI] [PubMed] [Google Scholar]
- Duclos A, Charbonnier F, Chambon P, Latouche JB, Blavier A, Redon R, Frebourg T, Flaman JM. Pitfalls in the use of DGV for CNV interpretation. Am J Med Genet A. 2011;155a:2593–2596. doi: 10.1002/ajmg.a.34195. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–247. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- Fryns JP, Vandenberghe K, Van den Berghe H. Prune-belly anomaly and large interstitial deletion of the long arm of chromosome 6. Ann Genet. 1991;34:127. [PubMed] [Google Scholar]
- Furthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev Biol. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- Haeri S, Devers PL, Kaiser-Rogers KA, Moylan VJ, Jr, Torchia BS, Horton AL, Wolfe HM, Aylsworth AS. Deletion of hepatocyte nuclear factor-1-beta in an infant with prune belly syndrome. Am J Perinatol. 2010;27:559–563. doi: 10.1055/s-0030-1248943. [DOI] [PubMed] [Google Scholar]
- Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-MacGilp S, Pi X, Roochun Y, Sharghi M, Tindal C, McMahon AP, Gottesman B, Little MH, Georgas K, Aronow BJ, Potter SS, Brunskill EW, Southard-Smith EM, Mendelsohn C, Baldock RA, Davies JA, Davidson D. The GUDMAP database - an online resource for genitourinary research. Development. 2011;138:2845–2853. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C, Harte R, Lin M, Howald C, Tanzer A, Derrien T, Chrast J, Walters N, Balasubramanian S, Pei B, Tress M, Rodriguez JM, Ezkurdia I, van Baren J, Brent M, Haussler D, Kellis M, Valencia A, Reymond A, Gerstein M, Guigo R, Hubbard TJ. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, Qi Y, Scherer SW, Lee C. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- Illumina. Infinium Genotyping Data Analysis. 2014 Retrieved from http://www.illumina.com/documents/products/technotes/technote_infinium_genotyping_data_analysis.pdf.
- Jennings RW. Prune belly syndrome. Semin Pediatr Surg. 2000;9:115–120. doi: 10.1053/spsu.2000.7556. [DOI] [PubMed] [Google Scholar]
- Kiviluoto S, Decuypere JP, De Smedt H, Missiaen L, Parys JB, Bultynck G. STIM1 as a key regulator for Ca2+ homeostasis in skeletal-muscle development and function. Skelet Muscle. 2011;1:16. doi: 10.1186/2044-5040-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, Blondel B, Breart G. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- Lloyd JC, Wiener JS, Gargollo PC, Inman BA, Ross SS, Routh JC. Contemporary epidemiological trends in complex congenital genitourinary anomalies. J Urol. 2013;190:1590–1595. doi: 10.1016/j.juro.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Manson SR, Austin PF, Guo Q, Moore KH. BMP-7 Signaling and its critical roles in kidney development, the responses to renal injury, and chronic kidney disease. Vitam Horm. 2015;99:91–144. doi: 10.1016/bs.vh.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Martin E, Caubit X, Airik R, Vola C, Fatmi A, Kispert A, Fasano L. TSHZ3 and SOX9 regulate the timing of smooth muscle cell differentiation in the ureter by reducing myocardin activity. PLoS One. 2013;8:e63721. doi: 10.1371/journal.pone.0063721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, Zhang P GUDMAP project. GUDMAP: the genitourinary developmental molecular anatomy project. J Am Soc Nephrol. 2008;19:667–671. doi: 10.1681/ASN.2007101078. [DOI] [PubMed] [Google Scholar]
- Mine N, Anderson RM, Klingensmith J. BMP antagonism is required in both the node and lateral plate mesoderm for mammalian left-right axis establishment. Development. 2008;135:2425–2434. doi: 10.1242/dev.018986. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman P, Fryns JP, Goddeeris P, Lauweryns JM. Pathogenesis of the prune-belly syndrome: a functional urethral obstruction caused by prostatic hypoplasia. Pediatrics. 1984;73:470–475. [PubMed] [Google Scholar]
- Murray PJ, Thomas K, Mulgrew CJ, Ellard S, Edghill EL, Bingham C. Whole gene deletion of the hepatocyte nuclear factor-1beta gene in a patient with the prune-belly syndrome. Nephrol Dial Transplant. 2008;23:2412–2415. doi: 10.1093/ndt/gfn169. [DOI] [PubMed] [Google Scholar]
- Online Mendelian Inheritance in Man, OMIM™. Johns Hopkins Univeristy; Baltimore, MD: MIM Number: 605921: 02/02/2017. Retrieved from http://www.omim.org/entry/605921. [Google Scholar]
- Peiffer DA, Le JM, Steemers FJ, Chang W, Jenniges T, Garcia F, Haden K, Li J, Shaw CA, Belmont J, Cheung SW, Shen RM, Barker DL, Gunderson KL. High-resolution genomic profiling of chromosomal aberrations using Infinium whole-genome genotyping. Genome Res. 2006;16:1136–1148. doi: 10.1101/gr.5402306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt KD, Harrow J, Harte RA, Wallin C, Diekhans M, Maglott DR, Searle S, Farrell CM, Loveland JE, Ruef BJ, Hart E, Suner MM, Landrum MJ, Aken B, Ayling S, Baertsch R, Fernandez-Banet J, Cherry JL, Curwen V, Dicuccio M, Kellis M, Lee J, Lin MF, Schuster M, Shkeda A, Amid C, Brown G, Dukhanina O, Frankish A, Hart J, Maidak BL, Mudge J, Murphy MR, Murphy T, Rajan J, Rajput B, Riddick LD, Snow C, Steward C, Webb D, Weber JA, Wilming L, Wu W, Birney E, Haussler D, Hubbard T, Ostell J, Durbin R, Lipman D. The consensus coding sequence (CCDS) project: Identifying a common protein-coding gene set for the human and mouse genomes. Genome Res. 2009;19:1316–1323. doi: 10.1101/gr.080531.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatikainen-Ahokas A, Hytonen M, Tenhunen A, Sainio K, Sariola H. BMP-4 affects the differentiation of metanephric mesenchyme and reveals an early anterior-posterior axis of the embryonic kidney. Dev Dyn. 2000;217:146–158. doi: 10.1002/(SICI)1097-0177(200002)217:2<146::AID-DVDY2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Haviland M, Woodard JR, Barone JG. Patterns of inheritance in familial prune belly syndrome. Urology. 2005;65:1227. doi: 10.1016/j.urology.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Rigler SL, Kay DM, Sicko RJ, Fan R, Liu A, Caggana M, Browne ML, Druschel CM, Romitti PA, Brody LC, Mills JL. Novel copy-number variants in a population-based investigation of classic heterotaxy. Genet Med. 2015;17:348–357. doi: 10.1038/gim.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh JC, Huang L, Retik AB, Nelson CP. Contemporary epidemiology and characterization of newborn males with prune belly syndrome. Urology. 2010;76:44–48. doi: 10.1016/j.urology.2009.12.072. [DOI] [PubMed] [Google Scholar]
- Saavedra-Matiz CA, Isabelle JT, Biski CK, Duva SJ, Sweeney ML, Parker AL, Young AJ, Diantonio LL, Krein LM, Nichols MJ, Caggana M. Cost-effective and scalable DNA extraction method from dried blood spots. Clin Chem. 2013;59:1045–1051. doi: 10.1373/clinchem.2012.198945. [DOI] [PubMed] [Google Scholar]
- Sadler TW. Langman’s Medical Embryology. Eleventh. Lippincott Williams & Wilkins; 1994. [Google Scholar]
- Seidel NE, Arlen AM, Smith EA, Kirsch AJ. Clinical manifestations and management of prune-belly syndrome in a large contemporary pediatric population. Urology. 2015;85:211–215. doi: 10.1016/j.urology.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Sekhobo JP, Druschel CM. An evaluation of congenital malformations surveillance in New York State: an application of Centers for Disease Control and Prevention (CDC) guidelines for evaluating surveillance systems. Public Health Rep. 2001;116:296–305. doi: 10.1093/phr/116.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, Gai X, Perin JC, Glessner JT, Xie H, Murphy K, O’Hara R, Casalunovo T, Conlin LK, D’Arcy M, Frackelton EC, Geiger EA, Haldeman-Englert C, Imielinski M, Kim CE, Medne L, Annaiah K, Bradfield JP, Dabaghyan E, Eckert A, Onyiah CC, Ostapenko S, Otieno FG, Santa E, Shaner JL, Skraban R, Smith RM, Elia J, Goldmuntz E, Spinner NB, Zackai EH, Chiavacci RM, Grundmeier R, Rappaport EF, Grant SF, White PS, Hakonarson H. High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res. 2009;19:1682–1690. doi: 10.1101/gr.083501.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Iura A, Terajima M, Liu F, Lyons K, Pan H, Zhang H, Yamauchi M, Mishina Y, Sun H. Deletion of BMP receptor type IB decreased bone mass in association with compromised osteoblastic differentiation of bone marrow mesenchymal progenitors. Sci Rep. 2016;6:24256. doi: 10.1038/srep24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford DA, Brunet LJ, Khokha MK, Economides AN, Harland RM. Cooperative activity of noggin and gremlin 1 in axial skeleton development. Development. 2011;138:1005–1014. doi: 10.1242/dev.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens FD, Gupta D. Pathogenesis of the prune belly syndrome. J Urol. 1994;152:2328–2331. doi: 10.1016/s0022-5347(17)31669-5. [DOI] [PubMed] [Google Scholar]
- Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub E, Spranger J. Etiology and pathogenesis of the prune belly syndrome. Kidney Int. 1981;20:695–699. doi: 10.1038/ki.1981.198. [DOI] [PubMed] [Google Scholar]
- Tsujimura T, Idei M, Yoshikawa M, Takase O, Hishikawa K. Roles and regulation of bone morphogenetic protein-7 in kidney development and diseases. World J Stem Cells. 2016;8:288–296. doi: 10.4252/wjsc.v8.i9.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Brenner-Anantharam A, Vaughan ED, Herzlinger D. Antagonism of BMP4 signaling disrupts smooth muscle investment of the ureter and ureteropelvic junction. J Urol. 2009;181:401–407. doi: 10.1016/j.juro.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hadley D, Liu R, Glessner J, Grant SF, Hakonarson H, Bucan M. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res. 2007;17:1665–1674. doi: 10.1101/gr.6861907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284:1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.