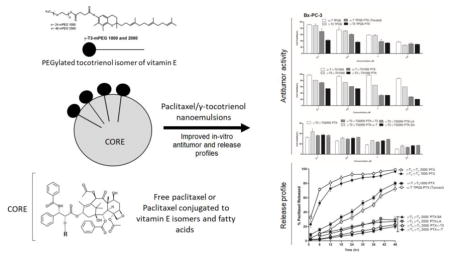
Abstract
Vitamin E TPGS is a tocopherol (α-T) based nonionic surfactant that was used in the formulation of the Tocosol™ paclitaxel nanoemulsion, which was withdrawn from phase III clinical trials. Unlike tocopherols, however, the tocotrienol (T3) isomers of vitamin E were found to have innate anticancer activity and were shown to potentiate the antitumor activity of paclitaxel. The primary objective of the present study was therefore to develop a paclitaxel nanoemulsions by substituting α-T oil core of Tocosol™ with γ-T3 in, and vitamin E TPGS with PEGylated γ-T3 as the shell, and test the nanoemulsions against Bx-PC-3 and PANC-1 pancreatic tumor cells. A secondary objective was to test the activity of paclitaxel when directly conjugated with the γ-T3 isomer of vitamin E. The synthesis of the conjugates was confirmed by NMR and mass spectroscopy. Developed nanoemulsions were loaded with free or lipid conjugated paclitaxel. Nanoemulsions droplets were < 300 nm with fastest release observed with formulations loaded with free paclitaxel when γ-T3 was used as the core. Substituting α-T with γ-T3 was also found to potentiate the anticancer activity of the nanoemulsions. Although marginal increase in activity was observed when nanoemulsions were loaded with free paclitaxel, a significant increase in activity was observed when lipid conjugates were used. The results from this study suggest that the developed paclitaxel nanoemulsions with either γ-T3, PEGylated γ-T3, or paclitaxel lipid conjugates may represent a more promising option for paclitaxel delivery in cancer chemotherapy.
Keywords: Nanoemulsion, Paclitaxel, Tocotrienol, PEGylation, Vitamin E TPGS, Drug Delivery, Cancer Chemotherapy, Nanotechnology
Graphical Abstract
1. Introduction
Vitamin E is a term used to represent a family of eight related α, β, γ, and δ tocopherols and tocotrienol isomers, which differ in the degree of methyl substitutions on their chroman moiety and the degree of saturation in their phytyl side chain (Sylvester, Kaddoumi, Nazzal, & El Sayed, 2010). While the tocopherols are known for their antioxidant activity (Galli et al., 2016), the tocotrienol isomers were found to display potent anticancer activity, which established a distinction in the health and therapeutic benefits between the tocotrienol and tocopherol isomers of vitamin E (Aggarwal & Nesaretnam, 2012; Sylvester et al., 2010). The poor water solubility of vitamin E isomers, however, limited their clinical use. To overcome this limitation, the α-tocopherol isomer of vitamin E was conjugated to PEG 1000, which is commercially known as D-α-tocopherol polyethylene glycol 1000 succinate or simply vitamin E TPGS or TPGS (Guo, Luo, Tan, Otieno, & Zhang, 2013). Vitamin E TPGS is currently being used as a pharmaceutical excipient for its solubilization capacity (Guo et al., 2013). During the early 2000s, a tocopherol-based product, known as Tocosol™, was developed as an alternative platform for paclitaxel delivery to reduce the side effects associated with the vehicle used in the commercial Taxol™ formulation (Constantinides et al., 2000). The development of Tocosol™, however, was terminated in phase III clinical trials due to low primary endpoint of overall response rate (ORR, 37% for Tocosol™) when compared to the control arm Taxol® (45%) (Ma & Mumper, 2013).
Tocosol™ paclitaxel was formulated with α-T as the oil core for paclitaxel solubilization and vitamin E TPGS as the primary emulsifier (Constantinides et al., 2000). However, since the tocotrienol isomers of vitamin E were shown to have an innate anticancer activity and were also shown to potentiate the efficacy of paclitaxel in-vitro (Ahn, Sethi, Krishnan, & Aggarwal, 2007), we hypothesized that substituting α-T oil core with the more pharmacologically active γ-tocotrienol (γ-T3) isomer in paclitaxel loaded nanoemulsions may improve the in vitro anticancer activity of paclitaxel. We also hypothesized that such activity may be further enhanced by replacing vitamin E TPGS as the surfactant stabilizer in nanoemulsion formulations with the PEGylated γ-T3 isomer, which was previously synthesized by our group (Fig. 1) (Abu-Fayyad & Nazzal, 2017). To address these hypotheses, a tocotrienol-based paclitaxel loaded nanoemulsions formulation was developed, which was tested for their in-vitro antitumor activity against Bx-PC-3 and PANC-1 human pancreatic cancer cell lines. In addition to enhancing the anticancer activity of paclitaxel by fortifying the nanoemulsions with the γT3 isomer of vitamin E, we also speculated that chemically conjugating paclitaxel to the polyunsaturated γ-T3 isomer might further potentiate its activity and improve entrapment in nanoemulsions. Although paclitaxel conjugates to vitamin E have already been reported (Fu et al., 2015), a paclitaxel conjugate to the γ-T3 isomer of vitamin E has not been previously reported. A secondary objective of the present study was therefore to present a detailed scheme for the synthesis of paclitaxel γ-T3 conjugates, and to compare the antitumor activity of nanoemulsions loaded with the paclitaxel γ-T3 conjugates to nanoemulsions loaded with paclitaxel conjugated to either α-T or stearic acid, which are fully saturated. Since the anticancer activity of polyunsaturated fatty acids has been well documented (Vaughan, Hassing, & Lewandowski, 2013), we also compared the anticancer activity of paclitaxel γ-T3 conjugate to paclitaxel conjugated to linoleic acid, a polyunsaturated omega-6 fatty acid, against human pancreatic cancer cell lines.
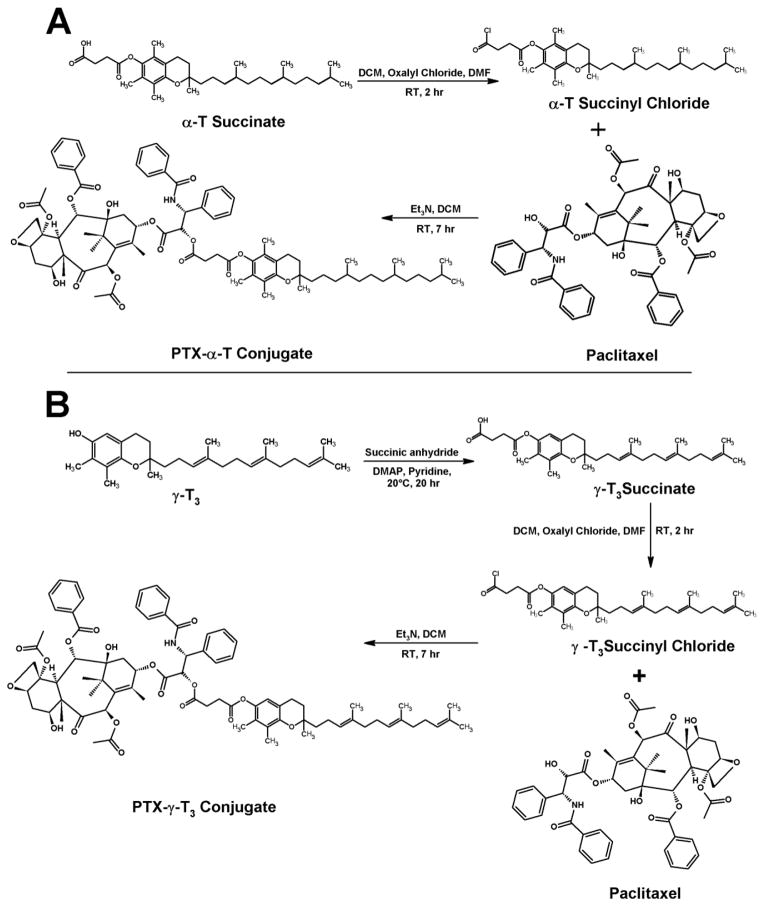
Fig. 1.
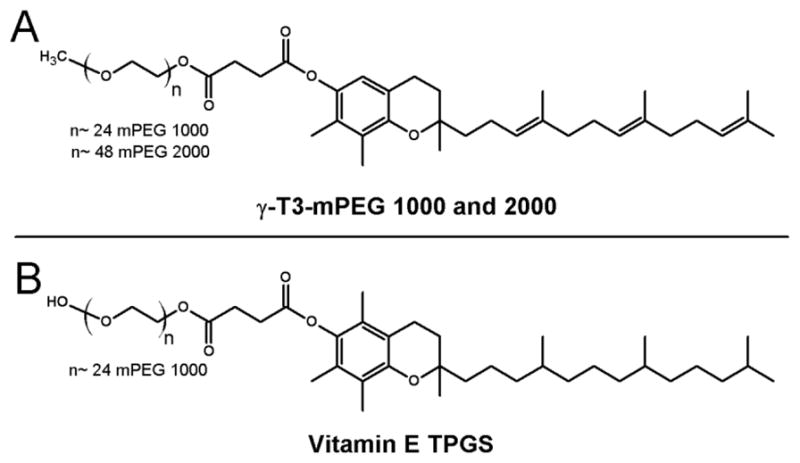
Chemical structures of (A) γ-T3-mPEG 1000 and 2000; and (B) vitamin E TPGS
2. Materials and Methods
2.1. Materials
Paclitaxel was obtained from LC Laboratories (Woburn, MA). Linoleoyl chloride, stearoyl chloride, and sodium oleate were from TCI America (Portland, OR). Vitamin E succinate was from Spectrum Chemical MFG Corp. (New Brunswick, NJ). 4-(Dimethylamino)pyridine (DMAP) was from EMD Chemicals Inc. (Gibbstown, NJ). γ-T3 was extracted from Tocotrol™ L50P, a Tocotrienol-rich fraction of palm oil (Fuji Health Science, Inc., Burlington, NJ) as reported in our previous work (Abu-Fayyad et al., 2015; Abu-Fayyad & Nazzal, 2017). Triethylamine, succinic chloride, oxalyl chloride and 1,4-dioxane anhydrous were from Alfa Aesar (Ward Hill, MA). Chloroform-d (CDCl3) was from Acros (Bridgewater, NJ). Ethyl acetate, methanol, acetonitrile, dichloromethane, and HPLC grade water were from Pharmco-AAPER (Brookfield, CT). PEGylated γ-T3 mPEG 1000 and 2000 were synthesized in our laboratory as detailed elsewhere (Abu-Fayyad et al., 2015; Abu-Fayyad & Nazzal, 2017). Vitamin E TPGS and Poloxamer 407 were from BASF Corporation (Florham Park, NJ). 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) was from Avanti Polar Lipids, Inc. (Alabaster, AL). Polyethylene Glycol (PEG) 400 was from the Dow Chemical Company (Midland, MI). Generic Taxol® was from Hospira, Inc. (Lake Forest, IL). All chemicals and solvents were of reagent grade or higher and were used as supplied without further modification.
2.2. Synthesis of the paclitaxel-lipid conjugates
Two different types of lipids were used to synthesize lipidated paclitaxel (PTX) in the current study; α-T and γ-T3 isomers of vitamin E, as well as stearic and linoleic acids. The detailed scheme for the synthesis of the PEGylated γ-T3 with the mPEG molecular weight of 1000 and 2000 (Fig. 1) were discussed in detail in our previous work (Abu-Fayyad et al., 2015; Abu-Fayyad & Nazzal, 2017). The synthesis schemes for PTX-lipid conjugation are outlined in Figs. 2 and 3.
Fig. 2.
Synthesis scheme for the conjugation of paclitaxel to the (A) α-Tocopherol (PTX-α-T) and (B) γ-Tocotrienol (PTX-γT3) isomers of vitamin E
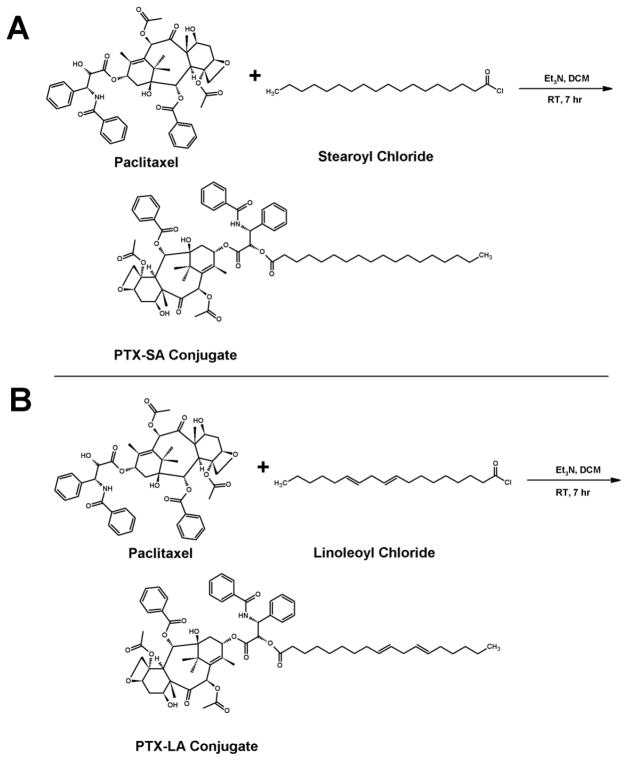
Fig. 3.
Synthesis scheme for the conjugation of paclitaxel to (A) Stearic acid (PTX-SA) and (B) Linoleic acid (PTX-LA)
2.2.1. Synthesis of the PTX-α-T conjugate
PTX-α-T was synthesized starting with α-T succinate. The succinate was converted first to the α-T succinyl chloride. Approximately 235 mg of α-T succinate and 85 mg oxalyl chloride were solubilized in 3 mL dichloromethane (DCM). 100 μL of dimethylformamide (DMF) was then added to the mixture. The mixture was stirred for 2 h at room temperature with the aid of IKA magnetic stirrer plate (IKA Works Inc., Wilmington, NC). Approximately 200 mg of PTX and an equivalent molar amount of α-T succinyl chloride were then solubilized in DCM. 30 μL of trimethylamine was then added to the mixture. The reaction mixture was stirred at room temperature for 7 h. The reaction residue was further purified by flash column chromatography on silica gel eluted with dichloromethane:ethanol (94:06). The solvent was removed using a Heidolph Laborota 4000 efficient rotary evaporator (Elk Grove Village, IL) to give off-white solid material.
2.2.2. Synthesis of the PTX-γ-T3 conjugate
PTX-γ-T3 was synthesized starting with free γ-T3. First, γ-T3 succinate was synthesized as follows; 200 mg of γ-T3 and 400 mg of succinic anhydride were weighted in 50 mL round bottom flask. 5 mL of anhydrous pyridine was then added along with 26.5 mg of 4-(Dimethylamino)pyridine (DMAP). The reaction mixture was stirred at room temperature for 20 h. 1 mL of water was then added to the reaction mixture, which was stirred for an additional 1 h. 100 mL of water was then added and extracted with ethyl acetate (4 × 50 mL). The extracted oily layers were washed with 5% HCL (3 × 5mL) and brine (2 × 10 mL). γ-T3 succinate was then converted to the chloride derivative using the same procedure for α-T succinyl chloride synthesis mentioned above. 200 mg of the synthesized γ-T3 succinyl chloride and an equivalent amount of PTX were then solubilized in DCM followed by addition of 30 μL triethylamine, and the reaction mixture was stirred at room temperature for 7 h. The workup and purification steps above for PTX-α-T were repeated for PTX-γ-T3.
2.2.2. Synthesis of the PTX-SA and PTX-LA conjugates
PTX-stearic acid (PTX-SA) and PTX-linoleic acid (PTX-LA) were synthesized in a single step. Approximately 80 mg of stearoyl chloride or linoleoyl chloride and 200 mg of PTX were stirred at room temperature for 12 h. The residues underwent the same workout protocol as reported for PTX-α-T above to afford white solid powders.
2.3. 1H-NMR and Mass spectrometry analysis of paclitaxel-lipid conjugates
Proton-NMR studies were carried out for the analysis of PTX-lipid conjugates. Samples were prepared in CDCl3 and analyzed using a JEOL Eclipse NMR spectrometer (JEOL USA, Inc., MA) operating at 400 MHz and 20°C. Delta™ NMR Data Processing Software (JEOL USA, Inc., MA) was used for data acquisition and spectral processing with chemical shifts reported in ppm (d). One-dimensional spectra were collected with 64 scans of 16 K points over 20 ppm and a recycle delay of 5s. JEOL AccuTOF™ time-of-flight mass spectrometer (JEOL Ltd., Tokyo, Japan) equipped with orthogonal spray electrospray ionization (ESI) ion source was used to analyze the PTX-lipid conjugates. HPLC grade methanol was used to dissolve the samples. 50 μL was injected through Rheodyne 6-port valve injector. The mass spectrometer was operated in positive-ion mode (ESI + ve) with needle voltage (2000 V). The atmospheric pressure interface potentials were set to the following values; orifice 1 = 55 V, ring lens voltage = 5 V and orifice 2 = 6 V. The detector voltage was set to 1900 V. Orifice 1 temperature was adjusted to 80°C with dissolving temperature at 250°C. Nebulizing and desolvation gas (N2) were adjusted to 2 and 5 L/min flow rate, respectively.
2.4. Preparation of tocotrienol-paclitaxel nanoemulsions
Paclitaxel-loaded and unloaded nanoemulsions were prepared by the solvent evaporation method. Briefly, α-T or γ-T3, free paclitaxel or paclitaxel-lipid conjugates, and vitamin E TPGS or PEGylated γ-T3 isomers were weighed into glass vials and solubilized with 0.5 mL dichloromethane. The samples were vortexed vigorously until a homogeneous phase was obtained. The solvent was left for evaporation, and the complete drying was ensured by flushing with N2 gas until a clear film formed on the bottom of the glass vials. Preheated distilled water was then added to each vial, and the samples were briefly vortexed and then subjected to 5 min sonication using an ultrasonic homogenizer (Model 150VT, Biologics, Inc., Manassas, VA). The list and composition of nanoemulsions that were prepared in the current study are provided in Table 1.
Table 1.
Composition and physical properties of the paclitaxel nanoemulsions, and their IC50 values against Bx-PC-3 and PANC-1pancreatic cancer cells
| Composition (mg)¥ | Physical Properties | IC50 (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Nanoemulsion | α-T | γ-T3 | PTX or PTX-conjugate | TPGS or γ-T3-PEG | Size (nm) | PDI | Zeta (mV) | EE (%) | Bx-PC-3 | PANC-1 |
| α-T TPGS | 10 | - | - | 10 | 246 ± 6 | 0.23 ± 0.02 | − 22 ± 2 | - | 2.2 | 1.4 |
| γ-T3 TPGS | - | 10 | - | 10 | 253 ± 8 | 0.18 ± 0.01 | − 23 ± 2 | - | 1.2 | 0.82 |
| α-T TPGS PTX (Tocosol)** | 10 | - | 1.6 | 10 | 263 ± 8 | 0.31 ± 0.05 | − 30 ± 3 | 98 ± 2 | 1.1 | 1 |
| γ-T3 TPGS PTX** | - | 10 | 1.6 | 10 | 271 ± 9 | 0.28 ± 0.07 | − 27 ± 2 | 98 ± 1 | 0.27 | 0.45 |
| α-T γ-T31000 | 10 | - | - | 10 | 261 ± 8 | 0.27 ± 0.03 | − 24 ± 2 | - | 8.3 | 11 |
| γ-T3 γ-T31000 | - | 10 | - | 10 | 254 ± 10 | 0.35 ± 0.06 | − 40 ± 3 | - | 1.7 | 1.4 |
| α-T γ-T31000 PTX** | 10 | - | 1.6 | 10 | 274 ± 3 | 0.29 ± 0.04 | − 25 ± 2 | 97 ± 2 | 1 | 1.7 |
| γ-T3 γ-T31000 PTX** | - | 10 | 1.6 | 10 | 282 ± 6 | 0.20 ± 0.06 | − 23 ± 2 | 0.47 | 0.55 | |
| γ-T3 γ-T32000 PTX** | - | 10 | 1.6 | 10 | 220 ± 6 | 0.25 ± 0.03 | − 42 ± 2 | 99 ± 1 | 0.08 | 0.34 |
| γ-T3 γ-T32000 PTX-α-T | - | 10 | 1.6* | 10 | 285 ± 6 | 0.19 ± 0.05 | − 34 ± 2 | 96 ± 1 | 0.15 | 0.98 |
| γ-T3 γ-T32000 PTX-γ-T3 | - | 10 | 1.6* | 10 | 290 ± 7 | 0.25 ± 0.04 | − 34 ± 2 | 96 ± 1 | 0.11 | 0.89 |
| γ-T3 γ-T32000 PTX-SA | - | 10 | 1.6* | 10 | 300 ± 8 | 0.32 ± 0.03 | − 32 ± 3 | 97 ± 1 | 0.12 | 0.95 |
| γ-T3 γ-T32000 PTX-LA | - | 10 | 1.6* | 10 | 310 ± 9 | 0.26 ± 0.04 | − 23 ± 3 | 96 ± 1 | 0.13 | 0.96 |
Concentration: α-T/γ-T3 (2.3 mM), PTX (187 μM) and TPGS/γ-T3-PEG (0.666 μM).
Paclitaxel (PTX) conjugates were weighted in the amount equivalent for free paclitaxel.
Significant enhancement in the anticancer activity upon paclitaxel addition to the nanoemulsions, replacement of α-T by γ-T3 isomer or γ-T3-mPEG1000 by γ-T3-mPEG2000 (P value < 0.05).
2.5. Physical characterization of the tocotrienol paclitaxel nanoemulsions
The mean particle size of the paclitaxel-loaded and unloaded nanoemulsions was measured by photon correlation spectroscopy (PCS) using a Nicomp™380 ZLS submicron particle size analyzer (Particle Sizing System, Port Richey, FL) at 25°C and a 90° laser light scattering. Samples were diluted with DI water to avoid multiple scattering and to achieve a count rate of 300 kHz. The volume-weighted mean diameter of the particles was calculated based on Stokes–Einstein law by curve fitting of the correlation function. For the calculations of particle size a refractive index (liquid index of refraction) of 1.33 was used. Zeta-potential of the nanoemulsions in water was measured using the same instrument under the zeta mode.
2.6. Entrapment efficiency
Entrapment efficiency (EE) of paclitaxel in the tocotrienol-paclitaxel nanoemulsions was determined using 1.5 mL Nanosep® centrifugal devices with a 30K MWCO (Pall Corporation, Port Washington, NY). Briefly, 500 μL of the nanoemulsions were pipetted into the reservoir upper part of the centrifugal device. The tubes were then capped and placed into a fixed-angle centrifuge rotor, and centrifuged at 10,000 rpm for 10 min (Eppendorf 5415, Hamburg, Germany). The amount of paclitaxel in the filtrate receiver compartment was analyzed by HPLC using a Kinetex™ C18 (5 μm, 150 × 4.6 mm) column (Phenomenex Inc., Torrance, CA). The mobile phase consisted of 80% acetonitrile and 20% water, which was run at a flow rate of 1 mL/min. Paclitaxel was detected at 227 nm λmax. EE (%) was calculated by calculating the ratio of free paclitaxel in the filtrate to the initial amount of paclitaxel added to the nanoemulsions.
2.7. In-vitro release studies
The In vitro release of paclitaxel from the nanoemulsions was measured using a dialysis bags with a 6–8K MWCO (Spectra/Por® 1, Spectrum Laboratories Inc., Rancho Dominguez, CA). Briefly, paclitaxel nanoemulsions (1 mg/mL) were loaded into dialysis bags and placed in 50 mL Falcon conical tubes containing the release medium (45 mL of PBS, pH 7.4 containing 0.5% v/v Tween 80). Tween 80 was added to maintain sink conditions. The conical tubes were then placed for 50 h in an oven at 37°C while rotating at 100 RPM. At predetermined time points, 1 mL of each sample was withdrawn and replaced with equal volume of releasing buffer. The samples were analyzed for paclitaxel content by HPLC as described above.
2.8. In-vitro cytotoxicity of the paclitaxel nanoemulsions
The in-vitro cytotoxicity study was carried out on the unloaded nanoemulsions (containing only the free and PEGylated isomers) and paclitaxel-loaded nanoemulsions. The cytotoxicity was evaluated against pancreatic Bx-PC-3 and PANC-1 cancer cell lines using a non-toxic single-step CellTiter-Blue® (CTB) assay (Promega, Madison, WI). CTB is a fluorescent based assay and thus more sensitive than the typical colorimetric MTT assays. Cells were purchased from ATCC™ (Manassas, VA). PANC-1 cells were maintained in DMEM medium (Corning, Cellgro, Manassas, VA) whereas BxPC-3 cells were maintained in RPMI Medium 1640 (Corning, Cellgro, Manassas, VA). Cell culture media were supplemented with 10% fetal bovine serum (Gemini Bio-Products, Sacramento, CA) and 1% penicillin–streptomycin (Corning, Cellgro, Manassas, VA). Cells (50 μL) were seeded at a density of 5000 cells/well into 96-well plates and incubated at 37°C with 5% CO2. After overnight incubation, an additional 50 μL of fresh medium containing the nanoemulsions were added to each well to maintain a final concentration of paclitaxel (or equivalent to the paclitaxel for the unloaded nanoemulsions) within the 0.5–25 μM range. After 72-hour incubation, 20 μL of CTB reagent was added to each well. The plates were incubated for 1 hour at 37°C with 5% CO2. Fluorescence was measured using a Synergy 4 plate reader (Bio-Tek Instruments, Winooski, VT). Cell viability was calculated as the percentage of cells remaining viable in reference to the untreated cells. The 50% inhibitory concentration (IC50) values were estimated using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA).
2.9. Statistical analysis
IC50 values (dose resulting in 50% cell growth inhibition) for the paclitaxel-loaded and unloaded nanoemulsions were determined by non-linear regression curve fit analysis using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). The statistical significance of the in-vitro anticancer activity of the nanoemulsions was analyzed by one-way analysis of variance with Tukey post-test calculation. A difference of P value < 0.05 was considered to be statistically significant.
3. Results and discussion
3.1. Synthesis and characterization of paclitaxel-lipid conjugates
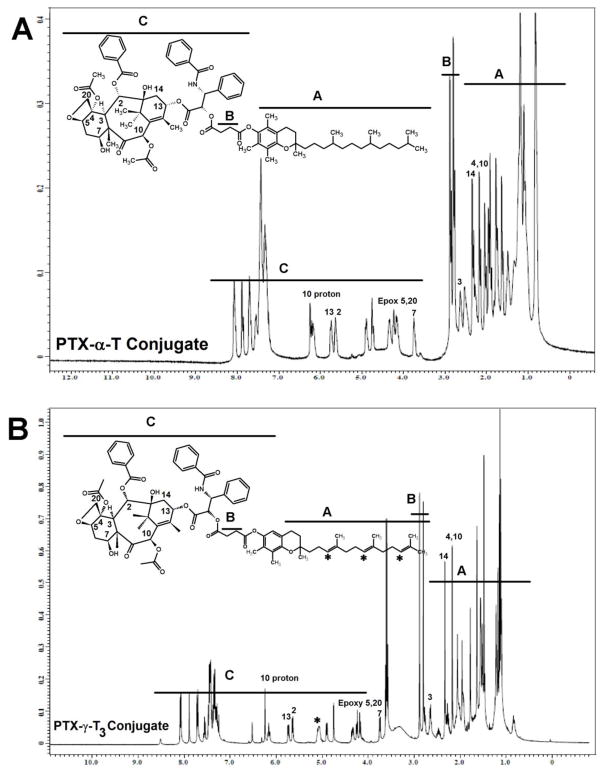
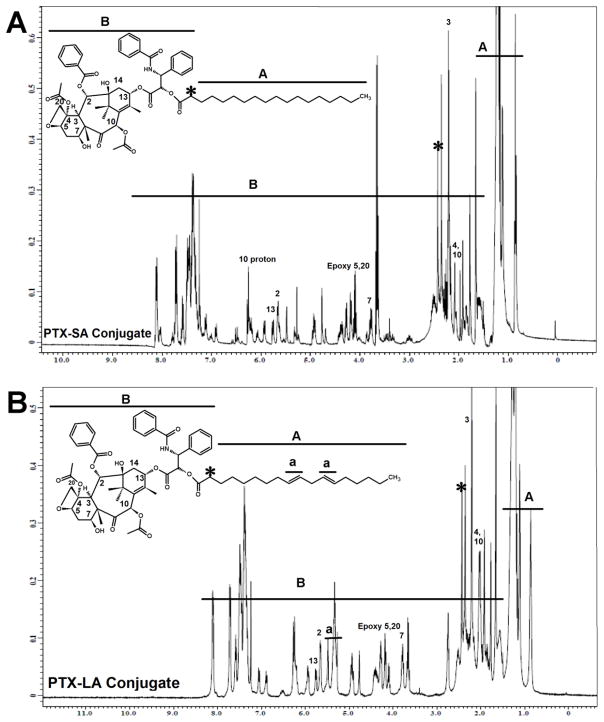
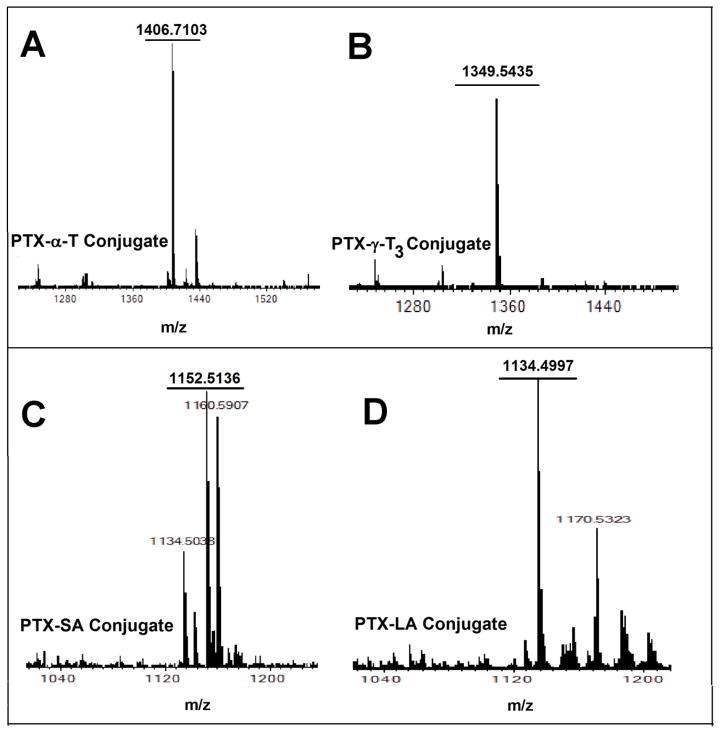
Two types of lipids were used in the current study to synthesize paclitaxel-lipid conjugates. Vitamin E isomers (α-T and γ-T3) and fatty acids (stearic and linoleic acids). The paclitaxel-lipid conjugates were synthesized using the acyl chloride derivatives of the isomers and fatty acids as depicted in the reaction schemes shown in Figs. 2 and 3, respectively. As discussed in the method section, PTX-α-T conjugate was synthesized by reacting the α-T succinate chloride with paclitaxel to give PTX-αT, a white powder product with a total yield of approximately 60% (~ 200 mg). The PTX-γ-T3 conjugate was synthesized by ring opening of succinic anhydride esterification reaction with γ-T3 followed by conjugation of the chloride derivative of γ-T3 with paclitaxel to give a brownish white solid material with a yield of approximately 60% (~ 200 mg, Fig. 2B). PTX-fatty acid conjugates were synthesized using acyl chloride derivatives of the fatty acids to give white solid materials with a yield of approximately 95% for both products (~ 240 mg, Fig. 3A and B). 1H-NMR spectra of the PTX-lipid conjugates are given in Figs. 4 and 5. The appearance of the succinyl protons linker between α-T/γ-T3 and PTX with an up-field shift at 2.77–2.89 ppm (m, 4H, COCH2CH2CO) due to the shielded effect of paclitaxel on the newly formed taxane conjugate, indicated successful esterification reaction. The downfield shifts of the isoserine protons of the N-Benzoyl-3-phenylisoserine side chain of PTX from 4.77 and 5.42 ppm to 5.27 and 5.53 ppm, respectively, due to the de-shielding effect of the carboxyl group of the fatty acid, indicated successful conjugation of fatty acids to PTX. The chemical shift at 2.35–2.45 ppm corresponds to the protons of the α-carbon of the fatty acids. Carbon chain signals were located at 0.83–2.18 ppm and 0.85–1.40 ppm for α-T/γ-T3 and SA/LA, respectively (Figs. 4 and 5). Ethenyl sp2 carbon protons of γ-T3 appeared at 5.11 ppm (Fig. 4B). The protons of the 4,10-acetyl groups of PTX gave chemical shift at 2.17 ppm (m, 6H, CH3CO). The chemical shift at 2.67 ppm was due to the proton on the carbon number 3 of PTX (s, 1H, CH). The proton of carbon number 13 was located at 5.76 ppm (s, 1H, CHOCO). The protons at carbons number 2 and 14 of paclitaxel gave chemical shift at 5.64 ppm (s, 1H, COOCH) and 2.3 ppm (m, 2H, HOCCH2CO), respectively. Chemical shifts at 4.17–4.33 correspond to the 5,20-epoxytaxene protons. Singlet chemical shift at 3.75 ppm was due to 7-OH carbon proton (s, 1H, CHOH). The protons of 7,10-carbons of paclitaxel yielded chemical shift at 3.75 ppm (s, 1H, CHOH) and 6.25 ppm (s, 1H, CHOH). The 16, 17, 18, 19 and 4,10 acetyl groups protons appeared within the region along with the α-T/γ-T3 carbon chain. The chemical shift region at 7.29–8.10 ppm was due to the aromatic protons of PTX. Time-of-flight mass spectrometer analysis (Fig. 6A, B, C, and D) further confirmed the synthesis of the PTX lipid conjugates. The average molecular weights of PTX-α-T, PTX-γ-T3, PTX-SA, and PTX-LA were observed with peaks at m/z of 1406.7, 1349.5, 1152.5, 1134.5, respectively, (Na+ adducts), which were in agreement with the expected MWs of 1366.7, 1346.6, 1120.4 and 1116.3 for PTX-α-T, PTX-γ-T3, PTX-SA and PTX-LA, respectively.
Fig. 4.
1H-NMR spectra in CDCl3 of paclitaxel conjugated to the (A) α-Tocopherol (PTX-α-T) and (B) γ-Tocotrienol (PTX-γT3) isomers of vitamin E
Fig. 5.
1H-NMR spectra in CDCl3 of paclitaxel conjugated to (A) Stearic acid (PTX-SA) and (B) Linoleic acid (PTX-LA)
Fig. 6.
ESI mass spectrum of paclitaxel conjugated to (A) α-Tocopherol (PTX-α-T); (B) γ-Tocotrienol (PTX-γT3); (C) Stearic acid (PTX-SA); and (D) Linoleic acid (PTX-LA)
3.2. Preparation and characterization of the tocotrienol-paclitaxel nanoemulsions
The compositions of the paclitaxel nanoemulsions (Table 1) were broadly based on the Tocosol™ formulation. Most distinctively, α-T was replaced with the γ-T3 isomer of vitamin E, and vitamin E TPGS was replaced by the PEGylated γ-T3 isomers. γ-T3 conjugates with mPEG 1000 and mPEG 2000 were used to study the impact of molecular weight variation on the physical properties of the nanoemulsions and potentially the in-vitro anticancer activity of paclitaxel.
Nanoemulsions were evaluated for particle size, polydispersity index (PDI), zeta potential, and entrapment efficiency (Table 1). As expected, nanoemulsions loaded with free paclitaxel had a larger particle size (263–283 nm) than unloaded nanoemulsions (246–261 nm). When the γ-T3 mPEG 1000 conjugate was replaced by the γ-T3 mPEG 2000 conjugate as the emulsifier, a significant decrease in particle size was observed, which was also anticipated due to the inverse relationship between the molecular weight of the PEG moiety and particle size (Fang, Shi, & Pei, 2005). The polydispersity index of all nanoemulsions was <0.4, which indicates a uniform distribution of the nanoemulsion droplets in the colloidal dispersion (Table 1). The larger payload of nanoemulsions loaded with the paclitaxel conjugates, however, caused an increase in particle size (285–310 nm) over the nanoemulsions loaded with the free paclitaxel (263–283 nm). Regardless of their size, all nanoemulsions had > 96% entrapment efficiency and zeta potential > 20 mV. The shelf stability of the nanoemulsions were assessed at refrigerated condition (4°C) and room temperature (25°C) for a period of 3 months. At refrigerated conditions, no significant changes were observed in particle size and physical appearance after the first and second months of storage. However, after the 3 months, separation and precipitation was observed. Nanoemulsions stored at room temperature were stable for 2 weeks, with no significant changes in size and the physical appearance.
3.3. In-vitro release studies
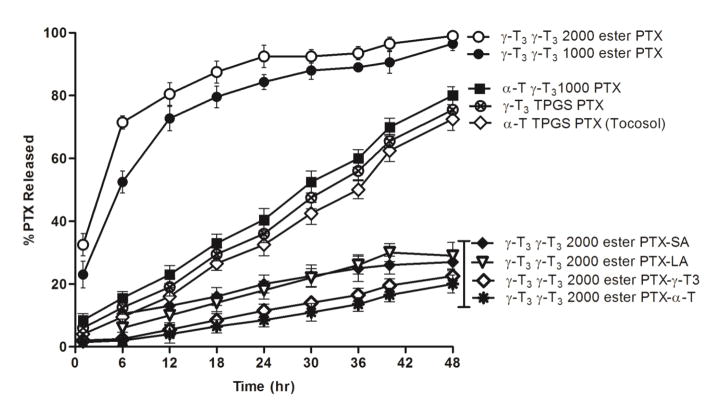
The in-vitro release of paclitaxel from the nanoemulsions was carried out at pH 7.4 using 0.5% tween 80 to provide sink conditions (Fig. 7). Nanoemulsions loaded with the paclitaxel-lipid conjugates had slower release profile than nanoemulsions loaded with the free paclitaxel, with only 20–30% of the drug released after 48 h. The slow release was expected due to the lipophilic nature of the conjugates compared to the free paclitaxel (Fig. 7). Among the conjugates, it also was observed that the vitamin E conjugates of paclitaxel had the slowest release profile, which was consistent with previous reports that demonstrated that the release of paclitaxel-lipid conjugates from nanoparticles was controlled by adjusting the length of the aliphatic chain of the lipids (Ansell et al., 2008). Faster release was observed for nanoemulsions loaded with the free paclitaxel where 70–100% of the drug was released after 48 h (Fig. 7). An interesting observation was that paclitaxel release from the nanoemulsions was depended on the type of the vitamin E isomer in the core. Slower release was observed from nanoemulsions where α-T was used to solubilize paclitaxel. A significantly faster release was observed when α-T was replaced with the γ-T3 isomer, regardless of the molecular weight of the PEG corona. Since α-T isomer is more hydrophobic than γ-T3 (Abu-Fayyad & Nazzal, 2017), it may lead to a stronger interaction with the lipophilic paclitaxel molecule, which may explain the slower release observed for paclitaxel from nanoemulsions made with α-T as the core.
Fig. 7.
In-vitro release profile of free and conjugated paclitaxel from nanoemulsions at pH 7.4 phosphate buffer over 48 h. Values are reported as the mean ± SD for triplicate samples
3.4. In-vitro cytotoxicity of paclitaxel-loaded nanoemulsions
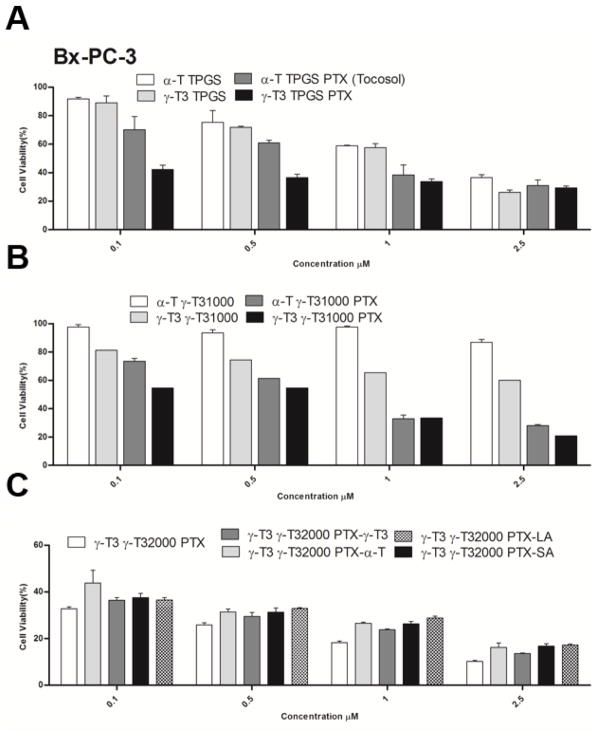
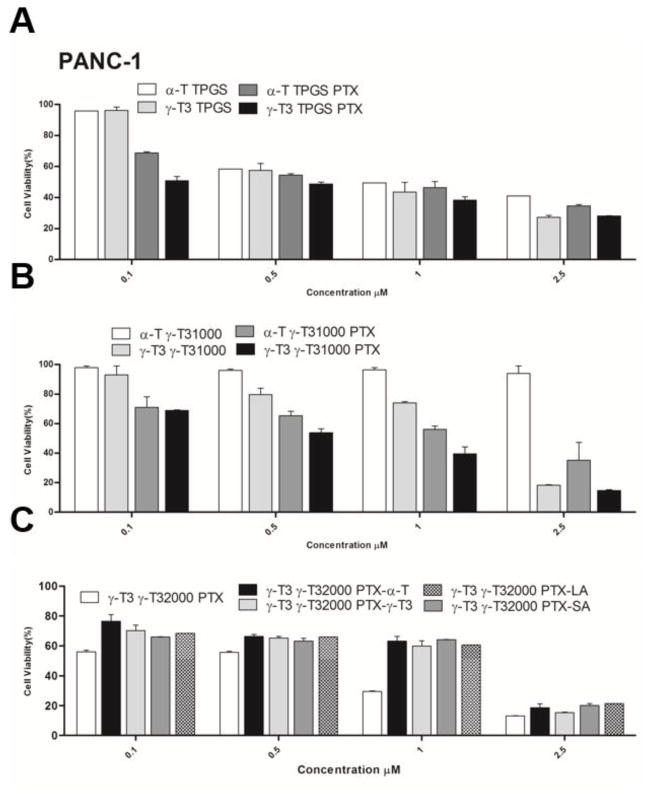
The anticancer activity of PTX-loaded and unloaded nanoemulsions was tested against Bx-PC-3 and PANC-1 pancreatic cancer cell lines (Figs. 8, 9 and Table 1). Nanoemulsions stabilized with TPGS showed dose-dependent increase in cytotoxicity against both cell lines regardless whether the core was made with α-T or γ-T3. Due to the high toxicity induced by the vehicle, cytotoxicity data at concentrations > 2.5 μM were omitted. Paclitaxel addition to the formulations significantly increased the anticancer activity of the nanoemulsions against the Bx-PC-3 cells (P value < 0.05). The effect of paclitaxel, however, was less evident when the nanoemulsions were tested against the PANC-1 pancreatic cells. The observed lower activity is supported by previous reports showing that paclitaxel has less anticancer activity against PANC-1 cells than the Bx-PC-3 cells (Awasthi et al., 2013). Replacement of the TPGS by γ-T3-mPEG1000 resulted in a reduction in the anticancer activity of the unloaded α-T nanoemulsion against both pancreatic cell lines. Although stipulations have been made on how TPGS induces cytotoxic activity (Neophytou, Constantinou, Papageorgis, & Constantinou, 2014), it is reasonable to assume that TPGS, as with sodium dodecyl sulfate (SDS) (Bondi et al., 2015), induce nonspecific toxicity on cells. Therefore, the observed reduction in activity implies that the γ-T3-mPEG1000 may serve as a less toxic excipient in the formulation of nanoemulsions. When α-T was replaced with γ-T3 in the γ-T3-mPEG1000-based nanoemulsions, a significant increase in the anticancer activity of the unloaded nanoemulsion against both cell lines was observed (P value < 0.05). This observation is consistent with our previous observation that the free γ-T3 isomer is significantly more active against breast and pancreatic cancer cells than the free α-T isomer of vitamin E (Alayoubi, Anderson, Satyanarayanajois, Sylvester, & Nazzal, 2013). Paclitaxel loading into the γ-T3-mPEG1000 based nanoemulsions further enhanced their anticancer activity against both cell lines, which was also higher when compared to the TPGS (Tocosol™) based formulations. A significant increase in the activity of paclitaxel was also observed when γ-T3-mPEG1000 was replaced by γ-T3-mPEG2000 (P value < 0.05), which could be attributed to their smaller particle size and thereby enhanced cellular uptake (Shang, Nienhaus, & Nienhaus, 2014; Zhang, Li, Lykotrafitis, Bao, & Suresh, 2009). While intracellular trafficking/cellular uptake was not evaluated, we postulate that the nanoemulsions will be internalized by an endocytic process. Gemcitabine-stearic acid conjugate loaded into lipid nanoparticles, for example, were shown to undergo clathrin-mediated endocytosis into the lysosomes of the cells where the conjugate is hydrolyzed (Wonganan et al., 2013).
Fig. 8.
In-vitro cell cytotoxicity study of nanoemulsions against human pancreatic cancer BxPC-3 cells. Cells were treated for 72 h with (A) α-T and γ-T3 TPGS-based nanoemulsions with or without free pacliaxel, (B) α-T and γ-T3 γT3-1000-based nanoemulsions with or without paclitaxel, and (C) paclitaxel lipids conjugates loaded into γT3-2000-based nanoemulsion. Values reported are the mean ± SD of triplicate samples
Fig. 9.
In-vitro cell cytotoxicity study of nanoemulsions against human pancreatic cancer PANC-1 cells. Cells were treated for 72 h with (A) α-T and γ-T3 TPGS-based nanoemulsions with or without free pacliaxel, (B) α-T and γ-T3 γT3-1000-based nanoemulsions with or without paclitaxel, and (C) paclitaxel lipids conjugates loaded into γT3-2000-based nanoemulsion. Values reported are the mean ± SD of triplicate samples
Unfortunately, paclitaxel-lipid conjugates did not demonstrate added advantage when tested in-vitro. They had comparable anticancer activity as the nanoemulsions loaded with the free paclitaxel against both cell lines. As in the earlier observations, they had lesser anticancer activity against PANC-1 cells than the Bx-PC-3 cells. It was reported that fatty acid conjugates of paclitaxel showed reduced in-vitro anticancer activity (Ali et al., 2001). Moreover, in the current study, the in-vitro release profile for paclitaxel was less than 30% after 48 hr, which might affect the availability of the drug in the cell culture media as the treatment time was 48–72 hr. This is further supported by the observations that other natural anticancer agents, like Illudin M, lost their activity against PANC-1 pancreatic cancer cells upon conjugation with docosahexaenoic acid (Schobert, Biersack, Knauer, & Ocker, 2008). No significant differences were observed between the types of lipids used for paclitaxel conjugation on their in-vitro activity. It is nonetheless probable that the conjugates will show differences in activity when tested in animal models. In-vivo testing of the molecules, although critical, was beyond the scope of the present work.
4. Conclusion
The paclitaxel nanoemulsions that were prepared in this study were broadly based on the α-T/TPGS composition of the Tocosol™ formulation. As expected from previous reports, substituting α-T with γ-T3 was advantageous due to the innate anticancer activity of the tocotrienol isomers of vitamin E. Substituting TPGS with the PEGylated T3 was also shown to reduce the cytotoxic effect of the vehicle, which may warrant further preclinical toxicological studies to confirm the superiority of the PEGylated T3 as excipients for paclitaxel delivery. While the inclusion of free paclitaxel significantly enhanced the cytotoxic effect of the nanoemulsions, conjugating paclitaxel to lipids suppressed the release of paclitaxel and its anticancer effect against tumor cells. Furthermore, no differences were observed in activity between the lipid conjugates, whether saturated or unsaturated, when tested in-vitro. It is not clear, however, if the paclitaxel conjugates would behave differently when tested in-vivo. Nonetheless, despite the experimental limitations of the present study, the results clearly suggest that nanoemulsions prepared using either γ-T3, PEGylated γ-T3, or paclitaxel lipid conjugates may represent a promising option for paclitaxel delivery in cancer chemotherapy.
Acknowledgments
This work was supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103424.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Fayyad A, Behery F, Sallam AA, Alqahtani S, Ebrahim H, El Sayed KA, … Nazzal S. PEGylated gamma-tocotrienol isomer of vitamin E: Synthesis, characterization, in vitro cytotoxicity, and oral bioavailability. Eur J Pharm Biopharm. 2015;96:185–195. doi: 10.1016/j.ejpb.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Abu-Fayyad A, Nazzal S. Synthesis, characterization, and in-vitro antitumor activity of the polyethylene glycol (350 and 1000) succinate derivatives of the tocopherol and tocotrienol isomers of Vitamin E. Int J Pharm. 2017;519(1–2):145–156. doi: 10.1016/j.ijpharm.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal B, Nesaretnam K. Vitamin E tocotrienols: life beyond tocopherols. Genes Nutr. 2012;7(1):1. doi: 10.1007/s12263-011-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem. 2007;282(1):809–820. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- Alayoubi AY, Anderson JF, Satyanarayanajois SD, Sylvester PW, Nazzal S. Concurrent delivery of tocotrienols and simvastatin by lipid nanoemulsions potentiates their antitumor activity against human mammary adenocarcenoma cells. Eur J Pharm Sci. 2013;48(3):385–392. doi: 10.1016/j.ejps.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Ali S, Ahmad I, Peters A, Masters G, Minchey S, Janoff A, Mayhew E. Hydrolyzable hydrophobic taxanes: synthesis and anti-cancer activities. Anticancer Drugs. 2001;12(2):117–128. doi: 10.1097/00001813-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Ansell SM, Johnstone SA, Tardi PG, Lo L, Xie S, Shu Y, … Mayer LD. Modulating the therapeutic activity of nanoparticle delivered paclitaxel by manipulating the hydrophobicity of prodrug conjugates. J Med Chem. 2008;51(11):3288–3296. doi: 10.1021/jm800002y. [DOI] [PubMed] [Google Scholar]
- Awasthi N, Zhang C, Schwarz AM, Hinz S, Wang C, Williams NS, … Schwarz RE. Comparative benefits of Nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer. Carcinogenesis. 2013;34(10):2361–2369. doi: 10.1093/carcin/bgt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CA, Marks JL, Wroblewski LB, Raatikainen HS, Lenox SR, Gebhardt KE. Human and Environmental Toxicity of Sodium Lauryl Sulfate (SLS): Evidence for Safe Use in Household Cleaning Products. Environ Health Insights. 2015;9:27–32. doi: 10.4137/ehi.s31765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides PP, Lambert KJ, Tustian AK, Schneider B, Lalji S, Ma W, … Quay SC. Formulation development and antitumor activity of a filter-sterilizable emulsion of paclitaxel. Pharm Res. 2000;17(2):175–182. doi: 10.1023/a:1007565230130. [DOI] [PubMed] [Google Scholar]
- Fang C, Shi B, Pei YY. Effect of MePEG molecular weight and particle size on in vitro release of tumor necrosis factor-alpha-loaded nanoparticles. Acta Pharmacol Sin. 2005;26(2):242–249. doi: 10.1111/j.1745-7254.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- Fu Q, Wang Y, Ma Y, Zhang D, Fallon JK, Yang X, … Liu F. Programmed Hydrolysis in Designing Paclitaxel Prodrug for Nanocarrier Assembly. Sci Rep. 2015;5(12023) doi: 10.1038/srep12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli F, Azzi A, Birringer M, Cook-Mills JM, Eggersdorfer M, Frank J, … Ozer NK. Vitamin E: emerging aspects and new directions. Free Radic Biol Med. 2016;2(16):30432–30434. doi: 10.1016/j.freeradbiomed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Guo Y, Luo J, Tan S, Otieno BO, Zhang Z. The applications of Vitamin E TPGS in drug delivery. Eur J Pharm Sci. 2013;49(2):175–186. doi: 10.1016/j.ejps.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Ma P, Mumper RJ. Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J Nanomed Nanotechnol. 2013;4(2):1000164. doi: 10.4172/2157-7439.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neophytou CM, Constantinou C, Papageorgis P, Constantinou AI. D-alpha-tocopheryl polyethylene glycol succinate (TPGS) induces cell cycle arrest and apoptosis selectively in Survivin-overexpressing breast cancer cells. Biochem Pharmacol. 2014;89(1):31–42. doi: 10.1016/j.bcp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Schobert R, Biersack B, Knauer S, Ocker M. Conjugates of the fungal cytotoxin illudin M with improved tumour specificity. Bioorg Med Chem. 2008;16(18):8592–8597. doi: 10.1016/j.bmc.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnology. 2014;12:5. doi: 10.1186/1477-3155-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester PW, Kaddoumi A, Nazzal S, El Sayed KA. The value of tocotrienols in the prevention and treatment of cancer. J Am Coll Nutr. 2010;29(3 Suppl):324S–333S. doi: 10.1080/07315724.2010.10719847. [DOI] [PubMed] [Google Scholar]
- Vaughan VC, Hassing MR, Lewandowski PA. Marine polyunsaturated fatty acids and cancer therapy. Br J Cancer. 2013;108(3):486–492. doi: 10.1038/bjc.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonganan P, Lansakara-P DSP, Zhu S, Holzer M, Sandoval MA, Warthaka M, Cui Z. Just getting into cells is not enough: Mechanisms underlying 4-(N)-stearoyl gemcitabine solid lipid nanoparticle’s ability to overcome gemcitabine resistance caused by RRM1 overexpression. Journal of Controlled Release. 2013;169(1):17–27. doi: 10.1016/j.jconrel.2013.03.033. doi: https://doi.org/10.1016/j.jconrel.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li J, Lykotrafitis G, Bao G, Suresh S. Size-Dependent Endocytosis of Nanoparticles. Adv Mater. 2009;21:419–424. doi: 10.1002/adma.200801393. [DOI] [PMC free article] [PubMed] [Google Scholar]