Abstract
Intensive pain rehabilitation programs for children with chronic pain are effective for many patients. However, characteristics associated with treatment response have not been well documented. Here we report trajectories of pain and functional impairment in patients with chronic pain up to one year after intensive pain rehabilitation and examine baseline factors associated with treatment response. Patients (n=253) with chronic pain and functional disability were assessed at 5 time points (admission, discharge, 1-month, 4-month, and 12-month follow-up). Individual trajectories were empirically grouped using SAS PROC TRAJ. For functional disability, two groups emerged: treatment responders (88%) and nonresponders (12%). Using a binomial logistic regression model to predict disability trajectory group, no baseline variables were significant predictors of disability trajectory group. For pain, three groups emerged: early treatment responders (35%), late treatment responders (38%), and nonresponders (27%). Using multinominal regression analyses to predict pain trajectory group, older age, higher pain scores, fewer social difficulties, higher anxiety levels, and lower readiness to change were characteristics that distinguished nonresponders from responders; no significant predictors distinguished the late responders from the early responders. These results provide key information on the baseline factors that influence intensive pain rehabilitation outcomes, including risk factors that predict treatment nonresponse. Our findings have implications for developing more targeted treatment interventions.
Keywords: chronic pain, child and adolescent, treatment response, intensive rehabilitation
Introduction
Recent studies provide evidence supporting an intensive pain rehabilitative approach to the management of complex pediatric chronic pain12, 19, 29. This approach encompasses daily physical, occupational, and psychological therapy focused on helping children return to premorbid levels of functioning through progressively engaging in previously avoided activities and taking a self-management approach to pain. Intensive pain rehabilitation is time intensive, costly (>30K)6, and often the last hope for families who have not experienced success in outpatient treatment. Given these constraints, identifying patients most likely to benefit as well as having an understanding of anticipated outcomes over time within the context of this treatment approach is imperative. In evaluating pain treatment success, both functional disability and pain intensity are considered key outcome measures23.
Few studies have examined trajectories of pain and disability over time among youth engaged in intensive pain rehabilitation. One study examined stability of treatment response from 3- to 12-month follow-up and qualitatively categorized patients into four groups: stable long-term improvers (46%), late improvers (14%), short-term improvers (19%), and unsuccessful treatment/nonresponders (22%)13. In examining group differences by baseline characteristics, no difference by age or pain diagnosis was observed, but some differences by psychosocial variables emerged. Stable long-term improvers had significantly higher school absences compared to nonresponders and short-term improvers had higher levels of generalized anxiety and depression than stable long-term improvers. A second study examined 24–42 month outcomes of intensive pain rehabilitation and identified two trajectories of treatment response for pain: patients with initially high pain ratings reporting large reductions in pain and patients with initially lower pain ratings showing smaller reductions1. Both response groups had similar rates of improvement in child school days missed, parent work days missed, and days hospitalized. Although these studies provide initial data characterizing trajectories of treatment response among patients who completed intensive pain rehabilitation, further work is needed. In addition to including previously examined factors of age, pain diagnosis, school functioning, anxiety, and depression, it is important to examine other known and influential variables. Higher levels of pain-related fear at admission to intensive pain rehabilitation has been associated with less improvements in functional disability during treatment26, and greater child readiness to change at baseline (higher contemplation scores) has been associated with greater improvements in functional disability during treatment20. Beyond these findings, recent theoretical models such as the resilience-risk model for pediatric chronic pain4 provide a framework for examining a number of key influences such as social functioning27, parent protective behavior17, 28, and pain catastrophizing2, 34 that have not yet been examined in this context and were thus considered important for inclusion in the current study.
The aim of the current study was to investigate trajectories of functional impairment and pain symptoms across and up to one year after intensive pain rehabilitation, and to identify characteristics at baseline that predict long term success or nonresponse in this treatment approach. Given that prior work has yielded two to four trajectories of treatment response, we took a parsimonious approach and hypothesized that for both disability and pain there would be at least two groups derived: treatment “responders” and “nonresponders.” Based on prior work for baseline differences, we anticipated that the nonresponder group would, at baseline, have higher levels of pain-related distress (catastrophizing, fear), worse social functioning, higher levels of generalized distress (anxiety, depression), lower levels of readiness to change, and higher levels of protective parenting behavior.
Methods
Setting and Participants
Participants were 280 children and adolescents who completed an intensive interdisciplinary pediatric pain rehabilitation day program from July 2009 to March 2014. In total, 301 patients enrolled in the program during that time period. Patients not included in the analysis did not consent to participate (n=9), were not approached due to a developmental delay (n=5), were immediately discharged from the program due to psychological or medical reasons (n=4), or left the program against medical advice (n=3). The pain rehabilitation program has historically targeted patients, ages 8–18 years, with persistent extremity pain with neuropathic features and significant impairment of mobility and limb use (e.g. Complex Regional Pain Syndrome), although more recently the patients served has expanded (as can be observed in Table 1). Program eligibility included failure to progress in conventional outpatient physical and cognitive behavioral therapies. While many patients had psychological challenges, those with active suicidality, psychosis, or an eating disorder prior to enrollment were not eligible for admission. All patients were evaluated through our multidisciplinary outpatient pain treatment clinic prior to admission.
Table 1.
Pain and disability treatment responders by pain diagnosis group
| Functional disability | Pain | ||||
|---|---|---|---|---|---|
|
| |||||
| Diagnosis | Early Responder % (n) | NR % (n) | Early Responder % (n) | Late Responder % (n) | NR % (n) |
| Localized musculoskeletal | 88.2% (15) | 11.8% (2) | 25.0% (7) | 39.3% (11) | 35.7% (10) |
| CRPS | 85.0% (96) | 15.0% (17) | 33.8% (46) | 38.2% (52) | 27.9% (38) |
| Idiopathic small fiber sensory neuropathy | 100% (17) | 0% (0) | 57.1% (12)a | 38.1% (8) | 4.8% (1)b |
| Widespread musculoskeletal | 84.6% (11) | 15.4% (2) | 31.8% (7) | 40.9% (9) | 27.3% (6) |
| Back | 93.3% (14) | 6.7% (1) | 33.3% (6) | 27.8% (5) | 38.9% (7) |
| Abdominal | 88.9% (8) | 11.1% (1) | 25.0% (3) | 41.7% (5) | 33.3% (4) |
| Headache | 100% (10) | 0% (0) | 37.5% (6) | 43.8% (7) | 18.8% (3) |
Note. ‘a’ superscripts indicate that the frequency of cases was significantly higher than expected, whereas ‘b’ superscripts indicate that the frequency of cases was significantly lower than expected.
Treatment Interventions
Program overview
When these data were collected the rehabilitation program entailed intensive daily physical, occupational and psychological therapies eight hours per day, five days per week for an average length of stay of three to four weeks. A typical treatment day began with a three one-hour blocks of individual physical therapy (PT), occupational therapy (OT), and psychological therapy (one hour each) followed by a two-hour period for studying and lunch. The subsequent two hours consisted of a one-hour of group physical or occupational therapy session and a one-hour of group psychological therapy session. For the final hour of the day, patients participated in either family psychotherapy (twice per week) or parent-observed individual physical or occupational therapy sessions (three times per week). A physician and nurse evaluated patients daily to ensure continued appropriateness for treatment (e.g., continued medical stability) and to address acute or ongoing medical issues.
Specific modalities
PT, OT, and psychotherapy focused on helping children return to premorbid levels of functioning through progressively engaging in previously avoided activities and taking a self-management approach to pain. Additional details on the program and its outcomes are reported elsewhere18, 19.
Physical therapy
PT aimed to maximize independence with mobility, functional tasks and exercise using a graded approach: promoting increased weight-bearing (stress loading), and improving strength, flexibility, coordination, balance and aerobic fitness. Tasks were linked to the child’s individualized functional goals such as playing a specific sport.
Occupational therapy
OT aimed to maximize independence and participation in self-care, school, and leisure activities. Progressive, individualized sensory re-education programs, including desensitization and sensory discrimination activities, were utilized to normalize responses to typical daily sensory stimuli, such as wearing a shoe or bathing. Patients also completed PT and OT home exercise programs each night to promote independence and generalization of skills acquired during the treatment day.
Psychological therapy
Psychological therapy followed a cognitive-behavioral model 35. Patients received daily individual and group-based cognitive behavioral therapy and families were actively incorporated into the program, with family therapy and parent education provided. Psychological therapy targets included: 1) teaching a self-management approach to pain, 2) addressing negative thinking and fears about pain, 3) engaging in valued activities and relationships in the presence of pain, and 4) reducing parental attention and protective responses to pain.
Post-discharge Follow-up Clinic
Patients returned for follow-up appointments approximately 1-, 4-, and 12-months post-discharge. At that time, patients were evaluated by a physician, psychologist, occupational therapist, and physical therapist individually, for one hour sessions each. Following these evaluations, the treatment team met and provided the family with feedback regarding current clinical status, goal attainment, and goal progression.
Procedures
The institutional review board approved the study. Pain intensity ratings at admission, discharge, and at follow-ups were collected during the nursing evaluation. Functional disability ratings were collected via patient self-report in a larger questionnaire packet of emotional and functional measures completed at admission (sent a few days prior to arrival), discharge (given to complete the day before discharge), and at follow-up visits (sent via mail a few days prior to their scheduled clinic appointment). All additional variables examined in this study were either extracted from the patient’s medical record or were also part of the questionnaire packet of emotional and functional measures administered at admission. The child and one parent are asked to complete them independently and bring them in. Standard outcome data are collected on a continuous, rolling basis, and several previous publications have reported on short-term outcomes19–21, 26 from this clinical program.
Measures
Outcomes over time
Pain intensity
During admission, discharge, and follow-up evaluations, children were asked to provide their typical pain intensity rating at rest on a standard 11-point numeric rating scale32 from 0 (no pain) to 10 (most pain possible).
Functional disability
The Functional Disability Inventory (FDI)33 is a child-completed scale that assesses difficulty in physical and psychosocial functioning due to physical health. The instrument consists of 15 items concerning perceptions of activity limitations during the past two weeks; total scores are computed by summing the items. Higher scores indicate greater disability. The FDI has good reliability and validity3. Internal consistency of this measure was 0.87, 0.92, 0.94, 0.94, and 0.93 across admission, discharge, and follow-up time-points.
Baseline variables
Demographic and pain condition characteristics
Basic demographic (e.g., age, gender) and medical information (e.g., pain diagnosis, duration of pain) were collected from patient charts.
Social and School Functioning
The Pediatric Quality of Life Inventory (PedsQL)30, 31 is a 23-item questionnaire that determines the overall health related quality of life of a child. A higher PedsQL score indicates better functioning. The social and school functioning subscales were examined. The social functioning subscale (5 items) assesses social difficulties (e.g., “Other teens tease me”, “I have trouble getting along with other teens”). The internal consistency of the social functioning subscale for this study was 0.76. The school functioning subscale (5 items) investigates how much children have an issue with paying attention in class, forgetting things, keeping up with schoolwork, and missing school due to not feeling well or due to doctor’s appointments. The internal consistency of the school functioning subscale for this study was 0.79.
Pain catastrophizing
The Pain Catastrophizing Scale (PCS-C, PCS-P)5, 7 assesses negative thinking associated with pain. It is comprised of 13 items rated on a 5-point scale. Items are summed to derive a total score. Higher scores indicated higher levels of catastrophic thinking. Internal consistency of the Total Score on this measure in this sample was 0.90.
Pain-related fear
The Fear of Pain Questionnaire (FOPQ-C)28 assesses self-reported perceptions of child pain-related fears and avoidance behaviors. Specific items include: “I avoid making plans because of my pain.” and “I worry when I am in pain.” It is comprised of 24 items rated on a 5-point scale. Items are summed to derive a total score and two subscale scores, Fear of Pain and Avoidance of Activities. Higher scores indicate higher levels of pain-related fear. The total score was used in this study. Internal consistency of the total score in this sample was 0.92 at admission.
Readiness to change
Pain Stages of Change Questionnaire for Adolescents (PSOCQ-A) and Parents (PSOCQ-P)8. Assesses adolescents’ readiness to adopt a self-management approach to pain and parents’ own levels of readiness to adopt this approach. PSOCQ-A yields three validated subscales: Precontemplation: Little perceived personal responsibility for managing pain; Contemplation: Awareness of personal responsibility for pain management, considering behavioral change; Action/Maintenance: Active involvement in learning or continued use of self- management strategies. PSOCQ-P yields four validated subscales: Precontemplation, Contemplation, Action, Maintenance. The measure has demonstrated reliability and validity. In the current sample, coefficient alphas for the PSOCQ-A subscales were 0.65 for Precontemplation, 0.86 for Contemplation, and 0.88 for Action/Maintenance. Coefficient alphas for the PSOCQ-P subscales were 0.72 for Precontemplation, 0.84 for Contemplation, 0.80 for Action, and 0.70 for Maintenance. For both scales, individuals were classified into one stage based on the highest score across stages. If there was tie between two stages, the patient/parent was categorized in the higher stage. This categorization was used in this study.
Depressive symptoms
The Children’s Depression Inventory (CDI)16 is a valid and reliable 27-item child self-report measure of depressive symptoms. The CDI has good reliability and validity and is widely used in assessments of children with medical conditions. Higher scores indicate higher levels of depression. Internal consistency of this measure was 0.87 at admission.
Anxiety symptoms
The Multidimensional Anxiety Scale for Children (MASC)22 is a 39-item, self-report inventory assessing anxiety in children. The factor structure of the MASC is stable across age and gender. Strong reliability and validity data exist for this measure22. Internal consistency of this measure was 0.90 at admission.
Statistical Analyses
All analyses were conducted in SPSS version 22 (SPSS Inc., Chicago, IL) and SAS 9.4 (SAS Institute, Cary, NC). Descriptive statistics were calculated for all demographic and study variables. Trajectory analyses were conducted to examine patterns of pain and disability from the start of treatment to one-year post-discharge utilizing data at admission, discharge, 1-mont follow-up, 4-month follow-up, and 1-year follow-up and potentially define clinically relevant groups of patients who could be classified as “responders” and “nonresponders” based on their trajectory of recovery (e.g., responder group would report low levels of disability after treatment). The SAS PROC TRAJ procedure 14 was used to determine models of pain and disability across pre-treatment and post-treatment time points. The TRAJ procedure is a mixture model that estimates a regression model for each discrete group within the population. PROC TRAJ can include missing observations as it uses all values available from each case to estimate an individual’s timeline. A logistic model of dropout probability was included for each time point to account for nonrandom attrition9. Model complexity and overall fit in PROC TRAJ is determined on the Bayesian information criterion (BIC), which are negative values in which values closer to 0 indicate a better fit. In addition to BIC values, visual inspection of graphic model curves is used to determine the number of trajectories, similar to cluster and exploratory factor analysis. PROC TRAJ provides individual fit estimates, probabilities that each child belongs to each of the modeled trajectory groups. Average probability for members of a trajectory group should be 0.7024. Trajectory group membership for each individual was then used as the dependent variable for the logistic and multinomial regression analyses examining baseline predictors of treatment response group status.
Logistic regression analyses were used to examine the relative risk associated with familiar baseline factors in relation to treatment responder group status. The regression model included demographic and pain characteristic (age, pain level at admission, duration of pain), functioning (disability, social, school), patient cognitive-affective (pain catastrophizing, pain-related fear, depression, anxiety, readiness to change), and parent cognitive-behavioral (pain catastrophizing, protective behavior, readiness to change) factors.
Final sample
Despite the robustness of the PROC TRAJ procedure, we wanted a stringent evaluation of pain and disability trajectories thus we omitted from our analyses any patient with less than 3 data points for pain or disability. Of the 280 patients who consented to participate, 253 patients had at least 3 pain data points (inclusive of baseline) to be included in trajectory analysis (90.4% of the sample). Among the 253 patients with sufficient pain ratings data, 194 patients had at least 3 functional disability data points (inclusive of baseline) to be included in trajectory analysis (69.2% of the sample). The discrepancy in size of these two groups can be attributed to data collection method. Disability was collected via questionnaire packets that patients completed at follow-up appointments or via mail whereas pain ratings were collected in person by a program clinician.
Results
Participant Characteristics
The 253 patients in this sample were predominantly Caucasian (92%) and females (84%) and ranged in age from 8 to 22 years (M=14.5, SD=2.7). Most patients reported neuropathic pain 61% (53% Complex Regional Pain Syndrome [CRPS]; 8% Non-CRPS), with remaining patients reporting musculoskeletal pain 27% (12% localized limb(s) or joint(s); 9% diffuse; 7% back), headache 6%, and functional abdominal pain 6%. Median duration of pain was 10.0 months (M=19.6, SD=22.1). The median length of stay in the pain rehabilitation program was four weeks, with follow-up evaluations completed at 1-, 4-, and 12-months post-discharge.
Preliminary and Descriptive Analyses
Of the 253 patients with pain ratings at 3 or more time points, pain ratings sample sizes were 253, 253, 237, 123, and 99, for admission, discharge, 1-, 4-, and 12-month follow-ups, respectively. Of the 194 patients with functional disability inventory scores sample sizes were 194, 182, 173, 106, and 91. We examined baseline differences between those included in analyses (n=253) and those excluded (n=27) with no significant differences emerging for admission duration of pain, disability, pain, age, or length of stay in the rehabilitation program. Moreover, we examined these same baseline variables between those who had 1-year follow-up pain data (n=99) and those that did not (n=154), with no significant differences between the two groups.
Pain Trajectories
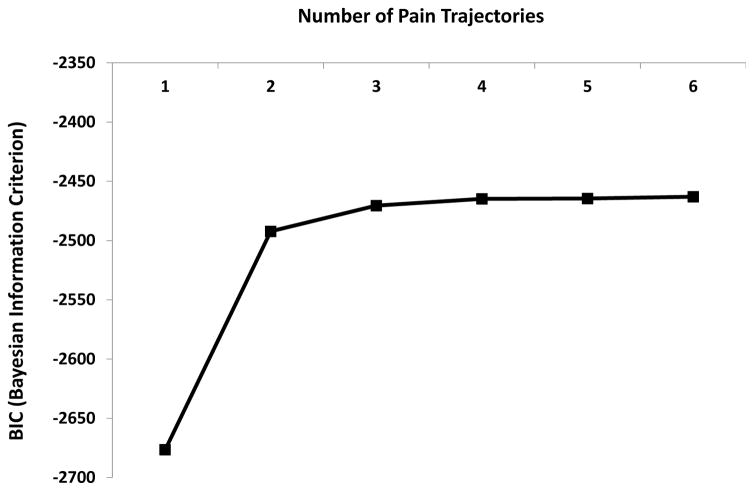
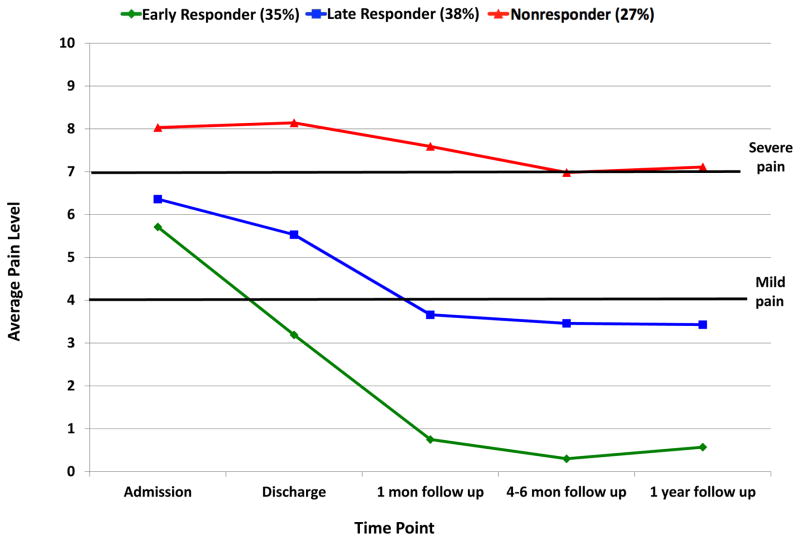
In the sample of 253 patients included in the pain trajectory analysis, we examined up to six trajectory solutions based on prior trajectory analysis work 25. The BIC values significantly improved from the 1- to 2-trajectory solution with a slight improvement with the 3-group solution, although there was small improvements in model fit with the addition of more trajectories (Figure 1), the 3-trajectory solution was most clinically meaningful. The groups are presented in Figure 2.
Early Treatment Responders-Pain (35%): presented at admission with moderate pain levels that decreased to mild pain at discharge, with a further decrease to virtually no pain at 1-month follow-up that maintained at the 1-year follow-up.
Late Treatment Responders-Pain (38%): presented at admission with moderate pain levels, had very little improvement in pain by treatment discharge, but reported significant improvements with mild pain at 1-month follow-up with these mild pain levels remaining stable through 1-year post-discharge.
Treatment Nonresponders-Pain (27%): presented at admission with severe pain, with pain levels virutually unchanged across all time points.
Figure 1.
Baeyesian Information Criterion (BIC) scores for pain trajectories.
Figure 2.
Trajectories of pain.
Mean fit estimates for the three trajectory groups were excellent: 0.93 (SD=0.12) for the early responder group, 0.85 (SD=0.15) for the late responder group, and 0.91 (SD=0.14) for the nonresponder group. SAS Proj Trac calculates a dropout probability for each patient at each timepoint. We examined differences in dropout probabilities at the 4-month and 1-year timepoints as several patients did not return for these two visits. The omnibus ANOVA was significant at 4-month f(2,239)=17.5, p<.01 and 12-month follow-up, f(2,120)=10.0, p<.01. In both cases the Early Treatment Responder-Pain group had a higher average dropout probability (4-month M=0.32, SD=0.14; 12-month M=0.38, SD=0.10) compared to the Late Responder-Pain group (4-month M=0.23, SD=0.12; 12-month M=0.26, SD=0.15) and Nonresponder-Pain group (4-month M=0.21, SD=0.13; 12-month M=0.26, SD=0.17). Thus it appears that patients who were doing quite well after 1-month follow-up were less likely to return for further clinical care at 4-month and 12-month post-discharge.
Functional Disability Trajectories
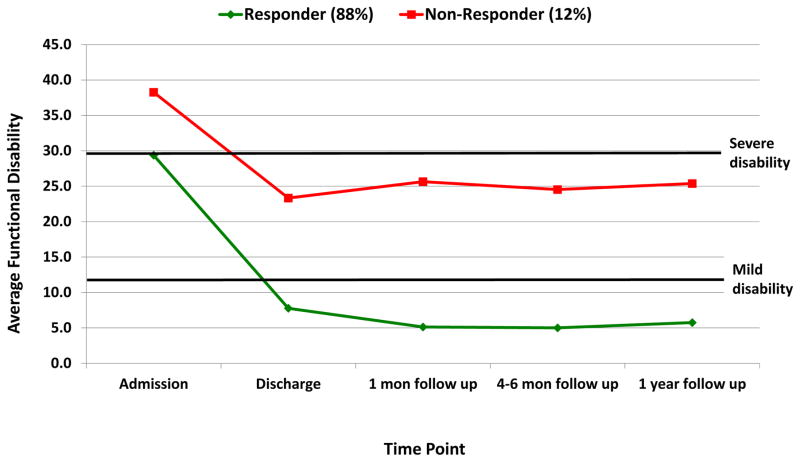
In the sample of 194 patients included in the functional disability trajectory analysis, we examined up to six trajectory solutions as was done for pain. The 2-trajectory solution was superior and the groups are depicted in Figure 3.
Treatment Responders-Functioning (88%): presented at admission with severe disability that decreased to mild disability at discharge and maintained through 1-year follow-up.
Treatment Nonresponders-Functioning (12%): presented at admission with very severe disability, with improvments in disability to moderate levels that remain unchanged across time.
Figure 3.
Trajectories of functional disability.
Mean fit estimates for the two trajectory groups were excellent: 0.98 (SD=0.07) for the responder group and 0.97 (SD=0.08) for the nonresponder group. Similar to pain, we examined differences in dropout probabilities at the 4-month and 1-year timepoints as several patients did not return for these two visits. The independent t-test was significant at 4-month t(171)=26.8, p<.01 and 12-month follow-up, t(104)=21.2, p<.01. In both cases the Treatment Responder-Disability group had a higher average dropout probability (4-month M=0.37, SD=0.05; 12-month M=0.37, SD=0.06) compared to the Nonresponder-Pain group (4-month M=0.03, SD=0.06; 12-month M=0.04, SD=0.05). Consistent with the pain trajectory groups, patients who were doing well after 1-month follow-up were less likely to return for further clinical care subsequently.
Trajectory Group Differences at Treatment Admission
Trajectory by pain diagnosis group
Using chi-square statistics, we examined the proportion of patients in each trajectory group by pain diagnosis. The overall chi-square statistic was non-significant for both functional disability trajectories χ2(6, n=194)=5.28, p=0.51 and pain trajectories χ2(6, n=253)=11.15, p=0.52. Using a z-test to compare column proportions, patients diagnosed with neuropathic pain were more likely to be classified as pain early responders (57.1%) versus nonresponders (4.8%). No other proportions were greater or smaller than predicted (see Table 1 for all frequencies).
Predictive model of baseline characteristics across pain trajectories
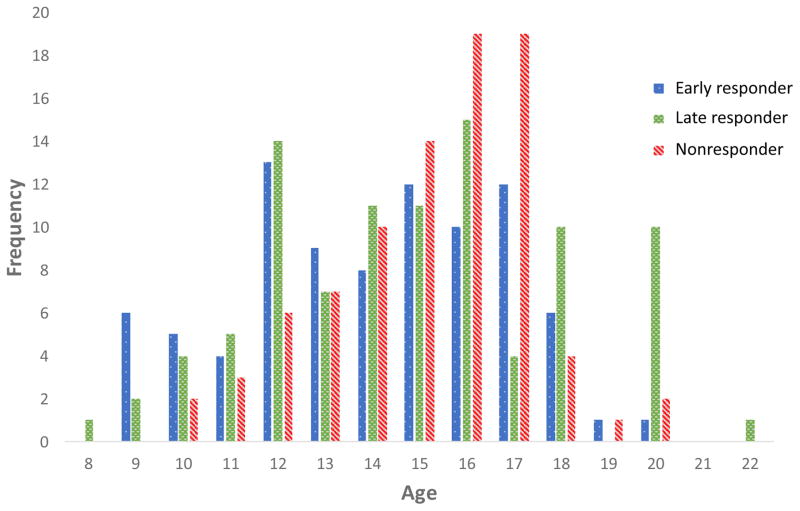
Age, pain (age, pain intensity, duration of pain), functional (disability, social functioning, school functioning), patient cognitive-affective (pain catastrophizing, pain-related fear, depressive symptoms, anxiety symptoms, readiness to change), and parent cognitive-behavioral (pain catastrophizing, protective behavior, readiness to change) parameters were examined at the bivariate level to ensure no multicollinearity prior to being entered into a multinomial logistic regression analysis. As anticipated, variables within domains were more strongly associated (e.g., functional disabiilty and school functioning), but none violated rule of multicollinearity (see Table 2). The results of the multinomial logistic regression model are reported in Table 3. The analysis was significant (χ2(34)=81.45, p<0.01 with five predictors of nonresponder (versus early responder) group status observed and no significant predictors of late responder (versus early responder) group status. Specifically, for every year older, the odds ratio of being in the nonresponder (versus early responder) group increased by 1.26. To examine if this upward progression of risk continues with age, we examined the frequency distribution of responder categories across age (depicted in Figure 4) noting that the highest proportion of nonresponders were ages 15–17 with a lower proportion among younger patients (ages 8–14) and older patients (ages 18–22). For pain intensity, a 1-point increase in average pain reported at baseline was associated with a 2-fold (2.29) increased risk of being in the nonresponder group. For every 1-point increase in social functioning (indicating better functioning) and 1-point increase in anxiety symptoms (indicating worse anxiety), the odds ratio of being in the nonresponder group increased by 1.0. Lastly, patients whose highest stage of change was categorized as either precontemplation or contemplation had a 9-fold increased risk of being in the pain nonresponder group.
Table 2.
Bivariate correlations among baseline factors.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age and Pain Characteristics | ||||||||||||||||
| 1. Age | -- | −.05 | .31** | .05 | −.002 | −.16 | −.02 | .13 | .24** | .07 | −.02 | .04 | .08 | −.07 | 14.6 | 2.46 |
| 2. Pain at admission | -- | −.13 | .49** | −.13 | −.37** | .30** | .22** | .12 | .01 | −.25** | .03 | .10 | −.05 | 6.69 | 1.96 | |
| 3. Duration of pain | -- | −.17* | .05 | −.01 | −.14 | −.09 | .07 | .06 | −.06 | −.20** | −.06 | .06 | 18.2 | 21.0 | ||
| Functioning | ||||||||||||||||
| 4. Functional disability | -- | −.38** | −.57** | −.57** | .48** | .30** | .28** | −.13* | .19* | .37** | −.06 | 31.0 | 10.3 | |||
| 5. Social functioning | -- | .43** | −.23** | −.39** | −.42** | −.42** | .13 | −.26** | −.16* | .03 | 63.7 | 19.8 | ||||
| 6. School functioning | -- | −.29** | −.50** | −.39** | −.28** | .08 | −.16* | −.42** | .20** | 44.0 | 23.5 | |||||
| Patient cognitive-behavioral factors | ||||||||||||||||
| 7. Pain catastrophizing | -- | .74** | .42** | .38** | −.34** | .24** | .22** | −.17* | 27.2 | 11.1 | ||||||
| 8. Pain-related fear | -- | .50** | .51** | −.26** | .28** | .47** | −.19* | 48.4 | 18.2 | |||||||
| 9. Depressive symptoms | -- | .55** | −.20* | .19* | .29** | −.14 | 53.1 | 11.8 | ||||||||
| 10. Anxiety symptoms | -- | .00 | .13 | .26** | −.03 | 50.4 | 13.1 | |||||||||
| 11. Readiness to change | -- | −.09 | −.07 | .11 | 1.97 | 0.73 | ||||||||||
| Parent cognitive-behavioral factors | ||||||||||||||||
| 12. Pain catastrophizing | -- | .31** | −.26** | 38.6 | 10.3 | |||||||||||
| 13. Protective behavior | -- | −.12 | 1.48 | 0.65 | ||||||||||||
| 14. Readiness to change | -- | 2.61 | 0.79 | |||||||||||||
Note.
p < 0.05,
p<0.01
Table 3.
Multinomial logistic regression results examining the differences between early pain responders vs. late responders and early pain responders vs. nonresponders (n=173)
| Variable | Late Respondera | Nonrespondera | ||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Age and Pain Characteristics | ||||
| Age | 1.06 | 0.89 – 1.26 | 1.26 | 1.00 – 1.58 |
| Pain at admission | 1.26 | 0.97 – 1.63 | 2.29 | 1.56 – 3.37 |
| Duration of pain | 1.00 | 0.98 – 1.02 | 0.99 | 0.96 – 1.01 |
| Functioning | ||||
| Functional disability | 1.04 | 0.98 – 1.10 | 1.07 | 1.00 – 1.15 |
| Social functioning | 1.02 | 0.99 – 1.05 | 1.05 | 1.01 – 1.08 |
| School functioning | 1.02 | 1.00 – 1.04 | 1.02 | 0.99 – 1.05 |
| Patient cognitive-affective factors | ||||
| Pain catastrophizing | 1.05 | 0.98 – 1.12 | 0.99 | 0.91 – 1.07 |
| Pain-related fear | .97 | 0.93 – 1.02 | 0.98 | 0.93 – 1.04 |
| Depressive symptoms | 1.01 | 0.96 – 1.06 | 1.00 | 0.94 – 1.06 |
| Anxiety symptoms | 1.04 | 1.00 – 1.08 | 1.02 | 1.02 – 1.14 |
| Readiness to change | ||||
| Pre vs. Act/Maintenance | 1.02 | 0.33 – 3.15 | 9.19 | 1.70 – 49.6 |
| Cont vs. Act/Maintenance | 2.21 | 0.82 – 5.97 | 9.96 | 2.00 – 49.6 |
| Parent cognitive-behavioral factors | ||||
| Pain catastrophizing | 1.00 | 0.96 – 1.05 | 0.99 | 0.94 – 1.05 |
| Protective behavior | 1.47 | 0.68 – 3.18 | 1.57 | 0.59 – 4.19 |
| Readiness to change | ||||
| Pre vs. Maintenance | 0.52 | 0.04 – 6.43 | 0.36 | 0.01 – 12.1 |
| Cont vs. Maintenance | 1.10 | 0.33 – 3.65 | 1.05 | 0.22 – 4.92 |
| Action vs. Maintenance | 0.86 | 0.24 – 3.02 | 1.06 | 1.06 – 5.20 |
Note. CI=Confidence Interval;
Responder group is the reference category; CIs with lower values ≥ 1.0 are bolded for emphasis.
Figure 4.
Frequency of patients across responder groups by age.
Predictive model of baseline characteristics across disability trajectories
Age, pain (age, pain intensity, duration of pain), functional (disability, social functioning, school functioning), patient cognitive-affective (pain catastrophizing, pain-related fear, depressive symptoms, anxiety symptoms, readiness to change), and parent cognitive-behavioral (pain catastrophizing, protective behavior, readiness to change) parameters were entered into a binary logistic regression analysis. The analysis was not significant (χ2(17)=22.12, p=0.18. No predictors of disability nonresponder (versus early responder) group status emerged.
Discussion
The results of this study indicate that the majority of children and adolescents in an intensive pain rehabilitation program perceive significant improvements in their functioning and pain during the first year after discharge. These rates of recovery are consistent with prior published work examining 1-year outcomes13. Beyond providing outcomes at 1-year post-discharge, this study provides the first empirically derived trajectories of treatment response across multiple time points for functioning and pain following an intensive pain rehabilitation program.
Functional Disability
Consistent with our hypotheses regarding functional disability, we found two trajectories: treatment responders and nonresponders. The overwhelming majority of patients (88%) exhibited an improvement in functioning at the end of treatment that persisted through one-year follow-up. Likely due to the small number of individuals classified as nonresponders, no baseline characteristics distinguished the two disability groups. These results provide convincing evidence for the impact of intensive pain rehabilitation programs on functional outcomes up to 1-year after treatment and are consistent with the recent meta-analysis of these programs for pediatric chronic pain11. These findings also suggest potentially examining the small subgroup of disability nonresponders more closely to examine additional clinical characteristics that may potentially drive this outcome. Additionally, it would be worthwhile to replicate this analysis with a larger cohort in the future to see if patterns of findings emerge with a larger subsample of nonresponders. Lastly, another approach to analysis may yield additional disability trajectory groups that are important to consider. For example, our results did not yield a group of patients who show initial significant improvements but then functionally decline. Although clinically we observe this subgroup, individuals with those characteristics did not emerge as a classifiable group and may be better captured via visual inspection or cut-off score categorization 13.
Pain Intensity
For pain, there were three trajectories that were fairly evenly distributed. Pain early responders (35%) essentially had a resolution of their pain by 1-month follow-up that continued at one year. The late responder group reported little improvement in pain at discharge and a significant decline at 1-month follow-up that stabilized in the mild pain range through one year after discharge.
Beyond the predictive influence of characteristics associated with pain problem complexity (high levels of pain) and older adolescent age, the most robust and modifiable risk factor was patient readiness to take a self-management approach to pain. Patients categorized as precontemplative (vs. action/maintenance) and contemplative (vs. action/maintenance) (via their highest score across the three PSOCQ-A stages) had a 9-fold increased risk of being categorized as a pain nonresponder, after controlling for several factors such as age, pain characteristics, functional impairment, patient cognitive-affective characteristics, and parent cognitive-behavioral characteristics. These results build upon prior work demonstrating that increased willingness to engage in a self-management treatment approach is associated with improved outcomes during intensive pain rehabilitation20. Moreover, its robust emergence in a multi-predictor model suggests that patients should be screened on readiness to change prior to enrollment. Those who are categorized as precontemplative or contemplative would benefit from 1) motivational interviewing to facilitate a shift in perspective/readiness and/or 2) education regarding how these perceptions may potentially impact their ability to fully benefit from pain rehabilitation treatment.
In addition to readiness to change, higher anxiety levels were associated with pain nonresponse and is consistent with prior work wherein greater psychiatric comorbidity is associated with poor treatment response13. Although exposure treatment is woven into the fabric of intensive pain rehabilitation via progressively increasing often feared and avoided activities, addressing more generalized anxiety symptoms via interoceptive exposure 10 may yield enhanced outcomes. Contrary to hypotheses, when controlling for all other factors, neither pain-related distress in the patient nor parent was associated with an increased risk of pain nonresponse. Unexpectedly, among several potential baseline factors associated with increased pain improvement, reporting more social difficulties emerged as a significant predictor. It is possible that the social milieu offered within this intensive pain rehabilitation program is particularly well-suited for those having more difficulties in this domain as they have the opportunity to connect with peers who are struggling with many of the same issues they have likely been facing. Moreover, it is possible that interactions among these risk factors amplify risk for patients who are, for example, highly anxious and thus less flexible in implementing self-management approaches to pain management.
Limitations
We note the following limitations. First, we had attrition across time and although we used a logistic model of dropout probability at each time point to account for nonrandom attrition, retaining all patients at each time point would have been ideal. Prospective controlled studies of psychiatric outpatient non-attendance suggest, for example, that those who miss appointments are more unwell, more socially impaired, and have a higher chance of subsequent admission than those who attend follow-up appointments15. Clinically, we find those returning for follow-up appointments at our clinic tend to represent patients and families who have a) been in close contact with our program due to continued difficulties and remain open to recommendations or b) attend follow-up appointments to share their successes and re-unite with staff. Patients/families who have elected to discontinue treatment with our service, on the other hand, are not represented in our study, and likely represent two segments: those who are doing quite well and have ‘moved on with their lives’ and those who are struggling and do not feel that the program has anything further to offer for their pain. In addition to statistically controlling for non-random attrition in our trajectory models, we only included patients who had data for at least 3 time points. Second, as for generalizability, our sample was predominantly Caucasian, female, and primarily composed of patients diagnosed with neuropathic pain or idiopathic small fiber sensory neuropathy conditions. As such, trajectories of treatment response should be examined across diverse ethnic and pain diagnostic groups, as well as males, to determine potential variability among patient subgroups. Third, despite effectively elucidating predictors of treatment response, this study did not examine the key components and optimal gradations of intensive pain rehabilitation. Each component of the interdisciplinary treatment is vital to its overall effectiveness; however, it would be useful to determine whether specific patient profiles at admission may be matched with tailored treatment approaches to optimize benefits and thus alter trajectories of treatment response detected in this study. Relatedly, our results did not account for the treatments patients may have received prior to engaging in and after discharging from our program. Although we began assessing both mothers and fathers in the program in 2013, we did not have sufficient data from enough families to include parent dyads in our analyses. This would be an important next step. Lastly, although we have previously demonstrated that treatment response within intensive pain rehabilitation is considerably more robust compared to standard multidisciplinary care 29, the current study does not have a control group where trajectories among standard care could be examined in comparison to the current trajectory groups.
Next steps
In the current study, we examined separate trajectories of treatment response for pain and disability. As anticipated in the context of intensive pain rehabilitation, this approach demonstrated considerable divergence in the proportion of patients within trajectory groups for pain compared to disability. These data are highly informative for clinicians and patient. It also elicits new questions regarding the interplay of these two variables. Using different analytic approaches, future studies can examine rate of change in both pain and disability to examine the temporal association of these two outcomes. Moreover, questions regarding the extent that improvement in pain overlap with or predict improvements in function can also be examined.
Summary
Intensive pain rehabilitation involves a significant commitment of resources from the patient, family, treating team, and others. Families are keenly interested and frequently ask, “Will this program make my child better?” They want to know if putting their lives on hold, and the associated financial cost, will be worth it. These data provide important outcome trajectories among those who enroll. This study suggests that the overwhelming majority of patients who enroll will regain day-to-day functional ability (88%) – they will be functioning better at discharge and those improvements will be maintained. For most families, that is not enough. They want to know if the pain will get better. These data demonstrate that approximately 35% of patients will report only mild pain at discharge, with another 38% reporting mild pain at 1-month follow-up. Thus, a full 73% of patients will report mild to no pain one month after treatment that maintains at 1-year follow-up. Moreover, we can now communicate to families that for the 27% of patient that do not report improvements in pain, the known modifiable risk factors are: higher anxiety symptoms and precontemplative or contemplative stage of change. It may be that incorporating a pretreatment motivational enhancement component to increase readiness to change may help patients get more out of intensive pain rehabilitation. This study provides an initial evaluation of key factors associated with treatment response and future work can determine how to best target these variables to maximize responsivity to treatment.
Perspective.
Deriving groups of individuals with differing treatment response trajectories stimulates new thinking regarding potential mechanisms that may be driving these outcomes.
Highlights.
Three pain response trajectories emerged: responder, late responder, nonresponder.
The strongest predictor of pain nonresponse was lower readiness to change.
Social milieu benefits for patients with social difficulties.
Targeted treatment of key factors can maximize responsivity.
Acknowledgments
The authors wish to thank the research assistants who worked on this study (Anne Pauler, Kelly Smith).
Footnotes
Disclosures: This study were supported by an NIH grant (K23 HD067202) awarded to LS, a Boston Children’s Hospital Office of Faculty Development Career Development Grant and NIH grant (K23 GM123372-01 awarded to CS, an NINDS grant (K24NS064050) awarded to DB, the Sara Page Mayo Endowment for Pediatric Pain Research and Treatment, and the Department of Anesthesiology, Perioperative and Pain Medicine at Boston Children’s Hospital. Drs. Simons, Sieberg, Conroy, Randall, Shulman, Borsook, Berde, Sethna, and Logan report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banez GA, Frazier TW, Wojtowicz AA, Buchannan K, Henry DE, Benore E. Chronic pain in children and adolescents: 24–42 month outcomes of an inpatient/day hospital interdisciplinary pain rehabilitation program. Journal of pediatric rehabilitation medicine. 2014;7:197–206. doi: 10.3233/PRM-140289. [DOI] [PubMed] [Google Scholar]
- 2.Benore E, D’Auria A, Banez GA, Worley S, Tang A. The Influence of Anxiety Reduction on Clinical Response to Pediatric Chronic Pain Rehabilitation. Clin J Pain. 2014 doi: 10.1097/AJP.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 3.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cousins LA, Kalapurakkel S, Cohen LL, Simons LE. Topical Review: Resilience Resources and Mechanisms in Pediatric Chronic Pain. Journal of pediatric psychology. 2015;40:840–845. doi: 10.1093/jpepsy/jsv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104:639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 6.Evans JR, Benore E, Banez GA. The Cost-Effectiveness of Intensive Interdisciplinary Pediatric Chronic Pain Rehabilitation. Journal of pediatric psychology. 2016;41:849–856. doi: 10.1093/jpepsy/jsv100. [DOI] [PubMed] [Google Scholar]
- 7.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. Pain. 2006;123:254–263. doi: 10.1016/j.pain.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 8.Guite JW, Logan DE, Simons LE, Blood EA, Kerns RD. Readiness to change in pediatric chronic pain: initial validation of adolescent and parent versions of the Pain Stages of Change Questionnaire. Pain. 2011;152:2301–2311. doi: 10.1016/j.pain.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haviland A, Jones B, Nagin D. Group-based trajectory modeling extended to account for nonrandom participant attrition. Sociological Methods & Research. 2011;40:367–390. [Google Scholar]
- 10.Hechler T, Dobe M, Damschen U, Blankenburg M, Schroeder S, Kosfelder J, Zernikow B. The pain provocation technique for adolescents with chronic pain: preliminary evidence for its effectiveness. Pain Med. 2010;11:897–910. doi: 10.1111/j.1526-4637.2010.00839.x. [DOI] [PubMed] [Google Scholar]
- 11.Hechler T, Kanstrup M, Holley AL, Simons LE, Wicksell R, Hirschfeld G, Zernikow B. Systematic Review on Intensive Interdisciplinary Pain Treatment of Children With Chronic Pain. Pediatrics. 2015;136:115–127. doi: 10.1542/peds.2014-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hechler T, Wager J, Zernikow B. Chronic pain treatment in children and adolescents: less is good, more is sometimes better. BMC pediatrics. 2014;14:262. doi: 10.1186/1471-2431-14-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirschfeld G, Hechler T, Dobe M, Wager J, von Lutzau P, Blankenburg M, Kosfelder J, Zernikow B. Maintaining lasting improvements: one-year follow-up of children with severe chronic pain undergoing multimodal inpatient treatment. Journal of pediatric psychology. 2013;38:224–236. doi: 10.1093/jpepsy/jss115. [DOI] [PubMed] [Google Scholar]
- 14.Jones B, Nagin D, Roeder K. SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29:374–393. [Google Scholar]
- 15.Killaspy H, Banerjee S, King M, Lloyd M. Prospective controlled study of psychiatric out-patient non-attendance. Characteristics and outcome. The British journal of psychiatry : the journal of mental science. 2000;176:160–165. doi: 10.1192/bjp.176.2.160. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacology bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- 17.Langer SL, Romano JM, Levy RL, Walker LS, Whitehead WE. Catastrophizing and Parental Response to Child Symptom Complaints. Children’s health care : journal of the Association for the Care of Children’s Health. 2009;38:169–184. doi: 10.1080/02739610903038750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logan D, Chiang G, Condon M, Firn E, Gaughan V, Hogan M, Leslie D, Olson K, Simons L, Berde C. Development of an intensive pain rehabilitation program for children and adolescents with complex regional pain syndrome. Pediatric Pain Letter. 2010;12:1–6. [Google Scholar]
- 19.Logan DE, Carpino EA, Chiang G, Condon M, Firn E, Gaughan VJ, Hogan M, Leslie DS, Olson K, Sager S, Sethna N, Simons LE, Zurakowski D, Berde CB. A Day-hospital Approach to Treatment of Pediatric Complex Regional Pain Syndrome: Initial Functional Outcomes. The Clinical journal of pain. 2012 doi: 10.1097/AJP.0b013e3182457619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan DE, Conroy C, Sieberg CB, Simons LE. Changes in willingness to self-manage pain among children and adolescents and their parents enrolled in an intensive interdisciplinary pediatric pain treatment program. Pain. 2012;153:1863–1870. doi: 10.1016/j.pain.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan DE, Sieberg CB, Conroy C, Smith K, Odell S, Sethna N. Changes in sleep habits in adolescents during intensive interdisciplinary pediatric pain rehabilitation. J Youth Adolesc. 2015;44:543–555. doi: 10.1007/s10964-014-0155-2. [DOI] [PubMed] [Google Scholar]
- 22.March J, Parker J, Sullivan K, Stallings P, Conners C. The multidimensional anxiety scale for children (MASC): Factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 23.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L PedImmpact. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. The journal of pain : official journal of the American Pain Society. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Nagin DS. Group-based trajectory modeling: an overview. Annals of nutrition & metabolism. 2014;65:205–210. doi: 10.1159/000360229. [DOI] [PubMed] [Google Scholar]
- 25.Sieberg CB, Simons LE, Edelstein MR, DeAngelis MR, Pielech M, Sethna N, Hresko MT. Pain prevalence and trajectories following pediatric spinal fusion surgery. The journal of pain : official journal of the American Pain Society. 2013;14:1694–1702. doi: 10.1016/j.jpain.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simons LE, Kaczynski KJ, Conroy C, Logan DE. Fear of pain in the context of intensive pain rehabilitation among children and adolescents with neuropathic pain: associations with treatment response. The journal of pain : official journal of the American Pain Society. 2012;13:1151–1161. doi: 10.1016/j.jpain.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons LE, Logan DE, Chastain L, Stein M. The relation of social functioning to school impairment among adolescents with chronic pain. Clin J Pain. 2010;26:16–22. doi: 10.1097/AJP.0b013e3181b511c2. [DOI] [PubMed] [Google Scholar]
- 28.Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. The journal of pain : official journal of the American Pain Society. 2011;12:677–686. doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Simons LE, Sieberg CB, Pielech M, Conroy C, Logan DE. What does it take? Comparing intensive rehabilitation to outpatient treatment for children with significant pain-related disability. Journal of pediatric psychology. 2013;38:213–223. doi: 10.1093/jpepsy/jss109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Medical care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 32.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143:223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of pediatric psychology. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 34.Wertli MM, Burgstaller JM, Weiser S, Steurer J, Kofmehl R, Held U. Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain: a systematic review. Spine. 2014;39:263–273. doi: 10.1097/BRS.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 35.Wetherell JL, Afari N, Rutledge T, Sorrell JT, Stoddard JA, Petkus AJ, Solomon BC, Lehman DH, Liu L, Lang AJ, Hampton Atkinson J. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain. 2011;152:2098–2107. doi: 10.1016/j.pain.2011.05.016. [DOI] [PubMed] [Google Scholar]