Abstract
Context
Higher bone marrow fat (BMF)1 is associated with osteoporosis and reduced hematopoiesis. Exogenous estradiol reduces BMF in older women, but effects of endogenous sex hormones are unknown.
Objective
To determine if endogenous sex hormones are associated with BMF in older men and women.
Design, setting and Participants
Cross-sectional study in the Age Gene/Environment Susceptibility (AGES) Reykjavik cohort. Participants using medications that may affect BMF were excluded.
Main Outcome Measures
Vertebral BMF was measured with magnetic resonance spectroscopy. Estradiol, testosterone and sex hormone binding globulin were measured on archived serum. Linear regression models were adjusted for age, total percent body fat and visit window.
Results
Analyses included 244 men and 226 women, mean age 81.5 (SD 4.1) years. Mean BMF was 54.1% (SD 8.6) (men) and 54.7% (SD 8.1) (women). In adjusted models, per 1 pg/ml increase in total estradiol, there was a statistically significant 0.26% decrease in BMF in men (95% CI: −0.41, −0.11) and a non-significant 0.20% decrease in women (95% CI: −0.55, 0.15), with no evidence of interaction by gender (p=0.88). Per 10 ng/dl increase in total testosterone, there was a significant 0.10% decrease in BMF in men (95% CI: −0.17, −0.03) and a non-significant 0.13% (95% CI: −0.79, 0.53) decrease in women, with no evidence of interaction by gender (p=0.97).
Conclusion
Higher bone marrow fat is associated with lower total estradiol and testosterone levels in older men, with a similar but statistically non-significant association in older women. Sex hormone levels appear to play a role in the regulation of bone marrow fat in older adults.
Keywords: Sex hormones, estradiol, testosterone, vertebral bone marrow fat
1. Introduction
Humans have little marrow fat at birth, but marrow adiposity increases with age, particularly during the third decade of life. Marrow fat in postmortem iliac crest bone biopsies increased from 40% at age 30 to 68% by age 100 years. Both the size and number of adipocytes appear to increase with age [1]. While these changes in bone marrow fat (BMF) have been recognized for years, appreciation of its role and functions has been a recent development. BMF may be an important determinant of skeletal health. Observational studies have shown that higher BMF is associated with lower bone density and with prevalent vertebral fractures, including our investigation of these associations in the Iceland Age Gene/Environment Susceptibility (AGES) Reykjavik cohort [2, 3]. BMF may also affect hematopoiesis and stem cell function [4]. Given the emerging evidence implicating marrow fat as an important factor involved in the regulation of both bone and stem cell function, better understanding of the mechanisms underlying marrow fat accumulation is essential.
Sex hormone levels may play an important regulatory role for marrow fat. Further, since total sex hormone levels differ by gender, dependence of BMF on sex hormones also might differ by gender. In rodents, ovariectomy increases BMF [5]. Estrogen administration prevents this increase in BMF [6]. Clinical trials in older women have demonstrated that exogenous estrogen reduces age-related increases in BMF [7, 8]. However, there are no studies to date of the effect of endogenous estrogen levels on marrow fat in humans. In addition, studies are not available on the relationship between testosterone levels and BMF. The objective of this study is to investigate the cross-sectional associations between endogenous sex hormone concentrations and vertebral marrow fat in older men and women, using data from the AGES-Reykjavik cohort.
2. Subjects and Methods
2. 1 Study Population
This is a cross sectional ancillary study in the AGES-Reykjavik cohort. AGES-Reykjavik, a population-based study of older men and women in Iceland, was designed to examine the genetic susceptibility and gene/environment interactions contributing to phenotypes of old age [9]. The first AGES-Reykjavik visit, conducted in 2002 to 2006, included 5,764 men and women aged 67 to 93. In 2007–2011, 3,411 participants returned for a second visit. At this 2nd AGES visit, bone marrow fat was measured in 301 participants in 2010–2011. In 2015, an additional 238 participants from the cohort of 3,411 participants seen at the second AGES visit had BMF measured. In 2016, estradiol, testosterone, and sex hormone binding globulin (SHBG) were measured on archived serum from participants who had BMF measured. Two participants did not have acceptable serum specimens. Participants were excluded (N= 66) who reported current use of medications that might affect bone marrow fat, including thiazolidinediones, oral glucocorticoids, treatments for osteoporosis (bisphosphonates, raloxifene, calcitonin, or parathyroid hormone), hormone therapy, tibolone, antiepileptics, and aromatase inhibitor therapy). One participant was excluded due to very high estradiol and testosterone levels. There were 470 participants with sex hormone levels available for these analyses.
The ancillary study was approved by the institutional review boards of the National Bioethics committee in Iceland, the National Institute of Aging, and the University of California, San Francisco (UCSF). All participants provided written informed consent.
2. 2 Outcome Variable: Vertebral Bone Marrow Fat
Participants attended a clinic visit that included measurement of BMF, whole body composition by DXA and a blood draw. Vertebral BMF was measured with a 1.5-T scanner (GE Healthcare, Milwaukee, Wisconsin) with an 8-channel cervical-thoracic-lumbar-spine coil (using the lower 3 elements; GE Healthcare). Single voxel MRS was acquired in individual vertebral bodies from L1–L4 using single voxel proton magnetic resonance spectroscopy (1H-MRS) based on point resolved spectroscopy (PRESS) sequence. The PRESS box was positioned in the middle of the vertebral body and the PRESS box size was kept the same for each vertebral level for all subjects. Two peaks were quantified: water around 4.65 ppm and bulk methylene protons (saturated lipids) around 1.3 ppm. Vertebral BMF is a ratio of fat to water plus fat, measured as a percentage. For the main analyses, an average BMF for the four vertebral levels (L1–L4) was calculated. The imaging center at AGES-Reykjavik used highly stringent and reproducible daily quality assurance tests based on GE’s System Performance Test (SPT). Weekly stability and calibration tests were performed.
2. 3 Exposure Variables: Estradiol, Testosterone, and SHBG Levels
Blood was drawn fasting within 2 weeks of the BMF visit. Serum was stored at −80C. Sex hormones were measured on the archived serum in January 2016 in one batch (Endoceutics Clinique, Quebec, Canada). Total estradiol and total testosterone were analyzed using gas chromatography/mass spectrometry (Shimadzu Nexera/Qtrap 6500) [10]. The lower limits of quantification for estradiol and testosterone were 1 pg/ml and 50 pg/ml. The inter-assay CV (%) at the LLOQ were 4.7 and 3.6 pg/ml for estradiol and testosterone. Values were extrapolated below the LLOQ using Analyst software (AB Sciex, Concord, Canada). GC/MS provides the most accurate readings in participants with low levels of estradiol and testosterone. Free estradiol and free testosterone were calculated using the Mazer equation [11]. SHBG was measured using ELISA (Asbach Medical Products) with inter-assay variations (CV %) of 7.7% and 8.8% at 15.995 nmol/L and 179.410 nmol/L respectively.
2.4 Covariates
Participants in the ancillary study also had DXA scans of the whole body, hip and spine. Scans were obtained with a GE Healthcare Lunar iDXA scanner, software version 11.4. Models were adjusted for total fat from DXA whole body scans since total fat influences levels of sex hormones and may influence BMF. Although hip and spine BMD are available for these participants, models were not adjusted for BMD. BMD is associated with marrow fat in this cohort [2] and is known to be associated with sex hormone levels [12]. However, BMD was not included as a covariate in adjusted models because it is not a confounder (i.e., a cause of sex hormone levels and marrow fat), but is rather an effect of these variables. Adjusting for an effect of exposure and outcome may introduce “collider” bias [13]. Because participants were seen for bone marrow fat measurements in two windows of time, 2010–2011 and 2015, the models were adjusted for visit window. Models were also adjusted for age at time of BMF measurement. Analysis was stratified by gender because of the large differences between older men and women in the levels of estradiol and testosterone.
2.5 Statistical Methods
Baseline characteristics of participants were summarized using means and standard deviations (SD). Scatterplots of sex hormones versus BMF and Pearson’s correlations were run separately for men and women. Linear regression was performed to analyze associations between bone marrow fat levels and sex hormone levels, stratified by gender. Hormone-specific multivariable models, also stratified by gender, controlled for age, visit window, and total percent fat. To assess for interaction, multivariable models for men and women combined were used, controlling for above variables plus gender and an interaction term between hormone level and gender. All analyses were conducted using STATA 14.1 (Stata Corporation, College Station, TX).
3. Results
3.1 Population Characteristics
The sample included 244 men and 226 women, who were 74 to 95 years old at BMF measurement. Mean BMF (L1–L4) was 54.1% (SD 8.6%) for men and 54.7% (SD 8.1%) for women. Two women were excluded from estradiol analyses due to high levels of total estradiol. Mean absolute endogenous total hormone levels were 4- and 16-fold higher among men than women, for estradiol and testosterone, respectively (Table 1).
Table 1.
Participant characteristicsa
| Men (n=244) | Women (n=226) | |
|---|---|---|
| Age, years | 82.4 ± 4.0 | 80.6 ± 4.1 |
| Vertebral bone marrow, % | 54.1 ± 8.6 | 54.7 ± 8.1 |
| Total percent fat, DXA % | 32.6 ± 6.1 | 41.4 ± 5.9 |
| Free estradiol, pg/ml b | 0.316 ± 0.115 | 0.073 ± 0.060 |
| Total estradiol, pg/ml b | 19.8 ± 7.1 | 4.9 ± 3.3 |
| Free testosterone, ng/dL | 42.4 ± 16.4 | 2.3 ± 1.6 |
| Total testosterone, pg/ml | 388.9 ± 167.2 | 24.1 ± 16.1 |
| SHBG, nmol/L | 79.8 ± 28.1 | 92.0 ± 39.7 |
Results are shown as mean± SD.
N=224 for women
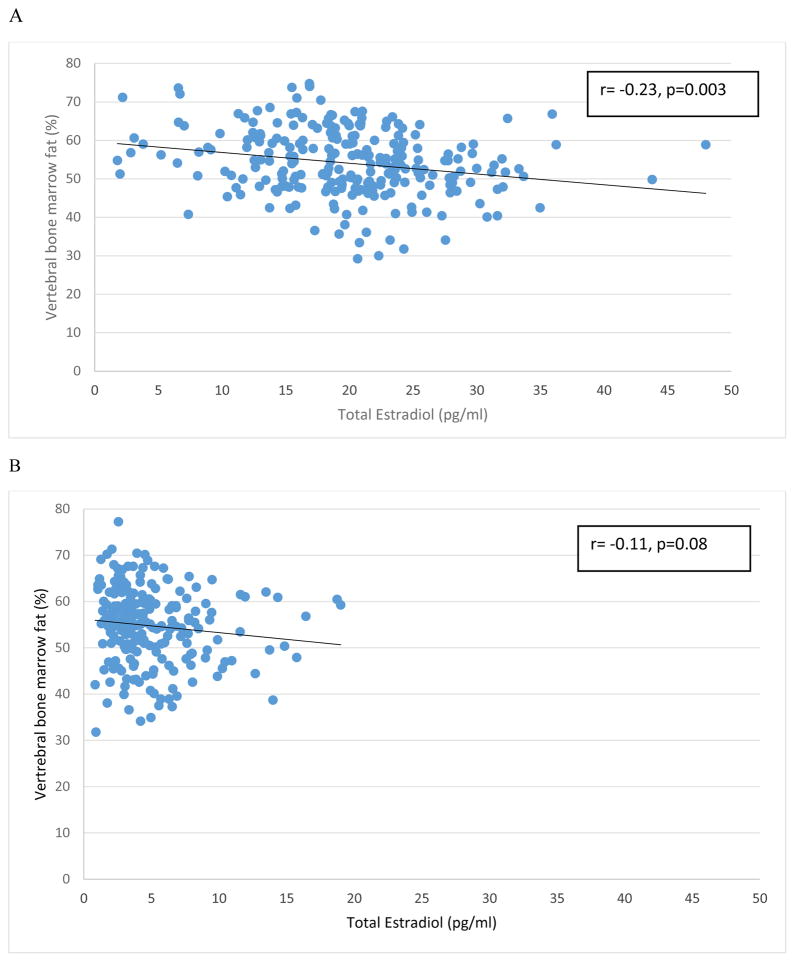
In unadjusted models, BMF was negatively associated with total estradiol in men [−0.28% difference in BMF (%) per 1 pg/ml increase in total estradiol (95% CI: −0.43%, −0.13%)] (Figure 1A). After adjusting for age, total percent fat, and visit window, BMF remained negatively associated in men [−0.26% (95% CI: −0.41%, −0.11%)] (Table 2). In women, BMF was also negatively associated with total estradiol (Figure 1B) but the association was not statistically significant in the unadjusted model or the adjusted model [−0.20% (95% CI: −0.55%, 0.15%)]. There was no evidence of interaction between gender and total estradiol (p for interaction = 0.88).
Figure 1.
Correlation between vertebral bone marrow fat (L1–L4) and total estradiol levels in men (A) and women (B). Regression line is shown on the scatterplot.
Table 2.
Unadjusted and adjusted differences in mean bone marrow fat (%) for given increase in hormone level among men and women
| Sex hormone | Men (n=244) | Women (n=226) | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted a | Unadjusted | Adjusted a | |||||
| Difference | 95% CI | Difference | 95% CI | Difference | 95% CI | Difference | 95% CI | |
|
Free estradiol
b Per 0.1 pg/ml |
−1.22 | −2.16 to −0.29 | −1.19 | −2.13 to −0.25 | −1.04 | −2.80 to 0.73 | −0.40 | −2.35 to 1.55 |
|
Total estradiol
b Per 1 pg/ml |
−0.28 | −0.43 to −0.13 | −0.26 | −0.41 to −0.11 | −0.29 | −0.61 to 0.03 | −0.20 | −0.55 to 0.15 |
|
Free testosterone Per 10 pg/ml |
−0.73 | −1.39 to −0.08 | −0.55 | −1.24 to 0.13 | −2.00 | −8.78 to 4.77 | −0.48 | −7.52 to 6.56 |
|
Total testosterone Per 10 ng/dl |
−0.11 | −0. 18 to −0.05 | −0.10 | −0.17 to − 0.03 | −0.16 | −0.81 to 0.50 | −0.13 | −0.79 to 0.53 |
|
SHBG Per 40 nmol/L |
−1.54 | −3.07 to −0.005 | −1.48c | −3.13 to 0.17 | 0.43 | −0.63 to 1.49 | 0.09c | −1.05 to 1.22 |
adjusted for age, visit window and total percent fat (DXA)
n=224 for women
p value for interaction between gender and SHBG = 0.03
Bold indicates p value< 0.05
BMF was negatively associated with total testosterone in men in unadjusted [−0.11% difference in BMF (%) per 10 ng/dl increase in total testosterone (95% CI: −0.18%, −0.05%); Figure 2A] and adjusted models [−0.10% (95% CI: −0.17%, −0.03%)]. BMF was also negatively associated with total testosterone in women (Figure 2B) but the association was not statistically significant in unadjusted or adjusted [−0.13% (95% CI: −0.79%, 0.53%)] models. There was no evidence of interaction between gender and total testosterone (p for interaction = 0.97).
Figure 2.
Correlation between vertebral bone marrow fat (L1–L4) and total testosterone levels in men (A) and women (B). Regression line is shown on the scatterplot.
BMF was negatively associated with free estradiol and free testosterone in unadjusted and adjusted models in men and women but results were only statistically significant in men. The adjusted associations between sex hormones and BMF for the individual vertebral levels were similar to the results for the average BMF (L1–L4) (Supplementary Figure 1).
4. Discussion
In the AGES-Reykjavik cohort of older adults, lower BMF was associated with higher endogenous estradiol and testosterone levels. This is the first study to report the relationship between endogenous sex steroid hormones and bone marrow fat. Our findings were statistically significant in men but not in women. The associations were modest, suggesting a limited role for physiological levels of sex hormones affecting BMF in this older age group.
It is important to interpret these findings in the context of an older population. Estradiol levels in postmenopausal women are much lower than in older men. The limited range of estradiol levels in these older women may be a factor in the lack of a statistically significant association with marrow fat in women. Higher endogenous estradiol may be a factor in the generally lower levels of marrow fat in premenopausal compared with postmenopausal women, an issue that could not be addressed in this cohort. It is also possible that sex steroids regulate marrow fat in a gender-specific manner. However, given the similar estimate of the association between estradiol and marrow fat and lack of significant gender interaction, we believe this to be less likely.
Previous clinical studies have reported that exogenous estradiol reduces BMF in postmenopausal women. [7,8] Syed et al compared paired iliac crest biopsy specimens obtained before and after one year of treatment with either transdermal estradiol or placebo in 56 postmenopausal women with osteoporosis. The investigators found that adipocyte volume/tissue volume (AV/TV) increased by 20% in placebo-treated women, and decreased by 24% in the estradiol-treated group (p<0.001). Furthermore, in a study in postmenopausal women evaluating the short-term effects of estrogen, marrow fat fraction decreased by 0.05% (from 0.48% to 0.43%) during two weeks of daily oral 17-β estradiol therapy [7]. This rapid reduction suggests that 17-β estradiol regulates bone marrow fat directly, not through effects on bone mass. To our knowledge, there are no previous studies of testosterone and marrow fat in humans.
Rodent studies suggest that lower endogenous estradiol is associated with higher marrow fat. This is the first study to report the relationship between endogenous sex steroid hormones and bone marrow fat. Marrow fat volume increases by approximately 3–10% after ovariectomy [5] in female rats. Human cross-sectional studies suggest that marrow fat increases in the decade after menopause by about 20 percent [8]. Before age 55, marrow fat content is higher in males than females. While marrow fat appears to increase sharply between the ages of 55 and 65 in women, marrow fat appears to gradually increase throughout life in men [14].
Although others have reported higher BMF in women compared with men in older adults, we found that BMF levels were similar in older men and women.
In our study, higher endogenous sex hormone levels in older adults were associated with lower bone marrow adiposity. However, in contrast to the relatively strong effects of exogenous estradiol, the correlations between endogenous estradiol levels and BMF that we observed in older adults were modest. We hypothesize that physiological levels of sex hormones have a limited influence on marrow fat in this older age group. Because the range of endogenous estradiol is particularly small in older women, it seems likely that variations in estradiol in older women play only a minor role in regulation of marrow fat. Our results might also have been attenuated due to non-differential measurement error in assessing marrow fat or hormone levels.
There are no previous studies of SHBG and marrow fat. Our results suggest a negative correlation between SHBG and marrow fat in men although not in women. This negative correlation is somewhat unexpected as one might anticipate that higher SHBG would result in lower levels of bioavailable estrogen and testosterone leading to a positive correlation between marrow fat and SHBG. However, our results are consistent with investigations of SHBG and other adipose depots, including total fat, visceral fat and liver fat, which report negative correlations, possibly through effects of inflammatory cytokines and adiponectin [15, 16]. The reason for the sex difference in the association between SHBG and marrow fat is not clear.
The strengths of our study include a large sample size (n=470) roughly balanced by sex, and state-of-the-art measurements of bone marrow fat and sex steroid hormone concentrations. Due to the cross-sectional study design, we are unable to assess temporal relationships between change in marrow fat and sex steroids. In addition, we were not able to consider physical activity level as a potential confounder of the association between bone marrow fat and hormone levels. Physical activity reduces marrow fat levels in rodents, and may be associated with changes in sex hormone levels in humans. Finally, this study was limited to older, white adults, and results may not be generalizable to other populations.
5. Conclusion
In conclusion, we observed a modest, but statistically significant, association between lower BMF and higher endogenous estradiol and testosterone levels in older men. There was a similar, but non-significant, association in older women. Our findings indicate a modest role for sex hormones in the regulation of marrow fat in older adults.
Supplementary Material
Highlights.
Lower estradiol is associated with higher vertebral marrow fat in older men.
Lower testosterone is associated with higher vertebral marrow fat in older men.
Similar, but statistically non-significant, associations are present in women.
Sex hormones appear to play a modest role in the regulation of marrow fat in older adults.
Acknowledgments
Funding:
This ancillary study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR057819 and 1R01AR065645). The AGES Reykjavik Study is supported by funding from the National Institutes of Health (Contract N01-AG-12100), the National Institute of Aging Intramural Research program, Hjartavernd (The Icelandic Heart Association), and the Althingi (The Icelandic Parliament).
Footnotes
BMF, Bone Marrow Fat
Disclosure Statement: All authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rozman C, et al. Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol. 1989;17(1):34–37. [PubMed] [Google Scholar]
- 2.Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, Vittinghoff E, Siggeirsdottir K, Sigurdsson G, Oskarsdottir D, Shet K, Palermo L, Gudnason V, Li X. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013 Jun;98(6):2294–300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005 Sep 23;6(3):945–51. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz AV. Marrow fat and bone: review of clinical findings. Front Endocrinol (Lausanne) 2015 Mar 30;6:40. doi: 10.3389/fendo.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sottile V, Seuwen K, Kneissel M. Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone) Calcif Tissue Int. 2004 Oct;75(4):329–37. doi: 10.1007/s00223-004-0224-8. [DOI] [PubMed] [Google Scholar]
- 6.Elbaz A, Rivas D, Duque G. Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology. 2009 Dec;10(6):747–55. doi: 10.1007/s10522-009-9221-7. [DOI] [PubMed] [Google Scholar]
- 7.Limonard EJ, Veldhuis-Vlug AG, van Dussen L, Runge JH, Tanck MW, Endert E, Heijboer AC, Fliers E, Hollak CE, Akkerman EM, Bisschop PH. Short-Term Effect of Estrogen on Human Bone Marrow Fat. J Bone Miner Res. 2015 Nov;30(11):2058–66. doi: 10.1002/jbmr.2557. [DOI] [PubMed] [Google Scholar]
- 8.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008 Sep;19(9):1323–30. doi: 10.1007/s00198-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saczynski JS, Jónsdóttir MK, Garcia ME, Jonsson PV, Peila R, Eiriksdottir G, Olafsdottir E, Harris TB, Gudnason V, Launer LJ. Cognitive impairment: an increasingly important complication of type 2 diabetes: the age, gene/environment susceptibility--Reykjavik study. Am J Epidemiol. 2008 Nov 15;168(10):1132–9. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson ME, et al. Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology. 2015;156:2492–502. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- 11.Mazer NA. A Novel Spreadsheet Method for Calculating the Free Serum Concentrations of Testosterone, dihydrotestosterone, Estradiol, Estrone, and Cortisol: with illustrative examples from male and female populations. Steroids. 2009;74(6):512–9. doi: 10.1016/j.steroids.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002 Jun;23(3):279–302. doi: 10.1210/edrv.23.3.0465. Review. [DOI] [PubMed] [Google Scholar]
- 13.Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002 Jan 15;155(2):176–84. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 14.Griffith JF, Yeung DK, Ma HT, Leung JC, Kwok TC, Leung PC. Bone Marrow Fat Content in the Elderly: A Reversal of Sex Difference Seen in Younger Subjects. J Magn Reson. 2012;36:225–30. doi: 10.1002/jmri.23619. [DOI] [PubMed] [Google Scholar]
- 15.Peter A, Kantartzis K, Machann J, Schick F, Staiger H, Machicao F, Stefan N. Relationships of Circulating Sex Hormone–Binding Globulin With Metabolic Traits in Humans. Diabetes. 2010;59(12):3167–3173. doi: 10.2337/db10-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simo R, et al. Novel Insights in SHBG Regulation and Clinical Implications. Trends in Endocrinology & Metabolism. 2015;7:376–83. doi: 10.1016/j.tem.2015.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.