Abstract
There is a notable lack of therapeutic alternatives for what is fast becoming a global epidemic of traumatic brain injury (TBI). Photobiomodulation (PBM) employs red or near-infrared (NIR) light (600-1100nm) to stimulate healing, protect tissue from dying, increase mitochondrial function, improve blood flow and tissue oxygenation. PBM can also act to reduce swelling, increase antioxidants, decrease inflammation, protect against apoptosis, and modulate microglial activation state. All these mechanisms of action strongly suggest that PBM delivered to the head should be beneficial in cases of both acute and chronic TBI. Most reports have used NIR light either from lasers or from light-emitting diodes (LEDs). Many studies in small animal models of acute TBI have found positive effects on neurological function, learning and memory, and reduced inflammation and cell death, in the brain. There is evidence that PBM can help the brain to repair itself by stimulating neurogenesis, upregulating BDNF synthesis, and encouraging synaptogenesis. In healthy human volunteers (including students and healthy elderly women) PBM has been shown to increase regional cerebral blood flow, tissue oxygenation and improve memory, mood and cognitive function. Clinical studies have been conducted in patients suffering from the chronic effects of TBI. There have been reports of improvements in executive function, working memory, and improved sleep. Functional magnetic resonance imaging has shown modulation of activation in intrinsic brain networks likely to be damaged in TBI (default mode network and salience network).
Keywords: photobiomodulation, low-level laser therapy, traumatic brain injury, stroke, chromophores, animal studies, clinical trials, human studies
1. Introduction
Photobiomodulation (PBM) formerly known as low-level laser (light) therapy (LLLT) is approaching its 50th anniversary, after being discovered by Endre Mester working in Hungary in 1967 (Hamblin et al. 2016). Originally thought to be a property of red lasers (600-700 nm), PBM has broadened to include near-infrared (NIR) wavelengths 760-1200 nm, and even blue and green wavelengths. Moreover the advent of inexpensive and safe light emitting diodes (LEDs) has supplanted the use of expensive lasers in many indications. The better tissue penetration properties of NIR light, together with its good efficacy, has made it the most popular wavelength range overall. The best-known medical applications of PBM have been for indications such as stimulation of wound healing (Hopkins et al. 2004; Kovacs et al. 1974), reduction of pain and inflammation in orthopedic and musculoskeletal conditions (Aimbire et al. 2006; Gam et al. 1993), and mitigation of cancer therapy side-effects (Zecha et al. 2016a; Zecha et al. 2016b). However in recent years there has been growing interest in the use of PBM in various brain disorders (Hamblin 2016b; Hennessy and Hamblin 2016; Naeser and Hamblin 2011; Naeser and Hamblin 2015). The almost complete lack of any adverse side-effects of PBM, coupled with growing disillusion with pharmaceutical drugs that affect brain function, have combined together to suggest an alternative physical therapy approach to improving brain function.
Traumatic brain injury (TBI) is caused by some type of trauma to the head, often resulting from road traffic accidents, assaults, falls, sports injuries, or blast injuries suffered in military conflict. TBI is classified as mild (loss of consciousness 0-30 minutes; altered mental state <24 hours; post-trauma amnesia <1 day); moderate (loss of consciousness 30 minutes to 24 hours; altered mental state >24 hours; post-trauma amnesia >1-7 days), or severe (loss of consciousness >24 hours; altered mental state >24 hours; post-trauma amnesia >7 days) (Blennow et al. 2016). There are three cases of TBI sustained each minute in the US (Faul et al. 2010). Repeated mild episodes of TBI (also known as concussions) even without loss of consciousness, may have devastating cumulative effects (Kamins and Giza 2016). Chronic traumatic encephalopathy is a recently recognized condition resulting from repeated head trauma, found in boxers, football players, and military personnel (McKee et al. 2016; Safinia et al. 2016). There is presently no accepted treatment for TBI, although some investigational approaches are being tested in both the acute (neuroprotection) and chronic (neurorehabilitation) settings (Loane and Faden 2010). One of these novel approaches is PBM or LLLT (Hamblin 2016a; Hamblin 2016b; Huang et al. 2012; Thunshelle and Hamblin 2016).
2. Mechanisms of action
Uncertainties about the mechanism of action of PBM at the molecular and cellular levels, have undoubtedly held back its acceptance in the wider biomedical community. However in recent years substantial progress has been made in this regard (de Freitas and Hamblin 2016). In the following section the state-of-the-art knowledge about the mechanisms of PBM is summarized. Figure 1 shows a graphical representation of the cellular and molecular mechanisms of PBM.
Figure 1. Molecular mechanisms of tPBM.
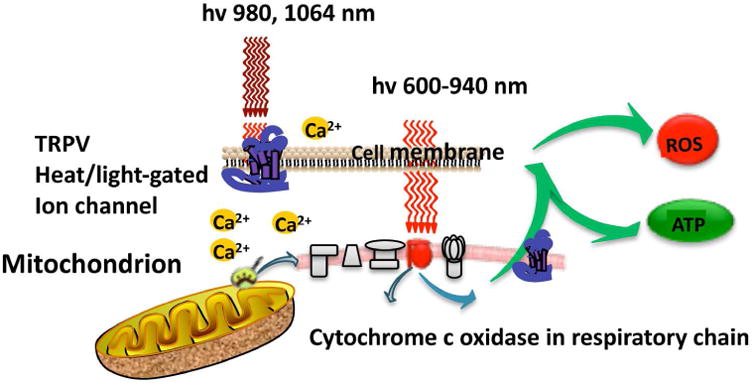
Light passes through the scalp and skull, where depending on the wavelength it is absorbed by two different chromophores. Red and NIR (up to 940nm) is primarily absorbed by cytochrome c oxidase in the mitochondrial respiratory chain of the cortical neurons. Longer wavelength NIR light (980nm, 1064nm) is primarily absorbed by heat and light-sensitive transient receptor potential ion channels. In both cases cell signaling and messenger molecules are upregulated as a result of stimulated mitochondrial activity, including reactive oxygen species (ROS), and adenosine triphosphate (ATP). hv is light, TRPV is transient receptor potential vanilloid (ion channels).
2.1 Chromophores
The first law of photobiology states that a photon must be absorbed by some molecule within the tissue to have any biological effect. The identity of these chromophores has been the subject of much scientific investigation and speculation. Largely due to the efforts of Tiina Karu in Russia, the enzyme cytochrome c oxidase (CCO) has been identified as a major chromophore of red/NIR light (Karu 1999; Karu and Kolyakov 2005; Karu et al. 2004a; Karu et al. 2004b). CCO is unit IV in the mitochondrial respiratory chain and has absorption peaks reaching well into the NIR spectral region (up to 900 nm) as well as in the red and blue regions. The most discussed hypothesis to explain exactly how photon absorption can stimulate the activity of CCO involves the photodissociation of inhibitory nitric oxide (NO) that can bind to the copper and heme centers in the enzyme and prevent oxygen from gaining access to the active sites (Lane 2006). In experimental models (such as isolated mitochondria) oxygen consumption and ATP production are increased, and the mitochondrial membrane potential is raised (Passarella et al. 1984).
A less well-appreciated mechanism involves light and heat-gated ion channels. These cation ion channels are thought to be members of the transient receptor potential (TRP) superfamily consisting of over 28 distinct members organized into six subfamilies, based on their primary amino acid structures (Caterina and Pang 2016). TRPV (vanilloid sub-family) members including TRPV1 (capsaicin receptor) have been shown to be activated by various wavelengths of light including green, red and NIR.
2.2 Cellular mechanisms
After the primary photon absorption event occurs, whether that the photons are absorbed by CCO, or by TRP ion channels a series of secondary events occurs. One of these events is the generation of reactive oxygen species (ROS), which are thought to be produced inside the mitochondria due to an increase in electron transport, and a rise in the mitochondrial membrane potential above the baseline levels (Suski et al. 2012). It should be noted that mitochondrial ROS can be produced when MMP is raised above normal, and also when ROS is reduced below normal. It is thought that the ROS produced when MMP is lowered (mitochondrial dysfunction) are more damaging than ROS produced when MMP is raised (mitochondrial stimulation). Nitric oxide is produced after PBM (Hamblin 2008), possibly by photodissociation from CCO where it inhibits oxygen consumption and electron transport (Lane 2006). Cyclic adenosine monophosphate (cAMP) (Gao and Xing 2009) and intracellular calcium are increased (Alexandratou et al. 2002). Many of these secondary mediators in the signaling pathways triggered by PBM, can induce activation of transcription factors, that go on to upregulate or downregulate expression levels of a large number of genes. One of the best-known transcription factors is NF-kB that can regulate expression of over one hundred genes including proteins with antioxidant, anti-apoptotic, pro-proliferation, and pro-migration functions. PBM (810 nm 3J/cm2) was shown to activate NF-kB in mouse embryonic fibroblasts via ROS production (Chen et al. 2011a). Since NF-kB is known to be a pro-inflammatory transcription factor, it might be thought that PBM would be pro-inflammatory. However it was shown that NF-KB was decreased in already activated (treated with Toll-like receptor ligands) inflammatory dendritic cells by PBM (810 nm 3J/cm2) (Chen et al. 2011b).
2.3 Tissue mechanisms
The changes in expression levels of proteins involved in antioxidant and redox-regulation, anti-apoptotic and pro-survival, cellular proliferation, etc mean that distinct changes in tissue homeostasis, healing and regeneration can be expected after PBM. For instance, structural proteins such as collagen are newly synthesized in order to repair tissue damage (Tatmatsu-Rocha et al. 2016). Cells at risk of dying in tissue that has been subjected to ischemic or other insults are protected (Sussai et al. 2010). Stem cells are activated to leave their niche, proliferate and differentiate (Oron and Oron 2016; Zhang et al. 2016). Pain and inflammation are reduced (Chow et al. 2009). Blood flow is increased (Samoilova et al. 2008) (possibly as a result of the release of NO (Mitchell and Mack 2013)), which also stimulates lymphatic drainage thereby reducing edema (Dirican et al. 2011).
2.4 Brain specific mechanisms
In addition to the foregoing, there are some PBM tissue mechanisms that are specific to the brain. One of the most important is an increase in cerebral blood flow often reported after transcranial photobiomodualtion (tPBM) (Salgado et al. 2015), leading to increased tissue oxygenation, and more oxidized CCO as measured by NIR spectroscopy (Rojas and Gonzalez-Lima 2013). tPBM has been shown to reduce activated microglia in the brains of TBI mice as measured by IBA1 (ionized calcium-binding adapter molecule-1) expression thus demonstrating reduced neuroinflammation (Khuman et al. 2012). tPBM has been shown to increase neurogenesis (formation of new brain cells derived from neuroprogenitor cells) (Xuan et al. 2014), and synaptogenesis (formation of new connections between existing brain cells) (Xuan et al. 2015) both in TBI mice. Figure 2 shows a graphical representation of a variety of these brain-specific tissue mechanisms.
Figure 2. Brain-specific mechanisms of tPBM.
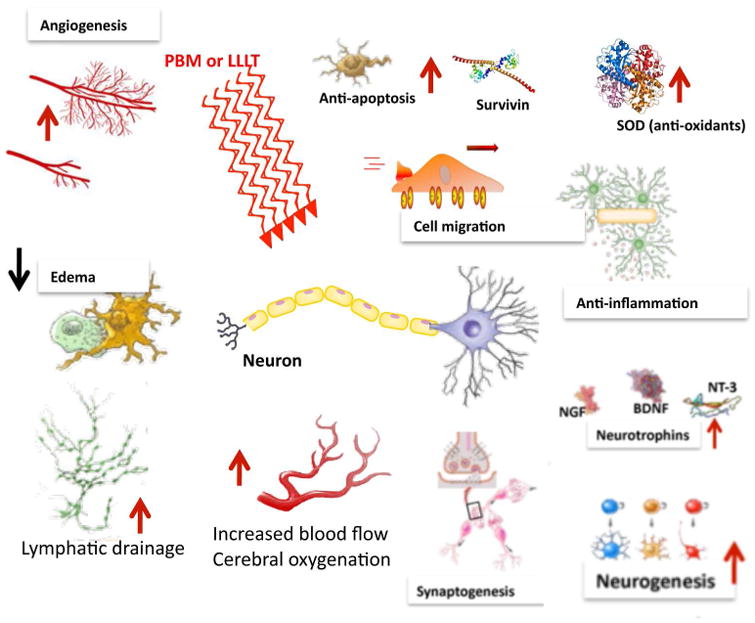
The gene transcription process described in Figure 1 can lead to decreases in neuronal apoptosis and excitotoxicity and lessening of inflammation and reduction of edema due to increased lymphatic flow, which together with protective factors such as antioxidants, will all help to reduce progressive brain damage. Increases in angiogenesis, expression of neurotrophins leading to activation of neural progenitor cells and more cell migration, and increased synaptogenesis may all contribute to the brain repairing itself from damage sustained in the trauma. AUC is area under the curve.
3. Transcranial photobiomodulation
Transcranial PBM is a growing approach to many different brain disorders that may be classified as sudden onset (stroke, TBI, global ischemia), neurodegenerative (Alzheimer's, Parkinson's, dementia), or psychiatric (depression, anxiety, posttraumatic stress disorder)(Hamblin 2016b; Hennessy and Hamblin 2016; Thunshelle and Hamblin 2016). In the following section some issues concerning where the light should be delivered, and the effects of PBM on uninjured mice and humans are addressed.
3.1 Light penetration
Several laboratories working in the field of tissue optics, have investigated the penetration of light of different wavelengths though the scalp and the skull, and to what depths into the parenchyma of the brain this light can penetrate. Answering the question “can light shone on the head sufficiently penetrate to reach the brain?” is difficult. The main reason is that at present it is unclear exactly what threshold of power density is necessary (expressed in mW/cm2) at some depth inside the brain to have a biological effect. There clearly must be a minimum value below which, the light can be delivered for an infinite time without having any effect, but whether this threshold is in the region of μW/cm2 or mW/cm2 is unknown at present.
Haeussinger et al. estimated that the mean penetration depth (5% remaining intensity) of NIR light through the scalp and skull was 23.6 + 0:7 mm (Haeussinger et al. 2011). Other studies have found comparable results with some variations depending on the precise location on the head and the precise wavelength studied (Okada and Delpy 2003; Strangman et al. 2014).
Jagdeo et al. (Jagdeo et al. 2012) used human cadaver heads (skull with intact soft tissue) to measure penetration of 830 nm light, and found that penetration depended on the anatomical region of the skull (0.9% at the temporal region, 2.1% at the frontal region, and 11.7% at the occipital region). Tedord et al. (Tedford et al. 2015) also used human cadaver heads to compare penetration of 660 nm, 808 nm, and 940 nm light. They found that 808 nm light penetrated best, and could reach a depth in the brain of 40–50 mm. Lapchak et al. compared the transmission of 810 nm light through the skulls (no soft tissue) of four different species, and found the mouse skull transmitted 40%, while for rat it was 21%, for rabbit it was 11.3 and for the human skull it was only 4.2% (Lapchak et al. 2015). Pitzschke and colleagues compared penetration of 670 nm and 810 nm light into the brain when delivered by a transcranial or a transphenoidal approach, and found that the best combination was 810 nm delivered transphenoidally (Pitzschke et al. 2015). Yaroslavsky et al. examined light penetration of different wavelengths through different parts of the brain tissue (white brain matter, gray brain matter, cerebellum, and brainstem tissues, pons, thalamus). Best penetration was found with wavelengths between 1000 and 1100 nm (Yaroslavsky et al. 2002). Henderson and Morries found that between 0.45% and 2.90% of 810 nm or 980 nm light penetrated through 3 cm of scalp, skull and brain tissue in ex vivo lamb heads (Henderson and Morries 2015a).
3.2 Local vs systemic effects of light
It is possible that the beneficial effects of PBM on the brain cannot be entirely explained by penetration of light through the scalp and skull into the brain itself, at a sufficient intensity to have an effect on the brain cells. The surface power density that can be safely applied to the head, is limited by heating of the skin. Perceptible heating of the skin starts to be felt when the power density is over about 500 mW/cm2, and can become severe at 1 W/cm2.
There has been one study that explicitly addressed whether direct transcranial PBM or indirect PBM is best for the brain. In a study of PBM for Parkinson's disease in a mouse model, Mitrofanis and colleagues compared the direct delivery of light to the mouse head, and they also covered up the head with aluminum foil so that the light was delivered to the remainder of the mouse body. They found that there was a highly beneficial effect on brain histology with light delivered to the head, but nevertheless there was also a statistically significant although less pronounced benefit (referred to as an “abscopal effect”) when the head was shielded from light. Moreover Oron and co-workers (Farfara et al. 2015) have shown that delivering NIR light to the mouse tibia (using either surface illumination or a fiber optic) resulted in improvements in memory and spatial learning in a transgenic mouse model of Alzheimer's disease. They proposed the mechanism involved PBM stimulating c-kit-positive mesenchymal stem cells (MSCs) that were normally resident in autologous bone marrow. These MSCs were proposed to be able to infiltrate the brain, and clear β-amyloid plaques (Oron and Oron 2016). It should be noted in general that the calvarial bone marrow of the skull contains substantial numbers of stem cells (Iwashita et al. 2003).
3.3 PBM for brain in uninjured animals
Several laboratories have reported that shining light onto the head of uninjured healthy mice or rats can improve various cognitive and emotional parameters. The first study reported that exposure of the middle aged (12 months) CD1 female mice to 1072 nm LED arrays (Michalikova et al. 2008) produced improved performance in a 3D maze compared to sham treated age-matched controls. Gonzalez-Lima and coworkers (Gonzalez-Lima and Barrett 2014) showed that transcranial PBM (9 mW/cm2 with a 660 nm LED array) delivered to rats induced dose-dependent increases in oxygen consumption (5% after 1 J/cm2 and 16% after 5 J/cm2) [113]. They also found that tPBM reduced fear renewal and prevented the reemergence of the extinguished conditioned fear-responses (Rojas et al. 2012).
3.4 PBM for enhancement of brain function in uninjured human volunteers
Gonzalez-Lima et al delivered transcranial PBM (1064 nm laser, 60 J/cm2 at 250 mW/cm2) to the forehead in uninjured human volunteers in a placebo-controlled, randomized study. The goal was to improve performance of cognitive tasks related to the prefrontal cortex, including a psychomotor vigilance task (PVT), a delayed match-to-sample (DMS) memory task, and improved mood as measured by the positive and negative affect schedule (PANAS-X) (Barrett and Gonzalez-Lima 2013). Subsequent studies in uninjured humans showed that tPBM with 1064 nm laser could improve performance in the Wisconsin Card Sorting Task (considered the gold standard test for executive function) (Blanco et al. 2015). They also showed that tPBM to the right forehead (but not the left forehead) could improve attention bias modification (ABM) in humans with depression (Disner et al. 2016).
Salgado et al. applied transcranial LED to enhance cerebral blood flow in healthy elderly women, as measured by transcranial Doppler ultrasound (TCD) of the right and left middle cerebral artery and basilar artery. Twenty-five non-institutionalized elderly women (mean age 72 years), with cognitive status > 24, were assessed using TCD before and after transcranial LED therapy. tPBM (627 nm, 70 mW/cm2, 10 J/cm2) was performed at four points of the frontal and parietal region for 30 s each twice a week for 4 weeks. There was a significant increase in the systolic and diastolic velocity of the left middle cerebral artery (25 and 30%, respectively) and the basilar artery (up to 17 and 25%), as well as a decrease in the pulsatility index and resistance index values of the three cerebral arteries analyzed (Salgado et al. 2015).
3.5 PBM for acute stroke
Transcranial PBM delivered to the head, has been investigated as a possible treatment for acute stroke (Lapchak 2010). Animal models such as rats and rabbits, were first used as laboratory models, and these animals had experimental strokes induced by a variety of methods and were then treated with light (usually 810 nm laser) within 24 h of stroke onset (Lampl 2007). In these studies intervention by tLLLT within 24 h had meaningful beneficial effects.
Treatment of acute stroke in human patients was then addressed in a series of three clinical trials called “Neurothera Effectiveness and Safety Trials” (NEST-1 (Lampl et al. 2007), NEST-2 (Huisa et al. 2013), and NEST-3 (Zivin et al. 2014)). The protocol used an 810 nm laser applied to the shaved head (20 separate points in the 10/20 EEG system) within 24 h of patients suffering an ischemic stroke. The first study, NEST-1, enrolled 120 patients between the ages of 40 to 85 years of age and found a significantly improved outcome (p < 0.05 real vs sham, NIH Stroke Severity Scale) 5 days after a single laser treatment had been administered (Lampl et al. 2007). This significantly improved status was still present 90 days post-stroke in 70% of the PBM patients (but only 51% of the sham patients). The second clinical trial, NEST-2, enrolled 660 patients, aged 40 to 90, who were randomly assigned to one of two groups (331 to PBM, 327 to sham) (Zivin et al. 2009). Significant improvements (p < 0.04) were found in the moderate and moderate-severe (but not for the severe) stroke patients. The last clinical trial, NEST-3, was planned for 1000 patients enrolled, but the study was prematurely terminated by the DSMB for futility (an expected lack of statistical significance) (Lapchak and Boitano 2016). Many commentators have asked how tPBM could work so well in the first trial, yet fail in the third trial. Insufficient light penetration, too long an interval between stroke onset and PBM, inappropriate stroke severity measurement scale, use of only one single tPBM treatment, and failure to illuminate different specific areas of the brain for individual patients, have all been suggested as contributory reasons (Hamblin 2016b). It is undoubtedly the case that the failure of NEST-3 has cast a cloud over the whole application of PBMT for TBI as well as for stroke. Many commentators have asked “Why are you testing PBMT for TBI, if it has been shown not to work for stroke?” The failure of the investigators not to take into account the anatomical location of the stroke (and also whether it was deep or superficial) was also likely to have played a role in the failure of NEST-3. It is logical that light should be applied to the same side of the head where the lesion was located, not both sides of the head (Naeser et al. 2012). In my opinion the use of a single application of PBMT also bore some of the responsibility. Although a single application of PBM to the head works very well for experimental animals (mice, rats, rabbits) who have suffered a stroke or a TBI, the same may not apply to humans.
4. Animal studies of PBM in acute TBI models
4.1 Studies from the Oron laboratory
Oron's group was the first (Oron et al. 2007) to demonstrate that a single exposure of the head of a mouse a few hours after creation of a TBI lesion using a NIR laser (808 nm) could improve neurological performance and reduce the size of the brain lesion. A weight-drop device was used to induce a closed-head TBI in the mice. An 808 nm diode laser with two energy densities calculated at the surface of the brain (1.2-2.4 J/cm2 delivered by 2 minutes of irradiation with 200mW laser power to the scalp) was delivered to the head 4 hours after TBI was induced. Neurobehavioral function was assessed by the neurological severity score (NSS). There was no significant difference between the control and laser-treated group in NSS between the power densities (10 vs 20 mW/cm2), and no significant difference at early time points (24 and 48 hours) post TBI. However, there was a significant improvement (27% lower NSS score) in the PBM group at times between 5 days and 4 weeks. The laser treated group also showed a smaller loss of cortical tissue than the sham group (Oron et al.). In another study (Oron et al. 2012) they varied the pulse parameters (CW, 100Hz, or 600Hz) and tested whether the tPBM was equally effective when delivered at 4, 6, or 8 hours post-TBI. They first established that a calculated dose to the cortical surface of 1.2 J/cm2 of 808nm laser at 200mW applied to the head, was more effective when delivered at 6 hours post TBI than at 8 hours. They then selected an even shorter time post-TBI (4 hours) and compared CW with 100Hz and 600Hz. At 56 days, more mice in the 100Hz group (compared to the CW and 600 Hz groups) had fully recovered. The 600Hz group had lower NSS scores than the CW and 100Hz groups up to 20 days. Magnetic resonance imaging (MRI) analysis demonstrated significantly smaller lesion volumes in PBM-treated mice compared to controls.
4.2 Studies from the Hamblin laboratory
Wu et al. (Wu et al. 2012) first explored the effect of varying the laser wavelengths of PBM had on closed-head TBI in mice. Mice were randomly assigned to a PBM treatment group with a particular wavelength, or to a sham treatment group as a control. Closed-head injury (CHI) was induced via a weight- drop apparatus. To analyze the severity of the TBI, the neurological severity score (NSS) was measured and recorded. The injured mice were then treated with varying wavelengths of laser light (665, 730, 810 or 980 nm) at an energy density of 36 J/cm2 directed onto the scalp at 4 hours post-TBI. The 665 nm and 810 nm laser groups showed significant improvement in NSS when compared to the control group between days 5 to 28. By contrast, the 730 nm and 980 nm laser groups did not show any significant improvement in NSS (Wu et al. 2012) (Figure 3). The tissue chromophore cytochrome c oxidase (CCO) is proposed to be responsible for the underlying photon absorption process that underlies many PBM effects. CCO has absorption bands around 665 nm and 810 nm while it has a low absorption region at the wavelength of 730 nm (Karu et al.). It should be noted that this particular study (Wu et al. 2012) found that the 980 nm did not produce the same positive effects as the 665 nm and 810 nm wavelengths did; nevertheless previous studies did find that the 980 nm wavelength was an active one for PBM (Anders et al. 2014). Wu et al. suggested that these dissimilar results may be due to differences in the energy density, irradiance etc. between the other studies and the Wu study (Wu et al. 2012). In particular a much lower dose of 980 nm might have been effective had it been tested (Wang et al. 2016). Ando et al. (Ando et al. 2011) next used the 810 nm wavelength produced by a Ga-Al-As diode laser delivered at parameters used in the Wu study, and varied the pulse modes of the laser. These modes consisted of either pulsed wave at 10 Hz or at 100 Hz (50% duty cycle) or continuous wave laser. They used a different mouse model of TBI induced with a controlled cortical impact device directly inflicting a lesion on the cortex via an open craniotomy. A single treatment with a power density of 50 mW/m2 and an energy density of 36 J/cm2 (duration of 12 minutes) was given via tLLLT to the closed head in mice at 4 hours post CCI. At 48 hours to 28 days post TBI, all laser treated groups had significant decreases in the measured neurological severity score (NSS) when compared to the controls. Although all laser treated groups had similar NSS improvement rates up to day 7, the PW 10 Hz group began to show even greater improvement beyond this point as seen in Figure 4. At day 28, the forced swim test for depression and anxiety was used and showed a significant decrease in the immobility time for the PW 10 Hz group. In the tail suspension test, which measures depression and anxiety, there was also a significant decrease in the immobility time at day 28, and also at day 1, in the PW 10 Hz group.
Figure 3. Effect of different laser wavelengths of tPBM in closed-head TBI in mice.
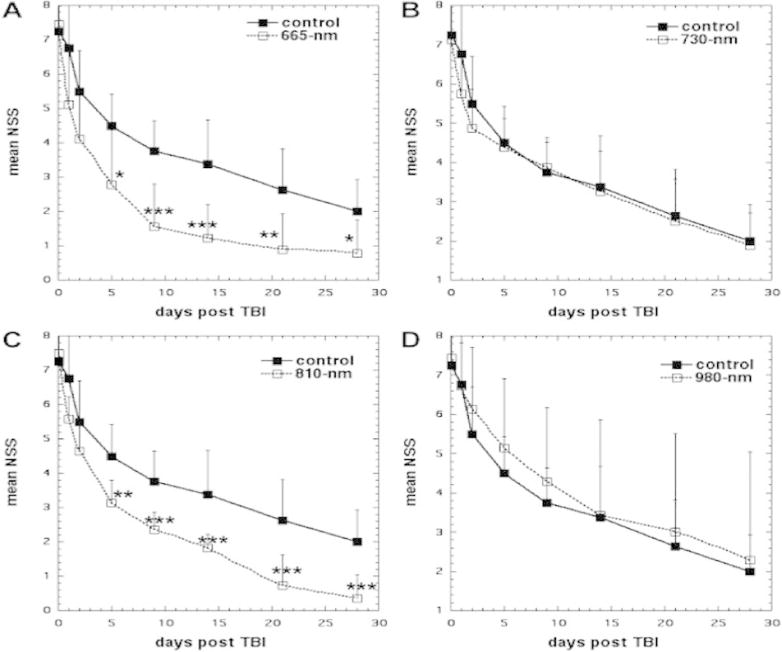
(A) Sham-treated control versus 665 nm laser. (B) Sham-treated control versus 730 nm laser. (C) Sham-treated control versus 810 nm laser. (D) Sham-treated control versus 980 nm laser. Points are means of 8–12 mice and bars are SD. *P < 0.05; **P < 0.01; ***P < 0.001 (one-way ANOVA). Reprinted with permission from (Wu et al. 2012)
Figure 4. Effects of pulsing in tPBM for CCI-TBI in mice.
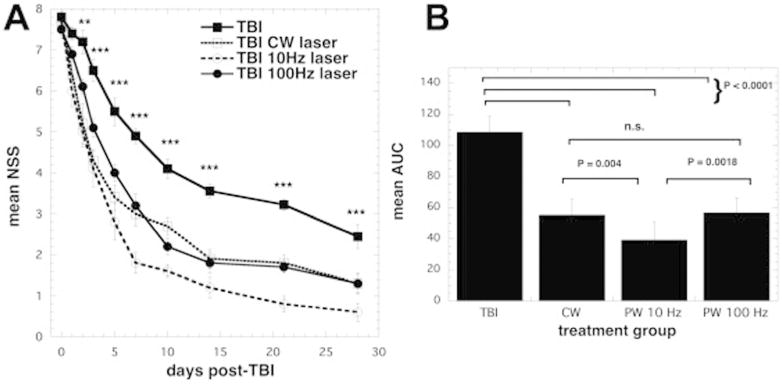
(A) Time course of neurological severity score (NSS) of mice with TBI receiving either control (no laser-treatment), or 810 nm laser (36 J/cm2 delivered at 50 mW/cm2 with a spot size of 0.78 cm2 in either CW, PW 10 Hz or PW 100 Hz modes. Results are expressed as mean +/- S.E.M ***P < 0.001 vs. the other conditions. (B) Mean areas under the NSS-time curves in the two-dimensional coordinate system over the 28-day study for the 4 groups of mice. Results are means +/- SD (n = 10). Reprinted from (Ando et al. 2011) (open access).
Studies using immunofluorescence staining of sections cut from mouse brains showed that tPBM increased neuroprogenitor cells (incorporating BrdU) in the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) at 7 days after the treatment (Xuan et al. 2014). The neurotrophin known as brain derived neurotrophic factor (BDNF) was also increased in the DG and SVZ at 7 days, while the protein marker (synapsin-1) for synaptogenesis and neuroplasticity was increased in the cortex at 28 days but not in the DG, SVZ or in any location at 7 days (Xuan et al. 2015). Learning and memory as measured by the Morris water maze was also improved by tPBM (Xuan et al. 2014).
4.3 Studies from the Wu laboratory
Zhang et al. (Zhang et al. 2014) first showed that secondary brain injury occurred to a worse degree in mice that had been genetically engineered to lack “Immediate Early Response” gene X-1 (IEX-1). When these mice were exposed to a gentle head impact (thought to closely resemble mild TBI in humans) they had a worse NSS than uninjured mice with the same TBI. Exposure of IEX-1 knockout mice to PBM (150 mW/cm2, 4 min, and 36 J/cm2) delivered at 4 hours post injury, restored the NSS to almost baseline levels, suppressed proinflammatory cytokine expression of interleukin (IL)-Iβ and IL-6, but upregulated TNF-α. The original lack of IEX-1 decreased ATP production, but exposing the injured brain to LLLT elevated ATP production back to near normal levels.
Dong et al. (Dong et al. 2015) asked whether the beneficial effects of PBM on TBI in mice could be enhanced by combining PBM with administration of metabolic substrates such as pyruvate and/or lactate. The goal was to even further improve mitochondrial function in the brain. This combinatorial treatment was able to reverse memory and learning deficits in TBI injured mice back to normal levels as well as leaving the hippocampal region completely protected from tissue loss; a stark contrast to control TBI mice that exhibited severe tissue loss from secondary brain injury.
4.4 Studies from the Whalen laboratory
Khuman et al (Khuman et al. 2012) delivered PBM (800nm) either directly to the injured brain tissue (through the craniotomy) or transcranially in mice beginning 60-80 min after CCI TBI. At a dose of 60J/cm2 (500mW/cm2) the mice showed increased performance in the Morris water maze (latency to the hidden platform, p<0.05, and probe trial, p<0.01) compared to non-treated controls. When PBM was delivered via open craniotomy there was reduced microgliosis at 48h (IbA-1+ cells, p<0.05). Little or no effect of tPBM on post-injury cognitive function was observed using lower or higher doses, a 4-h administration time point or 60J/cm2 at 7-days post-TBI.
4.5 Studies from the Whelan laboratory
Quirk et al (Quirk et al. 2012) studied Sprague-Dawley rats who had received a severe CCI TBI and were divided into three groups: real TBI, sham surgery, and anesthetization only. Each group received either real or sham PBM consisting of 670nm LED treatments of 15J/cm2, 50mW/cm2, 5min, given two times per day for 3 days (chemical analysis) or 10 days (behavioral analysis using a TruScan nose-poke device). Significant differences in task entries, repeat entries, and task errors were seen in the TBI rats treated with PBM vs untreated TBI mice, and in sham surgery mice treated with PBM vs untreated sham surgery mice. A statistically significant decrease was found in the pro-apoptotic marker Bax, and increases in the anti-apoptotic marker Bcl-2 and reduced glutathione (GSH) levels in tPBM TBI mice.
4.6 Studies from the Marques laboratory
Moreira et al used a different model of TBI (Moreira et al. 2009). Wistar rats received a craniotomy and a copper probe cooled in liquid nitrogen was applied to the surface of the brain to create a standardized cryogenic injury. They treated the rats with either a 780nm or 660nm laser at one of two different doses (3J/cm2 or 5J/cm2) twice (once immediately after the injury and again 3 hours later). Rats were sacrificed 6h and 24h after the injury. The 780nm laser was better at reducing levels of pro-inflammatory cytokines (TNFα, IL1β, IL6) particularly at early timepoints (Moreira et al. 2009). In a follow-up study using 3 J/cm2 (Moreira et al. 2011) these workers reported on the healing of the injuries in these rats at timepoints 6h, 1, 7 and 14 days after the last irradiation. Cryogenic injury created focal lesions in the cortex characterized by necrosis, edema, hemorrhage and inflammatory infiltrate. The most striking findings were: PBM-treated lesions showed less tissue loss than control lesions at 6h. During the first 24h the amount of viable neurons was significantly higher in the PBM groups. PBM reduced the amount of GFAP (glial fibrillary acidic protein, a marker of astrogliosis) and the numbers of leukocytes and lymphocytes, thus demonstrating its anti-inflammatory effect.
5. Patients with chronic TBI
The majority of studies of PBM for TBI in laboratory animals have been conducted in the acute setting, while the majority of human studies of PBM for TBI have been conducted in patients who have suffered head injuries at various times in the past (sometimes quite a long time ago).
5.1 Naeser case reports
In 2011 Naeser, Saltmarche et al., published the first report describing two chronic, TBI cases treated with tPBM (Naeser et al. 2011). A 500 mW CW LED source (mixture of 660 nm red and 870 nm NIR LEDs) with a power density of 22.2 mW/cm2 (area of 22.48 cm2), was applied all over the head, for 10 minutes at each placement location (13.3 J/cm2). In the first case study the patient reported that she could concentrate on tasks for a longer period of time (the time able to work at a computer increased from 20 minutes to 3 hours). She had a better ability to remember what she read, decreased sensitivity when receiving haircuts in the spots where PBM was applied, and improved mathematical skills after undergoing PBM. The second patient had statistically significant improvements compared to prior neuropsychological tests after 9 months of treatment. The patient had a 2 standard deviation (SD) increase on tests of inhibition and inhibition accuracy (9th percentile to 63rd percentile on the Stroop test for executive function and a 1 SD increase on the Wechsler Memory scale test for the logical memory test (83rd percentile to 99th percentile) (Naeser et al. 2011).
5.2 Naeser case series
Naeser et al then went on to report a case series containing a further eleven patients (Naeser et al. 2014). This was an open protocol study that examined whether scalp application of red and NIR LED could improve cognition in patients with chronic, mild TBI (mTBI). This study enrolled 11 participants ranging in age from 26 to 62 years (6 males, 5 females) who suffered from persistent cognitive dysfunction after mTBI. The injuries in the participants had been caused by motor vehicle accidents, sports related events and for one participant, an improvised explosive device (IED) blast. tPBM consisted of 18 sessions (Monday, Wednesday, and Friday for 6 weeks) and was started anywhere from 10 months to 8 years post-TBI. A total of 11 LED cluster heads (5.25 cm in diameter, 500 mW, 22.2 mW/cm2, 13 J/cm2) were applied for 10 minutes per set (5 or 6 LED placements per set, Set A and then Set B, in each session). Neuropsychological testing was performed pre-LED application and 1 week, 1 month and 2 months after the final treatment. They found that there was a significant positive linear trend for the Stroop Test for executive function, in trial 3 inhibition (p = 0.004); Stroop, trial 4 inhibition switching (p = 0.003); California Verbal Learning Test (CVLT)-II, total trials 1-5 (p = 0.003); CVLT-II, long delay free recall (p = 0.006). Improved sleep and fewer post-traumatic stress disorder (PTSD) symptoms, if present beforehand, were observed after treatment. Participants and family members also reported better social function and a better ability to perform interpersonal and occupational activities. Although these results were significant, the authors suggested that further placebo-controlled studies would be needed to ensure the reliability of this approach (Naeser et al. 2014).
Naeser has proposed (Naeser et al. 2016; Naeser et al. 2014) that specific scalp placements of the LED cluster heads may affect specific cortical nodes in the intrinsic networks of the brain, such as the default mode network (DMN), the salience network (SN), and the central executive network (CEN). These intrinsic networks are often dysregulated after TBI (Sharp et al. 2014). Naeser proposed that the specific areas of the head to receive light, to target cortical nodes in these networks were as follows:
For the DMN, placement of the LED cluster head on the midline of face, centered on the upper forehead and the front hairline, targeted the left and right mesial prefrontal cortex; and on a midline, scalp location half-way between the occipital protuberance and the vertex of the head, targeted the precuneus; and on left and right LED placements superior to the tip of each ear and posterior to each ear, targeted the inferior parietal cortex/angular gyrus areas.
For the SN, placement of LED cluster heads on the left and right temple areas, to target the anterior insula (but due to depth of insula, unknown if the photons reached the target); midline of the vertex of the head, to target the left and right presupplementary motor areas; and the LED cluster head placed on the midline of face, centered on the upper forehead and the front hairline, also targeted the left and right dorsal anterior cingulate cortex.
For the CEN, left and right scalp LED placements immediately posterior to the front hairline (on a line directly superior from the pupils of the eyes), targeted the dorso-lateral prefrontal cortex areas; and the left and right LED placements superior to the tip of each ear and posterior to each ear, also targeted the posterolateral inferior parietal cortex/angular gyrus areas (also treated as part of the DMN).
Further studies from Naeser and colleagues (Naeser et al. 2016) tested an intranasal LED (iLED) device. Two small iLEDs (one red and the other NIR) were clipped into each nostril and used at the same time for 25 min. The parameters were as follows: red, 633nm, 8mW CW, 1 cm2, energy density 12 J/cm2 (25 min); NIR 810nm, 14.2mW, pulsed 10Hz, 1cm2, 21.3J/cm2. The first mTBI participant (24-year old female) who had sustained four sports-related concussions (two during snowboarding and two during field hockey), received iLED PBM three times per week for 6 weeks. Significant improvements were observed in tasks measuring executive function and verbal memory as well as attention and verbal fluency. At 1 week after the 18th iLED treatment, the average total time asleep had increased by 61 min per night and her sleep efficiency (total sleep time divided by total time in bed) had increased by 11%. At 12 weeks after the last iLED treatment, she was able to discontinue all sleep medications that she had previously been using. The second, mTBI participant who received the intranasal only, LED treatment series is a 49 Yr. M (non-Veteran) who sustained mTBI in a MVA, 30 years prior to receiving the intranasal LED treatment series. He showed significant improvement on the Controlled Oral Word Association-FAS Test post- the iLED treatment series, improving by +1.3 SD and +1.5 SD at 1 and 2 months post- the 18th iLED treatment. His sleep data indicated he was already a good sleeper, at entry.
5.3 Bogdanova and Naeser studies
Bogdanova reported (Bogdanova et al. 2014) a case report of two patients (1 female) with moderate TBI (medical records and clinical evaluation) and persistent cognitive dysfunction (as measured by neuropsychological tests of executive function and memory). Patients received 18 sessions of transcranial LED therapy (3×/week for 6 weeks) using the mixed red/NIR cluster described above (Naeser et al. 2011).
Standardized neuropsychological tests for executive function, memory, depression, PTSD and sleep measures (PSQI, actigraphy) were administered to participants pre-(T1), mid-(T2), and one week (T3) post-PBM treatment. Both PBM treated cases (P1 and P2) showed marked improvement in sleep (actigraphy total sleep) 1 week post-LED treatment (T3), as compared to pre-treatment (T1). P1 also improved in executive function, verbal memory, and sleep efficiency; while P2 significantly improved on measures of PTSD (PCL-M) and depression. No adverse events were reported.
5.4 Studies from Henderson and Morries
Henderson and Morries (Henderson and Morries 2015b) used a high-power NIR laser (10-15 W at 810 and 980 nm) and applied it to the head to treat a patient with moderate TBI. The patient received 20 NIR applications over a 2-month period. They carried out anatomical magnetic resonance imaging (MRI) and perfusion single-photon emission computed tomography (SPECT). The patient showed decreased depression, anxiety, headache, and insomnia, whereas cognition and quality of life improved, accompanied by changes in the SPECT imaging.
They next reported (Morries et al. 2015) a series of ten patients with chronic TBI (average time since injury 9.3 years) where each patient received ten treatments over the course of 2 months using a high-power NIR laser (13.2 W/0.89 cm2 equivalent to 14.6 W/cm2 at 810nm; or 9 W/0.89 cm2 equivalent to 10.11 W/cm2 at 980nm). A continuous sweeping motion over the forehead was utilized to minimize skin heating and cover a larger area. Skin temperature increased no more than 3°C. Overall symptoms of headache, sleep disturbance, cognition, mood dysregulation, anxiety, and irritability improved. Symptoms were monitored by depression scales and a novel patient diary system specifically designed for this study. These authors have proposed that high power lasers are preferable for tPBM treatments because the photons can better reach the brain (Henderson and Morries 2015a).
5.5 Case study from Nawashiro
Nawashiro et al (Nawashiro et al. 2012) treated a single patient who had suffered a severe TBI. The patient survived but was left in a persistent vegetative state for 8 months after the accident. He showed no spontaneous movement of limbs and a CT scan of the head 8 months after the accident showed a focal low-density area in the right frontal lobe. The device had 23 individual 850nm LEDs (13mW each; total power 299mW, total area 57cm2). A treatment time of 30 min per session delivered 20.5 J/cm2 over the left and right forehead areas repeated twice daily (6h apart), for 73 days. Five days after beginning the PBM (after 10 treatments), the patient began to spontaneously move his left arm and hand, which had not occurred during the previous 8 months. Single-photon emission computed tomography with N-isopropyl-[123I]p-iodoamphetamine (IMP-SPECT) was performed twice. The IMP-SPECT scans showed a focal increase (20% higher) in cerebral blood flow in the uninjured left anterior frontal lobe 30 min after the last (146th) PBM treatment, compared to before PBM began.
6. Conclusion and future prospects
As was mentioned above, one of the most important questions to be answered when contemplating clinical treatment of TBI patients with tPBM, is what is the best time to administer the treatment? All the available reports of studies using PBM in laboratory animal models of TBI and stroke, and also in patients treated for stroke, have been in the acute phase where the overall goal of the intervention can be best described as neuroprotection. Not only that but there are several reports (Lapchak et al. 2007; Oron et al. 2012) that PBM for both TBI and stroke is most effective when it is delivered as soon as possible after the actual event (head impact or ischemic stroke). The protocols for the series of NEST clinical trials specified that patients should be treated with PBM within 24 hours of the stroke occurring. By contrast, all the clinical trials of PBM for patients with TBI, that have so far been carried out, have been with chronic TBI, after varying periods of time having elapsed after the original head injury, sometimes as long as 8 years. Although it would be generally supposed that tPBM would be effective when delivered to acute TBI patients, this has not yet been actually tested. If tPBM were to be used for acute TBI patients, then presumably the PBM should be delivered perhaps beginning at 4 to 6 hours post-TBI, for a limited number of times after the injury; perhaps once a day for 7 days?
The dosimetry and optimum delivery apparatus of tPBM is still uncertain. Although there is some consensus that wavelengths in the region of 800-900nm will penetrate the scalp and skull, other workers have used longer NIR wavelengths, 980nm, 1064nm, or 1072nm. Pulsing or CW is another unresolved question. The exact locations on the head that should receive the light are still unknown. Naeser has proposed (Naeser et al. 2016) some interesting considerations regarding the scalp placements of the tLEDS, and their effect on various intrinsic cortical networks of the brain. Targeted LED placements could promote better neuromodulation (activation/deactivation) in specific cortical nodes. It is possible that communication between nodes within one single network, and/or across networks could be improved. Moreover preliminary data indicate that intranasal, red plus near-infrared LEDs can also benefit TBI patients, although the degree to which light incident on the nasal mucosa, and possibly delivered transsphenoidally (Pitzschke et al. 2015) can penetrate directly into the brain, remains to be determined.
An advantage of intranasal and/or transcranial LED PBM therapy is that it can be performed in the home, for long-term use (Naeser et al. 2011). Also, 5 chronic, mild to moderately-severe dementia cases recently showed significant improvement on the Mini-Mental State Examination (p<0.003), and on the Alzheimer's Disease Assessment Scale-Cognitive subscale (p<0.023) after 12 weeks of daily, at-home, intranasal, near-infrared LED PBM treatments (810nm, pulsed at 10 Hz), and once-a-week in-office, tLED treatments applied to the cortical nodes of the Default Mode Network (Saltmarche et al. 2017). Anecdotally, there was also improved sleep, fewer angry outbursts, and less wandering. When all LED treatments were withdrawn after 12 weeks of active LED PBM treatment, there was precipitous decline in cognition and behavior. Thus, at-home, long-term use of iLED plus tLED PBM offers a potential therapy to mitigate the sequelae of Alzheimer's disease and possibly other neurodegenerative disorders, as well as TBI and stroke.
One highly distressing aspect of TBI symptomatology that has not so far been addressed by PBM, is that of post-traumatic epilepsy (PTE). TBI is the most significant cause of symptomatic epilepsy in people from 15 to 24 years of age. The frontal and temporal lobes are the most frequently affected regions, but imaging (MRI) often fails to show the precise cause. During PTE seizures there is an abnormal electrical discharge in the brain, with staring and unresponsiveness, stiffening or shaking of the body, legs, arms or head; strange sounds, tastes, visual images, feelings or smells; inability to speak or understand, etc (Cotter et al. 2017). Epilepsy has traditionally been considered to be a contra-indication for PBMT (Navratil and Kymplova 2002). However the knowledge that has recently been gained concerning the beneficial effects of PBMT on the damaged brain, suggests that this view may need to be critically revisited.
Moreover there is also potential of tPBM to treat a wide range of brain disorders only loosely associated with TBI, including Parkinson's disease (Purushothuman et al. 2013), depression, anxiety, post-traumatic stress disorder, autism spectrum disorder and so on (Hamblin 2016b).
The ongoing and accelerating clinical research efforts in testing PBM for TBI, are expected to lead to the answering of many of these questions in the coming years.
Acknowledgments
MRH was supported by US NIH grants R01AI050875 and R21AI121700, Air Force Office of Scientific Research grant FA9550-13-1-0068, US Army Medical Research Acquisition Activity grant W81XWH-09-1-0514, and US Army Medical Research and Materiel Command grant W81XWH-13-2-0067.
Footnotes
Conflict of Interest Statement: The author declares no conflict of interest
References
- Aimbire F, Albertini R, Pacheco MT, Castro-Faria-Neto HC, Leonardo PS, Iversen VV, Lopes-Martins RA, Bjordal JM. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed Laser Surg. 2006;24(1):33–37. doi: 10.1089/pho.2006.24.33. [DOI] [PubMed] [Google Scholar]
- Alexandratou E, Yova D, Handris P, Kletsas D, Loukas S. Human fibroblast alterations induced by low power laser irradiation at the single cell level using confocal microscopy. Photochem Photobiol Sci. 2002;1(8):547–552. doi: 10.1039/b110213n. [DOI] [PubMed] [Google Scholar]
- Anders JJ, Moges H, Wu X, Erbele ID, Alberico SL, Saidu EK, Smith JT, Pryor BA. In vitro and in vivo optimization of infrared laser treatment for injured peripheral nerves. Lasers Surg Med. 2014;46(1):34–45. doi: 10.1002/lsm.22212. [DOI] [PubMed] [Google Scholar]
- Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, Huang YY, Wu Q, Whalen MJ, Sato S, Obara M, Hamblin MR. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS ONE. 2011;6(10):e26212–26220. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13–23. doi: 10.1016/j.neuroscience.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Blanco NJ, Maddox WT, Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol. 2015 doi: 10.1111/jnp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, Zetterberg H. Traumatic brain injuries. Nat Rev Dis Primers. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- Bogdanova Y, Martin PI, Ho MD, Krengel MH, Ho VT, Yee MK, Knight JA, Hamblin MR, Naeser MA. LED Therapy Improves Sleep and Cognition In Chronic Moderate TBI: Pilot Case Studies. Archives Phys Med Rehab. 2014;95(10):e77. [Google Scholar]
- Caterina MJ, Pang Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals (Basel) 2016;9(4) doi: 10.3390/ph9040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR. Low-Level Laser Therapy Activates NF-kB via Generation of Reactive Oxygen Species in Mouse Embryonic Fibroblasts. PLoS ONE. 2011a;6(7):e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Huang YY, Sharma SK, Hamblin MR. Effects of 810-nm Laser on Murine Bone-Marrow-Derived Dendritic Cells. Photomed Laser Surg. 2011b;29(6):383–389. doi: 10.1089/pho.2010.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897–1908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- Cotter D, Kelso A, Neligan A. Genetic biomarkers of posttraumatic epilepsy: A systematic review. Seizure. 2017;46:53–58. doi: 10.1016/j.seizure.2017.02.002. [DOI] [PubMed] [Google Scholar]
- de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. Journal of Selected Topics in Quantum Electronics. 2016;22(3):7000417–7000434. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirican A, Andacoglu O, Johnson R, McGuire K, Mager L, Soran A. The short-term effects of low-level laser therapy in the management of breast-cancer-related lymphedema. Support Care Cancer. 2011;19(5):685–690. doi: 10.1007/s00520-010-0888-8. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Gonzalez-Lima F. Transcranial Laser Stimulation as Neuroenhancement for Attention Bias Modification in Adults with Elevated Depression Symptoms. Brain Stimul. 2016 doi: 10.1016/j.brs.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T, Zhang Q, Hamblin MR, Wu MX. Low-level light in combination with metabolic modulators for effective therapy of injured brain. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfara D, Tuby H, Trudler D, Doron-Mandel E, Maltz L, Vassar RJ, Frenkel D, Oron U. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer's disease. J Mol Neurosci. 2015;55(2):430–436. doi: 10.1007/s12031-014-0354-z. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths, 2002-2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- Gam AN, Thorsen H, Lonnberg F. The effect of low-level laser therapy on musculoskeletal pain: a meta-analysis. Pain. 1993;52(1):63–66. doi: 10.1016/0304-3959(93)90114-5. [DOI] [PubMed] [Google Scholar]
- Gao X, Xing D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci. 2009;16:4. doi: 10.1186/1423-0127-16-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Barrett DW. Augmentation of cognitive brain functions with transcranial lasers. Front Syst Neurosci. 2014;8:36. doi: 10.3389/fnsys.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeussinger FB, Heinzel S, Hahn T, Schecklmann M, Ehlis AC, Fallgatter AJ. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS One. 2011;6(10):e26377. doi: 10.1371/journal.pone.0026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR. The role of nitric oxide in low level light therapy. In: Hamblin MR, Anders JJ, Waynant RW, editors. Mechanisms for Low-Light Therapy II. Bellingham, WA: The International Society for Optical Engineering; 2008. [Google Scholar]
- Hamblin MR. Photobiomodulation or low-level laser therapy. J Biophotonics. 2016a;9(11-12):1122–1124. doi: 10.1002/jbio.201670113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016b;6:113–124. doi: 10.1016/j.bbacli.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR, de Sousa MV, Agrawal T. Handbook of Low Level Laser Therapy. Singapore: Pan-Stanford Publlshing; 2016. [Google Scholar]
- Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015a;11:2191–2208. doi: 10.2147/NDT.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson TA, Morries LD. SPECT Perfusion Imaging Demonstrates Improvement of Traumatic Brain Injury With Transcranial Near-infrared Laser Phototherapy. Adv Mind Body Med. 2015b;29(4):27–33. [PubMed] [Google Scholar]
- Hennessy M, Hamblin MR. Photobiomodulation and the brain: a new paradigm. J Optics. 2016 doi: 10.1088/2040-8986/19/1/013003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins JT, McLoda TA, Seegmiller JG, David Baxter G. Low-level laser therapy facilitates superficial wound healing in humans: a triple-blind, sham-controlled study. J Athl Train. 2004;39(3):223–229. [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Gupta A, Vecchio D, de Arce VJ, Huang SF, Xuan W, Hamblin MR. Transcranial low level laser (light) therapy for traumatic brain injury. J Biophotonics. 2012;5(11-12):827–837. doi: 10.1002/jbio.201200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisa BN, Stemer AB, Walker MG, Rapp K, Meyer BC, Zivin JA Nest, investigators. Transcranial laser therapy for acute ischemic stroke: a pooled analysis of NEST-1 and NEST-2. Int J Stroke. 2013;8(5):315–320. doi: 10.1111/j.1747-4949.2011.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita T, Tada T, Zhan H, Tanaka Y, Hongo K. Harvesting blood stem cells from cranial bone at craniotomy--a preliminary study. J Neurooncol. 2003;64(3):265–270. doi: 10.1023/a:1025684903137. [DOI] [PubMed] [Google Scholar]
- Jagdeo JR, Adams LE, Brody NI, Siegel DM. Transcranial red and near infrared light transmission in a cadaveric model. PLoS One. 2012;7(10):e47460. doi: 10.1371/journal.pone.0047460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamins J, Giza CC. Concussion-Mild Traumatic Brain Injury: Recoverable Injury with Potential for Serious Sequelae. Neurosurg Clin N Am. 2016;27(4):441–452. doi: 10.1016/j.nec.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23(4):355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol. 2004a;80(2):366–372. doi: 10.1562/2004-03-25-RA-123. [DOI] [PubMed] [Google Scholar]
- Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005;36(4):307–314. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004b;3(2):211–216. doi: 10.1039/b306126d. [DOI] [PubMed] [Google Scholar]
- Khuman J, Zhang J, Park J, Carroll JD, Donahue C, Whalen MJ. Low-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in mice. J Neurotrauma. 2012;29(2):408–417. doi: 10.1089/neu.2010.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs IB, Mester E, Gorog P. Stimulation of wound healing with laser beam in the rat. Experientia. 1974;30(11):1275–1276. doi: 10.1007/BF01945182. [DOI] [PubMed] [Google Scholar]
- Lampl Y. Laser treatment for stroke. Expert Rev Neurother. 2007;7(8):961–965. doi: 10.1586/14737175.7.8.961. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38(6):1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- Lane N. Cell biology: power games. Nature. 2006;443(7114):901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Taking a light approach to treating acute ischemic stroke patients: transcranial near-infrared laser therapy translational science. Ann Med. 2010;42(8):576–586. doi: 10.3109/07853890.2010.532811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Boitano PD. Transcranial Near-Infrared Laser Therapy for Stroke: How to Recover from Futility in the NEST-3 Clinical Trial. Acta Neurochir Suppl. 2016;121:7–12. doi: 10.1007/978-3-319-18497-5_2. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Boitano PD, Butte PV, Fisher DJ, Holscher T, Ley EJ, Nuno M, Voie AH, Rajput PS. Transcranial Near-Infrared Laser Transmission (NILT) Profiles (800 nm): Systematic Comparison in Four Common Research Species. PLoS One. 2015;10(6):e0127580. doi: 10.1371/journal.pone.0127580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Salgado KF, Chao CH, Zivin JA. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148(4):907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31(12):596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Alosco ML, Huber BR. Repetitive Head Impacts and Chronic Traumatic Encephalopathy. Neurosurg Clin N Am. 2016;27(4):529–535. doi: 10.1016/j.nec.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalikova S, Ennaceur A, van Rensburg R, Chazot PL. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: effects of low infrared light. Neurobiol Learn Mem. 2008;89(4):480–488. doi: 10.1016/j.nlm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Mitchell UH, Mack GL. Low-level laser treatment with near-infrared light increases venous nitric oxide levels acutely: a single-blind, randomized clinical trial of efficacy. Am J Phys Med Rehabil. 2013;92(2):151–156. doi: 10.1097/PHM.0b013e318269d70a. [DOI] [PubMed] [Google Scholar]
- Moreira MS, Velasco IT, Ferreira LS, Ariga SK, Abatepaulo F, Grinberg LT, Marques MM. Effect of laser phototherapy on wound healing following cerebral ischemia by cryogenic injury. J Photochem Photobiol B. 2011;105(3):207–215. doi: 10.1016/j.jphotobiol.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Moreira MS, Velasco IT, Ferreira LS, Ariga SK, Barbeiro DF, Meneguzzo DT, Abatepaulo F, Marques MM. Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat. J Photochem Photobiol B. 2009;97(3):145–151. doi: 10.1016/j.jphotobiol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Morries LD, Cassano P, Henderson TA. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr Dis Treat. 2015;11:2159–2175. doi: 10.2147/NDT.S65809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Hamblin MR. Potential for Transcranial Laser or LED Therapy to Treat Stroke, Traumatic Brain Injury, and Neurodegenerative Disease. Photomed Laser Surg. 2011;29(7):443–446. doi: 10.1089/pho.2011.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Hamblin MR. Traumatic Brain Injury: A Major Medical Problem That Could Be Treated Using Transcranial, Red/Near-Infrared LED Photobiomodulation. Photomed Laser Surg. 2015 doi: 10.1089/pho.2015.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Ho M, Martin PI. Improved language after scalp application of red/near-infrared light-emitting diodes: pilot study supporting a new, noninvasive treatment for chronic aphasia. Proc Soc Behav Sci. 2012;61:138–139. [Google Scholar]
- Naeser MA, Martin PI, Ho MD, Krengel MH, Bogdanova Y, Knight JA, Yee MK, Zafonte R, Frazier J, Hamblin MR, Koo BB. Transcranial, Red/Near-Infrared Light-Emitting Diode Therapy to Improve Cognition in Chronic Traumatic Brain Injury. Photomed Laser Surg. 2016;34(12):610–626. doi: 10.1089/pho.2015.4037. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed Laser Surg. 2011;29(5):351–358. doi: 10.1089/pho.2010.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin M, Knight JA, Meehan W, Baker EH. Significant improvements on cognitive performance post- transcranial, red/near-infrared LED treatments in chronic, mild TBI: Open-protocol study. J Neurotrauma. 2014 doi: 10.1089/neu.2013.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratil L, Kymplova J. Contraindications in noninvasive laser therapy: truth and fiction. J Clin Laser Med Surg. 2002;20(6):341–343. doi: 10.1089/104454702320901134. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Wada K, Nakai K, Sato S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed Laser Surg. 2012;30(4):231–233. doi: 10.1089/pho.2011.3044. [DOI] [PubMed] [Google Scholar]
- Okada E, Delpy DT. Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Appl Opt. 2003;42(16):2915–2922. doi: 10.1364/ao.42.002915. [DOI] [PubMed] [Google Scholar]
- Oron A, Oron U. Low-Level Laser Therapy to the Bone Marrow Ameliorates Neurodegenerative Disease Progression in a Mouse Model of Alzheimer's Disease: A Minireview. Photomed Laser Surg. 2016 doi: 10.1089/pho.2015.4072. [DOI] [PubMed] [Google Scholar]
- Oron A, Oron U, Streeter J, de Taboada L, Alexandrovich A, Trembovler V, Shohami E. low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2007;24(4):651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- Oron A, Oron U, Streeter J, De Taboada L, Alexandrovich A, Trembovler V, Shohami E. Near infrared transcranial laser therapy applied at various modes to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2012;29(2):401–407. doi: 10.1089/neu.2011.2062. [DOI] [PubMed] [Google Scholar]
- Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, Cingolani A. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175(1):95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Lovisa B, Seydoux O, Zellweger M, Pfleiderer M, Tardy Y, Wagnieres G. Red and NIR light dosimetry in the human deep brain. Phys Med Biol. 2015;60(7):2921–2937. doi: 10.1088/0031-9155/60/7/2921. [DOI] [PubMed] [Google Scholar]
- Purushothuman S, Nandasena C, Johnstone DM, Stone J, Mitrofanis J. The impact of near-infrared light on dopaminergic cell survival in a transgenic mouse model of parkinsonism. Brain Res. 2013;1535:61–70. doi: 10.1016/j.brainres.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Quirk BJ, Torbey M, Buchmann E, Verma S, Whelan HT. Near-infrared photobiomodulation in an animal model of traumatic brain injury: improvements at the behavioral and biochemical levels. Photomed Laser Surg. 2012;30(9):523–529. doi: 10.1089/pho.2012.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Bruchey AK, Gonzalez-Lima F. Low-level light therapy improves cortical metabolic capacity and memory retention. J Alzheimers Dis. 2012;32(3):741–752. doi: 10.3233/JAD-2012-120817. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol. 2013;86(4):447–457. doi: 10.1016/j.bcp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Safinia C, Bershad EM, Clark HB, SantaCruz K, Alakbarova N, Suarez JI, Divani AA. Chronic Traumatic Encephalopathy in Athletes Involved with High-impact Sports. J Vasc Interv Neurol. 2016;9(2):34–48. [PMC free article] [PubMed] [Google Scholar]
- Salgado AS, Zangaro RA, Parreira RB, Kerppers II. The effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. Lasers Med Sci. 2015;30(1):339–346. doi: 10.1007/s10103-014-1669-2. [DOI] [PubMed] [Google Scholar]
- Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, Lim L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed Laser Surg. 2017;35(8):432–441. doi: 10.1089/pho.2016.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoilova KA, Zhevago NA, Petrishchev NN, Zimin AA. Role of nitric oxide in the visible light-induced rapid increase of human skin microcirculation at the local and systemic levels: II. healthy volunteers. Photomed Laser Surg. 2008;26(5):443–449. doi: 10.1089/pho.2007.2205. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nat Rev Neurol. 2014;10(3):156–166. doi: 10.1038/nrneurol.2014.15. [DOI] [PubMed] [Google Scholar]
- Strangman GE, Zhang Q, Li Z. Scalp and skull influence on near infrared photon propagation in the Colin27 brain template. Neuroimage. 2014;85 Pt 1:136–149. doi: 10.1016/j.neuroimage.2013.04.090. [DOI] [PubMed] [Google Scholar]
- Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR. Relation between mitochondrial membrane potential and ROS formation. Methods Mol Biol. 2012;810:183–205. doi: 10.1007/978-1-61779-382-0_12. [DOI] [PubMed] [Google Scholar]
- Sussai DA, Carvalho Pde T, Dourado DM, Belchior AC, dos Reis FA, Pereira DM. Low-level laser therapy attenuates creatine kinase levels and apoptosis during forced swimming in rats. Lasers Med Sci. 2010;25(1):115–120. doi: 10.1007/s10103-009-0697-9. [DOI] [PubMed] [Google Scholar]
- Tatmatsu-Rocha JC, Ferraresi C, Hamblin MR, Damasceno Maia F, do Nascimento NR, Driusso P, Parizotto NA. Low-level laser therapy (904nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J Photochem Photobiol B. 2016;164:96–102. doi: 10.1016/j.jphotobiol.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med. 2015;47(4):312–322. doi: 10.1002/lsm.22343. [DOI] [PubMed] [Google Scholar]
- Thunshelle C, Hamblin MR. Transcranial Low-Level Laser (Light) Therapy for Brain Injury. Photomed Laser Surg. 2016;34(12):587–598. doi: 10.1089/pho.2015.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation of human adipose-derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbagen.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Xuan W, Ando T, Xu T, Huang L, Huang YY, Dai T, Dhital S, Sharma SK, Whalen MJ, Hamblin MR. Low-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengths. Lasers Surg Med. 2012;44:218–226. doi: 10.1002/lsm.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Agrawal T, Huang L, Gupta GK, Hamblin MR. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J Biophotonics. 2015;8(6):502–511. doi: 10.1002/jbio.201400069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Vatansever F, Huang L, Hamblin MR. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. Journal of biomedical optics. 2014;19(10):108003. doi: 10.1117/1.JBO.19.10.108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky AN, Schulze PC, Yaroslavsky IV, Schober R, Ulrich F, Schwarzmaier HJ. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys Med Biol. 2002;47(12):2059–2073. doi: 10.1088/0031-9155/47/12/305. [DOI] [PubMed] [Google Scholar]
- Zecha JA, Raber-Durlacher JE, Nair RG, Epstein JB, Elad S, Hamblin MR, Barasch A, Migliorati CA, Milstein DM, Genot MT, Lansaat L, van der Brink R, Arnabat-Dominguez J, van der Molen L, Jacobi I, van Diessen J, de Lange J, Smeele LE, Schubert MM, Bensadoun RJ. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 2: proposed applications and treatment protocols. Support Care Cancer. 2016a doi: 10.1007/s00520-016-3153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecha JA, Raber-Durlacher JE, Nair RG, Epstein JB, Sonis ST, Elad S, Hamblin MR, Barasch A, Migliorati CA, Milstein DM, Genot MT, Lansaat L, van der Brink R, Arnabat-Dominguez J, van der Molen L, Jacobi I, van Diessen J, de Lange J, Smeele LE, Schubert MM, Bensadoun RJ. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer. 2016b doi: 10.1007/s00520-016-3152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Dong T, Li P, Wu MX. Noninvasive low-level laser therapy for thrombocytopenia. Sci Transl Med. 2016;8(349):349ra101. doi: 10.1126/scitranslmed.aaf4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhou C, Hamblin MR, Wu MX. Low-level laser therapy effectively prevents secondary brain injury induced by immediate early responsive gene X-1 deficiency. J Cereb Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J, Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M, Streeter J, NeuroThera E, Safety Trial I. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40(4):1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- Zivin JA, Sehra R, Shoshoo A, Albers GW, Bornstein NM, Dahlof B, Kasner SE, Howard G, Shuaib A, Streeter J, Richieri SP, Hacke W investigators N. NeuroThera(R) Efficacy and Safety Trial-3 (NEST-3): a double-blind, randomized, sham-controlled, parallel group, multicenter, pivotal study to assess the safety and efficacy of transcranial laser therapy with the NeuroThera(R) Laser System for the treatment of acute ischemic stroke within 24 h of stroke onset. Int J Stroke. 2014;9(7):950–955. doi: 10.1111/j.1747-4949.2012.00896.x. [DOI] [PubMed] [Google Scholar]


