Abstract
Background
Recent epidemiological and experimental studies have shown that obesity is an important risk factor for colorectal cancer (CRC). Regular intake of high fat containing diet can promote obesity and metabolic syndrome by increasing the insulin resistance and inflammatory response which contribute to carcinogenesis. Previously, we have shown that inhibition of polyol pathway enzyme aldose reductase (AR) prevents carcinogens- and inflammatory growth factors-induced CRC. However, the effect of AR inhibition on high fat diet (HFD)-induced formation of intestinal polyps in Apc-deficient min (multiple intestinal neoplasia; ApcMin/+) mice is not known.
Method
Here, we examined the effect of AR inhibitor, fidarestat on HFD-induced formation of pre-neoplastic intestinal polyps in ApcMin/+ mice which is an excellent model of colon cancer.
Results
HFD were fed to APC Min/+ mice were fed with for 12 weeks caused a significant increase in the formation of polyps in the small and large intestines and fidarestat given along with the HFD prevented the number of intestinal polyps. Fidarestat also decreased the size of the polyps in the intestines of HFD- treated APC min mice. Further, the expression levels of beta-catenin, PCNA, PKC-β2, P-AKT, P-p65, COX-2 and iNOS in the small and large intestines of HFD-treated mice significantly increased and AR inhibitor (fidarestat) prevented it.
Conclusion
Our results thus suggest that fidarestat could be used as a potential chemopreventive drug for intestinal cancers due to APC gene mutations.
Keywords: Aldose reductase, fidarestat, APC min mice, high-fat diet, colon cancer, polyps
INTRODUCTION
Colorectal cancer (CRC) is one of the leading cause of cancer-related deaths the United States [1]. Recent studies indicate that lifestyle factors such as smoking, decreased physical activity and increased high-fat diet (HFD) and obesity could not only lead to diabetes and metabolic syndrome but are also a major risk factor for CRC. High consumption of dietary fats, especially, saturated fats can increase the formation of pro-carcinogenic markers such as PGE2, Cox-2, bile acids, and triglycerides which activate pro-inflammatory transcriptional factors such as NF-kB and PPAR leading to increased risk of cancer. Although, most (90%) of the tumors arise sporadically, the inherited cases constitute only 5 to 10% of all CRC cases [2–4]. The mutation in adenomatous polyposis coli (APC) gene was established in all CRCs with familial adenomatous polyposis (FAP) and in approximately 80% of sporadic CRCs [5–7]. In general, APC protein is associated with β –catenin and GSK3- β in the cell membrane and regulated by Wnt signaling pathway [5–7]. Abnormal regulation of the Wnt signaling pathway due to APC gene mutation leads to increased β –catenin translocation to the nucleus. The T cell factor (TCF)/lymphoid enhancer transcription factors (LEF) are activated by β-catenin leading to increased expression of genes such as cyclin-D and c-Myc that modulate cell proliferation and apoptosis [5–7]. Mouse with APC gene mutation (ApcMin/+) has an autosomal dominant heterozygous nonsense mutation of the mouse APC gene at codon 850, homologous to the human germ line and somatic APC mutations [8]. Therefore, ApcMin/+ mouse has been well recognized as the standard experimental model for the study of intestinal carcinogenesis because it allows the tumors to develop spontaneously in the intestinal tract. Further, several studies indicate that APC Min/+ mice develop intestinal polyps when treated with high-fat containing diet. This model is particularly advantageous for testing chemopreventive agents targeted at early stage tumorigenesis because adenomas grow to a grossly detectable size within a few months [8, 9].
We have shown earlier that the inhibition of aldose reductase (AR), a member of aldo–keto reductase super family (AKR1B1), prevents oxidative stress signals induced by growth factors, cytokines, and chemokines that lead to CRC cells growth [10–16]. We have shown that pharmacological inhibitors of AR or small interfering RNA (siRNA) of AR prevent the human CRC cells growth, invasion and migration in culture as well as in nude mice xenografts [10,14]. Further, we have also demonstrated that AR inhibition prevents azoxymethane –induced aberrant crypt foci (ACF) formation in BALB/c and db/db mice [12, 17], indicating that AR inhibitors such as fidarestat could be used to prevent risk of developing CRC in normal and obese mice. Although, our previous studies have shown that fidarestat prevents CRC growth in chemically-induced colon cancer and nude mice models, the effect of fidarestat in the prevention of polyps in a genetically altered mouse model is not known. Furthermore, the effect of fidarestat in preventing the risk of developing CRC in high-fat diet intake has not been investigated. Therefore, in this study, we evaluated the effect of fidarestat on HFD –induced intestinal adenoma formation in ApcMin/+ mice and our results suggest that fidarestat prevents the formation of intestinal polyps in small and large intestines of mice by decreasing the expression of various pro-carcinogenic markers such as beta-catenin, PCNA, PKC-β2, P-AKT, P-p65, COX-2 and iNOS. Our results thus suggest that fidarestat could prevent or delay the risk of formation of intestinal cancers in high –fat diet fed ApcMin/+ mice.
MATERIALS AND METHODS
Materials
Male C57BL/6J-ApcMin/J and female C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). For breeding, mice were housed in pathogen-free condition with free access to food and water at the institutional animal care facility. The animals were maintained in accordance to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and in accordance with the Institute’s ‘Guideline of the Animal Care and Use Committee’. Mice were kept in suspended cages ~10 cm above bedding trays with a 12 h light–dark cycle in the animal facility. Temperature and relative humidity were controlled at 21°C and 55% respectively. All the mice were acclimatized to the above conditions for 1 week with free access to standard laboratory rodent chow and drinking water and offsprings were genotyped as heterozygotes by PCR for the Apc gene by taking tail snips at weaning. Custom modified High-fat diet (HFD) AIN-76A containing 12% w/w Corn fat, 21% casein, 13% dextrose, 43% corn starch, 4.7% vitamin and mineral mix was purchased from Dyets, Inc., Bethlehem, PA. Fidarestat was obtained from Livwel Therapeutics Inc, CA, USA. Antibodies against proliferating cell nuclear antigen (PCNA), COX-2, β-catenin and phospho-protein kinase C (PKC) b2, phospho-AKT, and phospho-NF-κB P65 were obtained from Cell Signaling (Danvers, MA). All other reagents used were of analytical grade and were obtained from Sigma.
HFD-induced intestinal polyp formation
Approximately 6 weeks old wildtype (control) and ApcMin/+ mice (6–7/group, male) were divided into three treatment groups: (1) Wild type mice with control/normal diet (Con), (2) ApcMin/+ mice with HFD, and (3) ApcMin/+ mice with HFD and fidarestat (Fid). In group 3, mice were treated with fidarestat (50 mg/kg body wt, in drinking water) for entire period (12 weeks). All mice were euthanized at the end of the experiment, the small and large intestines were removed, flushed with saline, opened from distal to proximal end, and fixed flat between two pieces of filter paper in 10% buffered-formalin for 24 h. Intestines were stained with 0.2% methylene blue dissolved in saline and the number of polyps were counted in small and large intestine under the microscope.
Immunohistochemistry
After microscopic evaluation, small and large intestines were Swiss-rolled and embedded in paraffin. Immunohistochemical (IHC) analysis of serial sections (5 uM) of intestines was performed as described elsewhere [17]). Briefly, slides were warmed at 60°C for 1 h and deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Antigen retrieval was performed by boiling slides in 10 mM sodium citrate (pH 6.0) for 10 min followed by blocking of peroxidase with 3% H2O2. The sections were rinsed in phosphate-buffered saline twice and incubated overnight at 4 °C with blocking buffer (2% bovine serum albumin, 0.1% Triton X-100 and 2% normal goat serum). The sections were incubated with primary antibodies against PCNA, β-catenin, COX-2, iNOS, PKC-β2, phospho-AKT and phospho- NF-κB P65 for 1 h at room temperature. Antigen–antibody binding was detected by using DakoCytomation LSAB System-HRP kit. Sections were examined by bright-field light microscopy (EPI-800 microscope; Nikon, Tokyo, Japan) and photographed with a Nikon camera fitted to the microscope. Photomicrographs of the stained sections were acquired using an EPI-800 microscope (bright-field) connected to a Nikon camera.
Statistical analysis
Data presented as mean ± SEM and P-values were determined by unpaired Student’s t test using Microsoft Office Excel 2007 software. P < 0.05 was considered as statistically significant.
RESULTS
Inhibition of AR prevents intestinal pre-neoplastic polyps formation in HFD fed ApcMin/+ mice
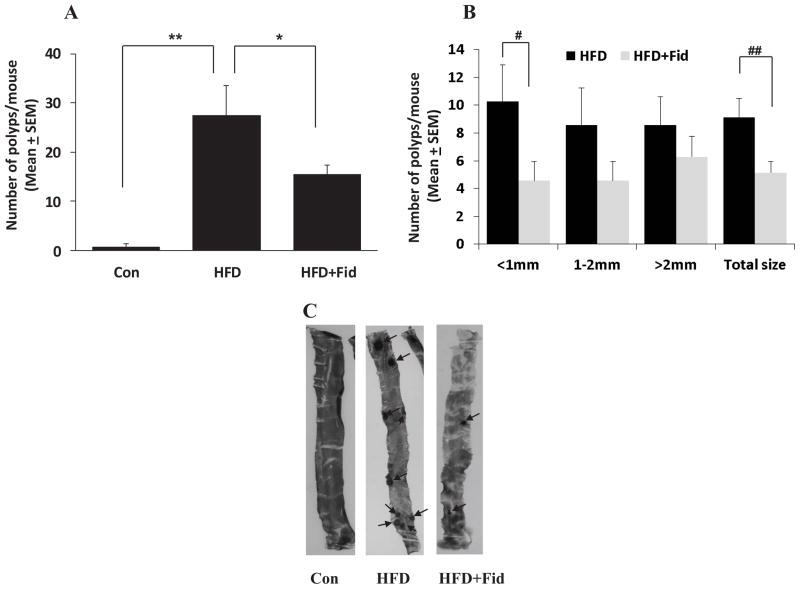
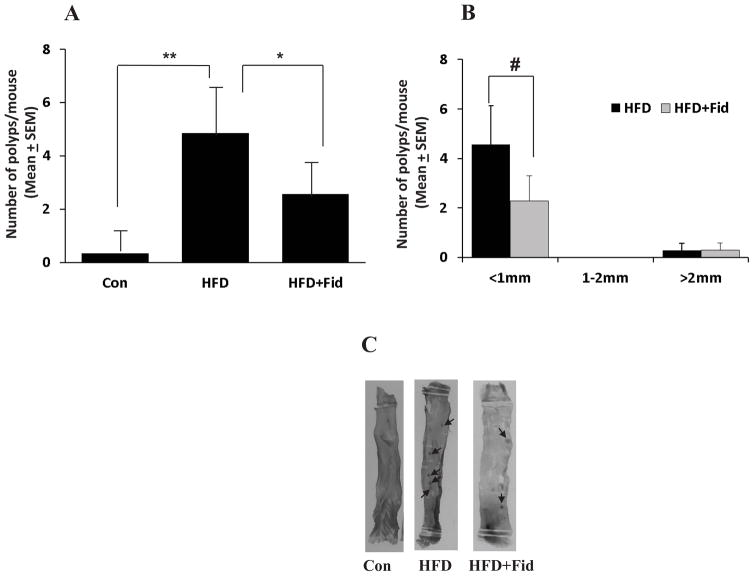
Since, obesity and diabetes are major risk factors for colon cancer [18–20], we investigated the effect of AR inhibitor on HFD-induced intestinal polyp formation in ApcMin/+ mice. The body weights of control and HFD mice with and without AR inhibitor were comparable and no significant changes were observed throughout the study (data not shown). At the early preneoplastic stage (12 weeks), mice were killed and their intestines were removed and analyzed microscopically for the presence of polyps. In ApcMin/+ mice, HFD caused significant increase in total number of polyps in small intestine (27.4 ± 6) and administration of fidarestat along with HFD significantly (p=0.0428) suppressed the formation of polyps (15.4 ± 1.8) (Figure 1A). To examine polyp size, we counted and classified polyps as being small (<1 mm in diameter), medium (between 1–2 mm in diameter) and large (>2 mm in diameter), and analyzed the effect of AR inhibition on polyp size in the small intestine. Overall, AR inhibition significantly reduced the number of polyps in small intestine. Interestingly, we found a significant reduction (p=0.037) in the number of small polyps with AR inhibition (Figure 1B). AR inhibition also reduced the number of medium and large polyps. We next investigated the effect of AR inhibitor on pre-neoplastic polyp formation in large intestine. The results shown in the Figure 2A indicate that 4.9 ± 1.6 polyps per mouse were formed in large intestine, and administration of fidarestat suppressed the formation of polyps (2.6 ± 0.95). Further, size of polyps in large intestine was also compared in HFD ± fidarestat-treated mice. AR inhibition reduced small polyps in large intestine of HFD-treated mice (Figure 2B). Collectively, these findings indicate that inhibition of AR by fidarestat prevents the formation pre-neoplastic intestinal polyps in HFD-diet fed ApcMin/+ mice.
Figure 1. Inhibition of AR prevents HFD-induced polyp formation in the small intestines of ApcMin/+ mice.
ApcMin/+ mice were divided into three groups: (i) control; (ii) HFD and (iii) HFD + fidarestat (50 mg/kg body wt, in drinking water) as described in the Methods. Mice were euthanized after 12 weeks and small intestines were stained and examined for polyp formation. A) Number of intestinal polyps and B) size of the intestinal polyps were determined by staining the intestines with methylene blue. Data represents mean ± SEM (n= 6–7); *P=0.001, #P= 0.0428, *P= 0.0375, ##P= 0.007, HFD alone vs HFD + Fid. Con, control; HFD, high fat diet; HFD + Fid, high fat diet + fidarestat.
Figure 2. Inhibition of AR prevents HFD-induced polyp formation in the large intestines of ApcMin/+ mice.
ApcMin/+ mice were divided into three groups: (i) control; (ii) HFD and (iii) HFD + fidarestat (50 mg/kg body wt, in drinking water) as described in the Methods. Mice were euthanized after 12 weeks and large intestines were stained and examined for polyp formation. A) Number of intestinal polyps and B) size of the intestinal polyps were determined by staining the intestines with methylene blue. Data represents mean ± SEM (n= 6–7); *P=0.001, #P= 0.117, HFD alone vs HFD + Fid. Con, control; HFD, high fat diet; HFD + Fid, high fat diet + fidarestat.
Inhibition of AR prevents the expression of pro-carcinogenic markers in HFD-fed ApcMin/+ mice
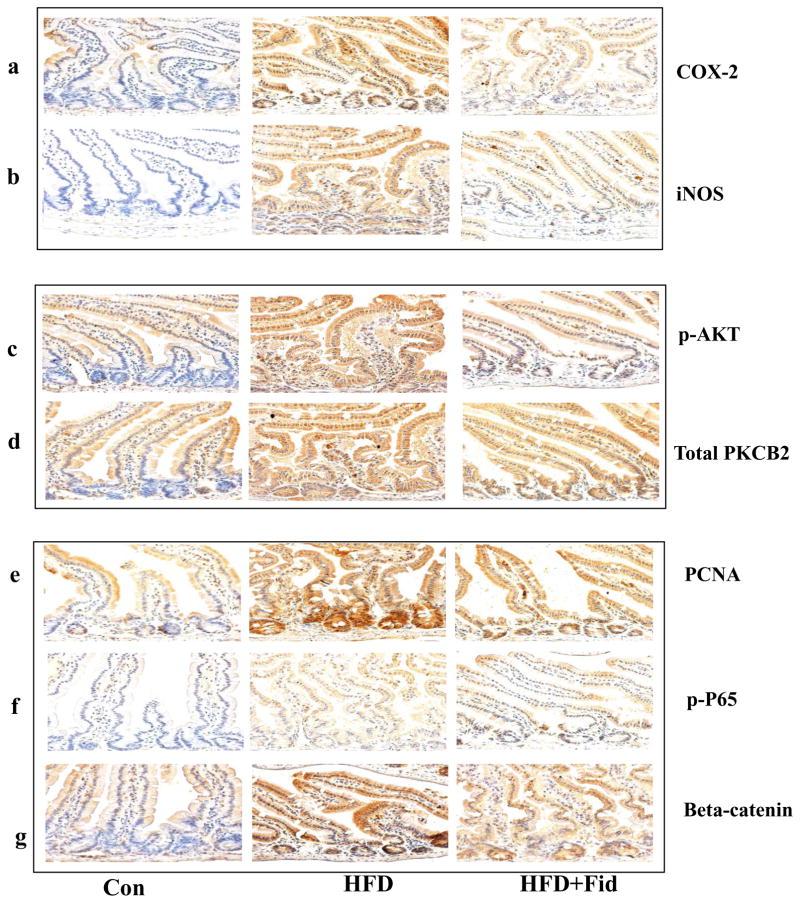
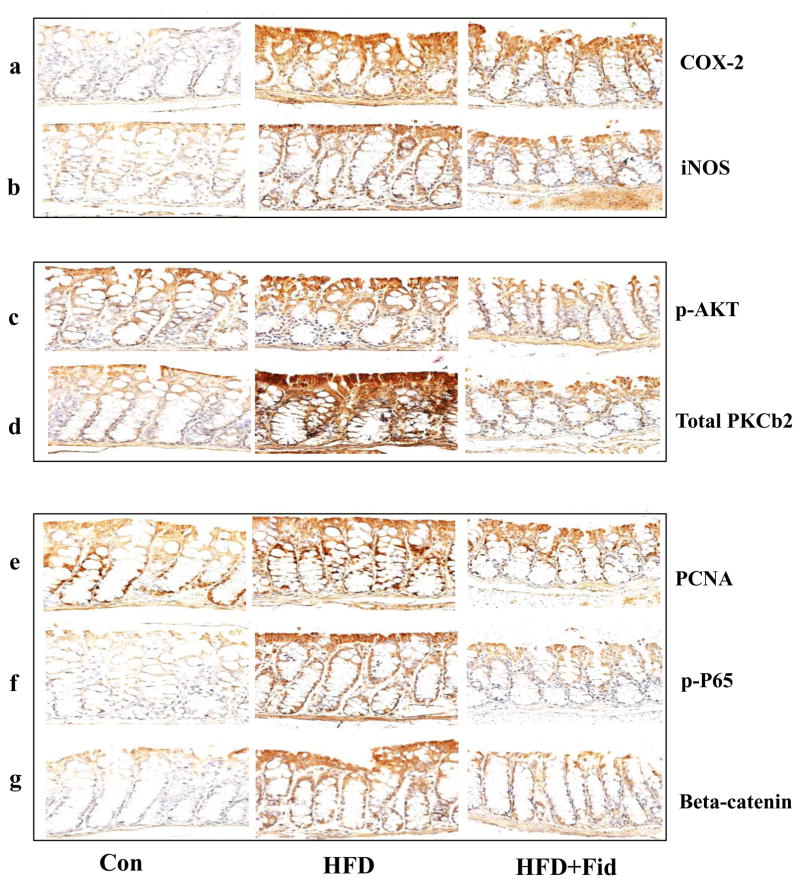
Since both COX-2 and iNOS are known to be involved in chronic inflammation, which creates a microenvironment that contributes to the development of pre-neoplastic lesions in the colon carcinogenesis and inhibitors of COX-2 and iNOS have been shown to reduce ACF formation in rodents [3, 21], we examined the effect of fidarestat on HFD-induced COX-2 and iNOS expression in the small and large intestines of ApcMin/+ mice. Immunohistochemical analysis suggests that the expression of COX-2 and iNOS were increased in the small intestine of ApcMin/+ mice fed with HFD and this increase was significantly prevented in the mice treated with fidarestat along with the HFD (Figure 3a and 3b). Similar results were observed in large intestines also (Figure 4a and 4b).
Figure 3. Inhibition of AR prevents the expression of inflammatory and pre-neoplastic markers in the small intestines of HFD-treated ApcMin/+ mice.
Histological sections of small intestines from HFD- and HFD+Fidarestat- treated mice were stained with antibodies against COX-2 (a), iNOS (b), phospho-AKT (c), PKCβ2 (d), PCNA (e), phospho- NF-κB (f) and β-catenin (g). Immunoreactivity of the antibody was assessed by dark brown staining in the intestinal cells, whereas the non-reactive areas displayed only the background color. Photomicrographs of the stained sections were acquired using an EPI-800 microscope (bright-field) connected to a Nikon camera (X400 magnification). A representative image from each group is shown. Con, control; HFD, high fat diet; HFD + Fid, high fat diet + fidarestat.
Figure 4. Inhibition of AR prevents the expression of inflammatory and pre-neoplastic markers in the large intestines of HFD-treated ApcMin/+ mice.
Histological sections of small intestines from HFD- and HFD+Fidarestat- treated mice were stained with antibodies against COX-2 (a), iNOS (b), phospho-AKT (c), PKCβ2 (d), PCNA (e), phospho- NF-κB (f) and β-catenin (g). Immunoreactivity of the antibody was assessed by dark brown staining in the intestinal cells, whereas the non-reactive areas displayed only the background color. Photomicrographs of the stained sections were acquired using an EPI-800 microscope (bright-field) connected to a Nikon camera (X400 magnification). A representative image from each group is shown. Con, control; HFD, high fat diet; HFD + Fid, high fat diet + fidarestat.
Inhibition of AR prevents HFD-induced phosphorylation of AKT and increase in PKC β2 in ApcMin/+ mice
Carcinogen-induced pre-neoplastic lesions in the intestinal epithelium are known to be associated with the activation of AKT and increase in PKC β2 that could cause hyperproliferation [22–24]. We therefore, examined the effect of AR inhibition on HFD-induced activation of AKT and increase in PKC β2 in both small and large intestine of ApcMin/+ mice. A dramatic increase in AKT phosphorylation and PKC β2 expression was observed in the HFD-treated mice, whereas inhibition of AR in HFD-treated mice showed a significant decrease in the phosphorylation of AKT and increase in PKC β2 in the small and large intestines (Figure 3c and 3d; Fig 4c and 4d). These results suggest that inhibition of AR prevents HFD-induced activation of AKT and increase in PKC β2 and expression of COX-2 and iNOS which could be considered as major contributors to intestinal polyp formation.
Inhibition of AR prevents HFD-induced PCNA, NF-κB (phospho-p65) and β-catenin expressions in ApcMin/+ mice
We next measured the expression of key proliferation markers, PCNA and β-catenin, and phospho-NF-κB P65 in mice small and large intestines. PCNA proteins serve as marker for survival and proliferation [12, 25, 26]. NF-κB which transcribes various inflammatory markers such as COX-2 and iNOS, is overexpressed in various forms of cancers [3, 12, 27]. Similarly, β-catenin during carcinogenesis translocates into the nucleus and interacts with T-cell factor/lymphoid enhancer factor family of transcription factors to promote the expression of various oncogenes [28, 29]. Our results also showed a significant increase in the expression of PCNA, β-catenin, and phospho-NF-κB P65 in the intestines of HFD-treated mice, and inhibition of AR prevented it (Figure 3e–g and 4e–g). Inhibition of AR by fidarestat decreased the HFD-induced expression of PCNA, β-catenin, and NF-κB, indicating that AR regulates the expression of the proteins that are known to promote intestinal polyp formation.
DISCUSSION
Obesity is a known cause of metabolic syndrome which includes Type II diabetes, hypertension, dyslipidemia and obesity-induced diabetes which could result in chronic inflammation, a risk of CRC (3,18–20). Although several studies indicate the significance of fat deposition, oxidative stress and inflammatory response in the development of HFD-induced pre-neoplastic polyps leading to CRC, the role of AR, a well-known lipid aldehyde metabolizing enzyme, that regulates oxidative and inflammatory signals in HFD-induced polyps formations in ApcMin/+ mice is not known. In this study, we have demonstrated that AR inhibitor prevents intestinal polyp formation in HFD-induced ApcMin/+ mice model. Further, we have shown that AR inhibition not only reduces the polyp number but also reduces the polyp size probably by inhibiting the expression of NF-kB –dependent inflammatory and carcinogenic markers in the intestine.
A number of studies indicate that antioxidants and flavonoids prevent intestinal polyp formation in HFD-induced ApcMin/+ mice. For example, curcumin an antioxidant with anti-inflammatory properties prevents HFD-induced pre-neoplastic polyps in both large and small intestines of ApcMin/+ mice [30–34]. Similarly, flavonoids such as chafuroside, caffeic-acid, sulforaphane, and epigallocatechin have also been shown to prevent diet-induced intestinal adenomas in ApcMin/+ mice [35–38]. Although, our current results are in consistence with the results from other antioxidant and flavonoid compounds, the use of AR inhibitor, fidarestat, has advantage over other natural products. The antioxidants such as curcumin and other flavonoids are relatively poorly absorbed in the body and at higher concentrations they act as oxidants. Fidarestat is a most potent AR small molecular inhibitor (Ki 9 nM) which has already undergone Phase-II clinical studies in the USA and Phase-III clinical studies in Japan for diabetic neuropathy and found to be safe without any major irreversible side effects [39]. Therefore, this drug could be developed rapidly for the prevention and therapy of CRC.
We have shown earlier that AR inhibitors prevent the cytokines-and growth factors-generated reactive oxygen species as well as oxidative stress –induced redox sensitive transcription factors –induced inflammatory signals that lead to the growth and metastasis of CRC in vitro and in nude mice xenograft models [10–16]. We have now investigated the effect of AR inhibition on various inflammatory markers in HFD-induced ApcMin/+ mice intestinal mucosa. Chronic inflammation mediated by increased inflammatory proteins such as COX-2 and iNOS is known to cause preneoplastic lesions and cancer [3, 21, 28]. Our result show that the increased expression of COX-2 and iNOS in the intestinal mucosa of HFD-induced ApcMin/+ mice is significantly inhibited in mice treated with AR inhibitor. Since, NF-κB is known to increase the expression of COX-2 and iNOS in various diseases including cancer, and antioxidants such as quercetin, curcumin and bardoxolone methyl prevent intestinal adenomas in diet-induced ApcMin/+ mice [30–34, 40, 41], we next examined the expression of NF-kB in the intestinal mucosa. In consistence with results from other antioxidants, AR inhibitor prevented the activation of NF-κB in the large and small intestines of HFD-induced ApcMin/+ mice. In addition, AR inhibition also decreased the expression of PCNA and β-catenin in the intestinal mucosa of HFD-treated ApcMin/+ mice. These findings are consistent with a previous study that indicated that curcumin inhibits the induction of COX-2 by TNF-α stimulus via the inhibition of NF-κB activation in human colon epithelial cells [42]. Further, our findings indicate that AR inhibition prevents the activation of AKT in the intestinal mucosa of HFD-treated ApcMin/+ mice. These findings are consistent with the studies that show that caffeine and green tea extracts prevent intestinal tumorigenesis in ApcMin/+ mice by preventing the β-catenin and AKT signaling [35–38].
In conclusion, we have shown a beneficial effect of AR inhibitor, fidarestat, in decreasing polyp number and size distribution in the HFD-induced ApcMin/+ mouse model of intestinal tumorigenesis. The inhibition of intestinal adenoma formation in diet-induced ApcMin/+ mice by AR inhibitor could be due to modulation of various inflammatory and carcinogenic markers such as PCNA, COX-2, iNOS, PKC β2, AKT, β-catenin, and NF-κB in the intestinal mucosa. Thus, our results suggest that fidarestat, which has already been tested in phase III clinical trial of diabetic neuropathy and found to be safe for human use, could be used to prevent the risk of formation of intestinal cancers in obese and high fat-consuming individuals.
Acknowledgments
Supported by funding from NIH/NCI CA129383 and NIH/NIDDK DK104786 grants.
Footnotes
Conflict of Interest: Authors declare no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–47. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 3.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 4.Nagy R, Sweet K, Eng C. Highly penetrant hereditary cancer syndromes. Oncogene. 2004;23:6445–70. doi: 10.1038/sj.onc.1207714. [DOI] [PubMed] [Google Scholar]
- 5.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–9. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 6.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 7.Kwong LN, Dove WF. APC and its modifiers in colon cancer. Adv Exp Med Biol. 2009;656:85–106. doi: 10.1007/978-1-4419-1145-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCart AE, Vickaryous NK, Silver A. Apc mice: models, modifiers and mutants. Pathol Res Pract. 2008;204:479–90. doi: 10.1016/j.prp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Mori H. Multistep carcinogenesis of the colon in Apc(Min/+) mouse. Cancer Sci. 2007;98:6–10. doi: 10.1111/j.1349-7006.2006.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–13. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava SK, Yadav UC, Reddy AB, Saxena A, Tammali R, Shoeb M, Ansari NH, Bhatnagar A, Petrash MJ, Srivastava S, Ramana KV. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem Biol Interact. 2011;191:330–8. doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena A, Shoeb M, Tammali R, Ramana KV, Srivastava SK. Aldose reductase inhibition suppresses azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Cancer Lett. 2014;355:141–7. doi: 10.1016/j.canlet.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxena A, Shoeb M, Ramana KV, Srivastava SK. Aldose reductase inhibition suppresses colon cancer cell viability by modulating microRNA-21 mediated programmed cell death 4 (PDCD4) expression. Eur J Cancer. 2013;49:3311–9. doi: 10.1016/j.ejca.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxena A, Tammali R, Ramana KV, Srivastava SK. Aldose reductase inhibition prevents colon cancer growth by restoring phosphatase and tensin homolog through modulation of miR-21 and FOXO3a. Antioxid Redox Signal. 2013;18:1249–62. doi: 10.1089/ars.2012.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tammali R, Saxena A, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents hypoxia-induced increase in hypoxia-inducible factor-1alpha (HIF-1alpha)and vascular endothelial growth factor (VEGF) by regulating 26 S proteasome-mediated protein degradation in human colon cancer cells. J Biol Chem. 2011;286:24089–100. doi: 10.1074/jbc.M111.219733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tammali R, Reddy AB, Saxena A, Rychahou PG, Evers BM, Qiu S, Awasthi S, Ramana KV, Srivastava SK. Inhibition of aldose reductase prevents colon cancer metastasis. Carcinogenesis. 2011;32:1259–67. doi: 10.1093/carcin/bgr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tammali R, Reddy AB, Ramana KV, Petrash JM, Srivastava SK. Aldose reductase deficiency in mice prevents azoxymethane-induced colonic preneoplastic aberrant crypt foci formation. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 19.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–91. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia. 2003;46:595–607. doi: 10.1007/s00125-003-1109-5. [DOI] [PubMed] [Google Scholar]
- 21.Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–70. [PubMed] [Google Scholar]
- 22.Murray NR, Davidson LA, Chapkin RS, Clay Gustafson W, Schattenberg DG, Fields AP. Overexpression of protein kinase C betaII induces colonic hyperproliferation and increased sensitivity to colon carcinogenesis. J Cell Biol. 1999;145:699–711. doi: 10.1083/jcb.145.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gökmen-Polar Y, Murray NR, Velasco MA, Gatalica Z, Fields AP. Elevated protein kinase C betaII is an early promotive event in colon carcinogenesis. Cancer Res. 2001;61:1375–81. [PubMed] [Google Scholar]
- 24.Rajamanickam S, Kaur M, Velmurugan B, Singh RP, Agarwal R. Silibinin suppresses spontaneous tumorigenesis in APC min/+ mouse model by modulating beta-catenin pathway. Pharm Res. 2009;26:2558–67. doi: 10.1007/s11095-009-9968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Luo H, Wang A. Expression of survivin and correlation with PCNA in osteosarcoma. J Surg Oncol. 2006;93:578–84. doi: 10.1002/jso.20507. [DOI] [PubMed] [Google Scholar]
- 26.Tan Z, Wortman M, Dillehay KL, Seibel WL, Evelyn CR, Smith SJ, Malkas LH, Zheng Y, Lu S, Dong Z. Small-molecule targeting of proliferating cell nuclear antigen chromatin association inhibits tumor cell growth. Mol Pharmacol. 2012;81:811–9. doi: 10.1124/mol.112.077735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319–27. [PubMed] [Google Scholar]
- 29.Nath N, Kashfi K, Chen J, Rigas B. Nitric oxide-donating aspirin inhibits beta-catenin/T cell factor (TCF) signaling in SW480 colon cancer cells by disrupting the nuclear beta-catenin-TCF association. Proc Natl Acad Sci U S A. 2003;100:12584–9. doi: 10.1073/pnas.2134840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, Williams ML, Steward WP, Gescher AJ. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11:535–40. [PubMed] [Google Scholar]
- 31.Pettan-Brewer C, Morton J, Mangalindan R, Ladiges W. Curcumin suppresses intestinal polyps in APC Min mice fed a high fat diet. Pathobiol Aging Age Relat Dis. 2011;1 doi: 10.3402/pba.v1i0.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Cui SX, Sun SY, Shi WN, Song ZY, Wang SQ, Yu XF, Gao ZH, Qu XJ. Chemoprevention of intestinal tumorigenesis by the natural dietary flavonoid myricetin in APCMin/+ mice. Oncotarget. 2016;7:60446–60460. doi: 10.18632/oncotarget.11108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YS, Li Y, Wang Y, Sun SY, Jiang T, Li C, Cui SX, Qu XJ. Naringin, a natural dietary compound, prevents intestinal tumorigenesis in Apc (Min/+) mouse model. J Cancer Res Clin Oncol. 2016;142:913–25. doi: 10.1007/s00432-015-2097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Notarnicola M, Tutino V, Caruso MG, Francavilla A. n-3 polyunsaturated fatty acids reverse the development of polyps in Apc(Min/+) transgenic mice. Oncol Rep. 2016;35:504–10. doi: 10.3892/or.2015.4359. [DOI] [PubMed] [Google Scholar]
- 35.Niho N, Mutoh M, Sakano K, Takahashi M, Hirano S, Nukaya H, Sugimura T, Wakabayashi K. Inhibition of intestinal carcinogenesis by a new flavone derivative, chafuroside, in oolong tea. Cancer Sci. 2006;97:248–51. doi: 10.1111/j.1349-7006.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MM. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21:921–7. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- 37.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20:506–8. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, Yang GY, Liu YY, Hou Z, Lin Y, Ma J, Shih WJ, Carothers AM, Yang CS. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–31. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 39.Hotta N, Toyota T, Matsuoka K, Shigeta Y, Kikkawa R, Kaneko T, Takahashi A, Sugimura K, Koike Y, Ishii J, Sakamoto N SNK-860 Diabetic Neuropathy Study Group. Clinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy: a 52-week multicenter placebo-controlled double-blind parallel group study. Diabetes Care. 2001;24:1776–82. doi: 10.2337/diacare.24.10.1776. [DOI] [PubMed] [Google Scholar]
- 40.Murphy EA, Davis JM, McClellan JL, Carmichael MD. Quercetin’s effects on intestinal polyp multiplicity and macrophage number in the Apc(Min/+) mouse. Nutr Cancer. 2011;63:421–6. doi: 10.1080/01635581.2011.535954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinh CH, Yu Y, Szabo A, Zhang Q, Zhang P, Huang XF. Bardoxolone Methyl Prevents High-Fat Diet-Induced Colon Inflammation in Mice. J Histochem Cytochem. 2016;64:237–55. doi: 10.1369/0022155416631803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]