Abstract
Objective
Patients with multiple chronic conditions face many stressors (e.g. financial, safety, transportation stressors) that are rarely prioritized for discussion with the primary care provider (PCP). In this pilot randomized controlled trial we examined the effects of a novel technology-based intervention called Customized Care on stressor disclosure.
Methods
The main outcomes were stressor disclosure, patient confidence and activation, as assessed by self-report and observational methods (transcribed and coded audio-recordings of the office visit). Results: Sixty patients were enrolled. Compared with care as usual, intervention patients were 6 times more likely to disclose stressors to the PCP (OR = 6.16, 95% CI [1.53, 24.81], p = 0.011) and reported greater stressor disclosure confidence (exp[B] = 1.06, 95% CI [1.01, 1.12], p = 0.028). No differences were found in patient activation or the length of the office visit.
Conclusion
Customized Care improved the likelihood of stressor disclosure without affecting the length of the PCP visit.
Practice implications
Brief technology-based interventions, like Customized Care could be made available through patient portals, or on smart phones, to prime patient-PCP discussion about difficult subjects, thereby improving the patient experience and efficiency of the visit.
Keywords: Multimorbidity, Patient priorities, Primary care, Patient-PCP communication, Stressors
1. Introduction
Patients with multiple chronic medical conditions frequently report poor communication with their primary care provider (PCP) [1,2]. The complexity of managing multiple conditions combined with the time-pressures PCPs typically face in the brief encounter make effective communication difficult (in the United States the average PCP encounter is about 20 min) [3]. As a result, PCPs miss cues about patients’ context and are often unaware of patients’ safety concerns, transportation difficulties, and other pragmatic stressors known to influence health [4,5]. When PCPs fail to recognize patients’ stressors, they may devise treatment recommendations that make adherence difficult [5,6] in part because patients are unlikely to get the supportive services (e.g., reliable transportation) they need to improve their health outcomes [7–12]. Moreover, when patients feel PCPs are not interested in their underlying stressors, they may lose trust in the PCP, which can further affect patient outcomes and lead to inefficiencies such as multiple office and emergency room visits and extra tests [13–15]. For their part, PCPs are often overwhelmed with knowing how best to utilize the precious time in a short office visit [16]. More efficient and effective communication is critical, but patients and PCPs need guidance.
One commonly proposed strategy to improve care for patients with multimorbidity is to allow patient priorities to drive care [17–19]. Attending to patient priorities requires a different approach to patient-primary care provider communication, starting with what to discuss first in the medical encounter. Unfortunately, PCPs have been given little guidance on how to assess patient priorities. Moreover, the more medical and mental health conditions a patient has, the less likely the PCP is to recognize patients’ priorities [20]. When patients do discuss their priorities, it is often relegated to the end of the visit, when the physician asks, with their hand already on the doorknob, “is there anything else you wanted to discuss?” This situation, in which the patient finally reveals the underlying concern, has been referred to as the “doorknob phenomenon” [21,22]. In certain circumstances, the late disclosure can lead to an entirely different diagnosis, in others it might alter the treatment plan. In this paper, we describe the results of a pilot randomized controlled study developed to examine the effects of Customized Care, a novel technology-based intervention designed to help patients disclose their stressors. Customized Care was developed with patient and PCP input to explicitly address three key barriers to patient-centered communication, described in further detail below.
1.1. Key barriers to communication
1.1.1. Medical care has arbitrary boundaries
There is often an unspoken assumption that biomedical needs should be the primary focus of the patient visit [23,24], yet the majority of PCPs believe patient stressors are just as important to address as medical conditions [25]. The arbitrary boundaries of medical care often marginalize if not ignore patients’ life circumstances that affect their health, and make it hard for patients to appreciate that their physicians want and need to know about their life circumstances. We thus developed our intervention to help patients tell their PCPs about commonly encountered daily stressors, identified in previous focus groups with patient stakeholders [26]. By encouraging and helping patients to mention their daily stressors, we expect patients to become more confident in their ability to discuss their stressors and, overall, become more active participants in the encounter with the PCP.
1.1.2. Prioritization is not an intuitive process
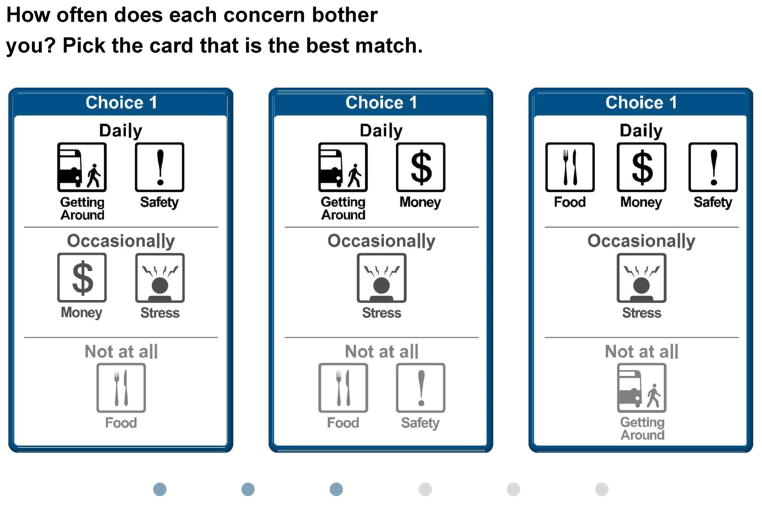
It can be extremely difficult for patients to identify which stressor, among various competing stressors, they will tell their PCP about. Indeed, recognizing which stressor ought to be prioritized does not always happen intuitively or quickly [27]. Making the process of prioritization more explicit can improve the process by which patients’ problems are elicited [28–30]. We developed our intervention to include a formalized Discussion Prioritization Tool (DPT). The computer-based DPT capitalizes on a type of preference measurement called conjoint analysis (CA). CA is a statistical technique, derived from market research and increasingly used for patient preference elicitation in health care [31–36]. It requires patients to make a series of trade-offs between competing options for treatment, or in this case, competing stressors (Fig. 1 shows an example of a CA trade-off task). In order to help patients clarify their priorities [37], we used a form of CA called adaptive best-worst CA [26,38].
Fig. 1.
Example of trade-off task shown in DPT.
1.1.3. There are substantial power-asymmetries between the patient and PCP
The third key barrier to patient-PCP communication is the historical power asymmetry between PCPs and patients, which makes it difficult for patients to set their own agenda [39]. Though PCPs are trained to elicit patients’ concerns, the primary goal of the discussion is typically to determine a diagnosis and treatment plan [40]. Because PCPs often drive the agenda in this way [41], PCPs are less likely to pay attention to the patient’s daily personal experiences and day-to-day stressors in the context of chronic disease [42]. Moreover, patients are not empowered to discuss their needs on their own terms and may fear being labeled as a ‘difficult patient’ if they do [43]. Keeping this in mind, our intervention not only primes the patient to consider common stressors but provides them with specific question prompts (Fig. 2). The question prompts were vetted by PCPs in preliminary work [26] and can help patients build their confidence to communicate their stressors. Question prompts have been shown to increase patient participation in decision making without undermining provider’s goals [44,45].
Fig. 2.
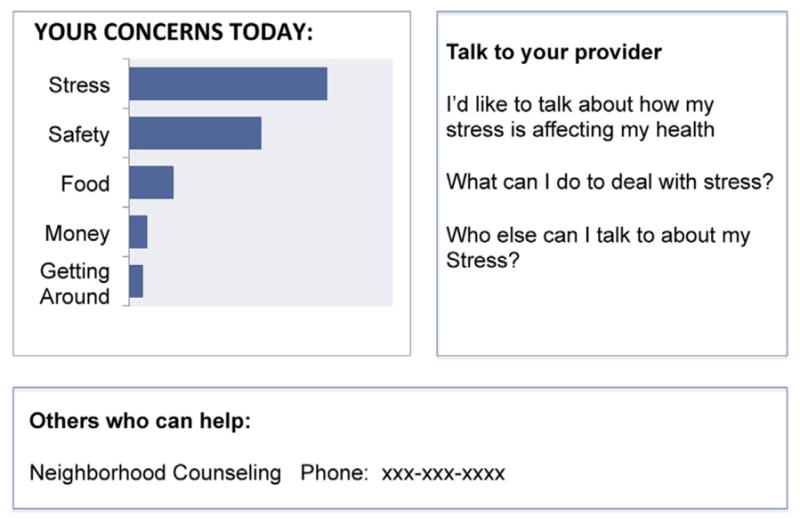
Example of QPL.
1.2. Study objectives and outcomes
We conducted a pilot, randomized controlled trial, using a pragmatic real world clinical trials approach [46], comparing Customized Care to care as usual. The main outcome was the likelihood that patients disclose their stressors in the patient-PCP visit. In addition, we assessed the following secondary outcomes: patient’s perceived confidence to disclose stressors after a PCP visit, patient activation during the visit, and the promptness with which patients disclosed their stressors in the patient-PCP visit.
2. Methods
2.1. Participant inclusion/exclusion criteria
Primary care patients and PCPs consented to participate in this study. PCP eligibility included all providers seeing patients at an urban primary care clinic that serves primarily low income patients and is operated by the University of Rochester Medical Center. Providers included physicians, as well as nurse practitioners (who are licensed to diagnose and treat independently in primary care), we excluded physicians in training (resident-physicians). Eligible patients included patients attending the clinic, age 40 and older, with diagnoses of two or more common, co-occurring chronic medical conditions (type 2 diabetes, cardiovascular disease, chronic obstructive pulmonary disease, asthma and osteoarthritis), plus depression or anxiety, as determined solely by documentation in the electronic medical record (EMR). Patient exclusion criteria included PCP-identified cognitive deficits or history of psychosis that would limit study participation, as well as inability to read and speak English.
2.2. Recruitment
In order to recruit PCPs to participate in the study, the research staff provided an overview of the study at regularly scheduled medical staff meetings. Additionally, flyers were posted in PCP areas and reminder e-mails regarding the study were sent. Interested PCPs were briefed on study procedures including audio-recording of the office visit, and were told that the study would focus broadly on patient-PCP communication among patients with multimorbidity.
Patients of consenting PCPs were recruited using an established protocol for identifying potential study participants in cooperation with the Greater Rochester Practice-Based Research Network (GR-PBRN) and approved by the University of Rochester’s Institutional Review Board. An EMR database of all consenting PCP patients who had two or more EMR-documented chronic medical conditions plus EMR-documented depression or anxiety was merged with the clinic appointment database generating a list of potentially eligible patients. PCPs were then requested to confirm eligibility and suitability of potential patients for the study (e.g., no cognitive deficits, no history of psychosis, able to read and speak English). Eligible patients were mailed a study information letter. Research staff then called eligible patients who had a scheduled, routine follow-up clinic appointment with their PCPs in the upcoming weeks. Based on these contacts, patients who were interested in the study were asked to arrive 30 min early to their appointment to meet with study staff.
2.3. Assessments and measures
Assessments occurred at three time points: 1. Immediately before a regularly scheduled office visit; 2. during the office visit (via audio-recording); and 3. immediately after the office visit. (Fig. 3).
Fig. 3.
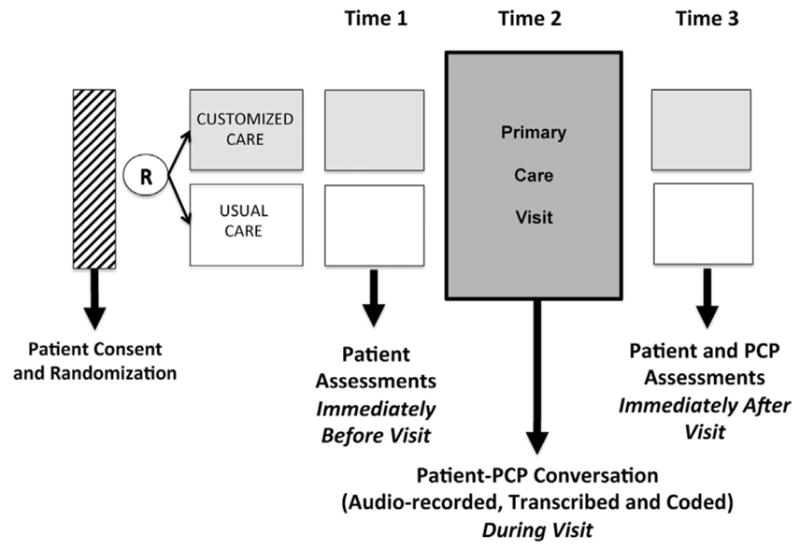
Patient and PCP Assessment Time Points.
At the first time point, before the regularly schedule office visit, patients completed a brief demographic questionnaire that included items on gender, age, education, race and ethnicity, employment, and household income. In addition, patients completed a medical conditions questionnaire that contained items to assess presence of 13 chronic medical conditions based on the Charlson comorbidity index [47].
The subsequent primary care office visit (time 2) was audio-recorded and the overall time of the office visit discussion was also documented. To assess primary patient communication outcomes, the audio-recordings were transcribed and coded using two coding schemes: the Patients’ Everyday Dilemmas (PED) Coding [26,48] and Active Patient Participation Coding (APPC) [49]. The Patients’ Everyday Dilemmas Coding System was developed by Wittink and colleagues as a way to categorize health-related stressors into three overarching content domains: Socio-emotional, safety, and health and social service challenges [26]. The APPC is a coding system developed by Street and colleagues that has been used in multiple studies [50,51]. Both coding systems code only patient utterances.
Immediately after the visit (time 3), patients completed questionnaires to assess patient confidence to disclose stressors using items adapted from the perceived competence scale [52,53]. Patients were asked to rank their confidence on a 5-point likert scale for the following 5 items: knowing which concerns are most important to me; discussing my concerns with my provider; talking with my provider about other concerns on top of my medical needs; determining what concerns are most important to me and asking my provider questions about what I can do outside of the medical office to improve my health. PCPs were asked to complete a brief demographics questionnaire (type of provider, gender, age and years in practice) at this time point.
2.4. Procedures
Upon arrival to the clinic, patients met with the research staff in the clinic waiting room and provided informed consent. Next, patients were randomized to intervention or usual care via an embedded computer program. Patients were given a brief tutorial on using a tablet computer and then completed assessments. Block randomization by PCP was used to ensure that approximately equal numbers of each participating PCP’s patients were assigned to the care as usual and intervention groups.
In the intervention arm, patients completed assessment measures and the DPT on the tablet computer. Upon completion of the DPT, a customized question prompt list was automatically generated, printed and provided to the patient to share with their PCP during their visit. Patients randomized to usual care completed measures on the tablet computer and then proceeded as usual with the primary care visit.
In both intervention and care as usual conditions, the ensuing routine patient-PCP office visit was audio-recorded. The research staff started the audio-recorder before the PCP entered the room. Audio-recorded visits were subsequently transcribed by a certified medical transcriptionist and all personal identifiers were removed. For the PED coding, two independent coders, who were unaware of the study's hypotheses and blind to treatment group, were trained in the coding method. Each coder coded all of the transcripts included in the study. Regularly scheduled consensus meetings facilitated by a research supervisor were convened to reconcile any differences in coding.
For APPC coding, two trained coders read transcripts while listening to audio-recordings of the medical encounters. Coders then categorized three types of active patient participation behaviors—questions, expressions of concern, and acts of assertiveness (e.g., stating preferences, making requests, introducing topics to discuss). Although the interactions were divided between the two coders (each taking a set), 12 (20%) of the encounters were coded by both coders. The mean ICC for the three APCC component scores was 0.87 (questions = 0.95, expressions of concern = 0.79, acts of assertiveness = 0.86). The overall ICC was 0.91%.
Immediately after the visit, patients completed items related to their perceived confidence to disclose stressors. PCP demographic information was also collected at this time. All study procedures were approved by the Institutional Review Board of the University of Rochester School of Medicine.
2.5. Analytic plan
2.5.1. Patient and provider demographics
Differences in patient characteristics at baseline between control and treatment groups were assessed with t-tests and chi-square to evaluate the randomization and identify any factors imbalanced across groups. Any factors differing between groups were then examined for the relationship with outcomes, to determine if they could be potential confounders of treatment effects and thus require control in subsequent analysis.
2.5.2. Patient likelihood to disclose stressors (primary outcome)
We used the Patient’s Everyday Dilemmas (PED) coding manual [48] scores to compare the intervention versus care as usual. Codes specifically primed by the DPT, including transportation, financial, safety, stress and/or food-related challenges, were scored as present (1) or absent (0). The impact of the DPT on likelihood of any DPT-primed code occurring was examined with a logistic regression model.
2.5.3. Confidence to disclose stressors and active patient participation (secondary outcomes)
To assess treatment group differences in patient confidence to discuss stressors, we used a Generalized Linear Model with gamma distribution and log link, as the confidence rating scores were heavily skewed. The exponentiated coefficient for the treatment group in this model reflects the ratio of confidence scores in the treatment group, over the control group; for instance, a value of 1.10 would indicate that the treatment group’s score was roughly 10% higher.
To assess group differences in patient activation, we used a Generalized Linear Model with negative binomial distribution and log link for the combined patient activation score as well as for the three different types of patient speech acts (question-asking, assertive responses, and expressions of concern), using the APPC [49]. In the case of a high proportion of participants with no reporting for a speech act, we used a zero-inflated regression.
2.5.4. Promptness of disclosure
The promptness with which patients disclosed a primed daily challenge within the visit was quantified as the text line number corresponding to the first PED code primed by the DPT (namely: transportation, financial, safety, stress or food-related challenges). Since promptness could only be measured for those who actually brought up a DPT-primed code, this outcome was examined only in those who mentioned a DPT-primed code, as described above (n = 37). Promptness, based on number of transcript lines or “speed” with which the code topic was broached, was modeled by Generalized Linear Model with gamma distribution and with log link, a classic distribution for time.
3. Results
3.1. Patient and PCP characteristics and visit length
Table 1 shows the basic characteristics of the total sample. Sixty patients were recruited and enrolled over six months for the study. Of the total patient sample, 44 (73.3%) were women and the mean (SD) age of participants was 55.4 (8.1) years. More than one half the sample were African American (n = 32 [54.2%]) and four participants (6.7%) were Hispanic. Most patients in the sample (45/60 [75.0%]) reported a household income less than $20,000 and the large majority (56/60 [93.3%]) reported a household income of less than $30,000 (the reported 2015 median household income in the U.S. was $55,775) [54]. The intervention and care as usual groups were generally well-matched, though women were over-represented in the intervention group (83.9% vs 62.1%; X2 = 3.64[1, N = 60], p = 0.06). Since gender could be plausibly related to outcomes and thus confound treatment estimates, all analyses included gender as a covariate.
Table 1.
Patient characteristics by treatment group (baseline).
| Characteristic | Total sample N = 60 (100.0%) | Intervention n = 31 (51.7%) | Care As Usual n = 29 (48.3%) | |
|---|---|---|---|---|
| Gender | Female | 44 (73.3) | 26 (83.9) | 18 (62.1) |
| Age | Mean (SD) | 55.4 (8.1) | 55.8 (7.8) | 55.0 (8.6) |
| Racea | Black/African American | 32 (54.2) | 17 (54.8) | 15 (53.6) |
| White | 18 (30.5) | 9 (29.0) | 9 (32.1) | |
| Other | 9 (15.2) | 5 (16.1) | 4 (14.2) | |
| Marital status | Now married | 14 (23.3) | 7 (22.6) | 7 (24.1) |
| Education | High school graduate or less | 31 (51.7) | 17 (54.8) | 14 (48.3) |
| Some college or more | 29 (48.3) | 14 (45.2) | 15 (51.7) | |
| Household income | <$19,999 | 45 (75.0) | 20 (64.5) | 25 (86.2) |
| >$20,000 | 15 (25.0) | 11 (35.5) | 4 (13.8) | |
| Medical conditions, number | Mean (SD) | 4.7 (1.6) | 4.9 (1.5) | 4.4 (1.7) |
Abbreviations: SD, standard deviation.
One care as usual participant did not provide data for this variable.
Patients were seen by 12 providers. All PCPs were trained in family medicine, 4 (33.3%) were NPs, 8 (66.7%) were women, and the average age was 48.7 years. Four PCPs had been in practice for 10 years or less, four had been in practice 11 years to 20 years, and four had been in practice longer than 20 years.
We found no significant difference in the overall length of the office visits by group, with an intervention mean visit time of 24.2 min (SD 7.2) vs. care as usual mean visit time of 22.9 min (SD = 7.7).
3.2. Patient likelihood to disclose stressors (primary outcome)
Patients in the intervention arm were more likely to bring up a stressor specifically primed by the intervention. In all, 37 (67.3%) participants mentioned one of the codes primed by the DPT. Of the 28 intervention group patients with transcriptions, 24 (85.7%) mentioned a primed code and of the 27 care as usual group patients, 13 (48.1%) mentioned a primed code. Results of the logistic regression model indicated the intervention group patients had approximately 6 times the odds (OR = 6.16, 95% CI [1.53, 24.81], p = 0.011) of mentioning a primed code compared with the care as usual group (Table 2).
Table 2.
Customized Care effects on outcomes.
| Outcome/Measure | Intervention M (SE) or% | Care as Usual M (SE) or% | Modela | ||
|---|---|---|---|---|---|
|
| |||||
| exp B (95% CI)b | Wald(df) | p | |||
| Discussion of primed stressors (Patient Everyday Dilemmas codes) | 85.7 | 48.1 | Logistic regression | ||
| 6.16 (1.53, 24.81) | 6.54 (1) | 0.011 | |||
| Confidence to disclose stressors | 24.7 (0.11) | 23.2 (0.58) | Gamma regression | ||
| 1.06 (1.01, 1.12) | 4.82 (1) | 0.028 | |||
| Disclosure promptness (first text line coded for primed stressors)c | 188.0 (36.5) | 341.2 (62.8) | Gamma regression | ||
| 0.55 (0.29, 1.02) | 3.56 (1) | 0.059 | |||
Abbreviations: M, mean; SE, standard error; exp B, exponentiated coefficient; CI, confidence interval; Wald, Wald chi square test; df, degrees of freedom.
‘Outcome’ as dependent variable, care as usual is reference group, gender included as covariate. Gamma regression is Generalized Linear Model with gamma distribution and log link.
The exp B for logistic regression is the odds ratio, exp B for gamma regression is the ratio of the intervention ‘outcome’ over care as usual ‘outcome.’ Values over 1 indicate intervention group is higher on outcome than control group, values below 1 indicate intervention group is lower on outcome than control group.
Conditional on presence of primed code.
3.3. Confidence to disclose stressors and patient activation (secondary outcomes)
We found evidence of treatment group differences in patient’s perceived confidence to disclose stressors. The mean confidence score after the visit for the treatment and care as usual group was 24.7 (SE = 0.11) and 23.2 (0.58), respectively. Results of the Generalized Linear Model indicated there was approximately a 6% higher confidence score for the intervention group (exp [B] = 1.06, 95% CI [1.01, 1.12], p = 0.028) than the care as usual group (Table 2).
With respect to patient activation, we found no significant differences between intervention (M = 30.6 [SE = 3.2) and care as usual (31.1 [3.4]) on the total patient activation scores as indicated by the Generalized Linear Model results (exp[B] = 0.97, 95% CI [0.57, 1.67], p = 0.92). Upon assessing the three types of speech acts independently, we did not find any differences for question-asking and assertive responses. The mean number of question-asking for the intervention was 11.4 (SE = 1.9) and for the control was 13.1 (1.8) with no significant differences found between groups in Generalized Linear Model analysis (exp[B] = 0.87, 95% CI [0.50,1.52], p = 0.63). Likewise, we found no significant differences between intervention (M = 16.9 [SE = 1.6) and care as usual (16.9 [1.9]) on the assertive responses as indicated by the Generalized Linear Model results (exp[B] = 0.99, 95% CI [0.57, 1.70], p = 0.96). For the third speech act analyzed, expressions of concern, the intervention mean was 2.3 (SE = 0.9) and the control mean was 1.1 (0.5). Due to the relatively high proportion of participants that had no expressions of concern, 32/55 (42.5%), we fit a zero-inflated negative binomial regression model. Results indicated that intervention group participants were less likely to have zero expressions of concern than the care as usual participants (B = −19.83, 95% CI [−23.70, −15.97], p <0.001). The results did not indicate a difference in number of expressions of concern by group (B = 0.18, 95% CI [−1.36, 1.72], p = 0.82).
3.4. Disclosure promptness (exploratory outcome)
Patients in the intervention were more prompt in bringing up the primed stressors evidenced by an earlier disclosure, quantified as the text-line of the first code in the transcript. Patients in the intervention had a median line number of 108 (M = 188.0, SD = 178.8) while the median line number for the usual care group was 310 (M = 341.2, SD = 226.6). Results of the Generalized Linear Model indicated there was approximately a 45% reduction in the number of text lines before the first primed stressor was coded for the intervention group (exp[B] = 0.55, 95% CI [0.29, 1.02], p = 0.059) as compared with the care as usual group (Table 2)
4. Discussion and conclusion
4.1. Discussion
Our intervention encourages patients to consider common stressors as valid topics of discussion. Customized care includes a discussion prioritization tool and question prompt list to help patients disclose their stressors to the PCP without affecting visit length. We found that patients who were exposed to the intervention were more likely to disclose their stressors and were confident in doing so. It is possible that the process of prioritization (making trade-off decisions between different stressors) led patients to spend more time thinking about the particular stressors and their relative impact on their health. Such explicit deliberation might lead patients to be more confident and capable of disclosing stressors to the PCP [29]. At the same time the effect does not appear to have made patients more activated in general: they were no more likely to ask questions, exhibit assertive responses or express concern than care as usual. Patients may need more intensive communication coaching to change the level of activation than the brief intervention provided [55]. While not statistically significant, we also found a trend towards more prompt stressor disclosure in the intervention. This finding suggests that the intervention may signal to patients that their stressors are important enough to bring up earlier rather than later in the visit.
Too frequently patients avoid discussing their priorities for fear of being labeled difficult [43] or they wait until the end of the office visit to mention an important concern, leaving little time to adequately discuss critical issues [56]. To prevent the doorknob phenomenon, interventions are needed to remove the barriers to patient-centered communication. Physician directed interventions have focused on techniques such as agenda setting [57] and early question asking [58] to avoid the doorknob phenomenon. While these techniques can be effective, other studies note that they occur infrequently in practice and may be difficult to maintain [59]. Encouraging the patient to set the agenda in a way that is unobtrusive and acceptable to physicians may be an effective adjunct to physician driven interventions.
There are several limitations to the present study. The results, as noted, were derived from a small sample of patients from one primary care clinic, and may not be generalizable to different populations of physicians and patients in different geographic locations. Furthermore, our intervention only primed patients to consider a specific set of stressors. It’s not known whether other issues might have been more difficult to prime. It is also possible, though unlikely given randomization procedures, that patients exposed to the intervention were more apt to be encouraged by the PCP to disclose stressors. Finally, it would have been useful to have asked qualitative follow-up questions to learn more about patient’s experiences with the intervention. None-the-less, the brief Customized Care intervention was successful in changing important communication outcomes.
4.2. Conclusion
While technology-based interventions hold great promise for improving the patient experience in primary care, successful adoption requires interventions to be unobtrusive and brief [56]. Customized Care improved the likelihood with which patients disclose stressors and the confidence with which they do so, without affecting the overall length of the PCP visit.
4.3. Practice implications
Primary care, at its best, focuses on the values and priorities of the patient with chronic conditions, as opposed to the disease process alone. Given that multimorbidity adds more complexity to the objectives of the primary care visit, the role of communication about patient’s priorities becomes paramount. PCPs may be reticent to bring up particularly difficult issues, (e.g. financial barriers, or end-of-life choices) for fear of offending the patient [61,62] or because they may have less knowledge about a subject (e.g. alternative and complementary treatments) than the patient [63]. Changing the conversation to include concerns that are difficult to bring up depends on procedures that trigger the conversation [55]. Technology-based tools can do so in a way that gives the patient the space and time to consider their options before talking with the PCP and these tools can prompt patients to use questions to which PCPs feel comfortable responding [59,60,64].
Brief technology-based interventions, like Customized Care, could be made available through patient portals, or on smart phones (so patients could use the tool a few days before a visit), to prime the patient-PCP discussion and potentially make the office visit more efficient. In addition, Customized Care could be adapted to a wide range of difficult conversations in primary care, including stigmatized conversations around mental or sexual health, cognitively straining decisions about what to prioritize among several treatment options, and decisions about end-of-life care. When combined with other comprehensive patient-centered practices, including supportive problem solving and increased access to community resources, interventions like Customized Care have the potential to improve patient-centered outcomes.
Acknowledgments
Funding
This work was supported by the National Institute of Mental Health (R34101236) and Leonard Salzman and Hendershot Research Development Funds, Department of Psychiatry, University of Rochester School of Medicine.
We wish to acknowledge the important contribution of Kim Van Orden, PhD for her help with early work on outcomes measures, the late Ely Dahan, PhD for his work on the early versions of the Discussion Prioritization Tool and Rachel Missel, BA and Emily Simon for their work coding the manuscripts. In addition, we wish to thank the primary care patients and providers who provided invaluable input and feedback in developing and testing the intervention.
Footnotes
Conflict of interest
There are no conflicts of interest to disclose. All study procedures were approved by the Institutional Review Board of the University of Rochester School of Medicine.
References
- 1.Jerant AF, von Friederichs-Fitzwater MM, Moore M. Patients' perceived barriers to active self-management of chronic conditions. Patient Educ Couns. 2005;57:300–307. doi: 10.1016/j.pec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Bayliss EA, Bosworth HB, Noel PH, Wolff JL, Damush TM, McIver L. Supporting self-management for patients with complex medical needs: recommendations of a working group. Chronic Illn. 2007;3:167–175. doi: 10.1177/1742395307081501. [DOI] [PubMed] [Google Scholar]
- 3.Shaw MK, Davis SA, Fleischer AB, Feldman SR. The duration of office visits in the United States, 1993 to 2010. Am J Manag Care. 2014;20:820–826. [PubMed] [Google Scholar]
- 4.Mjaaland TA, Finset A, Jensen BF, Gulbrandsen P. Physicians' responses to patients' expressions of negative emotions in hospital consultations: a video-based observational study. Patient Educ Couns. 2011;84:332–337. doi: 10.1016/j.pec.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Weiner SJ, Schwartz A. Contextual errors in medical decision making: overlooked and understudied. Acad Med. 2016;91:657–662. doi: 10.1097/ACM.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 6.Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1:189–199. [PMC free article] [PubMed] [Google Scholar]
- 7.Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 8.Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, van den Bos GA. Causes and consequences of comorbidity: a review. J Clin Epidemiol. 2001;54:661–674. doi: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- 9.Neeleman J, Ormel J, Bijl RV. The distribution of psychiatric and somatic III health: associations with personality and socioeconomic status. Psychosom Med. 2001;63:239–247. doi: 10.1097/00006842-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 10.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 11.McGaw JL. Whole patient care: reaching beyond traditional healthcare. Front Health Serv Manage. 2008;25:39–42. discussion 43, 45–46. [PubMed] [Google Scholar]
- 12.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition–multimorbidity. JAMA. 2012;307:2493–2494 307. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Street RL, Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns. 2009;74:295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Quail JM, Wolfson C, Lippman A. Unmet need and psychological distress predict emergency department visits in community-dwelling elderly women: a prospective cohort study. BMC Geriatr. 2011;11 doi: 10.1186/1471-2318-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Street RL., Jr How clinician-patient communication contributes to health improvement: modeling pathways from talk to outcome. Patient Educ Couns. 2013;92:286–291. doi: 10.1016/j.pec.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Covinsky KE. Multimorbidity, guidelines, and clinical inertia. JAMA Intern Med. 2014;174:819. doi: 10.1001/jamainternmed.2013.14406. [DOI] [PubMed] [Google Scholar]
- 17.Mangin D, Heath I, Jamoulle M. Beyond diagnosis: rising to the multimorbidity challenge. BMJ. 2012;344:e3526. doi: 10.1136/bmj.e3526. [DOI] [PubMed] [Google Scholar]
- 18.Reuben DB, Tinetti ME. Goal-oriented patient care–an alternative health outcomes paradigm. N Engl J Med. 2012;366:777–779. doi: 10.1056/NEJMp1113631. [DOI] [PubMed] [Google Scholar]
- 19.Tinetti ME, Naik AD, Dodson JA. Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9–10. doi: 10.1001/jamacardio.2015.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zulman DM, Kerr EA, Hofer TP, Heisler M, Zikmund-Fisher BJ. Patient-provider concordance in the prioritization of health conditions among hypertensive diabetes patients. J Gen Intern Med. 2010;25:408–414. doi: 10.1007/s11606-009-1232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis S. [Accessed 20.03.17];Treating the Doorknob Syndrome in Office Visits. 2017 https://www.allbusiness.com/treating-the-doorknob-syndrome-in-office-visits-4057870-1.html.
- 22.Finset A. When patients have more than one concern. Patient Educ Couns. 2016;99:671. doi: 10.1016/j.pec.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Wade DT, Halligan PW. Do biomedical models of illness make for good healthcare systems. BMJ. 2004;329:1398–1401. doi: 10.1136/bmj.329.7479.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wittink MN, Barg FK, Gallo JJ. Unwritten rules of talking to doctors about depression: integrating qualitative and quantitative methods. Ann Fam Med. 2006;4:302–309. doi: 10.1370/afm.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RWJF. Health Care's Blind Side: The Overlooked Connection Between Social Needs and Good Health. Robert Wood Johnson Foundation; 2011. [Google Scholar]
- 26.Wittink MN, Yilmaz S, Walsh P, Chapman B, Duberstein P. Customized Care: an intervention to improve communication and health outcomes in multimorbidity. Contemp Clin Trials Commun. 2016;4:214–221. doi: 10.1016/j.conctc.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai-Seale M, Stults C, Zhang W, Shumway M. Expressing uncertainty in clinical interactions between physicians and older patients: what matters? Patient Educ Couns. 2012;86:322–328. doi: 10.1016/j.pec.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaler RH, Sustein CR. Nudge Improving Decision About Health, Wealth, and Happiness, Updated Edition. Penguin; New York: 2009. [Google Scholar]
- 29.Kahneman D. Thinking, Fast and Slow. 1. Farrar, Straus and Giroux; New York: 2011. [Google Scholar]
- 30.Dowdy D, Bishai D, Chen AH. Setting clinical priorities: a framework for incorporating individual patient preferences. Patient Educ Couns. 2013;90:141–143. doi: 10.1016/j.pec.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Hawley ST. Conjoint analysis: a ‘new' way to evaluate patients' preferences. Patient. 2008;1:255–257. doi: 10.2165/01312067-200801040-00006. [DOI] [PubMed] [Google Scholar]
- 32.Halme K. Patients' preferences for generic and branded over-the-counter medicines: an adaptive conjoint analysis approach. Patient. 2009;2:243–255. doi: 10.2165/11314130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Wittink M, Tenhave T, Baron J, Gallo JJ. Towards patient-centered care for depression: conjoint methods to tailor treatment based on preferences. Patient. 2010;3:145–157. doi: 10.2165/11530660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson L, Loucks A, Bui C, Gipson G, Zhong L, Schwartzburg A, et al. Patient centered decision making: use of conjoint analysis to determine risk-benefit trade-offs for preference sensitive treatment choices. J Neurol Sci. 2014;344:80–87. doi: 10.1016/j.jns.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Jayadevappa R, Chhatre S, Gallo JJ, Wittink M, Morales KH, Malkowicz SB, et al. Treatment preference and patient centered prostate cancer care: design and rationale. Contemp Clin Trials. 2015;45:296–301. doi: 10.1016/j.cct.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Hampson LA, Allen IE, Gaither TW, Lin T, Ting J, Osterberg EC, et al. Patient-centered treatment decisions for urethral stricture: conjoint analysis improves surgical decision-making. Urology. 2017;99:246–253. doi: 10.1016/j.urology.2016.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witteman HO, Scherer LD, Gavaruzzi T, Pieterse AH, Fuhrel-Forbis A, Dansokho SC, et al. Design features of explicit values clarification methods: a systematic review. Med Decis Making. 2016;36:453–471. doi: 10.1177/0272989X15626397. [DOI] [PubMed] [Google Scholar]
- 38.Dehan E, Lambrechts S, Kaplan R, Saigal C. Treatment Preferences Derived Using Adaptive Best-Worst Conjoint (ABC) Analysis. Society for Medical Decision Making; Phoenix AZ: 2012. [Google Scholar]
- 39.Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94:291–309. doi: 10.1016/j.pec.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 40.Voigt I, Wrede J, Diederichs-Egidi H, Dierks ML, Junius-Walker U. Priority setting in general practice: health priorities of older patients differ from treatment priorities of their physicians. Croat Med J. 2010;51:483–492. doi: 10.3325/cmj.2010.51.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roter D, Hall J. Doctors Talking with Patients/Patients Talking with Doctors. Praeger Publishers; Westport, CT: 2005. [Google Scholar]
- 42.Marvel MK, Epstein RM, Flowers K, Beckman HB. Soliciting the patient's agenda: have we improved? JAMA. 1999;281:283–287. doi: 10.1001/jama.281.3.283. [DOI] [PubMed] [Google Scholar]
- 43.Frosch DL, May SG, Rendle KA, Tietbohl C, Elwyn G. Authoritarian physicians and patients' fear of being labeled ‘difficult' among key obstacles to shared decision making. Health Aff (Millwood) 2012;31:1030–1038. doi: 10.1377/hlthaff.2011.0576. [DOI] [PubMed] [Google Scholar]
- 44.Blumenthal-Barby JS. ‘That's the doctor's job': overcoming patient reluctance to be involved in medical decision making. Patient Educ Couns. 2017;100:14– 17. doi: 10.1016/j.pec.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Coulter A. Patient engagement–what works? J Ambul Care Manage. 2012;35:80–89. doi: 10.1097/JAC.0b013e318249e0fd. [DOI] [PubMed] [Google Scholar]
- 46.Helms PJ. ‘Real world' pragmatic clinical trials: what are they and what do they tell us? Pediatr Allergy Immunol. 2002;13:4–9. doi: 10.1034/j.1399-3038.2002.00194.x. [DOI] [PubMed] [Google Scholar]
- 47.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 48.Wittink M, Walsh P, Yilmaz S, Chapman B, Duberstein P. Assessing Patients' Disclosure of Day-to-day Health-related Challenges: Development of the Patients' Everday Dilemnas Coding Scheme. University of Rochester School of Medicine; 2016. [Google Scholar]
- 49.Street RL, Jr, Millay B. Analyzing patient participation in medical encounters. Health Commun. 2001:61–73. doi: 10.1207/S15327027HC1301_06. [DOI] [PubMed] [Google Scholar]
- 50.Street RL, Jr, Gordon HS, Ward MM, Krupat E, Kravitz RL. Patient participation in medical consultations: why some patietns are more involved than others. Med Care. 2005;43:960–969. doi: 10.1097/01.mlr.0000178172.40344.70. [DOI] [PubMed] [Google Scholar]
- 51.Street RL, Jr, Slee C, Kalauokalani DK, Dean DE, Tancredi DJ, Kravits RL. Improvng physician-patient communication about cancer pain with a tailored education-coaching intervention. Patient Educ Couns. 2010:42–47. doi: 10.1016/j.pec.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams GC, Deci EL. Internalization of biopsychosocial values by medical students: a test of self-determination theory. J Pers Soc Psychol. 1996;70:767–779. doi: 10.1037//0022-3514.70.4.767. [DOI] [PubMed] [Google Scholar]
- 53.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21:1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Census Bureau. Household Income: 2015. 2016 Retrieved from: https://www.census.gov/library/publications/2016/acs/acsbr15-02.html.
- 55.Alegría M, Polo A, Gao S, Santana L, Rothstein D, Jimenez A, et al. Evaluation of a patient activation and empowerment intervention in mental health care. Med Care. 2008;46:247–256. doi: 10.1097/MLR.0b013e318158af52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown RF, Butow PN, Dunn SM, Tattersall MH. Promoting patient participation and shortening cancer consultations: a randomised trial. Br J Cancer. 2001;85:1273–1279. doi: 10.1054/bjoc.2001.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frankel RM, Salyers MP, Bonfils KA, Oles SK, Matthias MS. Agenda setting in psychiatric consultations: an exploratory study. Psychiatr Rehabil J. 2013;36:195–201. doi: 10.1037/prj0000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heritage J, Robinson JD, Elliott MN, Beckett M, Wilkes M. Reducing patients' unmet concerns in primary care: the difference one word can make. J Gen Intern Med. 2007;22:1429–1433. doi: 10.1007/s11606-007-0279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson JD, Tate A, Heritage J. Agenda-setting revisited: when and how do primary-care physicians solicit patients' additional concerns? Patient Educ Couns. 2016;99:718–723. doi: 10.1016/j.pec.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Young HM, Nesbitt TS. Increasing the capacity of primary care through enabling technology. J Gen Intern Med. 2017;32:398–403. doi: 10.1007/s11606-016-3952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roelens K, Verstaelen H, Van Egmond K, Temmerman M. A knowledge, attitudes, and practice survey among obstetrician-gynaecologists on intimate partner violence in Flanders, Belgium. BMC Public Health. 2006;6:238. doi: 10.1186/1471-2458-6-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunlay SM, Foxen JL, Cole T, Feely MA, Loth AR, Strand JJ, et al. A survey of clinician attitudes and self-reported practices regarding end-of-life care in heart failure. Palliative Med. 2014:260–267. doi: 10.1177/0269216314556565. [DOI] [PubMed] [Google Scholar]
- 63.Chao MT, Handley MA, Quan J, Sarkar U, Ratanawongsa N, Schillinger D. Disclosure of complimentary health approaches among low income and racially diverse safety net patients with diabetes. Patient Educ Couns. 2015;98:1360–1366. doi: 10.1016/j.pec.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snyder CF, Wu AW, Miller RS, Jensen RE, Bantug ET, Wolff AC. The role of informatics in promoting patient-centered care. Cancer J. 2011:211–218. doi: 10.1097/PPO.0b013e318225ff89. [DOI] [PMC free article] [PubMed] [Google Scholar]