Abstract
Concepts organize our experiences and allow for meaningful inferences in novel situations. Acquiring new concepts requires extracting regularities across multiple learning experiences, a process formalized in mathematical models of learning. These models posit a computational framework that has increasingly aligned with the expanding repertoire of functions associated with the hippocampus. Here, we propose the Episodes-to-Concepts (EpCon) theoretical model of hippocampal function in concept learning and review evidence for the hippocampal computations that support concept formation including memory integration, attentional biasing, and memory-based prediction error. We focus on recent studies that have directly assessed the hippocampal role in concept learning with an innovative approach that combines computational modeling and sophisticated neuroimaging measures. Collectively, this work suggests that the hippocampus does much more than encode individual episodes; rather, it adaptively transforms initially-encoded episodic memories into organized conceptual knowledge that drives novel behavior.
Keywords: hippocampus, concept learning, episodic memory, attention, prediction error, computational modeling
Concepts define the relationships between similar objects; they represent combinations of features shared by objects of the same kind and allow us to recognize new instances of a concept when first encountered. Concepts also serve as the basis for inference about properties that have not or cannot be directly observed. To acquire a concept, we must experience multiple instances across unique episodes and learn both what features are common to concept exemplars and what features differentiate between concepts. Both of these operations, extracting commonalities across related experiences and distinctly representing similar experiences, are akin to episodic memory functions associated with the hippocampus [1–3]. In particular, the hippocampus is thought to perform pattern separation to differentiate overlapping experiences into distinct memory representations [1, 2]. Pattern separation is complemented by memory integration, in which the hippocampus is thought to encode features of the current experience along with shared information from previously encoded experiences resulting in integrated memory representations that highlight commonalities across experiences [3, 4]. In other words, what concept acquisition requires largely overlaps with coding strategies attributed to the hippocampus.
The theoretical convergence between concept formation and episodic memory posits a role for the hippocampus in acquiring concepts. While initial patient work suggested otherwise [5, 6], subsequent findings indicate that the hippocampus plays a key role in representing concepts. For example, “concept cells” in the hippocampus show high selectivity to conceptual rather than perceptual features of events [7] and a recent report report showed hippocampal lesions impair concept learning [8]. Here, we review neuroimaging research that has begun to reveal the precise hippocampal mechanisms that support concept formation and use [9–14]. The success of this research has depended on the emergence of sophisticated analytic approaches that combine mathematical accounts of psychological learning theories with representational approaches to neuroimaging. We propose the Episodes-to-Concepts (EpCon) theoretical model of concept formation in the hippocampus, which links evidence from episodic memory and category learning.
Building concepts in the hippocampus
It is well established that the hippocampus is critical for rapidly encoding and retrieving experiences to and from memory [15, 16]. However, within the past decade, theories of hippocampal function have broadened beyond memory for single episodes [17, 18] to suggest that the hippocampus plays the more general role of building flexible representations that span multiple experiences [3], are sensitive to goal states [19, 20], and guide novel decisions [21–23]. We propose that this expanded functional repertoire situates the hippocampus as an ideal site for the formation of new conceptual knowledge. Central to this proposal is the EpCon theoretical framework that details how the hippocampus transforms episodic memories to organized concepts.
EpCon is motivated by the striking parallel between hippocampal-based memory processes and a computational model of concept learning named SUSTAIN [24, 25]. SUSTAIN posits that during new learning, conceptual representations are formed through a dynamic interaction of selective attention and memory (corresponding hippocampal processes are noted in italics, each of which will be described later):
When presented with a stimulus, attention is directed to stimulus feature dimensions that are diagnostic for the task goal according to the current state of knowledge (attentional biasing).
The attention-weighted feature information then promotes retrieval of similar prior learning experiences (pattern completion). These memories are used to predict a concept label.
Depending on the prediction outcome (memory-based prediction error), a new distinct memory is created that binds together the current stimulus and the correct concept (pattern separation), and/or an existing concept representation is updated to incorporate the new stimulus (integration). This updated knowledge state then influences attentional strategy on subsequent learning experiences.
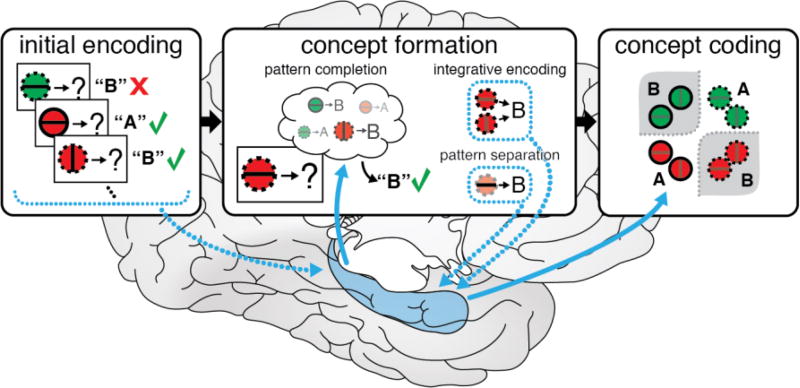
As learning continues, this process iterates. Pattern completion retrieves previously integrated representations that highlight the common features diagnostic of the concept, which, in turn are updated with new information from the current experience. Irrelevant features are dropped from concept representations, and concept exemplars are organized according to their similarity on the most relevant features, with the most typical exemplars taking a central position in representational space. By learning what features are common to concept exemplars and what features differentiate between concepts, this adaptive process transforms initially-encoded episodic memories into organized conceptual knowledge representations (Figure 1).
Figure 1.
The Episodes-to-Concepts (EpCon) theoretical model of concept formation in the hippocampus. Initially, each new learning experience consisting of stimulus features (e.g., dotted outline, red fill, and vertical center) and concept label (e.g., B) is encoded as a distinct memory (dotted blue lines represent hippocampal encoding). After encoding these initial experiences, memory integration processes soon dominate learning: Pattern completion processes retrieve related memories (solid blue lines depict hippocampal retrieval) that are used to predict a concept label. Feedback then leads to integration across experiences (e.g., red items with dotted outlines are associated with concept B) and/or distinct representation of the current experience through pattern separation. Concept formation continues as learning progresses, with more complex integrated representations that span experiences retrieved through pattern completion and encoded through memory integration. This adaptive process culminates in conceptual coding in which the learned integrated representations capture the structure of the concept. Brain illustration by Margaret Schlichting.
The component processes of this theoretical framework for concept learning map onto the hippocampal functions of pattern separation and completion, memory integration, and memory-based prediction error, and the framework is further influenced by the fact that hippocampal encoding is biased by attention. The EpCon model is thus a theoretical bridge between SUSTAIN’s formalism of concept learning and the functions of the hippocampus. It is important to note that concept learning is supported by many brain regions (see [26] for a recent review); EpCon serves to highlight how the hippocampus is an important player in concept learning’s broader neural substrate. Below, we review the evidence for EpCon by highlighting the complementary hippocampal functions that are implicated in concept formation.
Memory integration
Memory integration arises when the current experience shares features with previously-encoded experiences, which may trigger hippocampal pattern completion resulting in the retrieval of related memories. The current experience may then be encoded into the reactivated memory trace, resulting in an updated representation that captures both the features of the current experience as well as those of the retrieved memory [1, 3, 27, 28]. A wave of recent findings has converged on the existence of such integrated representations in the hippocampus that support complex inference behaviors [29–34].
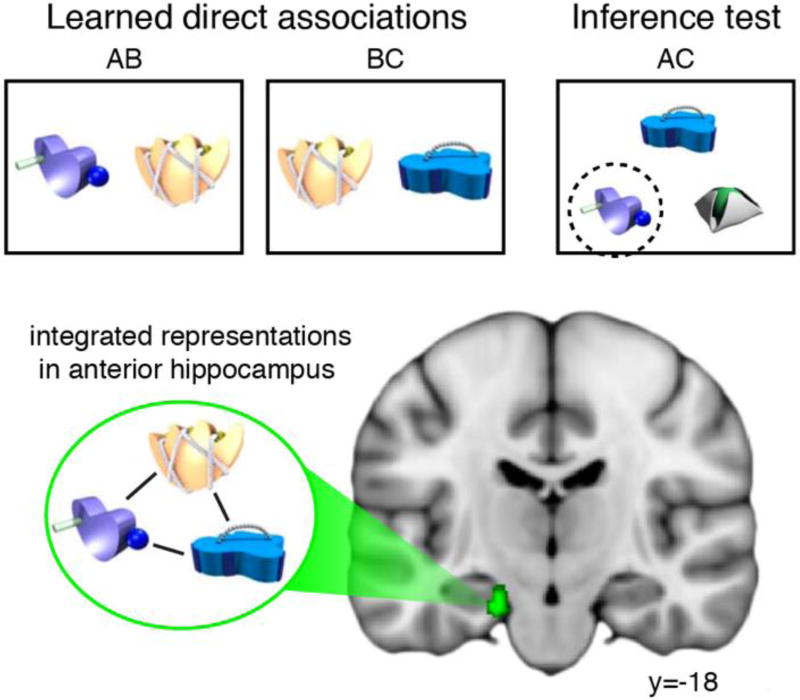
In particular, one recent human fMRI study by Schlichting and colleagues [32] targeted the specific nature of integrated representations in the hippocampus. In this study, participants learned pairs of novel objects that shared one common object (AB and BC) before making inference judgments about the objects indirectly linked by the shared object (AC; Figure 2). Critically, participants viewed each object before and after learning, allowing investigation of learning-related changes in the neural representations for the A and C items with representational similarity analysis (RSA). This analysis revealed that neural patterns in anterior hippocampus for indirectly-associated A and C items showed greater similarity after learning (Figure 2). By quantifying the learning-related changes at the level of individual elements of episodes (i.e., A and C objects), these findings provide compelling evidence for memory integration during encoding. Although these findings are limited to memory representations formed for overlapping experiences, it follows that integrative encoding mechanisms characterized in this study likely underlie the formation of more complex representations including multi-step chains of associations [35]. Importantly, this work characterizes how individual learning experiences can be extended and shaped to include features from related experiences, a process that is fundamental to the formation of new concepts [24].
Figure 2.
Associative inference paradigm and RSA results from Schlichting et al. [32]. Participants learned direct associations (AB and BC) before being tested on an indirect inference (AC). Participants were cued with a C object and selected the indirectly associated A object (circled object). RSA measures showed evidence of integrated representations (i.e., increased similarity between A and C objects post- versus pre-learning) in left anterior hippocampus. Figure adapted from [32].
Memory-based prediction error
Errors are critical to learning concepts; whether generated through internal evaluation or surprise or provided by external feedback, models of concept learning leverage prediction errors and mismatch signaling to guide how prior knowledge is updated with new information [24]. The importance of error signals to learning is paralleled in memory theories that suggest that the hippocampus, in particular subregion CA1, serves as a comparator that detects when new experiences deviate from memory-based expectations [1, 36]. Indeed, both rodent [37] and human [31, 38–40] work has implicated CA1 in signaling novelty, mismatch, or errors. This memory-based prediction error extends to expectations derived from conceptual knowledge [41]. For example, anterior hippocampus engagement is greater during encoding of conceptually-novel word pairs (e.g. “purple banana”) that are later remembered [42].
Hippocampal prediction error signals are thought to trigger encoding processes that lead to pattern separation, in the case of large errors, or forge integrative links between the current experience and prior memory, in the case of smaller errors [3, 43, 44]. Recent rodent work has shown increased CA1 activity and plasticity in the presence of novelty [37]. Such novelty-related encoding would lead to binding of activity patterns that reflect not only perceptually-available content, but also reactivated memory content leading to integrated representations. In humans, CA1 mismatch signaling during encoding of overlapping experiences has been shown to predict subsequent success in inferring relationships between indirectly-related memory elements [31]. Importantly, such CA1 mismatch signaling increases across repetitions of overlapping, but not non-overlapping pairs, consistent with memory-based prediction error [40].
One recent study, in particular, examined memory-based prediction error in the hippocampus during concept learning [11]. In this study, participants learned to categorize visual objects into categories based on a combination of feature dimensions. Learning performance was quantified with SUSTAIN [24] to derive trial-by-trial predictions of decision uncertainty, a latent signal that can trigger encoding of new information with existing knowledge [36]. This model-based uncertainty measure correlated with anterior hippocampus engagement throughout learning. These findings suggest that the hippocampus signals more than novelty, rather it indicates the degree that current experience deviates from existing conceptual knowledge.
Attentional biasing
Models of concept learning posit that selective attention is a key mechanism that shapes representations during learning by biasing encoding to concept-relevant features and ignoring irrelevant dimensions [24]. A similar view of attention is found in theoretical accounts of memory, whereby top-down attention biases hippocampal encoding and retrieval according to current goal states [45, 46]. That is, attention is not a hippocampal computation per se, but rather acts to impact hippocampal function. Neural evidence of attention’s influence on hippocampal engagement has been mixed [47, 48]; rather, attention may act on representations in activity patterns across the hippocampus. In the rodent hippocampus, the same environment is remapped to different spatial codes depending on what features matter for the animal’s current goal state [49, 50]. Specifically, when presented with an odor-based cue for a food reward that varied in location trial-to-trial, hippocampal place cells dynamically reconfigured to represent the location of the rewarded odor on every trial [49]. These findings suggest attention rapidly influences the information encoded in hippocampal representations.
Two recent human fMRI studies [19, 20] have demonstrated that hippocampal representations are shaped by different tasks that require distinct attentional strategies. In these studies, visual search of room images for a style of wall art evoked distinct hippocampal patterns relative to searching the same room for a specific room layout. Critically, this remapping due to attentional state was tied to memory behavior: Task-relevant information was better remembered when the hippocampus was in a task-specific encoding state [20]. These findings offer compelling evidence that attention enhances encoding and retrieval of distinct hippocampal representations. Although this work only tested the contribution of attention to memory processes, it is clear that attentional strategy can bias hippocampal coding and motivates the notion that similar attention-hippocampus interactions are at play during concept learning.
Directly relating hippocampal function to concept formation with model-based fMRI
Several recent studies have directly tested the parallels between formal computational models of concept learning and hippocampal representation of concepts using model-based fMRI. These studies motivate the EpCon model by demonstrating the links between the hippocampal mechanisms reviewed above (memory integration, memory-based prediction error, and attentional biasing) and concept formation.
Davis, Love, and Preston, 2012
Davis and colleagues [12] tested the hypothesis that the hippocampus dynamically recruits and shapes representations during concept learning. They explored this hypothesis with rule-plus-exception category learning in which multidimensional visual stimuli were mapped onto categories according to a unidimensional rule with the exception of two items that violated the rule.
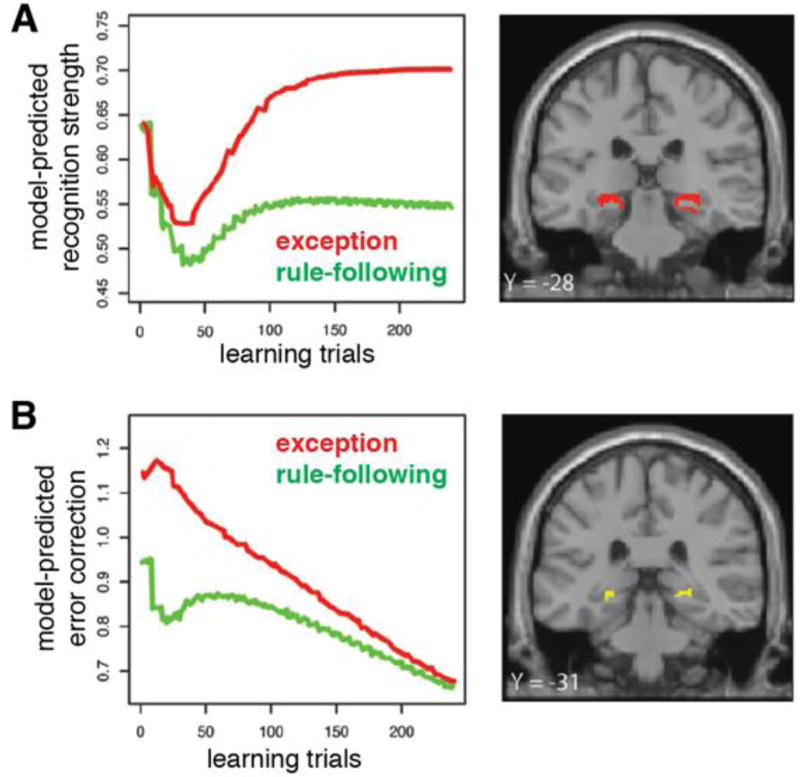
Davis et al. derived quantitative predictions for hippocampal engagement throughout learning with SUSTAIN. According to SUSTAIN, exception items require the formation of distinct representations that distinguish exceptions from rule-following items, whereas rule-following items are supported by abstracted representations that capture their average features through a process of integration. Davis et al. proposed a hippocampal role in representing both exception and rule-following items and predicted that during learning, hippocampal activation would track recognition strength, a model measure that indicates the extent that a test item activates SUSTAIN’s category representations. In the rule-plus-exception task, SUSTAIN’s recognition strength is characterized by two aspects (Figure 3A): 1) It increases over learning as stored category representations are updated to better represent the structure of the learning task, and 2) exception items are supported by distinct representations that show greater recognition strength than rule-following items. These trial-by-trial predictions of recognition strength were directly incorporated into fMRI analyses as parametric regressors. As predicted, activation throughout learning in the hippocampal body and tail significantly tracked the recognition strength predictions (Figure 3A). In other words, how SUSTAIN’s flexible category representations are differentially informative to rule-following and exception items throughout learning was reflected in hippocampal engagement.
Figure 3.
SUSTAIN-based measures of concept formation during a rule-plus-exception category learning task [12] and corresponding statistical maps of the hippocampus. A) Recognition strength varies across learning trials and is greater for exception (red) versus rule-following (green) items. Trial-by-trial activation in bilateral hippocampus (red regions) correlated with recognition strength. B) Error correction correlated with activation in bilateral hippocampus (yellow regions) during learning trial feedback. Figure adapted from [12].
Davis et al. also investigated memory-based prediction error during feedback. Specifically, they derived a model measure, error correction, that indicated the difference between SUSTAIN’s predicted category and the actual category. Error correction serves the important role of dictating how much category representations should be updated after each trial. Much like recognition strength, error correction changes over learning and differs between rule-following and exception items (Figure 3B). By including trial-by-trial predictions of error correction as parametric regressors, Davis et al. found that feedback-related activity in posterior hippocampus tracked this measure of memory-based prediction error signaling (Figure 3B).
The Davis et al. study offers a direct argument for hippocampal involvement in concept formation. A key prediction of SUSTAIN is that during learning, representations are flexibly adapted to capture the nature of new concepts. And, a rule-plus-exception paradigm provides a strong test of this representational flexibility, with distinct item-specific representations supporting exceptions and abstracted prototype-like representations capturing rules [13]. The Davis et al. findings suggest that such representations are formed in the hippocampus: Rule-following representations emerge throughout learning by integrating over overlapping experiences and distinct exception item representations result from pattern separation.
Mack, Love, and Preston, 2016
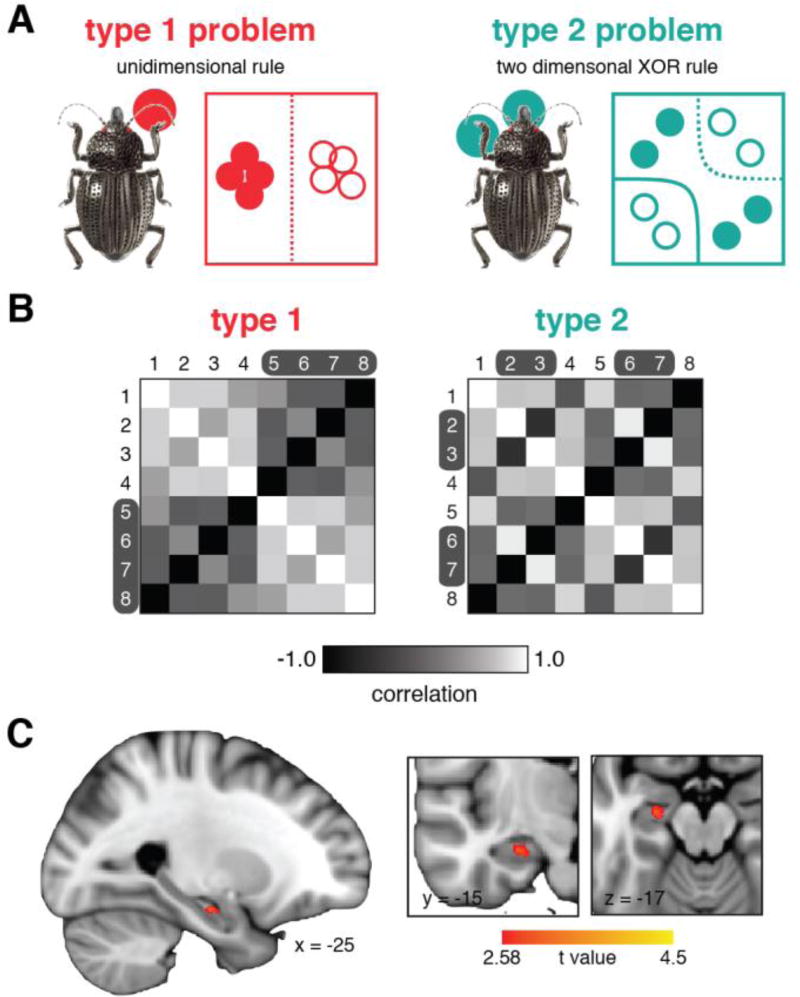
Conceptual knowledge supports flexible adaptation to different learning goals. Mack et al. [14] asked how conceptual representations of visual objects in the hippocampus are flexibly encoded to reflect changing goal states. In this study, participants first learned to categorize a set of multidimensional objects into one of two categories before learning to categorize the same set of objects in a new, orthogonal category structure. The two learning problems were defined by a unidimensional rule and a two-dimensional XOR rule with each problem relying on distinct stimulus dimensions (Figure 4A). This paradigm, therefore, required participants to change attentional strategies between problems to form new conceptual representations that best supported the changing learning goals.
Figure 4.
Mack et al. [14] learning problem schematics, model predictions, and corresponding neural results. A) Participants learned to classify the same set of multidimensional objects (beetles with different legs, antennae, and mandibles) according to two different learning problems. B) SUSTAIN-based predictions of the similarity between object representations in the two problems. Lighter cells correspond to higher similarity. C) Neural representations in left anterior hippocampus corresponded with the conceptual reorganization between learning problems as predicted by SUSTAIN. Figure adapted from [14].
Mack et al. leveraged the quantitative predictions of SUSTAIN to perform a model-based analysis of fMRI data recorded during the two learning problems. Specifically, participant-specific model parameter estimates were used to quantify the nature of the object representations learned within the context of the changing problems. This was accomplished by using the fitted model to predict how similar each pair of objects were within each learning problem. It was expected that the same two items could be similar or different depending on the learning problem, and even that items in the same category could be highly dissimilar depending on the learned attentional biases and conceptual representations. The resulting similarity matrices (Figure 4B) demonstrated that the model predicted very different underlying conceptual representations across the problems even though the same visual objects were present in both problems.
The key question posed by Mack et al. [14] was if SUSTAIN’s prediction of conceptual reorganization across the two learning problems was evident in neural representations in the hippocampus. To answer this question, they performed model-based RSA to compare the neural similarity of hippocampal activation patterns for all pairs of visual objects for each learning problem, resulting in problem-specific neural similarity matrices. If hippocampal representations reorganize in the face of changing learning goals, these neural similarity matrices should correspond with the model-based similarity matrices. This is exactly what was found; anterior hippocampus showed a reorganization in neural representations across the learning problems that matched SUSTAIN’s concept reorganization (Figure 4C). These findings demonstrate that as goals change and new concepts must be learned, hippocampal representations reorganize in concert with changing attentional strategy to reflect the relevant information for the current goal.
It is important to note that these highlighted studies [12, 14] were possible only by leveraging the quantitative predictions of how conceptual representations are formed and organized by learning as formalized in a computational model. The predictive power of this approach stems from a comprehensive mechanistic account of concept learning that combines the computations of selective attention, memory-based prediction error, and memory integration. By leveraging computational models, the latent processes and representations of psychological learning theory can be linked to the neural substrate of concept formation.
A role for anterior hippocampus?
Notably, the work reviewed here implicates anterior hippocampus in concept formation. Not only has this region been shown to form integrated neural codes that capture commonalities across individual experiences [14, 31, 32], it has also been associated with uncertainty during concept learning [11]. Relatedly, more complex memory functions that rely on integrating and organizing prior experiences such as autobiographical memory [51], schematic representation [52], and imagining the future [53, 54] have been distinctly associated with anterior hippocampus. Anatomically, anterior hippocampus is well suited for the operations that mediate concept formation. Place fields in anterior hippocampus have broad receptive fields [55], potentially allowing for representations that generalize across episodes and behavioral relevance [56]. Anterior hippocampus also has anatomical connections to anterior temporal and medial prefrontal cortices [57], areas that may be involved in the retrieval of previously-learned conceptual/schematic information during new learning [56]. Although future studies are needed to fully characterize the functional properties of anterior hippocampus, and how they differ from posterior hippocampus, the current evidence suggests it may play an important role in concept formation.
Conclusion
The research on concept learning and related processes reviewed here is in line with other recent work suggesting that the hippocampus plays a much broader role in cognition that originally thought [17, 18]. The hippocampus seems to be the brain’s integrative code builder, binding together elements that share spatial, temporal, or conceptual features to form relational codes that capture the commonalities and organization of our experiences. The hippocampus, of course, is not the only region implicated in concept formation. An important question is how the hippocampus interacts with other brain regions to support the acquisition of knowledge from individual episodes both immediately during learning [14] and over time through consolidation [58, 59]. The goal of the EpCon model discussed here, however, is to bridge an influential set of computational and neurobiological theories of learning and memory [1–3, 24, 25, 36], most notably SUSTAIN [24] and its neural framework [14, 25]. In doing so, EpCon provides a means to isolate the computations the hippocampus performs not only in the service of concept learning, but cognition more generally.
Highlights.
The hippocampus integrates across experiences to support complex behaviors.
Activation patterns in the hippocampus are influenced by selective attention.
These hippocampal processes align with formal accounts of concept learning.
Recent fMRI evidence supports a role for the hippocampus in concept formation.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council [Discovery Grant to M.L.M]; National Institute of Mental Health [F32-MH100904 to M.L.M; R01-MH100121 to A.R.P]; the Leverhulme Trust [RPG-2014-075 to B.C.L]; the Wellcome Trust [Senior Investigator Award WT106931MA to B.C.L]; and the National Institute of Child Health and Human Development [1P01HD080679 to B.C.L].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Reilly RC, Rudy JW. Conjunctive Representations in Learning and Memory: Principles of Cortical and Hippocampal Function. Psychol. Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- 2.Norman K, O’Reilly R. Modeling Hippocampal and Neocortical Contributions to Recognition Memory: A Complementary-Learning-Systems Approach. Psychol. Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 3.Schlichting ML, Preston AR. Memory integration: Neural mechanisms and implications for behavior. Curr. Opin. Behav. Sci. 2015;1:1–8. doi: 10.1016/j.cobeha.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schapiro AC, Turk-Browne NB, Botvinick MM, Norman KA. Complementary learning systems within the hippocampus: a neural network modelling approach to reconciling episodic memory with statistical learning. Philos. Trans. R. Soc. B Biol. Sci. 2017;372:20160049. doi: 10.1098/rstb.2016.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowlton BJ, Squire LR. The learning of categories: parallel brain systems for item memory and category knowledge. Science. 1993;262:1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- 6.Squire LR, Knowlton BJ. Learning about categories in the absence of memory. Proc. Natl. Acad. Sci. U. S. A. 1995;92:12470–12474. doi: 10.1073/pnas.92.26.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quiroga RQ, Kreiman G, Koch C, Fried I. Sparse but not “Grandmother-cell” coding in the medial temporal lobe. Trends Cogn. Sci. 2008;12:87–91. doi: 10.1016/j.tics.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal A, Duke D, Bowles B, Gilboa A, Rosenbaum RS, Köhler S, McRae K. Abnormal semantic knowledge in a case of developmental amnesia. Neuropsychologia. 2017;102:237–247. doi: 10.1016/j.neuropsychologia.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the Emergence of Conceptual Knowledge during Human Decision Making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schapiro AC, Kustner LV, Turk-Browne NB. Shaping of object representations in the human medial temporal lobe based on temporal regularities. Curr. Biol. 2012;22:1622–1627. doi: 10.1016/j.cub.2012.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis T, Love BC, Preston AR. Striatal and hippocampal entropy and recognition signals in category learning: Simultaneous processes revealed by model-based fMRI. J. Exp. Psychol. Learn. Mem. Cogn. 2012;38:821–839. doi: 10.1037/a0027865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis T, Love BC, Preston AR. Learning the exception to the rule: Model-based fMRI reveals specialized representations for surprising category members. Cereb. Cortex. 2012;22:260–273. doi: 10.1093/cercor/bhr036. [DOI] [PubMed] [Google Scholar]
- 13.Davis T, Xue G, Love BC, Preston AR, Poldrack RA. Global neural pattern similarity as a common basis for categorization and recognition memory. J. Neurosci. 2014;34:7472–84. doi: 10.1523/JNEUROSCI.3376-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack ML, Love BC, Preston AR. Dynamic updating of hippocampal object representations reflects new conceptual knowledge. Proc. Natl. Acad. Sci. 2016;113:13203–13208. doi: 10.1073/pnas.1614048113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Shohamy D, Turk-Browne NB. Mechanisms for widespread hippocampal involvement in cognition. J. Exp. Psychol. Gen. 2013;142:1159–70. doi: 10.1037/a0034461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eichenbaum H, Cohen NJ. Can We Reconcile the Declarative Memory and Spatial Navigation Views on Hippocampal Function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aly M, Turk-Browne NB. Attention Stabilizes Representations in the Human Hippocampus. Cereb. Cortex. 2015;26:bhv041. doi: 10.1093/cercor/bhv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aly M, Turk-Browne NB. Attention promotes episodic encoding by stabilizing hippocampal representations. Proc. Natl. Acad. Sci. 2016;113:420–429. doi: 10.1073/pnas.1518931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutishauser U, Ye S, Koroma M, Tudusciuc O, Ross IB, Chung JM, Mamelak AN. Representation of retrieval confidence by single neurons in the human medial temporal lobe. Nat. Neurosci. 2015:1–12. doi: 10.1038/nn.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wimber M, Alink A, Charest I, Kriegeskorte N, Anderson MC. Retrieval Induces Adaptive Forgetting of Competing Memories via Cortical Pattern Suppression. Nat. Publ. Gr. 2015;18:582–589. doi: 10.1038/nn.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack ML, Preston AR. Decisions about the past are guided by reinstatement of specific memories in the hippocampus and perirhinal cortex. Neuroimage. 2016;127:144–157. doi: 10.1016/j.neuroimage.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love BC, Medin D, Gureckis TM. SUSTAIN: A network model of category learning. Psychol. Rev. 2004;111:309–332. doi: 10.1037/0033-295X.111.2.309. [DOI] [PubMed] [Google Scholar]
- 25.Love BC, Gureckis TM. Models in search of a brain. Cogn. Affect. Behav. Neurosci. 2007;7:90–108. doi: 10.3758/cabn.7.2.90. [DOI] [PubMed] [Google Scholar]
- 26.Seger CA, Peterson EJ. Categorization=decision making+generalization. Neurosci. Biobehav. Rev. 2013;37:1187–1200. doi: 10.1016/j.neubiorev.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer AC, Karlsson MP, Nathe AR, Carr MF, Frank LM. Experience-Dependent Development of Coordinated Hippocampal Spatial Activity Representing the Similarity of Related Locations. J. Neurosci. 2010;30:11586–11604. doi: 10.1523/JNEUROSCI.0926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenzie S, Frank AJ, Kinsky NR, Porter B, Rivière PD, Eichenbaum H. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 2014;83:202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeithamova D. Flexible Memories: Differential Roles for Medial Temporal Lobe and Prefrontal Cortex in Cross-Episode Binding. J. Neurosci. 2010 doi: 10.1523/JNEUROSCI.3250-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeithamova D, Dominick AL, Preston AR. Hippocampal and Ventral Medial Prefrontal Activation during Retrieval-Mediated Learning Supports Novel Inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlichting ML, Zeithamova D, Preston AR. CA1 subfield contributions to memory integration and inference. Hippocampus. 2014;1260:1248–1260. doi: 10.1002/hipo.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlichting ML, Mumford JA, Preston AR. Learning-related representational changes reveal dissociable integration and separation signatures in the hippocampus and prefrontal cortex. Nat. Commun. 2015;6:8151. doi: 10.1038/ncomms9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collin SHP, Milivojevic B, Doeller CF. Memory hierarchies map onto the hippocampal long axis in humans. Nat. Neurosci. 2015;18:1562–1564. doi: 10.1038/nn.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berens SC, Bird CM. The role of the hippocampus in generalizing configural relationships. Hippocampus. 2017;27:223–228. doi: 10.1002/hipo.22688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horner AJ, Bisby Ja, Bush D, Lin W-J, Burgess N. Evidence for holistic episodic recollection via hippocampal pattern completion. Nat. Commun. 2015;6:7462. doi: 10.1038/ncomms8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Larkin MC, Lykken C, Tye LD, Wickelgren JG, Frank LM. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus. 2014;24:773–783. doi: 10.1002/hipo.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: hippocampal retrieval success and CA1 mismatch detection. Learn. Mem. 2011;18:523–528. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: A high-resolution fMRI study of the human hippocampus. Hippocampus. 2012;22:389–398. doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeithamova D, Manthuruthil C, Preston AR. Repetition suppression in the medial temporal lobe and midbrain is altered by event overlap. Hippocampus. 2016;26:1464–1477. doi: 10.1002/hipo.22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poppenk J, Walia G, McIntosh AR, Joanisse MF, Klein D, Köhler S. Why is the meaning of a sentence better remembered than its form? An fMRI study on the role of novelty-encoding processes. Hippocampus. 2008;18:909–918. doi: 10.1002/hipo.20453. [DOI] [PubMed] [Google Scholar]
- 42.Reggev N, Bein O, Maril A. Distinct Neural Suppression and Encoding Effects for Conceptual Novelty and Familiarity. J. Cogn. Neurosci. 2016;28:1455–1470. doi: 10.1162/jocn_a_00994. [DOI] [PubMed] [Google Scholar]
- 43.Shohamy D, Wagner AD. Integrating Memories in the Human Brain: Hippocampal-Midbrain Encoding of Overlapping Events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Kesteren MTR, Ruiter DJ, Fernández G, Henson RN. How schema and novelty augment memory formation. Trends Neurosci. 2012;35:211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Chun MM, Turk-Browne NB. Interactions between attention and memory. Curr. Opin. Neurobiol. 2007;17:177–184. doi: 10.1016/j.conb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Hardt O, Nadel L. Cognitive maps and attention. Prog. Brain Res. 2009;176:181–194. doi: 10.1016/S0079-6123(09)17610-0. [DOI] [PubMed] [Google Scholar]
- 47.Uncapher MR, Rugg MD. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. J. Neurosci. 2009;29:8270–8279. doi: 10.1523/JNEUROSCI.1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dudukovic NM, Preston AR, Archie JJ, Glover GH, Wagner AD. High-resolution fMRI reveals match enhancement and attentional modulation in the human medial temporal lobe. J. Cogn. Neurosci. 2011;23:670–82. doi: 10.1162/jocn.2010.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muzzio IA, Kentros C, Kandel E. What is remembered? Role of attention on the encoding and retrieval of hippocampal representations. J. Physiol. 2009;587:2837–2854. doi: 10.1113/jphysiol.2009.172445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenton AA, Lytton WW, Barry JM, Lenck-Santini P-P, Zinyuk LE, Kubík S, Bures J, Poucet B, Muller RU, Olypher AV. Attention-like modulation of hippocampus place cell discharge. J. Neurosci. 2010;30:4613–4625. doi: 10.1523/JNEUROSCI.5576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheldon S, McAndrews MP, Pruessner J, Moscovitch M. Dissociating patterns of anterior and posterior hippocampal activity and connectivity during distinct forms of category fluency. Neuropsychologia. 2016;90:148–158. doi: 10.1016/j.neuropsychologia.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 52.Gutchess AH, Schacter DL. The neural correlates of gist-based true and false recognition. Neuroimage. 2012;59:3418–3426. doi: 10.1016/j.neuroimage.2011.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The Future of Memory: Remembering, Imagining, and the Brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat. Rev. Neurosci. 2016;17:173–182. doi: 10.1038/nrn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strange Ba, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 56.Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- 58.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 59.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013;23:R764–73. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]