Abstract
Macrophages are common targets for infection and innate immune activation by many pathogenic viruses including the neurotropic Theiler’s Murine Encephalomyelitis Virus (TMEV). As both infection and innate activation of macrophages are key determinants of viral pathogenesis especially in the central nervous system (CNS), an analysis of macrophage growth factors on these events was performed. C3H mouse bone-marrow cells were differentiated in culture using either recombinant macrophage colony stimulating factor (M-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF), inoculated with TMEV (BeAn) and analyzed at various times thereafter. Cytokine RNA and protein analysis, virus titers, and flow cytometry were performed to characterize virological parameters under these culture conditions. GM-CSF-differentiated macrophages showed higher levels of TMEV viral RNA and proinflammatory molecules compared to infected M-CSF -differentiated cells. Thus, GM-CSF increases both TMEV infection and TMEV-induced activation of macrophages compared to that seen with M-CSF. Moreover, while infectious viral particles decreased from a peak at 12 hours to undetectable levels at 48 hours post infection, TMEV viral RNA remained higher in GM-CSF- compared to M-CSF-differentiated macrophages in concert with increased proinflammatory gene expression. Analysis of a possible basis for these differences determined that glycolytic rates contributed to heightened virus replication and proinflammatory cytokine secretion in GM-CSF compared to M-CSF-differentiated macrophages. In conclusion, we provide evidence implicating a role for GM-CSF in promoting virus replication and proinflammatory cytokine expression in macrophages, indicating that GM-CSF may be a key factor for TMEV infection and the induction of chronic TMEV-induced immunopathogenesis in the CNS.
Keywords: Theiler’s Murine Encephalomyelitis Virus, GM-CSF, M-CSF, macrophage, cytokines
Graphical abstract
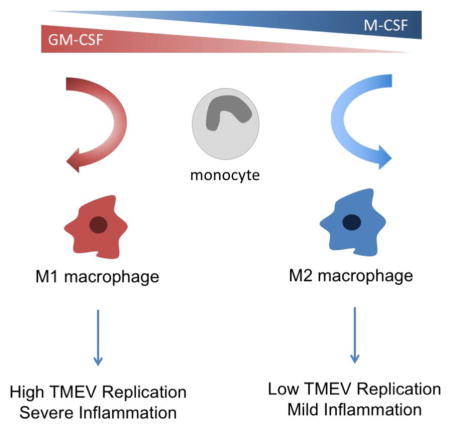
1. INTRODUCTION
Bone-marrow derived monocytes/macrophages are targets for infection and replication by viruses such as Human Immunodeficiency virus (HIV) [9, 12, 35], Dengue [50, 59], Ebola [16], RSV, Influenza A and Theiler’s Murine Encephalomyelitis (TMEV) [42, 54]. Macrophages are composed of very heterogeneous and plastic populations, whose differentiation from monocytes is driven primarily by two macrophage growth factors: macrophage-colony stimulating factor (M-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) [51, 55, 62]. The state of macrophage differentiation at the time of virus infection is important, as virus interactions with different macrophage subpopulations can result in alternative disease outcomes [69].
M-CSF and GM-CSF exert different influences on phenotype and function of macrophages [55]. Macrophages differentiated with these factors are relatively quiescent until triggered by appropriate microbe-derived ligands (pathogen associated molecular patterns, PAMPs) that stimulate various pathogen recognition receptors (PRRs) expressed on macrophages [4]. For instance, M-CSF has been shown to prime macrophages to respond to PAMPs with an anti-inflammatory profile including IL-10 secretion [23]. In contrast, GM-CSF primes monocytes to respond with an inflammatory macrophage phenotype [23, 32]. Inflammatory macrophages express proinflammatory cytokines such as IL-12, IL-23, TNF-α, IL-1α, IL-1β, and CCL2 [13, 46, 47, 70] when exposed to various PAMPs [61]. In addition to proinflammatory cytokine production in macrophages, GM-CSF increases rates of nitric oxide production, phagocytosis, antigen presentation, cell survival, and proliferation in response to PAMPs [6]. Based on these differences, studies have shown that GM-CSF and M-CSF differentiated macrophages can shape the outcome of inflammation-driven diseases by affecting the polarization states of macrophages [8, 32, 34, 52].
Macrophages have been shown to play an important role in virus-induced CNS disease [1, 19, 74]. Monocytes infected in the periphery are able to cross the blood brain barrier by the “Trojan horse” pathway, and differentiate into macrophages once in the tissue under the influence of local factors including macrophage growth factors, cytokines and microbial materials. Of particular relevance to the present studies, both M-CSF and GM-CSF are produced within the CNS parenchyma during CNS virus infection by microglia and astrocytes respectively [27, 57]. Moreover, GM-CSF produced by infiltrating T cells provides an additional stimulus to monocyte differentiation in the CNS [20, 64, 67]. Under these influences, monocyte-derived macrophages produce a distinct array of proinflammatory cytokines, chemokines and toxic molecules that either promote anti-microbial immunity or, if dysregulated, damage CNS tissue [38].
The neurotropic virus, TMEV, is a murine single-stranded RNA picornavirus. Attenuated forms of the virus are used to study biphasic demyelinating disease as a model for the human demyelinating disease, multiple sclerosis [17, 71]. In the TMEV model, viral RNA copies have been detected in high numbers in the spinal cord up to six months after infection despite the lack of infectious virions, indicating a propensity for the persistence of TMEV genomes in the CNS [41, 53, 74]. An explanation for the disparity between viral RNA and infectious virus has not yet been elucidated during TMEV persistence [25], but chronically elevated levels of viral RNA represent a source of persistent innate immune activation and potential reactivation of viral production. Previous studies have shown that TMEV infects and replicates in monocytes/macrophages [11, 41, 42, 54]. More specifically, evidence suggests that TMEV replicates in terminally differentiated pro-inflammatory macrophages, but not undifferentiated macrophages [39, 40, 42]. In Src homology region 2 domain-containing phosphatase 1 (SHP-1)-deficient mice, our lab established that intracranial or peritoneal infection of suckling mice with the attenuated BeAn strain of TMEV resulted in macrophage-mediated CNS demyelinating disease that was not seen in wild type (WT) mice [10, 11, 54], demonstrating a key role of innate immune activation of macrophages in the demyelinating process. Our previous in vivo studies found that monocyte-derived macrophages (CD45hiCD11b+F4/80lo) infiltrated the CNS of SHP-1-deficient mice at significantly higher levels than wild type mice during TMEV infection, and that the infiltrating SHP-1-deficient macrophages had a 5-fold increase of TMEV RNA compared to wild type mice, indicating TMEV replicated at a higher rate in SHP-1-deficient macrophages [10]. Our studies also found that the TMEV-infected SHP-1-deficent macrophages are more M1-like, with significantly higher levels of IL-6 and IL-1β compared to wild type TMEV-infected macrophages [10, 72].
Since both GM-CSF and M-CSF have been shown to play different roles in disease and macrophage differentiation and function including in the CNS [7, 44, 55, 69], it was important to probe the influence these growth factors on TMEV infection, RNA persistence, and innate activation of macrophages in vitro. The latter was particularly relevant to our previous in vivo studies suggesting that the pathogenic infiltrating macrophages were M1-like, and therefore might be variably influenced by these factors. Our analysis shows that GM-CSF- and M-CSF- differentiated macrophages develop unique phenotypes that respond to TMEV infection in distinct ways. In particular, GM-CSF differentiated macrophages infected with TMEV had higher levels of viral RNA, infectious virus, and proinflammatory molecules compared to infected M-CSF -differentiated cells. Importantly, while both M-CSF and GM-CSF differentiated macrophages ceased to produce infectious TMEV viral particles over time, TMEV genomes uniquely persisted in GM-CSF-differentiated macrophages suggesting a pathogenic role for GM-CSF in TMEV persistence and inflammation seen in CNS TMEV infections. Of particular interest, we found that the glycolytic rate of GM-CSF macrophages was significantly higher than in M-CSF macrophages, suggesting that TMEV required glycolysis in inflammatory macrophages for efficient replication. This observation may provide a potential mechanism for increased TMEV replication and proinflammatory activity in GM-CSF-derived macrophages in TMEV-induced macrophage-mediated disease.
2. MATERIALS AND METHODS
2.1 Animals
Homozygous wild type mice were produced from congenic C3FeLe.B6 a/a-Ptpn6/J wild type mice (Jackson Laboratories, Bar Harbor, ME) All animal experiments were performed under approval from the Institutional Animal Care and Use Committee (IACUC) at SUNY Upstate Medical University.
2.2 Bone marrow-derived macrophages
Bone marrow-derived macrophages were prepared by cell harvest from femurs and tibias of two-to-three week old wild type mice. Cells were cultured in complete medium containing 10% fetal bovine serum (FBS; Tissue Culture Biologicals, Long Beach, CA; No. 101), 1% Penicillin Streptomycin (Corning Cellgro, Manassas, VA; No. 30-02 CI) in DMEM with 4.5g/L glucose, L-glutamine and sodium pyruvate (Corning Cellgro, Manassas, VA; No. 10-013-CV). Medium was supplemented with 20ng/ml recombinant mouse M-CSF or GM-CSF (R&D Systems, Minneapolis, MN; No. 416-ML-050 or 415-ML-050, respectively), and cells were differentiated into macrophages for 6 days at 37°C.
2.3 Macrophage infections
BeAn TMEV was obtained from ATCC (Manassas, VA; No. VR-995) and propagated in BHK-21 cells (ATCC; No. CCL-10). Plaque assays were performed to determine viral titer as plaque-forming units per milliliter (PFU/ml). Bone marrow-derived macrophages were washed with serum-free DPBS and infected with BeAn TMEV at an MOI of 10 or mock infected with serum-free DMEM. Cells were gently agitated every 15 minutes, and after adsorption of TMEV for one hour at 37°C virus was removed and cells were cultured in complete medium containing 20ng/ml recombinant mouse M-CSF or GM-CSF. At 1, 12, 24 and 48h time points supernatant and cells were spun down to collect adherent and nonadherent cells. Supernatants were removed and stored at -80°C and cell pellets were suspended in RNA STAT-60 (Tel-Test, Inc., Friendswood, TX) and stored at -80°C until further processing for RNA extraction/analysis. For the kinetics study, bone-marrow-derived macrophages were grown, infected and collected as described at 1, 12, and 24h post-infection. For the 2-DG assay, bone marrow derived macrophages were isolated, grown and inoculated with TMEV as previously described. After inoculation, 50mM of 2-DG was added to the complete medium, and supernatants were removed 12 h.p.i. and stored at -80°C.
2.4 RNA analysis
RNAs were purified from cell pellets by extraction using the RNeasy Mini Kit (Qiagen, Germantown, MD; No. 74104), and were analyzed by a custom designed Quantigene 2.0 Multiplex Assay (Affymetrix, Inc., Santa Clara, CA) for the 1, 12, and 24h time points. The Affymetrix QuantiGene Plex 2.0 Assay (a multiplex bead-based assay) was used to measure the expression of 41 genes of interest (including 38 target genes and 3 reference genes). The plate was read using the BioRad BioPlex 200 instrument using settings of 100ul volume; 60 seconds timeout; and 100 Bead Events/Bead regions. Fluorescent readings from blank wells were subtracted from fluorescent values for each mRNA of interest. Values exceeding background were then normalized to the geometric mean signal derived from three reference genes in each sample: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Hypoxanthine-guanine phosphoribosyltransferase (HPRT1) and TATA binding protein 1 (TBP1). These normalized ratios were then scaled to positive integer values by multiplying them by a constant (10,000).. The 48h time point was run on a separate custom designed Quantigene 2.0 Multiplex assay looking at genes of interest and TMEV RNA expression. Results from the 48h time point were analyzed as described above.
2.5 TMEV Real-Time qPCR
RNAs from 1, 12, and 24h time points were purified by RNeasy Mini Kit (Qiagen, Germantown, MD; No. 74104) and converted to cDNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA; No. 170-8891) following manufacturer’s instructions. Real-time qPCR was done on Step One Plus Real Time PCR System (Applied Biosystems, Foster City, CA) using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA; No. 4367659). The PCR parameters were 3 min. at 95°C and 40 cycles at 95°C for 15 sec and 51°C for 60sec. The primers were used at 300uM. Relative gene expression levels were calculated during the logarithmic amplification phase by comparison to mock controls, and standardized to GAPDH. The primer pairs used in the study were: TMEV forward: TGGTCGACTCTGTGGTTACG, reverse: GCCGGTCTTGCAAAGATAGT; and GAPDH, forward: ACCACCATGGAGAAGGC, reverse: GGCATGGACTGTGGTCATGA (Integrated DNA Technologies, Coralville, IA).
2.6 TMEV titers
Released infectious TMEV particles were measured by standard plaque assay on a confluent monolayer of BHK-21 cells. Cells were washed with DPBS and infected with serial dilutions of supernatants from infected macrophages for 1 hour in a 37°C. After the incubation, the BHK-21 cells were washed in DMEM with 1% FBS, and overlaid with 3 mLs of 2% agarose (Sigma Aldrich, St. Louis, MO; No. A9045) in DMEM supplemented with 2% FBS and incubated at 37°C. Three days after infection, the monolayer was fixed with methanol, stained with 0.1% crystal violet, and plaques were counted to calculate pfu/ml.
2.7 Multiplex cytokine bead assays
Production of select cytokine, chemokine and growth factors was measured in supernatants from 1, 12, 24h TMEV and mock infected macrophages by the Bio-Plex Pro Mouse Cytokine 23-Plex Immunoassay (Bio-Rad, Hercules, CA; No. M60009RDPD) according to manufacturer’s instructions on a BioRad BioPlex 200 instrument.
2.8 IL-6 cytokine assay
Production of IL-6 was measured in culture supernatants from 1, 12 and 24 h TMEV- and mock-infected macrophages by DuoSet ELISA kit (R&D Systems, Minneapolis, MN; No. DY406) according to manufacturer’s instructions.
2.9 Glycolysis assay
GM-CSF and M-CSF differentiated bone marrow-derived macrophages were re-plated at day 5 onto 96-well seahorse plates, cultured for two days, the media was changed to Seahorse base media on day 7, and the Seahorse glycolysis stress test was preformed using the XFe96 analyzer (Seahorse Biosciences). The glycolysis assay was run by measuring the extracellular acidification rate (ECAR) after the following treatments: 10mM glucose, 2μM oligomycin, and 50mM 2-DG. The rate of basal glycolysis was determined by subtracting the ECAR before the glucose injection from the ECAR after the injection of glucose.
2.10 Statistical analyses
GraphPad Prism 5 was used to perform statistical analyses. For Figures 2A, 2B and 4B, two-way anova with Bonferroni post-test was performed to compare measurements between groups across time- points. One-way anova with a Bonferroni post-test was used to compare measurements between time-points within one group. For Figures 1, 3 and 4A, 5, and 6A–C, a two-tailed unpaired student t-test was used to compare measurements between two groups within one time-point. Error bars on graphs represent mean ± SEM.
FIGURE 2.
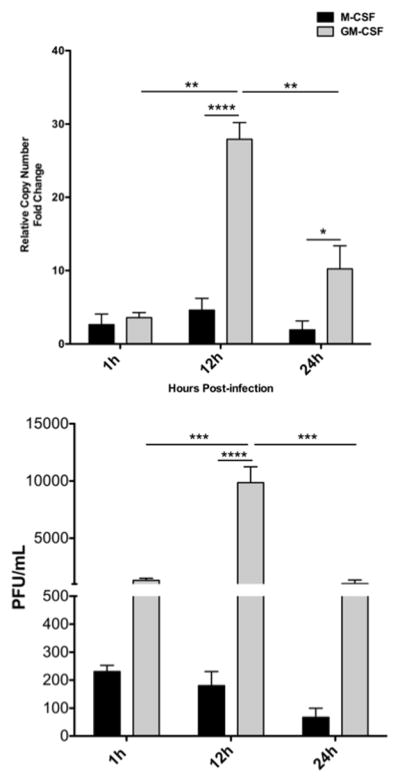
Kinetics of TMEV genome replication and infectious virus production. GM-CSF or M-CSF cultured bone marrow-derived macrophages were were infected with TMEV (A) RNA was extracted from and subjected to an RNA multiplex assay (n = 3). *p< 0.05 ****p < 0.001. (B) For titers, a plaque assay was performed using serially diluted supernatant to measure pfu/mL (n = 3). ***p < 0.005, ****p < 0.001
FIGURE 4.
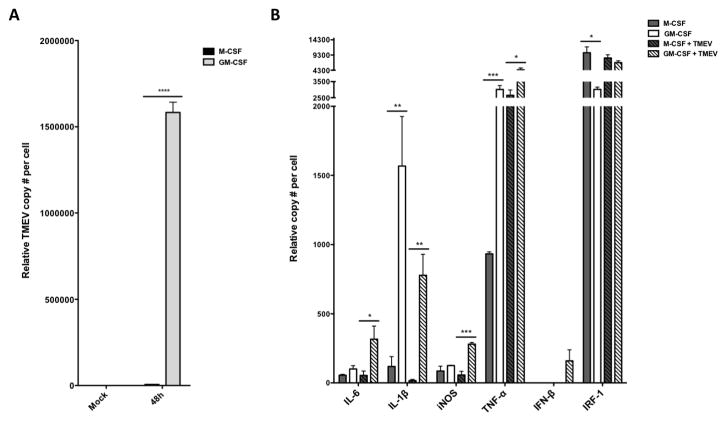
TMEV and cytokine RNA levels in macrophages 48h post-infection.. Murine bone marrow-derived macrophages differentiated in GM-CSF or M-CSF were infected with TMEV for 48h and RNA was extracted. (A) TMEV genome copies and (B) pro-inflammatory cytokine gene expression levels were measured using an RNA multiplex bead-based assay (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
FIGURE 1.
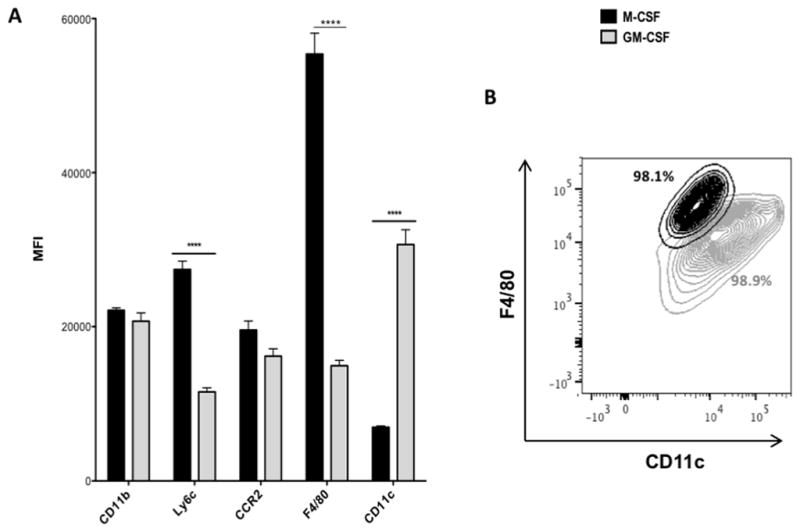
Flow cytometry profile of bone marrow-derived macrophages. Bone marrow-derived macrophages were differentiated in M-CSF or GM-CSF. Cells were labeled with fluorescently-labeled antibodies against CD11b, Ly6c, CCR2, F4/80, and CD11c, and surface expression was detected by flow cytometry. (A) Average MFI levels +/− SEM, ****p <0.0001. (B) Representative scatter plot depicting F4/80 and CD11c profiles of macrophages, including percentages of total cells.
FIGURE 3.
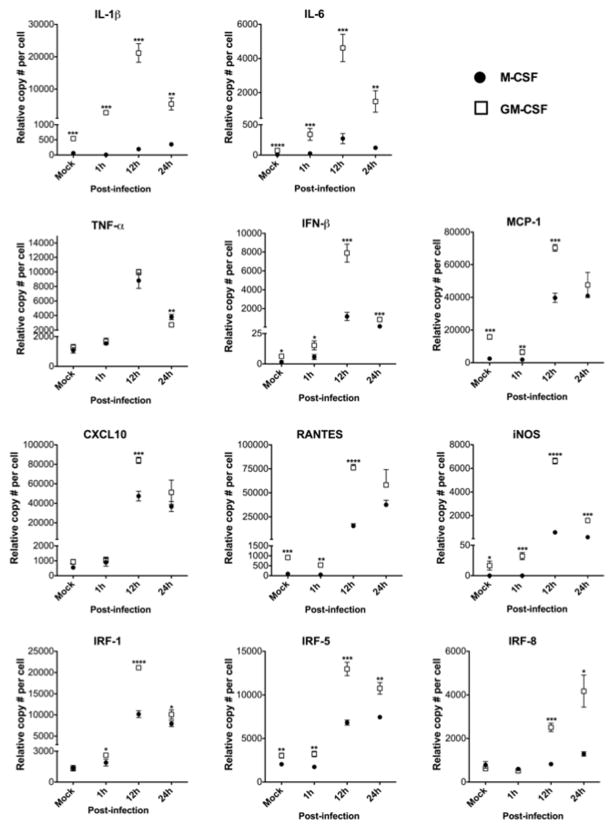
Kinetics of macrophage gene expression. RNA levels measured from TMEV infected M-CSF or GM-CSF cultured macrophages (n = 3). *p < 0.05, **p < 0.005, ***p < 0.001, ****p <0.0001.
FIGURE 5.
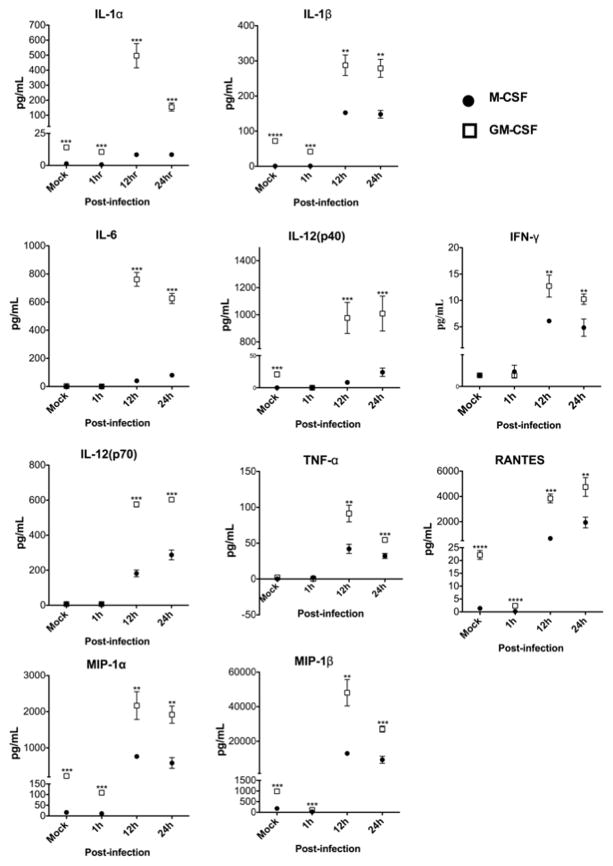
Kinetics of macrophage cytokine production. Murine bone marrow-derived macrophages differentiated in GM-CSF or M-CSF were infected with TMEV. Post-infection protein levels in supernatants were measured with a cytokine multiplex bead-based assay (n = 3). *p ≤ 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
FIGURE 6.
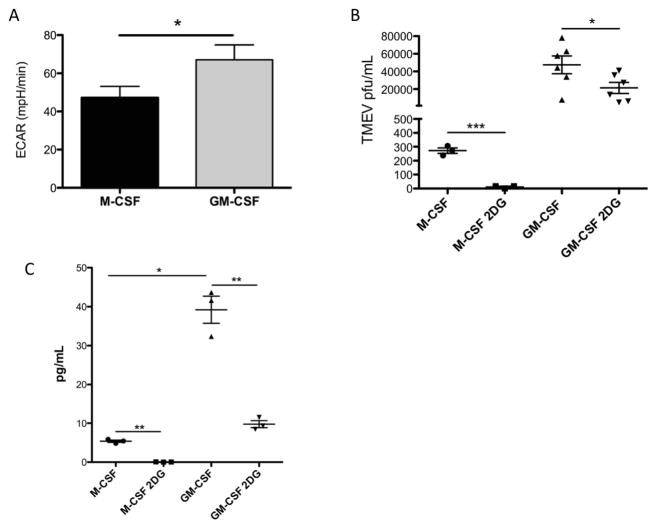
Glycolysis and TMEV replication. Murine bone-marrow macrophages were differentiated in GM-CSF or M-CSF. (A) Basal glycolysis levels of GM-CSF and M-CSF differentiated macrophages were measured by Seahorse (B) Macrophages were infected with TMEV and then treated with 2-DG. Supernatants were collected 12 h.p.i. and titered for TMEV virion production. (C) IL-6 protein levels were measured by ELISA in supernatants from TMEV-infected macrophages treated with 2-DG 12 h.p.i. *p < 0.05, **p < 0.01, ***p < 0.005
3. RESULTS
3.1 Bone marrow-derived macrophages differentiated with GM-CSF or M-CSF develop into phenotypically distinct cell populations
It is established that M-CSF and GM-CSF exert distinct influences on the differentiation of monocytes into macrophages. (12, [6, 31, 32, 56] To characterize the phenotypes of macrophages derived under the influence of these cytokines, bone marrow-derived cells were differentiated in M-CSF or GM-CSF, and surface expression of the macrophage-specific molecules CD11b, Ly6C, F4/80, CCR2 and CD11c were analyzed by flow cytometry (Fig 1A). M-CSF-differentiated macrophages displayed significantly higher expression of Ly6C and F4/80 compared to macrophages cultured in GM-CSF, while GM-CSF-derived macrophages expressed significantly higher levels of CD11c. Thus, two distinct populations could be observed in a cytokine-specific manner based on surface expression levels of F4/80 and CD11c (Fig 1B). Decreased levels of F4/80 observed in GM-CSF-derived macrophages suggested that GM-CSF-differentiated macrophages may be less mature than M-CSF-derived cells, or alternatively, GM-CSF-derived macrophages trended towards a dendritic cell phenotype in the myeloid cell differentiation spectrum.
3.2 GM-CSF promotes greater replication of TMEV than M-CSF in macrophages
To determine if cytokine-specific differentiation of macrophages impacts macrophage susceptibility to TMEV infection, we performed a kinetic analysis of TMEV genome copies in either M-CSF or GM-CSF-differentiated macrophages. M-CSF and GM-CSF bone marrow-derived macrophages were infected with TMEV at a MOI of 10, and cell-associated genomes were analyzed at various time points from 1 to 24 hours after infection. Analysis of TMEV RNA genomes showed a significant increase TMEV RNA replication in GM-CSF-differentiated macrophages between 1h and 12h post-infection. A significant decrease in TMEV-replication occurred in GM-CSF-differentiated macrophages between 12 and 24 hours post-inoculation. No significant change in TMEV replication was seen in M-CSF-differentiated macrophages between these timepoints. Comparatively, TMEV replication occurred at significantly higher levels in GM-CSF- compared to M-CSF-differentiated macrophages at 12h and 24h post-inoculation (Fig. 2A), indicating that GM-CSF-differentiated macrophages may be more supportive of TMEV replication than M-CSF-differentiated cells. The significant decrease in TMEV RNA levels after 12h P.I. in GM-CSF-differentiated macrophages is in agreement with previous reports that the highest rate of TMEV replication in macrophages occurs at approximately 8–10h post-inoculation of macrophages [41].
To determine if the differences in TMEV RNA levels corresponded to relative levels of infectious virion production in M-CSF or GM-CSF-differentiated cells, viral titers were measured (Fig 2B). Virus titers peaked at 12h and significantly decreased from 12h to 24h after infection in GM-CSF-differentiated macrophages. This result was in contrast to M-CSF-differentiated macrophages that did not show significant differences in infectious virion production from 1h to 12 h.p.i., and trended lower from 12h to 24h.. Between groups, infectious virus levels at 12h were significantly higher in GM-CSF- compared to M-CSF-differentiated macrophages. Further, infectious virions produced in GM-CSF-differentiated macrophages trended higher at 1h and 24h time points compared to virus produced from M-CSF-differentiated macrophages. Overall, these data suggest that GM-CSF may promote macrophage differentiation that is more favorable to TMEV replication, compared to M-CSF.
3.3 Proinflammatory Activation of GM-CSF- and M-CSF-differentiated macrophages infected with TMEV
To further characterize growth factor-specific differences in macrophage-virus interactions, bone marrow-derived cells were differentiated in M-CSF or GM-CSF and either mock-infected or infected with TMEV at an MOI of 10. RNA was extracted from cells collected at 1, 12, and 24h P.I., and mock-infected cells were harvested at 24h. RNA levels of genes traditionally associated with proinflammatory activity were quantified by an RNA multiplex bead assay (Fig. 3). In mock-infected cells, GM-CSF-derived macrophages displayed increased levels of proinflammatory genes (IL-1β, IL-6, MCP-1, RANTES, and iNOS) compared to M-CSF, indicating that GM-CSF may constitutively promote an overall increased macrophage activation state compared to M-CSF. Upon TMEV infection, GM-CSF-differentiated macrophages were induced to a greater proinflammatory state throughout the course of infection. At 1h post-inoculation., these proinflammatory genes were significantly increased in GM-CSF-derived macrophages compared to M-CSF. Interestingly, genes encoding proteins of the interferon system (IFN-β and IRF-1) were also induced to higher levels in GM-CSF-differentiated macrophages consistent with the potential induction of anti-viral responses and the eventual decrease in viral particle production after 12 hours of infection. Nearly all genes assessed were significantly increased in GM-CSF-treated macrophages compared to M-CSF at 12h P.I. (IL-1β, IL-6, MCP-1, CXCL10, RANTES, iNOS, IRF-1, IRF-5 and IRF8), and this trend continued at 24h post-inoculation for IL-1β, IL-6, iNOS, IRF-1, IRF-5 and IRF8.
In order to further confirm the increase in activation and pro-inflammatory molecule production, an alternate RNA multiplex approach was used at 48h post-inoculation with TMEV. In agreement with the above kinetic analysis of TMEV replication, infected GM-CSF-differentiated macrophages showed significantly higher numbers of TMEV genomes compared with M-CSF-differentiated cells analyzed by the multiplex bead approach (Figure 4A). Interestingly, released viral particles were undetectable by plaque assay in either GM-CSF or M-CSF-differentiated macrophages at 48 hours post infection (data not shown). However, in concert with the presence of TMEV genomes in GM-CSF-differentiated macrophages, mRNA for IL-6, IL-1β, iNOS and TNF-α were much higher in GM-CSF-differentiated macrophages compared to M-CSF differentiated macrophages infected with TMEV for 48h (Fig. 4B). Taken together, these data indicate that GM-CSF-differentiated macrophages may be activated by TMEV infection to produce greater amounts of TMEV genomes and proinflammatory cytokines.
3.4 Secretion of proinflammatory chemokines and cytokines in GM-CSF- and M-CSF-differentiation macrophages infected with TMEV
To quantify levels of proinflammatory protein production in infected GM-CSF and M-CSF-differentiated macrophages, macrophages were mock- or TMEV-inoculated and secreted cytokine levels were measured in supernatants collected at 1, 12, and 24h P.I. by a cytokine multiplex bead assay (Fig. 5). Basal levels of cytokines and chemokines including IL-1α, IL-β, IL-12 (p40), MIP-1α, MIP-1β, and RANTES were constitutively increased in GM-CSF compared to M-CSF-differentiated macrophages. At 1h P.I., IL-1α, IL-β, MIP-1α, MIP-1β, and RANTES were further increased in GM-CSF differentiated macrophages compared to M-CSF differentiated macrophages. At 12h P.I., all cytokines and chemokines measured, including IFN-γ and IL-6, were significantly increased in GM-CSF-cultured macrophages compared to M-CSF-differentiated cells. Secreted cytokine and chemokine protein levels from GM-CSF-differentiated macrophages also remained significantly higher relative to M-CSF at 24h P.I. Taken together along with mRNA expression levels (Fig. 3 and 4B), GM-CSF appears to promote macrophages to respond to TMEV infection with a greater activation state than M-CSF. Moreover, the increased activation state of GM-CSF-derived macrophages corresponds with an overall increased viral burden (TMEV genomes) following TMEV infection.
3.5 Glycolysis contributes to increased TMEV replication in macrophages
Because previous studies have demonstrated a dependence of virus replication on glycolysis [30, 68], and the previous reports of higher glycolytic rates in GM-CSF compared to M-CSF macrophages [2, 49, 63], a possible role for glycolysis in increased TMEV replication in GM-CSF-differentiated macrophages compared to M-CSF glycolysis was addressed. In agreement with previously reported studies mentioned above, GM-CSF differentiated macrophages had significantly higher basal glycolysis compared to M-CSF differentiated macrophages (Fig 6A). To determine if glycolysis affected TMEV replication in M-CSF and GM-CSF macrophages, we inoculated these cultures with TMEV and then added 2-deoxyglucose (2-DG), an inhibitor of glycolysis and, consequently, macrophage activation [18, 63, 65, 73]. Importantly, optimal TMEV replication was significantly dependent on glycolysis in both M-CSF and GM-CSF macrophages (Fig 6B). TMEV replication, while significantly decreased, was not entirely inhibited in 2DG-treated GM-CSF-differentiated macrophages suggesting a additional mechanisms promoting TMEV replication in GM-CSF-differentiated cells in addition to glycolysis. Finally,, we also determined TMEV-induced secretion of the proinflammatory cytokine IL-6 was significantly inhibited by 2-deoxyglucose in both M-CSF and GM-CSF-differentiated macrophages (Fig 6C). Taken together these results suggested that both TMEV replication and proinflammatory cytokine secretion by macrophages may be highly dependent on glycolysis, and that increased levels of glycolysis in GM-CSF compared to M-CSF macrophages may be a significant contributing factor in the observed increase levels of TMEV and proinflammatory cytokine production by GM-CSF macrophages relative to M-CSF macrophages.
4. DISCUSSION
M-CSF and GM-CSF are major growth factors involved in monocyte/macrophage differentiation both peripherally and in the CNS [3, 26, 33, 60]. Indeed, our data suggests that bone marrow cells cultured in M-CSF or GM-CSF differentiate into phenotypically distinct macrophage populations in vitro. These unique populations appear to provide varying levels of permissiveness for TMEV replication and innate immune activation evidenced by increased RNA genomes, infectious virions and cytokine production in GM-CSF-differentiated macrophages compared to M-CSF. Finally, we show that glycolysis is significantly higher in GM-CSF compared to M-CSF macrophages, and that increased glycolysis may contribute to increased TMEV replication and cytokine secretion by GM-CSF macrophages. Overall, our data suggests that GM-CSF may promote a higher state of pro-inflammatory activation in macrophages, both constitutively and in response to TMEV infection, compared to M-CSF by promoting a glycolytic state in macrophages.
Our data are in agreement with previous studies that suggest the differentiation state of macrophages affects the susceptibility of the cells to TMEV infection. Early work by Jelachich et. al. showed varying susceptibility to TMEV by transformed macrophage cell lines RAW264.7, P388D1 and M1, with the latter being completely resistant [39]. Later studies by Jelachich et. al. showed that the M1 cell line becomes susceptible to TMEV infection after differentiation with M-CSF [40]. In addition, studies by Himeda, et. al. using the attenuated TMEV DA strain, demonstrated that persistent infection in the J774 macrophage cell line may be due to downregulation of interferons and upregulation of granulocyte colony- stimulating factor, IL-10 and B-lymphocyte chemoattractant indicating further that macrophages are key targets in TMEV-induced CNS inflammatory disease in agreement with our studies [37].
The concept of macrophage differentiation states affecting susceptibility to virus infection and replication is not novel to TMEV, and human immunodeficiency virus (HIV) is a prominent example of the effects of M-CSF and GM-CSF on macrophage-virus interactions. Early HIV studies suggested that GM-CSF-derived macrophages displayed higher rates of HIV replication compared to M-CSF-derived macrophages [48]. Later research showed that M-CSF was sufficient to increase HIV replication in macrophages. However, it appears that GM-CSF enables HIV to infect cells at a less mature stage of macrophage differentiation relative to M-CSF, and may allow monocytes/macrophages to resist apoptosis upon infection [14].
West Nile Virus (WNV) is another example of a virus that infects monocytes/macrophages and drives an inflammatory activation state of those cells in the CNS, but it is unclear if M-CSF or GM-CSF regulate WNV infection of monocytes/macrophages in vivo [28]. Indeed, multiple factors that control macrophage differentiation associated with a particular tissue microenvironment or pathogen in question are likely to affect virus replication and immune activation. For example, activated glial cells in the CNS produce pro-and anti-inflammatory cytokines in response to infection and innate microbial recognition [15, 29, 36, 58]. In addition, cells undergoing apoptosis during infection release molecules that can affect monocyte differentiation [3]. Therefore, it is likely that the microenvironment within different tissues affects monocyte/macrophages in ways that affect virus infection, innate inflammation, and tissue-specific pathology. We believe that the availability of M-CSF and GM-CSF may be predominant factors determining the outcome of in tissue-specific virus infections that involve macrophage tropism and macrophage-mediated tissue damage.
We have previously investigated the function of the macrophage-enriched protein tyrosine phosphatase, SHP-1, in controlling macrophage-mediated demyelination following TMEV infection, and we found that CD45+ macrophages isolated from spinal cords of TMEV-infected SHP-1-deficent mice were skewed towards a pro-inflammatory phenotype similar to that described here in GM-CSF-differentiated macrophages [72]. Interestingly, SHP-1 has been implicated in playing a role in regulating both GM-CSF- and M-CSF-induced signaling in macrophages [43]. We speculate that the effects of macrophage regulators, including SHP-1, on GM-CSF or M-CSF signaling in macrophages in specific virus infections, will determine the outcomes of virus-induced macrophage-mediated diseases. Because inflammatory macrophages play a primary role in the demyelinating phase of disease in our model, we hypothesize that monocytes migrate into the CNS during TMEV infection and come under the influence of GM-CSF most likely secreted by resident CNS cells including astrocytes. We further hypothesize that the secreted GM-CSF skews monocyte differentiation towards a M1 macrophage phenotype that supports both TMEV replication and pro-inflammatory cytokine production as presented in this report. The precise pathological role of GM-CSF in TMEV-induced CNS demyelination will be tested in future studies.
In regards to a possible mechanism as to why GM-CSF-differentiated macrophages support higher levels of TMEV replication, it is well established in the literature that GM-CSF differentiation of bone-marrow precursor cells requires an upregulation of glycolysis to increase production of metabolites important for cell growth, differentiation, and proinflammatory potential [18, 63, 65, 73]. With regards to triggering proinflammatory activity, GM-CSF-differentiated bone-marrow macrophages that have been activated through TLRs for more than 12 hours switch exclusively to Warburg metabolism (i.e. glycolysis) [21]. Because glycolysis has been shown to be required for efficient replication of viruses including dengue virus, cytomegalovirus, Semliki Forest virus, Sindbis, Adenovirus, and Hepatitis C virus and poliovirus, the Warburg effect may be purposely enhanced by viral infections to increase energy and metabolite production required for virus replication and inhibition of apoptosis in host cells [5, 22, 24, 30, 45, 66].
We propose that the increased ability of GM-CSF-differentiated macrophages to replicate TMEV and secrete proinflammatory cytokines is due to the increased rate of basal glycolysis relative to that seen in M-CSF macrophages. Future investigations on the role of GM-CSF and M-CSF in TMEV disease and pathology in vivo will need to be performed to substantiate the implications of the present studies for CNS disease. For instance, it will be important to measure levels of GM-CSF and M-CSF in the CNS during TMEV infection, and determine their individual roles in TMEV-induced disease using various immunological and molecular approaches in infected mice. Of particular interest is whether GM-CSF is a critical determinant of TMEV RNA persistence and inflammatory activity in monocyte-derived macrophages and perhaps resident macrophages (microglia) in the CNS.
HIGHLIGHTS.
TMEV replicates in GM-CSF-differentiated macrophages
TMEV induces proinflammatory cytokines in GM-CSF-differentiated macrophages.
TMEV-induced glycolysis is higher in GM-CSF-differentiated macrophages
Acknowledgments
KMS, NBW and PTM contributed equally to the experimental design and analyses of this study. KMS was the primary author, with editing by PTM and NBW. SBM was responsible for the metabolic design and Seahorse analysis in Figure 6A.
Funding source: This work was supported by National Institutes of Health Grant R01 NS072051.
Footnotes
Author Disclosure Statement: No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexaki A, Wigdahl B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 2008 doi: 10.1371/journal.ppat.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiel E, Everts B, Fritz D, et al. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. The Journal of Immunology. 2014;193:2821–2830. doi: 10.4049/jimmunol.1302498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashhurst TM, van Vreden C, Niewold P, King NJC. The plasticity of inflammatory monocyte responses to the inflamed central nervous system. Cell Immunol. 2014;291:49–57. doi: 10.1016/j.cellimm.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton GM. A calculated response: control of inflammation by the innate immune system. J Clin Invest. 2008;118:413–420. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker Y, Grossowicz N, Bernkopf H. Metabolism of Human Amnion Cell Cultures Infected with Poliomyelitis Virus. I. Glucose Metabolism During Virus Synthesis.¶. Proceedings of the Society for Experimental Biology and Medicine. 1958 doi: 10.3181/00379727-97-23650. [DOI] [PubMed] [Google Scholar]
- 6.Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56:947–958. [PubMed] [Google Scholar]
- 7.Byrd D, Shepherd N, Lan J, et al. Primary human macrophages serve as vehicles for vaccinia virus replication and dissemination. Journal of Virology. 2014;88:6819–6831. doi: 10.1128/JVI.03726-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrieri PB, Provitera V, De Rosa T, et al. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing-remitting multiple sclerosis: a correlation with clinical activity. Immunopharmacol Immunotoxicol. 1998;20:373–382. doi: 10.3109/08923979809034820. [DOI] [PubMed] [Google Scholar]
- 9.Cassol E, Alfano M, Biswas P, Poli G. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. Journal of leukocyte …. 2006 doi: 10.1189/jlb.0306150. [DOI] [PubMed] [Google Scholar]
- 10.Christophi GP, Hudson CA, Panos M, et al. Modulation of Macrophage Infiltration and Inflammatory Activity by the Phosphatase SHP-1 in Virus-Induced Demyelinating Disease. Journal of Virology. 2008;83:522–539. doi: 10.1128/JVI.01210-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christophi GP, Massa PT. Central neuroinvasion and demyelination by inflammatory macrophages after peripheral virus infection is controlled by SHP-1. Viral Immunology. 2009;22:371–387. doi: 10.1089/vim.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology. 2009;6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey S, Eguinoa A, Puyana-Theall K, et al. Granulocyte macrophage-colony stimulating factor stimulates both association and activation of phosphoinositide 3OH-kinase and src-related tyrosine kinase(s) in human myeloid derived cells. The EMBO Journal. 1993;12:2681–2690. doi: 10.1002/j.1460-2075.1993.tb05929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowe SM, Lopez A. GM-CSF and its effects on replication of HIV-1 in cells of macrophage lineage. Journal of Leukocyte Biology. 1997;62:41–48. doi: 10.1002/jlb.62.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Cusick MF, Libbey JE, Patel DC, et al. Infiltrating macrophages are key to the development of seizures following virus infection. Journal of Virology. 2013;87:1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlmann F, Biedenkopf N, Babler A, et al. Analysis of Ebola Virus Entry Into Macrophages. Journal of Infectious Diseases. 2015;212(Suppl 2):S247–57. doi: 10.1093/infdis/jiv140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dal Canto MC, Miller SD. Theiler’s Murine Encephalomyelitis Virus (TMEV)-Induced Demyelination: A Model for Multiple Sclerosis. 1996:1–9. doi: 10.1006/meth.1996.0123. [DOI] [PubMed] [Google Scholar]
- 18.Del Prete A, Zaccagnino P, Di Paola M, et al. Role of mitochondria and reactive oxygen species in dendritic cell differentiation and functions. Free Radic Biol Med. 2008;44:1443–1451. doi: 10.1016/j.freeradbiomed.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Dogan R-NE, Elhofy A, Karpus WJ. Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J Immunol. 2008;180:7376–7384. doi: 10.4049/jimmunol.180.11.7376. [DOI] [PubMed] [Google Scholar]
- 20.El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nature Immunology. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everts B, Amiel E, Huang S, Smith AM. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKK [epsiv] supports the anabolic demands of dendritic cell activation. Nature. 2014 doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Findlay JS, Ulaeto D. Semliki Forest virus and Sindbis virus, but not vaccinia virus, require glycolysis for optimal replication. Journal of General Virology. 2015;96:2693–2696. doi: 10.1099/jgv.0.000226. [DOI] [PubMed] [Google Scholar]
- 23.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 24.Fontaine KA, Sanchez EL, Camarda R. Dengue virus induces and requires glycolysis for optimal replication. Journal of …. 2015 doi: 10.1128/JVI.02309-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox JG, Barthold S, Davisson M, Newcomer CE. The Mouse in biomedical research: diseases 2006 [Google Scholar]
- 26.Franco R, Fernández-Suárez D. Alternatively activated microglia and macrophages in the central nervous system. Progress in Neurobiology. 2015;131:65–86. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Frei K, Nohava K, Malipiero UV, et al. Production of macrophage colony-stimulating factor by astrocytes and brain macrophages. Journal of Neuroimmunology. 1992;40:189–195. doi: 10.1016/0165-5728(92)90133-6. [DOI] [PubMed] [Google Scholar]
- 28.Getts DR, Terry RL, Getts MT, et al. Ly6c+ inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. Journal of Experimental Medicine. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghoshal A, Das S, Ghosh S, et al. Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia. 2007;55:483–496. doi: 10.1002/glia.20474. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin CM, Xu S, Munger J. Stealing the Keys to the Kitchen: Viral Manipulation of the Host Cell Metabolic Network. Trends in Microbiology. 2015;23:789–798. doi: 10.1016/j.tim.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature Reviews Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends in Immunology. 2002 doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nature Reviews Immunology. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton JA. GM-CSF as a target in inflammatory/autoimmune disease: current evidence and future therapeutic potential. Expert Rev Clin Immunol. 2015;11:457–465. doi: 10.1586/1744666X.2015.1024110. [DOI] [PubMed] [Google Scholar]
- 35.Hao Z, Mitsuya H, Johns DG, Broder S. Replication of human immunodeficiency virus in monocytes. Granulocyte/macrophage colony-stimulating factor (GM-CSF) potentiates viral production yet enhances …. The Journal of …. 1989 doi: 10.1084/jem.169.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herder V, Iskandar CD, Kegler K, et al. Dynamic Changes of Microglia/Macrophage M1 and M2 Polarization in Theiler’s Murine Encephalomyelitis. Brain Pathol. 2015;25:712–723. doi: 10.1111/bpa.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Himeda T, Okuwa T, Muraki Y, Ohara Y. Cytokine/chemokine profile in J774 macrophage cells persistently infected with DA strain of Theiler’s murine encephalomyelitis virus (TMEV) Journal of Neurovirology. 2010;16:219–229. doi: 10.3109/13550284.2010.484040. [DOI] [PubMed] [Google Scholar]
- 38.Hosking MP, Lane TE. The Role of Chemokines during Viral Infection of the CNS. PLoS Pathog. 2010;6:e1000937. doi: 10.1371/journal.ppat.1000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jelachich ML, Bandyopadhyay P, Blum K, Lipton HL. Theiler’s Virus Growth in Murine Macrophage Cell Lines Depends on the State of Differentiation. Virology. 1995;209:437–444. doi: 10.1006/viro.1995.1276. [DOI] [PubMed] [Google Scholar]
- 40.Jelachich ML, Bramlage C, Lipton HL. Differentiation of M1 Myeloid Precursor Cells into Macrophages Results in Binding and Infection by Theiler’s Murine Encephalomyelitis Virus and Apoptosis. 1999 doi: 10.1128/jvi.73.4.3227-3235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jelachich ML, Lipton HL. Restricted Theiler’s murine encephalomyelitis virus infection in murine macrophages induces apoptosis. J Gen Virol. 1999;80(Pt 7):1701–1705. doi: 10.1099/0022-1317-80-7-1701. [DOI] [PubMed] [Google Scholar]
- 42.Jelachich ML, Reddi HV, Trottier MD, et al. Susceptibility of peritoneal macrophages to infection by Theiler’s virus. Virus Research. 2004;104:123–127. doi: 10.1016/j.virusres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Jiao H, Yang W, Berrada K, et al. Macrophages from motheaten and viable motheaten mutant mice show increased proliferative responses to GM-CSF: detection of potential HCP substrates in GM-CSF signal transduction. Exp Hematol. 1997;25:592–600. [PubMed] [Google Scholar]
- 44.Jibin Li ID. The Effects of Macrophage Polarity on Influenza Virus Replication and Innate Immune Responses. J Clin Cell Immunol. 2015 doi: 10.4172/2155-9899.1000297. [DOI] [Google Scholar]
- 45.Karniely S, Weekes MP, Antrobus R, et al. Human Cytomegalovirus Infection Upregulates the Mitochondrial Transcription and Translation Machineries. MBio. 2016;7:e00029. doi: 10.1128/mBio.00029-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karpus WJ, Kennedy KJ, Fife BT, et al. Anti-CCL2 treatment inhibits Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Journal of …. 2006 doi: 10.1080/13550280600873819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. doi: 10.1182/blood-2008-07-168575. bloodjournal.hematologylibrary.org.dbgateway.nysed.gov. [DOI] [PMC free article] [PubMed]
- 48.Kitano K, Abboud CN, Ryan DH, et al. Macrophage-active colony-stimulating factors enhance human immunodeficiency virus type 1 infection in bone marrow stem cells [see comments] Blood. 1991;77:1699–1705. [PubMed] [Google Scholar]
- 49.Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kyle JL, Beatty PR, Harris E. Dengue virus infects macrophages and dendritic cells in a mouse model of infection. Journal of Infectious Diseases. 2007 doi: 10.1086/518007. [DOI] [PubMed] [Google Scholar]
- 51.Lacey DC, Achuthan A, Fleetwood AJ, et al. Defining GM-CSF- and Macrophage-CSF-Dependent Macrophage Responses by In Vitro Models. The Journal of Immunology. 2012;188:5752–5765. doi: 10.4049/jimmunol.1103426. [DOI] [PubMed] [Google Scholar]
- 52.Lang RA, Metcalf D, Cuthbertson RA, et al. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987;51:675–686. doi: 10.1016/0092-8674(87)90136-X. [DOI] [PubMed] [Google Scholar]
- 53.Lipton HL, Kumar A, Trottier M. Theiler’s virus persistence in the central nervous system of mice is associated with continuous viral replication and a difference in outcome of infection of infiltrating macrophages versus oligodendrocytes. Virus Research. 2005 doi: 10.1016/j.virusres.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 54.Lipton HL, Twaddle G, Jelachich ML. The predominant virus antigen burden is present in macrophages in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Journal of Virology. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louis C, Cook AD, Lacey D, et al. Specific Contributions of CSF-1 and GM-CSF to the Dynamics of the Mononuclear Phagocyte System. The Journal of Immunology. 2015;195:134–144. doi: 10.4049/jimmunol.1500369. [DOI] [PubMed] [Google Scholar]
- 56.Jelachich ML, HLL CB. Differentiation of M1 Myeloid Precursor Cells into Macrophages Results in Binding and Infection by Theiler’s Murine Encephalomyelitis Virus and Apoptosis. Journal of Virology. 1999;73:3227. doi: 10.1128/jvi.73.4.3227-3235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malipiero UV, Frei K, Fontana A. Production of hemopoietic colony-stimulating factors by astrocytes. J Immunol. 1990;144:3816–3821. [PubMed] [Google Scholar]
- 58.Marques CP, Cheeran MC-J, Palmquist JM, et al. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. The Journal of Immunology. 2008;181:6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marques RE, Guabiraba R, Del Sarto JL, et al. Dengue virus requires the CC-chemokine receptor CCR5 for replication and infection development. Immunology. 2015;145:583–596. doi: 10.1111/imm.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell AJ, Roediger B, Weninger W. Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell Immunol. 2014 doi: 10.1016/j.cellimm.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Reviews Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Na YR, Hong JH, Lee MY, et al. Proteomic Analysis Reveals Distinct Metabolic Differences Between Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) and Macrophage Colony Stimulating Factor (M-CSF) Grown Macrophages Derived from Murine Bone Marrow Cells. Mol Cell Proteomics. 2015;14:2722–2732. doi: 10.1074/mcp.M115.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pearce EJ, Everts B. Dendritic cell metabolism. Nature Reviews Immunology. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rasouli J, Ciric B, Imitola J, et al. Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-β Therapy. The Journal of Immunology. 2015;194:5085–5093. doi: 10.4049/jimmunol.1403243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rehman A, Hemmert KC, Ochi A, et al. Role of fatty-acid synthesis in dendritic cell generation and function. The Journal of Immunology. 2013;190:4640–4649. doi: 10.4049/jimmunol.1202312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ripoli M, D’Aprile A, Quarato G. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1α-mediated glycolytic adaptation. Journal of …. 2010 doi: 10.1128/JVI.00769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rostami A, Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J Neurol Sci. 2013;333:76–87. doi: 10.1016/j.jns.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez EL, Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479–480:609–618. doi: 10.1016/j.virol.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sang Y, Miller LC, Blecha F. Macrophage Polarization in Virus-Host Interactions. J Clin Cell Immunol. 2015 doi: 10.4172/2155-9899.1000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terry RL, Getts DR, Deffrasnes C, et al. Inflammatory monocytes and the pathogenesis of viral encephalitis. Journal of Neuroinflammation. 2012;9:1–1. doi: 10.1186/1742-2094-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsunoda I, Fujinami RS. Neuropathogenesis of Theiler’s Murine Encephalomyelitis Virus Infection, An Animal Model for Multiple Sclerosis. J Neuroimmune Pharmacol. 2009;5:355–369. doi: 10.1007/s11481-009-9179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watson NB, Schneider KM, Massa PT. SHP-1-Dependent Macrophage Differentiation Exacerbates Virus-Induced Myositis. The Journal of Immunology. 2015;194:2796–2809. doi: 10.4049/jimmunol.1402210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaccagnino P, Saltarella M, Maiorano S. An active mitochondrial biogenesis occurs during dendritic cell differentiation. … of biochemistry & cell …. 2012 doi: 10.1016/j.biocel.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 74.Monocytes/macrophages isolated from the mouse central nervous system contain infectious. Theiler’s murine encephalomyelitis virus (TMEV) 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]