Abstract
Background
The impact of prenatal ambient air pollution on child asthma may be modified by maternal stress, child sex and exposure dose and timing.
Objective
We prospectively examined associations between co-exposure to prenatal particulate matter with an aerodynamic diameter of less than 2.5 microns (PM2.5) and maternal stress on childhood asthma (n=736).
Methods
Daily PM2.5 exposure during pregnancy was estimated using a validated satellite-based spatio-temporally resolved prediction model. Prenatal maternal negative life events (NLEs) were dichotomized around the median (high: NLE≥3; low: NLE<3). We employed Bayesian distributed lag interaction models (BDLIMs) to identify sensitive windows for prenatal PM2.5 exposure on children’s asthma by age 6, and determine effect modification by maternal stress and child sex.
Results
BDLIMs identified a critical window of exposure (19–23 weeks gestation, cumulative OR=1.15, 95%CI=1.03–1.26; per IQR (1.7 μg/m3) increase in prenatal PM2.5 level) during which children concomitantly exposed to prenatal PM2.5 and maternal stress had increased risk of asthma. No significant association was seen in children born to women reporting low prenatal stress. When examining modifying effects of prenatal stress and fetal sex, we found that boys born to mothers with higher prenatal stress were most vulnerable (19–21 weeks gestation, cumulative OR=1.28, 95%CI=1.15–1.41; per IQR increase in PM2.5).
Conclusion
Prenatal PM2.5 exposure during sensitive windows is associated with increased risk of child asthma, especially in boys concurrently exposed to elevated maternal stress.
Keywords: particulate matter, ambient air pollution, negative life events, prenatal stress, childhood asthma, sex- and temporal-specific effects
Introduction
A wealth of data prospectively links childhood ambient air pollution exposure to childhood respiratory disorders, including asthma onset and morbidity and reduced lung function.(1–3) Conversely, improvements in children’s lung function and growth trajectory have been seen with modest reductions in air pollution exposure.(4) Emerging evidence highlights the importance of prenatal particulate matter with an aerodynamic diameter of less than 2·5 microns (PM2.5) on subsequent airway dysfunction, suggesting that ambient air pollution exposure begins to influence the respiratory system in utero.
Lung development commences prenatally through a sequence of carefully orchestrated stages.(5) When the fetus is exposed to noxious stimuli, fetal programming is altered and lung cells may undergo dysfunctional remodeling. Maternal environmental exposures may thus alter fetal lung development, predisposing the fetus to future respiratory disease. Associations between prenatal environmental exposures and postnatal respiratory disorders may depend on timing of exposure as well as dose.
Moreover, environmental exposures rarely occur in isolation and the co-occurrence of multiple exposures may modify alterations in lung development, especially in susceptible sub-populations. Psychosocial stress has been associated with childhood respiratory disorders, including wheeze(6) and asthma.(7) Populations disproportionately exposed to ambient air pollution may also be more likely to experience social stress, which may enhance air pollution effects.(8) Interactive effects of air pollution and stress on respiratory outcomes have been shown in older children, however these associations have not been examined starting in utero.(9)
Boys appear particularly vulnerable to the independent effects of prenatal air pollution and stress exposure.(7, 10) Chemical (e.g., air pollution) and non-chemical (e.g., stress) exposures can disrupt similar or overlapping pathways leading to respiratory disease. For example, prenatal air pollution and stress exposures can both induce fetal oxidative stress, which is thought to play a central role in the programming of respiratory disorders, including asthma.(11, 12) The male fetus may be at increased risk for oxidative injury, thus they may have an exaggerated response to both prenatal air pollution and stress exposures. Furthermore, animal and human studies demonstrate sex differences in lung development.(13, 14) For example, delayed fetal breathing and surfactant production in males may also enhance their vulnerability to toxic exposures during fetal development.
We previously implemented distributed lag models (DLMs), a data driven approach, to identify windows of vulnerability for the effect of prenatal PM2.5 exposure on asthma risk in children. This prior analysis examined sex-specific effects by fitting a DLM stratified by sex and demonstrated that higher prenatal PM2.5 exposure between 16–25 weeks gestation was associated with increased risk of asthma in boys but not girls. Motivated by this finding, our group next implemented Bayesian distributed lag interaction models (BDLIMs) to extend the traditional constrained DLM framework (15, 16) to simultaneously estimate windows of vulnerability and effect modification between subgroups (e.g., sex) confirming our finding of sex-specific effects.(17)
Herein we apply this novel approach to examine complex interactions among prenatal PM2.5 exposure, psychological stress in pregnancy, and infant sex in relation to asthma development in early childhood. We hypothesized that children born to mothers with higher exposure to PM2.5 in pregnancy and increased prenatal stress would be particularly at risk for developing asthma. We also posited that boys would be particularly vulnerable to these synergistic effects.
Methods
Study Participants
Subjects were from the Asthma Coalition on Community, Environment and Social Stress (ACCESS) project, a pregnancy cohort originally designed to examine the effects of both chemical and non-chemical exposures, on urban childhood asthma risk.(18) Briefly, English- or Spanish-speaking women receiving prenatal care at two Boston hospitals and affiliated community health centers were recruited at 28.4 ± 7.9 weeks gestation between August 2002 and July 2009. Of those approached and deemed eligible, 989 (78.1%) agreed to enroll and 955 gave birth to a singleton infant and continued follow up. There were no significant differences in race/ethnicity, education, and income between those enrolled and those who declined enrollment. Procedures were approved by human studies committees at the Brigham and Women’s Hospital and Boston Medical Center; written consent was obtained in the subject’s primary language.
Daily Prenatal PM2·5 Levels
Maternal residence over gestation, collected at enrollment, confirmed across pre-enrollment gestation, and updated if they moved, was used to derive daily air pollution exposure over the entire pregnancy. As detailed previously, residence-specific estimates of daily PM2.5 were determined using a novel spatiotemporal model incorporating moderate resolution imaging spectroradiometer satellite-derived aerosol optical depth (AOD) measurements at a 10 × 10 km spatial resolution and layering this remote sensing data with traditional land use regression (LUR) predictors.(19) Day-specific calibrations of AOD data were performed using ground PM2.5 measurements from 78 monitoring stations covering New England and LUR and meteorologic variables (temperature, wind speed, visibility, elevation, distance to major roads, percent open space, point emissions, and area emissions). Thus, the model incorporates highly resolved spatial information from the LUR data and spatiotemporal data from the remote sensing satellite. Data were available to derive estimates for the entire gestation of each participant (model predictions available beginning January 1, 2000).
Daily data from grid cells with both monitor and AOD values was used to calibrate the AOD-PM2.5 relationship using mixed models with random slopes for day, nested within region. For days without AOD data (due to cloud coverage, snow, etc), the model was fit with a smooth function of latitude and longitude and a random intercept for each cell. The “out of sample” 10-fold cross validation R2 for daily values was 0.83 and 0.81 for days with and without available AOD data, respectively. Daily levels were averaged into weekly exposure profiles to reduce potential noise due to day-to-day PM2.5 variation.
Negative life events
Prenatal maternal stress was measured using the Crisis in Family Systems-Revised (CRISYS-R) survey, validated in English and Spanish and administered within 2 weeks of enrollment.(20, 21) Mothers were asked to endorse life events experienced in the past 6 months across 11 domains (e.g., financial, legal, career, relationships, safety in the home, safety in the community, medical issues pertaining to self, medical issues pertaining to others, home issues, authority, and prejudice) and to rate each as positive, negative or neutral. The numbers of domains with ≥1 negative event were summed to create a negative life events (NLEs) domain score (ranged 0 to 9 in our sample), with higher scores indicating greater stress.(7) Prenatal stress was dichotomized as low stress (NLE scores=0–2) and high stress (NLE scores≥3) based on the median value.
Asthma onset
Maternal-reported clinician-diagnosed asthma was ascertained during telephone and in-person interviews at approximately 3-month intervals for the first 24 months of life and then annually up to age 6 years. Mothers were asked, “Has a doctor or nurse ever said that your child had asthma?” The majority of children received a diagnosis of asthma after age 3 years (78.7%).(10)
Covariates
Maternal age at enrollment, race/ethnicity, education, and pre-pregnancy height and weight were ascertained by questionnaire. Internal validation of self-reported and measured height and weight showed good agreement.(22) Maternal body mass index (BMI) was calculated by dividing weight by height squared (kilograms per meter squared). Obesity was defined as a BMI≥30 kg/m2. Mothers who reported smoking at baseline and/or in the third trimester were classified as prenatal smokers; postnatal smoke exposure was documented based on maternal report of smoking and/or whether others smoked in the home at each postpartum interview.
Statistical Analysis
Prior research links prenatal air pollution exposure to preterm birth which, in turn, is linked to respiratory disease in children.(23, 24) Thus, we restrict our analyses to 736 mothers and their singleton children with exposure data (PM2.5 and stress) born at ≥37 weeks gestation to minimize over-adjustment for a pathway variable.
To examine associations between prenatal PM2.5 and childhood asthma and modification by child sex and prenatal stress, we used Bayesian distributed lag interaction models (BDLIMs), which extends the traditional constrained distributed lag model (DLM) framework that identifies critical windows(10) and additionally accounts for effect modifications.(17) We first conducted BDLIM in the overall sample, then examined effect modification by prenatal stress and child sex. In addition to critical windows, BDLIM estimates the cumulative effect of PM2.5 exposure over pregnancy for each sex-stress combination, accounting for critical windows and within-window effects. BDLIM partitions the distributed lag function into two components: 1) the weights that identify critical windows of susceptibility, and 2) the coefficients that identify the magnitude of the within-window effects. Each sex-stress combination can have either the same, or different, sensitive windows (weights) and within-window effects (magnitude of effects).(17) This allows situations where subgroups have the same critical window but different within window effects or vice versa, which are not allowed in the standard DLM framework. When the weights are constant over time, the BDLIM is equivalent to a model with mean exposure interacted with child sex and prenatal stress group. When the weights vary by time, the model identifies time periods with greater weight (i.e., potential sensitive windows) that will graphically appear as a bump during which exposure is significantly associated with the asthma outcome. This approach determines whether the weights and the coefficients are the same or different for each group. The model quantifies the likelihood of each pattern of heterogeneity and estimates the association between exposure and outcome under the effect modification pattern that is best supported by the data.
While being able to examine the interaction, the BDLIM incorporates the data from all exposure time points simultaneously and assumes that the association between the outcome and exposure varies smoothly as a function of time while controlling for exposure at all other time points. It also allows the ability to yield an estimation of a cumulative effect across all time points (in our case, across the entire pregnancy) accounting for both the critical windows and the strength of within window associations for each stress or sex group, by calculating the sum of the product of weight and point effect estimate for each time point. Standard controls (maternal age, and child’s sex) as well as potential confounders (maternal race/ethnicity, education, pre-pregnancy obesity and prenatal/postnatal smoking) were included in analyses. All analyses were implemented in R statistical software (v3.3.1, Vienna, Austria).
Results
Mothers were largely ethnic minorities (Black 30%, Hispanic 54%) with ≤12 years of education (66%) (Table 1). Median maternal age at enrollment was 26 years (IQR 22–31). The majority of mothers were non-smokers (86%) and non-obese (72%). There were 110 asthma cases (15%). Boys were more likely than girls to be diagnosed with asthma (18 vs 12%; chi-square test p=0.02). Children born to mothers with high prenatal maternal stress [NLE≥3 (n=308) vs NLE<3 (n=428)] were more likely to develop asthma (19 vs 12%, p=0.01) and were more likely exposed to prenatal maternal smoking (19 vs 11%, p<0.01). The distribution of other covariates did not vary by prenatal maternal stress level. There were no sex-specific significant differences in terms of gestational age at birth, maternal age, obesity, prenatal stress, and PM2.5 exposure.
Table 1.
ACCESS participant characteristics (n=736)
| All children (n=736) | High Prenatal Stress NLEd ≥3 (n=308) |
Low Prenatal Stress NLEd <3 (n=428) |
||||
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 374 | 50.8 | 165 | 53.6 | 209 | 48.8 |
| Female | 362 | 49.2 | 143 | 46.4 | 219 | 51.2 |
| Ever had asthmaa up to 6 years old (n, %) | ||||||
| No | 626 | 85.1 | 249 | 80.8 | 377 | 88.1 |
| Yes | 110 | 15.0 | 59 | 19.2 | 51 | 11.9 |
| Race/Ethnicity (n, %) | ||||||
| Black | 218 | 29.6 | 96 | 31.2 | 122 | 28.5 |
| Hispanic | 395 | 53.7 | 157 | 51.0 | 238 | 55.6 |
| White/Other | 123 | 16.7 | 55 | 17.8 | 68 | 15.9 |
| Maternal education (n, %) | ||||||
| >12 yrs | 251 | 34.1 | 106 | 34.4 | 145 | 33.9 |
| ≤12 yrs | 485 | 65.9 | 202 | 65.6 | 283 | 66.1 |
| Pre- and postnatal tobacco smoke exposure statusb (n, %) | ||||||
| Never | 532 | 72.3 | 203 | 65.9 | 329 | 76.9 |
| Prenatal only | 35 | 4.8 | 13 | 4.2 | 22 | 5.1 |
| Postnatal only | 100 | 13.6 | 46 | 14.9 | 54 | 12.6 |
| Both prenatal and postnatal | 69 | 9.4 | 46 | 14.9 | 23 | 5.4 |
| Maternal obesityb (n,%) | ||||||
| No | 531 | 72.2 | 214 | 69.5 | 317 | 74.1 |
| Yes | 205 | 27.8 | 94 | 30.5 | 111 | 25.9 |
| Maternal age at enrollment (years; median, IQR) | 25.5 | 22.3–30.7 | 25.3 | 22.0–31.0 | 25.8 | 22.5–30.6 |
| Averaged prenatal PM2.5 level (μg/m3; median, IQR) | 11.2 | 10.2–11.9 | 11.2 | 10.2–11.9 | 11.2 | 10.2–11.8 |
| Prenatal negative life events (NLE) scorec (median, IQR) | 2 | 1–4 | 4 | 3–5 | 1 | 0–2 |
Ever self-reported doctor-diagnosed asthma, eczema, and/or hay fever
Combination of prenatal maternal smoking and postnatal maternal and/or household smoking status
Pre-pregnancy obesity: ≥30 kg/m2.
Assessed using Crisis in Family Systems-Revised (CRISYS-R) survey (40); multi-item survey summarized into a continuous score.
Bayesian distributed lag interaction models (BDLIMs)
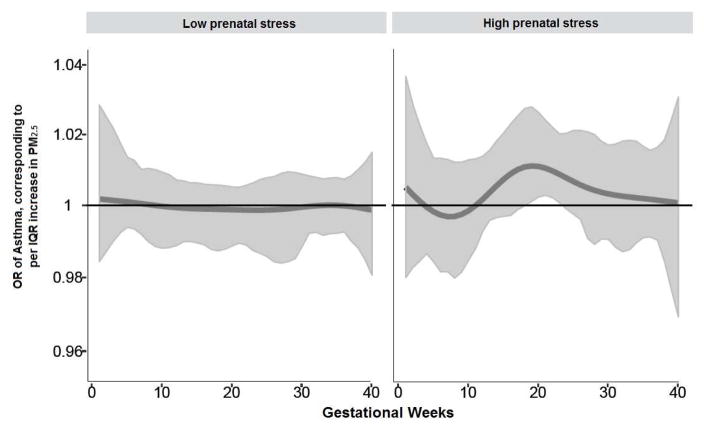
The cumulative effect of weighted prenatal PM2.5 across pregnancy on child asthma risk was estimated by BDLIMs, which accounted for both the time-varying sensitive window and the within-window effects throughout the pregnancy (cumulative OR=1.17, 95% CI=1.04–1.30; per IQR (1.7 μg/m3) increase in prenatal PM2.5 level). Figure 1 demonstrates the association between prenatal PM2.5 and children’s asthma modified by prenatal maternal stress (NLE<3 vs NLE≥3), after adjustment for maternal age at enrollment, race/ethnicity, education, pre-pregnancy obesity, prenatal and postnatal tobacco smoke exposure, and child sex. We observed a significant sensitive window between 19–23 weeks gestation in children concomitantly exposed to high prenatal maternal stress; no significant association was seen in children born to women reporting low prenatal stress. The estimated cumulative effects of PM2.5 exposure across pregnancy, accounting for sensitive windows and time-varying effects determined by BDLIMs, was significant in the high prenatal stress group only (OR=1.15, 95%CI=1.03–1.26; per IQR (1.7 μg/m3) increase in PM2.5). The model with different windows and different within-window effects (pattern as described in the Statistical Analysis section) indicates strong support for a PM2.5 and stress interaction (the normalized posterior density was 0.95, which can be interpreted as a probability that this was the best fitting pattern of effect modification).
Figure 1. Associations between prenatal PM2·5 exposure and children’s asthma: interaction by stress.
Odds ratios (95% CIs) per IQR (1.7 μg/m3) increase in PM2·5 estimated by BDLIM demonstrating the relationship of prenatal stress with children’s asthma diagnosed by age 6 years. Models were adjusted for maternal age, race/ethnicity, education, pre-pregnancy obesity, and prenatal/postnatal smoking and child sex. The x-axis demarcates the gestational age in weeks. The y-axis represents the odds ratio (OR) of developing asthma by age 6 years of age in relation to an IQR (1.7 μg/m3) increase in PM2.5 exposure, assuming week-specific effects. The solid line represents the predicted OR, and the gray area indicates the 95% confidence interval (CI). A sensitive window is identified when the estimated pointwise 95% CI does not include one.
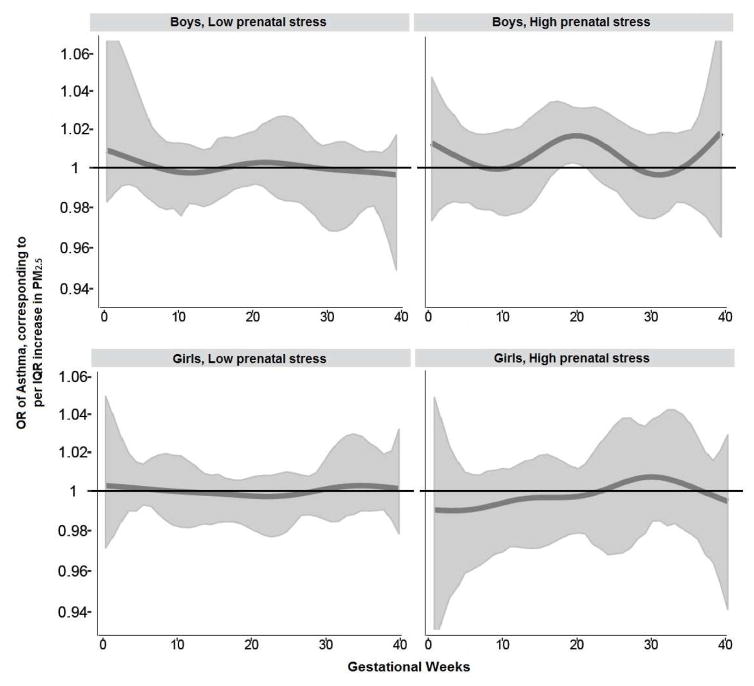
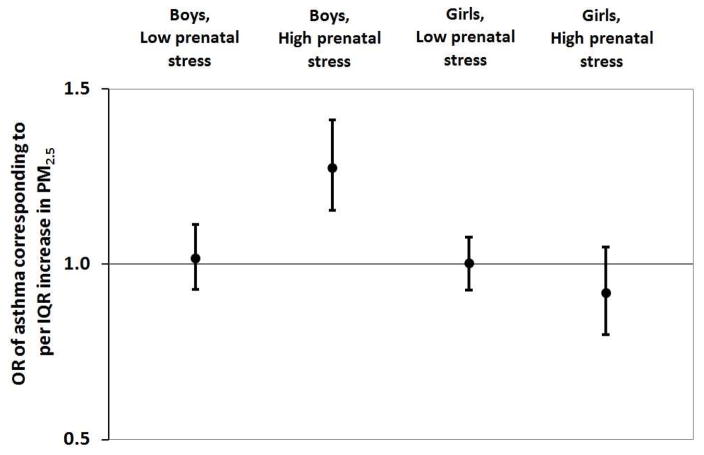
Figure 2 demonstrates the association between prenatal PM2.5 and children’s asthma modified by prenatal maternal stress (NLE<3 vs NLE≥3) and child sex. BDLIMs demonstrated that associations between higher PM2.5 exposure at 19–21 weeks gestation and increased odds of asthma among boys born to mothers reporting high prenatal stress, whereas no significant window was found in boys born to mothers with low prenatal stress. The estimated cumulative effects of PM2.5 exposure across pregnancy, accounting for sensitive windows and time-varying effects determined by BDLIMs, was also significant in boys born to mothers experiencing high stress (OR=1.28, 95%CI=1.15–1.41; per IQR (1.7 μg/m3) increase in PM2.5) (Figure 3). No significant associations were found among girls, regardless of prenatal stress level. The BDLIM suggested that effect modification by child sex and prenatal stress was attributable to difference in both the sensitive windows and the magnitude of the within-window association for different sex-stress combinations (normalized posterior densities was 0.92 indicating strong support for an interaction).
Figure 2. Associations between prenatal PM2.5 exposure and children’s asthma: interaction by stress and child sex.
Odds ratios (95% CIs) per IQR (1.7 μg/m3) increase in PM2.5 estimated by BDLIM demonstrating the relationship of prenatal PM2·5, stress and sex with children’s asthma diagnosed by age 6 years. Models were adjusted for maternal age at enrollment, race/ethnicity, education, pre-pregnancy obesity, and prenatal/postnatal smoking and child sex. The x-axis demarcates the gestational age in weeks. The y-axis represents the odds ratio (OR) of developing asthma by age 6 years of age in relation to an IQR (1.7 μg/m3) increase in PM2.5 exposure, assuming week-specific effects. The solid line represents the predicted OR, and the gray area indicates the 95% confidence interval (CI). A sensitive window is identified when the estimated pointwise 95% CI does not include one.
Figure 3. Cumulative effect (OR of asthma) of PM2·5 across pregnancy on childhood asthma.
Cumulative effect of PM2.5 across pregnancy on childhood asthma onset estimated by Bayesian distributed lag interaction models, accounting for both sensitive windows and within-window effects. The model was adjusted for maternal age, race/ethnicity, education, pre-pregnancy obesity and prenatal/postnatal smoking and child sex. Maternal stress assessed using Crisis in Family Systems-Revised (CRISYS-R) survey; multi-item survey summarized into a continuous score, which was dichotomized around median to create High (NLE ≥3) versus Low (NLE <3) categories.
Discussion
These analyses combine highly temporally resolved ambient PM2.5 exposure estimates with advanced statistical modeling to determine susceptible windows of exposure to asthma and identify higher-risk subgroups. This is the first study to prospectively demonstrate synergistic effects of prenatal maternal stress and PM2.5 exposure on childhood asthma risk. Children born to mothers reporting elevated stress in pregnancy and with higher PM2.5 exposures between 19–23 weeks gestation were significantly more likely to develop asthma. The model examining 3-way interactions found that among children co-exposed in utero to higher PM2.5 and maternal stress, boys were most vulnerable with a sensitive window of exposure identified between 19–21 weeks gestation.
While previous work by our group linked increased prenatal maternal stress(7) and increased prenatal PM2.5 exposure in mid-gestation(10) to asthma outcomes in separate analyses, understanding the impact of co-occurring exposures had not been previously described. Using pre-specified exposure windows, such as mean PM2.5 over the full gestation period or a selected trimester, can result in biased estimates and can identify incorrect critical windows. In contrast, data-driven methods that use temporally-resolved exposure data, such as DLM and BDLIM, are generally unbiased.(25) Use of BDLIMs expands on previously applied DLMs to identify critical windows while allowing for inclusion of interaction terms. By sharing information across subgroups on the timing of windows and the within-window effects, BDLIM provides a more powerful method of detecting windows of vulnerability and transient effects under interaction. Thus, it provides a temporal understanding of the effect of multiple toxins on respiratory outcomes and suggests specific time points of lung development during which these exposures may interact to negatively impact respiratory development.
For all co-exposed children (i.e., higher PM2.5 and increased stress in utero), the dentified sensitive window of PM2.5 exposure coincides with the canalicular stages of fetal lung development. Distal airway formation and proximal to distal airway epithelial differentiation occurs during the canalicular stage of development. Perturbation during this phase of development may result in airway growth restriction, consistent with previously reported CT findings in asthmatics(26) and lung function findings associated with prenatal air pollution exposure.(3) Notably, airway epithelium is a major source of IL-25, IL-33 and thymic stromal lymphopoietin, known regulators of immune-mediated inflammatory diseases such as asthma. For example, IL-25 induces eosinophilic inflammation, airway hyperresponsiveness and airway remodeling, evidenced by subepithelial collagen deposition, goblet cell hyperplasia and angiogenesis.(27) Angiogenesis and vascular development are hallmarks of the canalicular stage of development - by 24 weeks gestation the blood gas barrier is the same thickness as in adults.(28) Alterations in vascular bed structure such as increases in sub-mucosal vascular density and permeability, leading to increased blood flow and potential edema and tissue inflammation, have also been documented in asthmatic patients.(29)
Our finding that boys are the most vulnerable to air pollution and stress co-exposure during fetal development is consistent with established evidence of delayed lung maturation in boys(30) and supported by previous work by our group demonstrating a differential effect of prenatal air pollution or maternal stress on child respiratory outcomes.(7, 10) Sex-differences in lung development, such as delayed fetal breathing and surfactant production in males - critical determinants of lung development - may make males more susceptible to the deleterious effects of prenatal exposures.(31) Animal models demonstrate that prenatal exposure to noxious stimuli result in increased airway inflammation, decreased alveolarization and altered angiogenesis in males as compared to females.(32, 33) Further, female sex hormones and sex-specific placental responsiveness may be protective against oxidative stress, a mechanism that is thought to play a central role in the association between maternal stress or air pollution and child respiratory outcomes.(34) In murine models, inducing oxidative stress during the canalicular phase of lung development results in reductions in peripheral airway number, branching complexity and alveolarization. Taken together, these studies suggest that male sex is a risk factor for environmentally-induced alterations in prenatal lung development.
Our study has several strengths. First, these analyses included a reasonably large sample of lower income, ethnically mixed inner city pregnant women at increased risk for exposure to both ambient air pollution and maternal stress during pregnancy, and their children who are also at greater risk for asthma. Second, we prospectively assessed maternal stress using validated questionnaires and particulate air pollution using validated hybrid spatiotemporal land use regression models incorporating satellite-derived AOD measures based on maternal residences throughout pregnancy. These exposure estimates were applied using data-driven, advanced statistical methods to objectively identify susceptible windows during pregnancy. Finally, this is the first study to consider the sex-specific effects of co-occurring PM and maternal stress exposures on children’s asthma risk.
Some limitations are also worth noting. Our study focuses on the associations between co-exposure to prenatal ambient air pollution and maternal stress on asthma risk. We leverage methods that take advantage of highly temporally resolved (i.e., daily) estimate of ambient particulate matter derived through spatio-temporal modeling of ambient PM2.5 integrating remote-sensing satellite and land use data (35, 36). We do not adjust for indoor air pollution which is a significant exposure, particularly in inner-city homes. Some studies demonstrate that indoor air pollution, largely from cooking and tobacco smoke exposure, can be two to three times higher than ambient levels (37, 38). Studies also find that variations in indoor source particles are largely uncorrelated with variations in outdoor source particles (39). Thus, while particles of indoor origin are an important predictor of respiratory health in and of themselves, they are unlikely to confound associations between ambient particulate matter and asthma. Notably, we did adjust for tobacco smoke exposure, a major contributor to indoor pollution, in these analyses and associations remained significant.
Additionally, while we were able to adjust for some important confounders and effect modifiers, others such as prenatal maternal diet were not available in the dataset. Second, our outcome of asthma was maternal-reported physician diagnosed asthma, which may have resulted in misclassification. However, the majority of diagnoses (78.6%) were made in children over 3 years of age, reducing the likelihood that these diagnoses were representing other wheezing respiratory illnesses. Third, while we examined the interaction between PM air pollution and maternal stress exposures, the effects of the concurrent exposure to other airborne toxins were not considered. Future work should consider the interactive effects of multiple pollutants, with and without concurrent exposure to stress, in order to more fully inform our understanding of the respiratory health effects of ambient air pollution. Lastly, since our cohort represented a vulnerable population of inner city mothers and children, our findings may not be generalizable to other US communities.
In summary, we find that children concurrently exposed to higher levels of prenatal maternal stress and ambient air pollution have an increased risk of asthma and identify a mid-gestation period corresponding to the canalicular phase of fetal lung development as a sensitive window of co-exposure. In addition, boys were most vulnerable to the joint effects of PM2.5 and maternal stress. Our findings highlight the importance of considering complex interactions to more fully characterize susceptible subgroups.Future mechanistic studies aimed at elucidating the etiology of these associations will likely need to consider biomarkers of interrelated systems (i.e., immune, autonomic, neuroendocrine and oxidation systems). With the development of high-throughput systems-wide technologies, future research in this area might include metabolomics, epigenomics as well as integrated multi-omics approaches.
Key Messages.
BDLIMs demonstrate that prenatal PM2.5 exposure during the pseudoglandular and canalicular phases of lung development increase risk of childhood asthma.
Co-exposure to prenatal maternal stress modifies the effect of prenatal PM2.5 on childhood asthma and refines the window of sensitivity to the canalicular phase of lung development.
Boys are particularly susceptible to prenatal PM2.5 and maternal stress co-exposure.
Acknowledgments
The Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project has been funded by the National Institutes of Health grants R01 ES010932, U01 HL072494, and R01 HL080674 (Wright RJ, PI), and phenotyping and biostatistical support was funded by P30 ES023515, P30 ES000002, and T32 ES007142. During preparation of this manuscript AL was supported by K23 HL135349 and MJR was supported by T32 HD049311-09. The authors declare they have no competing financial interests.
Abbreviations
- ACCESS
Asthma Coalition on Community, Environment, and Social Stress
- AOD
aerosol optical depth
- BDLIM
Bayesian distributed lag interaction model
- BMI
body mass index
- CRYSIS
Crisis in Family Systems-Revised Survey
- DLM
distributed lag model
- IQR
interquartile range
- LUR
land use regression
- NLEs
negative life events
- OR
odds ratio
- PM2.5
particulate matter with an aerodynamic diameter of less than or equal to 2.5 microns
Footnotes
Conflicts of interest: None
Contributions: AL, HH, YC, RJW designed the study. IK, JS modeled air pollution exposures. HH, YC, AW, BC developed the BDLIMs. HH, YC analyzed the data and drew the figures. AL, HH, YC, SB, MR, RJW interpreted the data. AL, HH, YC, SB, MR, IK, AW, JS, SC, BC, ROW, RJW wrote the report. All authors approved the final version to be published and accept responsibility for all aspects of the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright RJ, Brunst KJ. Programming of respiratory health in childhood: influence of outdoor air pollution. Current opinion in pediatrics. 2013;25(2):232–9. doi: 10.1097/MOP.0b013e32835e78cc. [DOI] [PubMed] [Google Scholar]
- 2.Jedrychowski WA, Perera FP, Maugeri U, Majewska R, Mroz E, Flak E, et al. Long term effects of prenatal and postnatal airborne PAH exposures on ventilatory lung function of non-asthmatic preadolescent children. Prospective birth cohort study in Krakow. The Science of the total environment. 2015;502:502–9. doi: 10.1016/j.scitotenv.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latzin P, Roosli M, Huss A, Kuehni CE, Frey U. Air pollution during pregnancy and lung function in newborns: a birth cohort study. The European respiratory journal. 2009;33(3):594–603. doi: 10.1183/09031936.00084008. [DOI] [PubMed] [Google Scholar]
- 4.Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of improved air quality with lung development in children. The New England journal of medicine. 2015;372(10):905–13. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copland I, Post M. Lung development and fetal lung growth. Paediatric respiratory reviews. 2004;5(Suppl A):S259–64. doi: 10.1016/s1526-0542(04)90049-8. [DOI] [PubMed] [Google Scholar]
- 6.Mathilda Chiu YH, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. American journal of respiratory and critical care medicine. 2012;186(2):147–54. doi: 10.1164/rccm.201201-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee A, Mathilda Chiu YH, Rosa MJ, Jara C, Wright RO, Coull BA, et al. Prenatal and postnatal stress and asthma in children: Temporal-and sex-specific associations. The Journal of allergy and clinical immunology. 2016;138:740–7. doi: 10.1016/j.jaci.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen E, Schreier HM, Strunk RC, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environmental Health Perspectives. 2008;116:970–5. doi: 10.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam T, Urman R, Gauderman WJ, Milam J, Lurmann F, Shankardass K, et al. Parental stress increases the detrimental effect of traffic exposure on children’s lung function. American journal of respiratory and critical care medicine. 2011;184(7):822–7. doi: 10.1164/rccm.201104-0720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu HL, Chiu YM, Coull BA, Kloog I, Schwartz J, Lee A, et al. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children: Identifying Sensitive Windows and Sex Differences. American journal of respiratory and critical care medicine. 2015 doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorgensen A. Oxidatively generated DNA/RNA damage in psychological stress states. Danish medical journal. 2013;60(7):B4685. [PubMed] [Google Scholar]
- 12.Gidron Y, Russ K, Tissarchondou H, Warner J. The relation between psychological factors and DNA-damage: a critical review. Biological psychology. 2006;72(3):291–304. doi: 10.1016/j.biopsycho.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Torday JS, Nielsen HC. The sex difference in fetal lung surfactant production. Experimental lung research. 1987;12(1):1–19. doi: 10.3109/01902148709068811. [DOI] [PubMed] [Google Scholar]
- 14.Ishak N, Sozo F, Harding R, De Matteo R. Does lung development differ in male and female fetuses? Experimental lung research. 2014;40(1):30–9. doi: 10.3109/01902148.2013.858197. [DOI] [PubMed] [Google Scholar]
- 15.Gasparrini A. Modeling exposure-lag-response associations with distributed lag non-linear models. Statistics in medicine. 2014;33(5):881–99. doi: 10.1002/sim.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanobetti A, Wand MP, Schwartz J, Ryan LM. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics (Oxford, England) 2000;1(3):279–92. doi: 10.1093/biostatistics/1.3.279. [DOI] [PubMed] [Google Scholar]
- 17.Wilson AC, Yueh-Hsiu, Hsu Hsiao-Hsien, Wright Robert, Wright Rosalind, Coull Brent. Bayesian Distributed Lag Interaction Models to Identify Perinatal Windows of Vulnerability in Child Health. Biostatistics (Oxford, England) 2017 doi: 10.1093/biostatistics/kxx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright RJ, Suglia SF, Levy J, Fortun K, Shields A, Subramanian S, et al. Transdisciplinary research strategies for understanding socially patterned disease: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Ciencia & saude coletiva. 2008;13(6):1729–42. doi: 10.1590/s1413-81232008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloog IKP, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45:6267–75. [Google Scholar]
- 20.Berry CA, Quinn KA, Portillo N, Shalowitz MU. Reliability and validity of the Spanish version of the Crisis in Family Systems-Revised. Psychological Reports. 2006;98:123–32. doi: 10.2466/pr0.98.1.123-132. [DOI] [PubMed] [Google Scholar]
- 21.Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Services Research. 1998;33(5):1382–402. [PMC free article] [PubMed] [Google Scholar]
- 22.Wright RJ, Fisher K, Chiu YH, Wright RO, Fein R, Cohen S, et al. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. American journal of respiratory and critical care medicine. 2013;187(11):1186–93. doi: 10.1164/rccm.201208-1530OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS medicine. 2014;11(1):e1001596. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environmental research. 2012;117:100–11. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Wilson A, Chiu Y-HM, Hsu H-HL, Wright RO, Wright RJ, Coull BA. Potential for Confounding Bias When Estimating the Association Between Prenatal Exposure to Air Pollution and Children’s Health Outcomes. American journal of epidemiology. 2017 doi: 10.1093/aje/kwx184. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Hartley R, Khan UT, Singapuri A, Hargadon B, Monteiro W, et al. Quantitative computed tomography-derived clusters: redefining airway remodeling in asthmatic patients. The Journal of allergy and clinical immunology. 2014;133(3):729–38. e18. doi: 10.1016/j.jaci.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao X, Sun Y, Wang W, Sun Y. Interleukin (IL)-25: Pleiotropic roles in asthma. Respirology (Carlton, Vic) 2016;21(4):638–47. doi: 10.1111/resp.12707. [DOI] [PubMed] [Google Scholar]
- 28.Hislop A. Developmental biology of the pulmonary circulation. Paediatric respiratory reviews. 2005;6(1):35–43. doi: 10.1016/j.prrv.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Keglowich LF, Borger P. The Three A’s in Asthma - Airway Smooth Muscle, Airway Remodeling & Angiogenesis. The open respiratory medicine journal. 2015;9:70–80. doi: 10.2174/1874306401509010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gortner L, Shen J, Tutdibi E. Sexual dimorphism of neonatal lung development. Klinische Padiatrie. 2013;225(2):64–9. doi: 10.1055/s-0033-1333758. [DOI] [PubMed] [Google Scholar]
- 31.Torday JS, Nielsen HC, de Fencl MM, Avery ME. Sex differences in fetal lung maturation. The American review of respiratory disease. 1981;123(2):205–8. doi: 10.1164/arrd.1981.123.2.205. [DOI] [PubMed] [Google Scholar]
- 32.Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. American journal of physiology Lung cellular and molecular physiology. 2016;311(2):L481–93. doi: 10.1152/ajplung.00047.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Naeem E, Tian J, Lombardi V, Kwong K, Akbari O, et al. Sex-specific perinatal nicotine-induced asthma in rat offspring. American journal of respiratory cell and molecular biology. 2013;48(1):53–62. doi: 10.1165/rcmb.2011-0344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minghetti L, Greco A, Zanardo V, Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26(3):259–62. doi: 10.3109/14767058.2012.733751. [DOI] [PubMed] [Google Scholar]
- 35.Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, et al. A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmospheric Environment. 2014;95:581–90. doi: 10.1016/j.atmosenv.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy PM2.5 exposure, premature birth and birth weight in Massachusetts. Environ Health. 2012;11:40. doi: 10.1186/1476-069X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace LA, Mitchell H, O’Connor GT, Lippmann M, Kattan M, Koenig J, et al. Particle concentration in inner-city homes of children with asthma: the effect of smoking, cooking and outdoor pollution. Environ Health Perspect. 2003;111:1265–72. doi: 10.1289/ehp.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, et al. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117:294–98. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson WE, Mage DT, Grant LD. Estimating separately personal exposure to ambient and nonambient particulate matter for epidemiology and risk assessment. Why and how. J Air Waste manage Assoc. 2000;50:1167–1183. doi: 10.1080/10473289.2000.10464164. [DOI] [PubMed] [Google Scholar]
- 40.Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998;33(5 Pt 1):1381–402. [PMC free article] [PubMed] [Google Scholar]