Abstract
Traditional methods of health surveillance often under-represent racial and ethnic minorities. Our objective was to use geospatial analysis and emergency claims data to estimate local chronic disease prevalence separately for specific racial and ethnic groups. We also performed a regression analysis to identify associations between median household income and local disease prevalence among Black, Hispanic, Asian, and White adults in New York City. The study population included individuals who visited an emergency department at least once from 2009–2013. Our main outcomes were geospatial estimates of diabetes, hypertension, and asthma prevalence by Census tract as stratified by race and ethnicity. Using emergency claims data, we identified 4.9 million unique New York City adults with 28.5% of identifying as Black, 25.2% Hispanic, and 6.1% Asian. Age-adjusted disease prevalence was highest among Black and Hispanic adults for diabetes (13.4% and 13.1%), hypertension (28.7% and 24.1%), and asthma (9.9% and 10.1%). Correlation between disease prevalence maps demonstrated moderate overlap between Black and Hispanic adults for diabetes (0.49), hypertension (0.57), and asthma (0.58). In our regression analysis, we found that the association between low income and high disease prevalence was strongest for Hispanic adults, whereas increases in income had more modest reductions in disease prevalence for Black adults, especially for diabetes. Our geographically detailed maps of disease prevalence generate actionable evidence that can help direct health interventions to those communities with the highest health disparities. Using these novel geographic approaches, we reveal the underlying epidemiology of chronic disease for a racially and culturally diverse population.
Keywords: minority health, chronic disease, public health surveillance, emergency department, geographic information systems, administrative claims
INTRODUCTION
Traditional health surveillance methods, such as telephone-based surveys, are not only limited by small sample sizes, but substantially under-represent many racial and ethnic minorities.[1] Therefore, it is extremely difficult to identify the underlying epidemiology of chronic disease prevalence as stratified by race and ethnicity.[2] However, we have recently demonstrated the feasibility of using emergency department data to obtain a large population-based sample and identify the local prevalence of common chronic diseases like diabetes, hypertension, and asthma at a neighborhood level.[3] In this study, we use these approaches to study chronic disease prevalence separately for specific racial and ethnic groups. To address health disparities, preventable differences in disease prevalence can be analyzed not only between-groups, but also within-groups to understand which specific communities experience the highest health burdens.
The advantages of emergency department data are that it is already widely available in existing databases and captures a large study sample. In any given year, one in five adults reports visiting an emergency department at least once, providing a 20% population sample with just one year of data.[4] With multiple years of data, it is easy to obtain health records for a majority of the entire population.[3] Furthermore, this sample of emergency department patients has a substantial representation of minority groups, who often face the poorest health outcomes and access to health care.[5] Thus, emergency claims data can become a powerful tool for studying the intersection of disease with race and ethnicity and evaluating within-group health disparities.[6]
The objective of this study was to investigate how large scale emergency claims data could be used to enhance chronic disease surveillance among racial and ethnic minorities. We applied our novel geographic method of emergency department surveillance to identify the local prevalence of diabetes, hypertension and asthma among Black, Hispanic, and Asian groups. These conditions were chosen because they were included in the original validation study of this method. We used detailed patient level address data to identify the local geographic patterns of chronic disease prevalence by racial and ethnic group. Finally, we also investigated how socioeconomic factors such as household income affect chronic disease prevalence in these different racial and ethnic groups.
METHODS
Study Design and Setting
To compare the local geographic patterns of chronic disease prevalence by race and ethnicity, we used a recently validated method of identifying individuals with diseases like diabetes, hypertension, and asthma using emergency claims data.[3] The study period was 2009–2013, and our study setting was New York City, a diverse urban setting with approximately 22.7% Black residents, 28.7% Hispanic residents, 12.9% Asian residents, and 33.1% White residents based on data from the American Community Survey 2009–2013. Chronic disease prevalence was assessed at the Census tract level using geocoded addresses. We then determined how associations between local chronic disease prevalence and median household income differed by race and ethnicity.
Data Sources
SPARCS Database
The New York State Department of Health established the Statewide Planning and Research Cooperative System (SPARCS) to collect administrative claims data from inpatient hospitalizations, emergency department visits, and other health care utilization across all insurance types. Along with demographics and diagnosis codes, it includes unique identifiers that can track visits by the same patient across different hospitals. It also contains address data, which can be geocoded to identify the exact location of a patient’s residence.
American Community Survey
To analyze the association of local chronic disease prevalence with median household income, we used Census tract level data from the American Community Survey from 2009–2013. These values were compared to rates of chronic disease by Census tract to determine how disease prevalence changed with increasing income at a local level.
Participants
We included all adult patients aged 18 years and older who visited an emergency department located in a general acute care hospital in New York State between 2009 and 2013. Included patients had geocodable home address located in New York City. We excluded patients from correctional facilities or transferred from nursing homes and other health care facilities to determine disease prevalence for the non-institutionalized adult population.
Patients who had multiple emergency department visits at the same or across several hospitals during the study period were only counted as a single observation by collapsing emergency department visits using unique identifiers in SPARCS. The result was a population that represented unique New York City residents who had accessed emergency care at least once during the study period.
We used race and ethnicity flags in SPARCS to stratify patients. The specific racial and ethnic groups analyzed were non-Hispanic Black adults (hereon referred to as Black), Hispanic adults (including any race), non-Hispanic Asian adults (hereon referred to as Asian), and non-Hispanic White adults (hereon referred to as White). To account for inconsistencies in categorizations of race and ethnicity, we conservatively only included patients consistently categorized as Black, Hispanic, Asian, or White across multiple emergency visits. We excluded patients whose racial classification was only marked as other or was missing, in addition to any patient for which available racial and ethnic classification data was conflicting.
Main Outcome
Our outcome measure was the local prevalence of chronic disease by New York City Census tract for Black, Hispanic, Asian, and White adults. After accounting for repeat emergency department visits by the same individual, all primary and secondary ICD-9 diagnosis codes for each unique patient were analyzed for the presence of diabetes (250), hypertension (401 to 405), and asthma (493). To calculate chronic disease prevalence, the number of unique patients with any diagnosis code for each condition was divided by the total number of unique patients by Census tract. This disease prevalence was age-adjusted using the direct method described by the Centers for Disease Control and Prevention and four age stratifications (18 to 24, 25 to 44, 45 to 64, 65 and older).[7] Geospatial smoothing techniques described below were used to map rates using age-adjusted counts.
Statistics
Population characteristics and our main outcome were first summarized for each of race and ethnicity in our study. Age strata, sex, insurance status, in addition to overall unadjusted and age-adjusted disease prevalence are reported for each group. Given the large sample sizes, all differences were statistically significant, thus only differences in proportions greater than 5% are reported in the results.
In our analysis, we excluded 50 Census tracts that were non-residential areas, e.g., parks and airports, based on zero or low population counts in the American Community Survey data. In addition, we excluded Census tracts that had fewer than 30 unique individuals in emergency department data that met inclusion criteria for the study. This additional exclusion was performed to reduce the bias of inaccurate disease prevalence estimates due to limited data.
To smooth local rates of chronic disease prevalence by Census tract, we used Spatial Empirical Bayes modeling with first-order rook spatial weights.[8] This process started with the underlying age-adjusted count data for rates of chronic disease by Census tract. Then, we used the rates calculated in adjacent neighboring Census tracts that shared a border segment to determine the final smoothed local estimate of chronic disease prevalence in each Census tract using a Spatial Empirical Bayes modeling approach. This smoothing was performed to reduce error from small sample sizes and use neighboring Census tracts to provide enhanced estimates of local chronic disease prevalence.
To compare the geographic distribution of high and low chronic disease prevalence by race and ethnicity, we analyzed the correlation between geospatially smoothed estimates of diabetes, hypertension, and asthma prevalence among Black, Hispanic, Asian, and White adults by Census tract. The Pearson product moment correlation was used to compare estimates with 99.92% confidence intervals using a Bonferroni correction for 18 pairwise correlations. By convention, correlations were categorized as very weak (0.00–0.19), weak (0.20–0.39), moderate (0.40–0.59), strong (0.60–0.79), and very strong (0.80–1.00).
Finally, we compared the association between local chronic disease prevalence and income by race and ethnicity. To perform this analysis, we used linear regression of median household income by Census tract and the geospatially smoothed estimates of diabetes, hypertension, and asthma prevalence for Black, Hispanic, Asian, and White adults. The regression coefficients in this analysis were reinterpreted to determine what absolute change in disease prevalence would result from a change in median household income of $100,000 by Census tract. (Regression equation: [Chronic Disease Prevalence] = coefficient [Income by Census tract] + error). We also compared the relative effect size of this income difference by dividing the absolute change in disease prevalence by the average disease prevalence for each racial and ethnic group. Statistically significant differences were analyzed using 99.96% confidence intervals using a Bonferroni correction for 12 separate linear regressions. We also reported the coefficient of determination (R2) as a measure of the variance in chronic disease prevalence explained by median household income for each race and ethnicity.
Statistical analyses were performed in Stata 14.2 (StataCorp: College Station, TX. 2015). Geospatial analysis was performed using GeoDa 1.8.12 (Luc Anselin: Arizona State University: Tempe, AZ. 2016). Maps were created in ArcGIS Desktop 10.3.1 (ESRI: Redlands, CA. 2015). Our study protocol was approved by the Institutional Review Board at the New York University School of Medicine.
RESULTS
Study Population
Using large scale emergency claims data, we identified 4.9 million unique adults in New York City who had visited an emergency department at least once during 2009–2013. This represents a substantial proportion of the estimated 6.8 million adults that reside in New York City. In our study population, 28.5% of adults were Black, 25.2% were Hispanic, 6.1% were Asian, 24.4% were White, and 15.9% were excluded because of inadequate or conflicting data on race and ethnicity. There were a higher proportion of Black and Hispanic adults than Asian and White adults aged 18 to 24, and a higher proportion of White adults than minorities aged 65 years and older (Table 1). In addition, there was a substantially higher proportion of White adults with private insurance or Medicare compared to racial and ethnic minorities, who had higher rates of Medicaid and uninsurance. Compared to Census estimates, there were proportionally more Black residents and fewer Asian residents in our study sample than the general population.
Table 1.
Population Characteristics Using Emergency Department Surveillance by Race and Ethnicity in New York City
| Characteristics | Black Adults | Hispanic Adults | Asian Adults | White Adults |
|---|---|---|---|---|
| Sample | 1,386,859 | 1,226,694 | 295,139 | 1,188,220 |
| Proportion | 28.5% | 25.2% | 6.1% | 24.4% |
| Age | ||||
| 18–24 | 18.0% | 19.9% | 13.0% | 11.8% |
| 25–44 | 38.6% | 41.8% | 43.0% | 35.8% |
| 45–64 | 30.0% | 26.1% | 27.6% | 25.7% |
| 65 & Up | 13.4% | 12.2% | 16.4% | 26.7% |
| Gender | ||||
| Male | 42.0% | 44.4% | 40.9% | 45.7% |
| Female | 58.0% | 55.6% | 59.1% | 54.3% |
| Insurance | ||||
| Private | 29.4% | 22.6% | 26.7% | 46.7% |
| Medicare | 13.1% | 12.1% | 12.6% | 25.8% |
| Medicaid | 30.6% | 37.1% | 39.2% | 14.0% |
| Uninsured | 26.9% | 28.2% | 21.5% | 13.5% |
| Unadjusted Prevalence | ||||
| Diabetes | 12.2% | 10.9% | 11.4% | 9.6% |
| Hypertension | 26.2% | 19.8% | 20.7% | 26.1% |
| Asthma | 9.9% | 9.8% | 3.5% | 5.0% |
| Age-Adjusted Prevalence | ||||
| Diabetes | 13.4% | 13.1% | 11.7% | 7.9% |
| Hypertension | 28.7% | 24.1% | 21.2% | 20.9% |
| Asthma | 9.9% | 10.1% | 3.6% | 4.9% |
Main Outcome Measures
The unadjusted diabetes prevalence was highest among Black adults, whereas the unadjusted hypertension prevalence was nearly equivalent between Black and White adults. Unadjusted asthma prevalence was highest among Black and Hispanic adults. After age-adjustment using the direct method, adjusted diabetes prevalence was still highest among Black adults (13.4%); however, was nearly matched by adjusted diabetes prevalence among Hispanic adults (13.1%). Age-adjusted hypertension prevalence was much higher among Black adults (28.7%) and was no longer equivalent with White adults (20.9%). Finally, age-adjusted asthma prevalence continued to be highest among Black and Hispanic adults (9.9% and 10.1%).
Geographic Distribution of Chronic Disease
We mapped the geographic distribution of these chronic diseases using geospatially smoothed rates for each racial and ethnic group. For comparison, we show age-adjusted diabetes prevalence among Black (Figure 1), Hispanic (Figure 2), and Asian adults (Appendix). For comparison, age-adjusted diabetes prevalence among White adults is also found in the appendix. These Census tract level maps demonstrate substantial differences in the concentration of chronic disease prevalence among the different racial and ethnic groups.
Fig. 1.
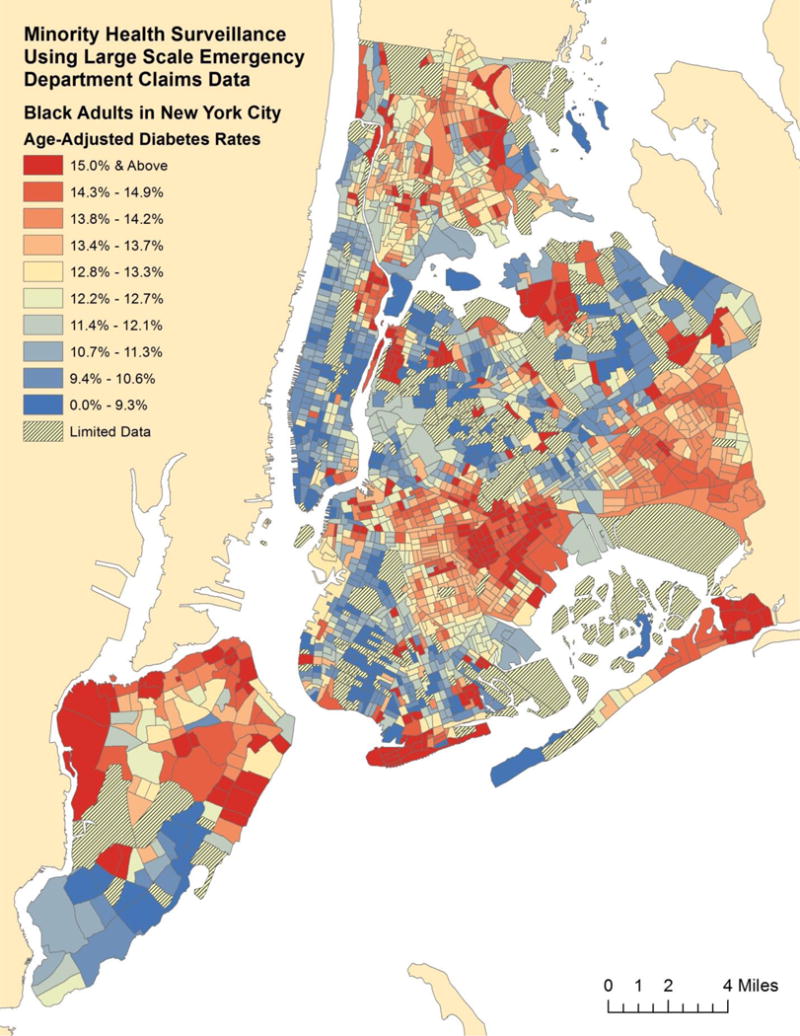
Age-Adjusted Diabetes Prevalence Estimates for Black Adults by Census Tract in New York City
Fig. 2.
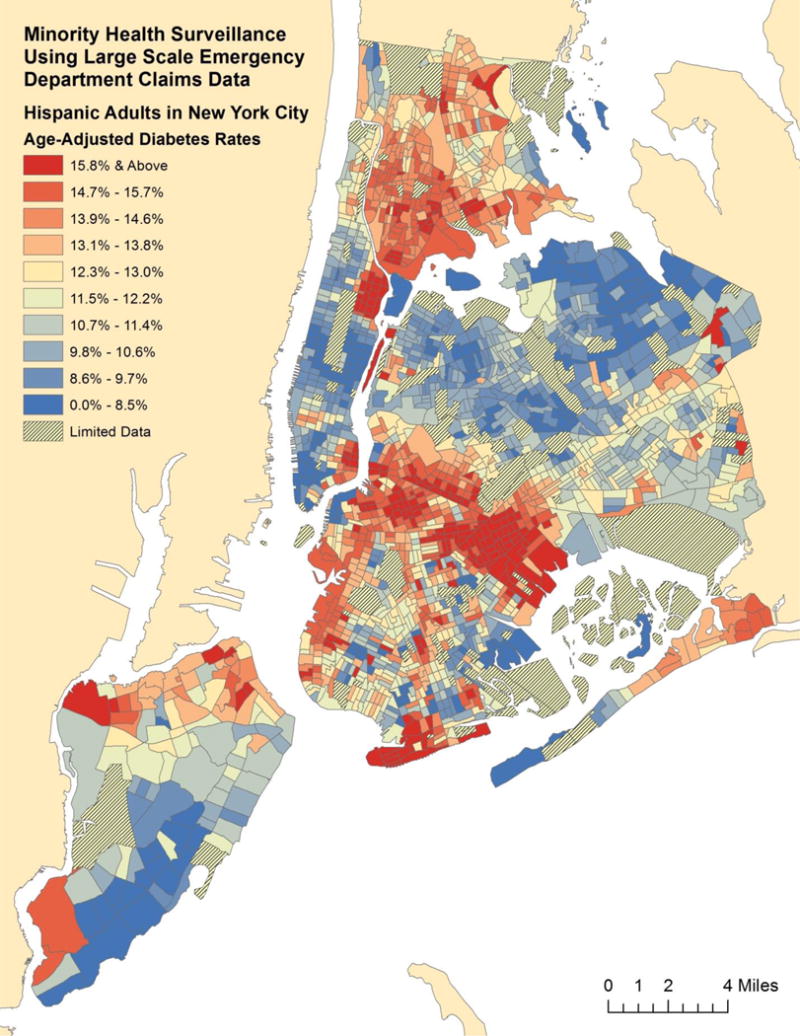
Age-Adjusted Diabetes Prevalence Estimates for Hispanic Adults by Census Tract in New York City
Furthermore, our analysis of correlations by race and ethnicity identifies the differences in the geographic distribution of chronic disease prevalence (Table 2). For diabetes prevalence, correlations between Black and Hispanic groups and between Asian and White groups were moderate (0.49 and 0.48), meaning that the overlap of high and low prevalence areas for diabetes between these groups was moderate. Other pairwise correlations between diabetes prevalence by race and ethnicity were weak, meaning that there was little overlap of high and low prevalence areas for diabetes between other groups. We found a similar pattern of correlation by race and ethnicity for hypertension prevalence. However, for asthma prevalence, correlations between Black and Hispanic groups and between Hispanic and White groups were moderate (0.58 and 0.41). Whereas all other pairwise correlations were weak or very weak.
Table 2.
Correlation of Chronic Disease Prevalence Between Race and Ethnicities by Census Tract in New York City
| Disease | Black Adults | Hispanic Adults | Asian Adults | White Adults |
|---|---|---|---|---|
| Diabetes | ||||
| Black Adults | – | 0.49 (0.43–0.55) | 0.35 (0.27–0.42) | 0.32 (0.25–0.39) |
| Hispanic Adults | 0.49 (0.43–0.55) | – | 0.26 (0.18–0.34) | 0.22 (0.15–0.29) |
| Asian Adults | 0.35 (0.27–0.42) | 0.26 (0.18–0.34) | – | 0.48 (0.41–0.54) |
| White Adults | 0.32 (0.25–0.39) | 0.22 (0.15–0.29) | 0.48 (0.41–0.54) | – |
| Hypertension | ||||
| Black Adults | – | 0.57 (0.51–0.61) | 0.31 (0.23–0.38) | 0.26 (0.19–0.33) |
| Hispanic Adults | 0.57 (0.51–0.61) | – | 0.22 (0.14–0.30) | 0.17 (0.10–0.24) |
| Asian Adults | 0.31 (0.23–0.38) | 0.22 (0.14–0.30) | – | 0.54 (0.47–0.60) |
| White Adults | 0.26 (0.19–0.33) | 0.17 (0.10–0.24) | 0.54 (0.47–0.60) | – |
| Asthma | ||||
| Black Adults | – | 0.58 (0.52–0.62) | 0.19 (0.10–0.27) | 0.31 (0.24–0.38) |
| Hispanic Adults | 0.58 (0.52–0.62) | – | 0.19 (0.11–0.27) | 0.41 (0.34–0.47) |
| Asian Adults | 0.19 (0.10–0.27) | 0.19 (0.11–0.27) | – | 0.24 (0.16–0.31) |
| White Adults | 0.31 (0.24–0.38) | 0.41 (0.34–0.47) | 0.24 (0.16–0.31) | – |
Notes: Correlations compare the overlap of high and low prevalence areas between racial and ethnic groups. Parentheses with 99.92% confidence intervals.
Association of Chronic Disease Prevalence with Income
We found that the prevalence of all chronic diseases studied decreased the most among Hispanic adults with an increase in median household income compared to other groups (Table 3). For a $100,000 increase in income, diabetes prevalence among Hispanic adults decreased by 5.0 percentage points, hypertension prevalence decreased by 5.6 percentage points, and asthma prevalence decreased by 3.8 percentage points. In addition, the coefficient of determination was consistently higher among Hispanic adults for the association of income with all chronic diseases studied. This finding suggests that a greater proportion of the variation in chronic disease prevalence is explained by income for Hispanic adults than other groups. On a relative basis, diabetes prevalence dropped only to 0.80× of its average value among Black adults with a $100,000 increase in income, whereas it dropped much lower on a relative basis among Hispanic, Asian, and White adults (0.61×, 0.61×, and 0.65× respectively).
Table 3.
Decrease in Age-Adjusted Disease Prevalence by Race and Ethnicity Based on Change in Median Household Income by Census Tract in New York City
| Increase in Income by $100,000 | Black Adults | Hispanic Adults | Asian Adults | White Adults |
|---|---|---|---|---|
| Absolute Change in Disease Prevalence by Percentage | ||||
| Diabetes | −2.6% (−3.2% to −2.1%) | −5.0% (−5.6% to −4.5%) | −4.5% (−5.5% to −3.6%) | −2.8% (−3.2% to −2.3%) |
| Hypertension | −3.8% (−4.7% to −3.0%) | −5.6% (−6.3% to −4.9%) | −3.3% (−4.4% to −2.2%) | −1.9% (−2.7% to −1.0%) |
| Asthma | −2.6% (−3.2% to −2.1%) | −3.8% (−4.4% to −3.1%) | −1.0% (−1.4% to −0.5%) | −1.7% (−2.0% to −1.4%) |
| Relative Change in Disease Prevalence by Ratio | ||||
| Diabetes | 0.80× (0.76–0.84) | 0.61× (0.57–0.66) | 0.61× (0.53–0.70) | 0.65× (0.59–0.70) |
| Hypertension | 0.87× (0.84–0.90) | 0.77× (0.74–0.80) | 0.84× (0.79–0.90) | 0.91× (0.87–0.95) |
| Asthma | 0.73× (0.68–0.79) | 0.63× (0.56–0.70) | 0.73× (0.62–0.85) | 0.65× (0.58–0.71) |
| Coefficient of Determination (R2) | ||||
| Diabetes | 0.09 | 0.24 | 0.10 | 0.13 |
| Hypertension | 0.07 | 0.20 | 0.04 | 0.02 |
| Asthma | 0.09 | 0.11 | 0.03 | 0.11 |
Notes: Parentheses with 99.58% confidence intervals.
DISCUSSION
By using geospatial analysis and emergency claims data, we demonstrated that it is possible to enhance the surveillance of chronic disease among racial and ethnic minority adults. With five years of data, we identified a substantial majority of all adult New York City residents and used diagnosis codes to create geographically detailed maps of diabetes, hypertension, and asthma prevalence as stratified by race and ethnicity. The advantage of applying these novel geographic methods to existing emergency claims databases is that it provides an incredibly large population sample with a strong representation by minority groups.[5]
Our study results identified specific geographic areas with a higher prevalence of chronic disease for each racial and ethnic group. To begin an investigation of the socioeconomic factors that might explain these health disparities, we also analyzed the influence of income differences on these geographic distributions of diabetes, hypertension, and asthma prevalence. We found that the income effects were much more profound for Hispanic adults compared to other groups. In comparison, increases in income were associated with smaller relative decreases in chronic disease prevalence among Black adults, especially for diabetes. These findings are evidence that race and ethnicity can significantly modify the association of chronic disease prevalence and income at a local geographic level.[9] Though health disparities research has demonstrated that many minorities have been isolated in low income areas, our results suggest that disparities in chronic disease prevalence may not be driven by income alone. Future studies should investigate how other socioeconomic factors such as education, housing and residential stability and environmental factors such as proximity of healthy and unhealthy food sources may influence the presence of these within-group geographic disparities in chronic disease prevalence.
Though health disparities are largely attributed to socioeconomic disparities, our findings suggest that simply improving incomes may not reduce disease burden substantially across all races and ethnicities.[10] The development of disease is also driven by other social, behavioral, and cultural factors.[11] Effective interventions to reduce health disparities may require approaches that are tailored for communities that have the highest burden of disease.[12] Our novel geographic approach can help identify these areas, which may have shared culture and behavioral patterns as well as shared geography and disease risk. Our geographically detailed maps provide actionable evidence of where to geographically target interventions by identifying which neighborhoods experience the worst health outcomes.[13] These findings can also spur investigations of what specific health challenges these geographic areas face, which can help shape the interventions for minority communities that face the highest health disparities.[14]
Overall, we found that the geographic distribution of chronic disease prevalence were not strongly correlated between specific racial and ethnic groups. This lack of correlation suggests that areas of high and low chronic disease prevalence are not located in the same parts of the city for different racial and ethnic groups. These within-group health disparities demonstrate that the neighborhoods that face the highest health burdens differ depending on the race or ethnicity studied. This finding may be driven largely by the fact that certain races and ethnicities cluster within specific neighborhoods in New York City.[15] However, we found that for diabetes and hypertension, geographic areas of high and low disease prevalence were moderately correlated between Black and Hispanic adults. But for asthma, disease maps also had moderate overlap between Hispanic and White adults.[16] These differences among diseases suggest that environmental influences whether physical or social may differ depending on the condition studied.[17, 18] Understanding the underlying epidemiology of each chronic disease is a critical first step in reducing health disparities, and it is important to identify the geographic variation in disease burden that exists within each racial and ethnic group.[19]
Limitations
Our study was observational, therefore associations cannot be taken as evidence of causation. SPARCS is also known to have some issues with accuracy of race and ethnicity coding by certain hospitals that contribute data.[20] We overcame some of these limitations by ignoring observations where classification of race and ethnicity was ambiguous. However, these exclusions may have introduced bias in our chronic disease prevalence estimates. We also found that collapsing data across hospitals help filled in missing data as some hospitals frequently listed race as other without categorizing ethnicity for Hispanic adults. We captured a diverse population, but Asians were notably underrepresented in our sample. Our study also did not include children who have very different patterns of chronic diseases. Finally, claims data provided by hospitals can contain other types of coding errors. In our experience, these methods may produce erroneous results when examining individual observations, but the large sample size generated can smooth over the relatively small number of these inconsistencies.[3]
Supplementary Material
Acknowledgments
This study was funded by a grant K23DK110316 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) for geographic and community-based research on chronic disease disparities among minority adults.
Funding: This study was funded by the National Institute of Diabetes and Digestive and Kidney Disease (grant number K23DK110316) for geographic and community-based research on chronic disease disparities among minority adults.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest: David C. Lee, Stella S. Yi, Jessica K. Athens, Andrew J. Vinson, Stephen P. Wall, and Joseph E. Ravenell declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1.Boland M, Sweeney MR, Scallan E, Harrington M, Staines A. Emerging advantages and drawbacks of telephone surveying in public health research in Ireland and the U.K. BMC public health. 2006;6:208. doi: 10.1186/1471-2458-6-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piccolo RS, Duncan DT, Pearce N, McKinlay JB. The role of neighborhood characteristics in racial/ethnic disparities in type 2 diabetes: results from the Boston Area Community Health (BACH) Survey. Social science & medicine (1982) 2015;130:79–90. doi: 10.1016/j.socscimed.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DC, Long JA, Wall SP, Carr BG, Satchell SN, Braithwaite RS, et al. Determining Chronic Disease Prevalence in Local Populations Using Emergency Department Surveillance. American journal of public health. 2015 Sep;105(9):e67–74. doi: 10.2105/ajph.2015.302679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Health, United States 2013: With Special Feature on Prescription Drugs. Hyattsville, MD: National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
- 5.Lee DC, Doran KM, Polsky D, Cordova E, Carr BG. Geographic variation in the demand for emergency care: A local population-level analysis. Healthcare (Amsterdam, Netherlands) 2016;4(2):98–103. doi: 10.1016/j.hjdsi.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Lee DC, Long JA, Sevick MA, Yi SS, Athens JK, Elbel B, et al. The local geographic distribution of diabetic complications in New York City: Associated population characteristics and differences by type of complication. Diabetes research and clinical practice. 2016;119:88–96. doi: 10.1016/j.diabres.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected US population Healthy People Statistical Notes. Hyattsville, Maryland: National Center for Health Statistics; Jan, 2001. [PubMed] [Google Scholar]
- 8.Anselin L, Syabri I, Kho Y. GeoDa: An Introduction to Spatial Data Analysis. Geographical Analysis. 2006;38(1):5–22. doi: 10.1111/j.0016-7363.2005.00671.x. [DOI] [Google Scholar]
- 9.Suchindran S, Vana AM, Shaffer RA, Alcaraz JE, McCarthy JJ. Racial differences in the interaction between family history and risk factors associated with diabetes in the National Health and Nutritional Examination Survey, 1999–2004. Genetics in medicine: official journal of the American College of Medical Genetics. 2009;11(7):542–7. doi: 10.1097/GIM.0b013e3181a70917. [DOI] [PubMed] [Google Scholar]
- 10.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health affairs (Project Hope) 2002;21(2):60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 11.Kindig DA, Asada Y, Booske B. A population health framework for setting national and state health goals. JAMA: the journal of the American Medical Association. 2008;299(17):2081–3. doi: 10.1001/jama.299.17.2081. [DOI] [PubMed] [Google Scholar]
- 12.Williams IC, Utz SW, Hinton I, Yan G, Jones R, Reid K. Enhancing diabetes self-care among rural African Americans with diabetes: results of a two-year culturally tailored intervention. The Diabetes educator. 2014;40(2):231–9. doi: 10.1177/0145721713520570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davila-Payan C, DeGuzman M, Johnson K, Serban N, Swann J. Estimating prevalence of overweight or obese children and adolescents in small geographic areas using publicly available data. Preventing chronic disease. 2015;12:E32. doi: 10.5888/pcd12.140229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro FG, Shaibi GQ, Boehm-Smith E. Ecodevelopmental contexts for preventing type 2 diabetes in Latino and other racial/ethnic minority populations. Journal of behavioral medicine. 2009;32(1):89–105. doi: 10.1007/s10865-008-9194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim S, Harris TG. Neighborhood Contributions to Racial and Ethnic Disparities in Obesity Among New York City Adults. American journal of public health. 2015;105(1):159–65. doi: 10.2105/ajph.2013.301782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corburn J, Osleeb J, Porter M. Urban asthma and the neighbourhood environment in New York City. Health & place. 2006;12(2):167–79. doi: 10.1016/j.healthplace.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117(2):417–24. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- 18.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner-city asthma epidemic. The Journal of allergy and clinical immunology. 2015;135(3):655–62. doi: 10.1016/j.jaci.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhotra K, Baltrus P, Zhang S, McRoy L, Immergluck LC, Rust G. Geographic and racial variation in asthma prevalence and emergency department use among Medicaid-enrolled children in 14 southern states. The Journal of asthma: official journal of the Association for the Care of Asthma. 2014;51(9):913–21. doi: 10.3109/02770903.2014.930479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.New York State Department of Health. Facility Race and Ethnicity Concordance Reports. 2012 https://www.health.ny.gov/statistics/sparcs/reports/race_eth/. Accessed October 4th 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


