Abstract
The endosomal sorting complex required for transport (ESCRT) machinery is composed of five multi-subunit protein complexes, which act cooperatively at specialized endosomes to facilitate the movement of specific cargoes from the limiting membrane into vesicles that bud into the endosome lumen. Over the past decade, numerous proteins, lipids, and RNAs have been shown to be incorporated into intralumenal vesicles (ILVs), but the mechanisms by which these unique cargoes are captured are only now becoming better understood. Here, we discuss the potential roles that the ESCRT machinery plays during cargo sorting at multivesicular endosomes (MVEs).
Keywords: ESCRT machinery, multivesicular endosome, cargo sorting, intralumenal vesicle, ubiquitin
1. Introduction
Eukaryotic cells have evolved elaborate mechanisms to sense environmental cues, rapidly initiate responses mediated by cell surface transmembrane receptors, and ultimately downregulate signaling via receptor sequestration within endosomal compartments. This pathway was originally described several decades ago in elegant electron microscopy-based studies using labeled epidermal growth factor (EGF)1–4, which is internalized rapidly into cells and deposited into multivesicular endosomes (MVEs; also referred to as multivesicular bodies/MVBs). Identification of MVEs at the ultrastructural level is made relatively simple by their characteristic morphology: membrane-bound organelles that harbor small intralumenal vesicles (ILVs). To generate these unique membrane compartments, eukaryotes use a set of protein complexes collectively known as the Endosomal Sorting Complex Required for Transport (ESCRT) machinery, which were defined originally in a series of genetic screens using yeast5–10. In the absence of ESCRT function, transmembrane cargoes are unable to efficiently reach the lumen of the vacuole/lysosome, resulting in the formation of an aberrant prevacuolar organelle in yeast termed the ‘class E compartment’. Since their initial discovery, the field has begun to reach consensus regarding the roles of the ESCRT complexes at the endosome limiting membrane, which include cargo selection, membrane deformation to generate nascent, inward-budded vesicles, cargo sequestration within these newly formed vesicles, and narrowing of the vesicle bud neck to ultimately facilitate release of ILVs into the lumen of the endosome.
During the characterization of these processes, several model cargoes were identified as substrates of the ESCRT machinery. Perhaps most famously, post-translational modification of the EGF receptor by ubiquitin was shown to play an integral role in its endosomal sorting and degradation11–14. Together with other seminal discoveries linking ubiquitin to the ESCRT machinery at MVEs, the trajectory for the field was set, and a unifying mechanism that supports the entry of numerous, unrelated cargoes into the endosome lumen was defined8,15–18. More recently, additional cargoes that do not rely on ubiquitin modification have also been shown to enter ILVs, including proteins (both soluble and transmembrane), specialized lipids, and nucleic acids, suggesting that multiple modes of cargo recognition exist19–24. Furthermore, the fate of MVEs has been expanded beyond lysosomal fusion and ILV degradation. In particular, some MVEs are specialized to facilitate the dissemination of cargoes to the extracellular environment, fusing with the plasma membrane to release their ILVs (exosomes)25.
The spatial distribution of MVEs can vary widely between cell types, and probes specific to the organelle have been challenging to identify, as they regularly undergo fusion with lysosomes and other membrane compartments. Similarly, the size and content of MVEs are not uniform and depend on cell cycle stage, nutrient availability, and other environmental conditions. Components of the ESCRT machinery may represent the best markers available, given their intimate role in MVE biogenesis. In general, mammalian MVEs tend to accumulate near the center of cells at steady state26, in a manner dependent on dynein-mediated transport27, which positions them well to receive biosynthetic cargoes from the perinuclear trans-Golgi network. However, a large number of cargoes also originate at the cell surface, entering the endosomal system via clathrin-dependent and clathrin-independent endocytosis, phagocytosis, and micropinocytosis28–31. Based on live cell total internal reflection microscopy (TIRFM), components of the ESCRT machinery have been observed at clathrin-coated structures at or near the plasma membrane, suggesting that cargoes are sorted rapidly upon cellular internalization32. Thus, MVE formation likely initiates at multiple subcellular locations to sequester cargoes destined for degradation or exocytosis. Perhaps not surprisingly, growth factor stimulation augments the rate of MVE formation, enabling a rapid response to limit cell signaling that is initiated upon receptor binding33–35.
Based on genetic and biochemical evidence, more than two dozen ESCRT proteins have been implicated in the deposition of cargoes into ILVs36. In many cases, structural and functional evidence points to mechanisms by which these factors participate in cargo sorting. For example, early acting ESCRT complexes (ESCRT-0, ESCRT-I, and ESCRT-II) harbor ubiquitin-binding domains, which play a key role in cargo selection37. However, it remains unclear how (and to what extent) substrates of the pathway, but not core components of the ESCRT machinery, become selectively enriched within ILVs. Here, we will focus on this issue and discuss potential models to explain how ESCRT-mediated cargo sorting is efficiently achieved.
2. Ubiquitin-mediated cargo recognition and sorting by the ESCRT machinery
Post-translational modification of integral membrane proteins by ubiquitin serves as a key sorting signal for endocytosis and targeting to MVEs. In particular, members of the Cbl family of E3 ubiquitin ligases have been tied to the internalization and ESCRT-dependent degradation of dozens of activated cell surface receptors11,38–40. Although the addition of a single ubiquitin moiety has been shown to be sufficient to enable ESCRT-mediated protein sorting at MVEs41, many cargoes including EGF receptor undergo polyubiquitin modification (predominantly via K63-linkages), which may enhance their rate of sequestration within ILVs18,42–44. Several components of the ESCRT machinery contain ubiquitin binding domains (UBDs), which act as receptors for ubiquitin-modified cargoes (Figure 1). In general, the UBDs found on ESCRT subunits exhibit only modest affinity (Kd of ~70–510 μM) for cargoes45, but the avidity of these interactions may be sufficient to play a significant role in cargo capture and retention, especially when considering that integral membrane proteins are constrained to two dimensional movement within a bilayer46. The two subunits of ESCRT-0 (Hrs and STAM) contain two UBDs each. The double ubiquitin interacting motif (DUIM) of Hrs can bind simultaneously to two ubiquitin molecules47, each with an affinity of ~120 μM48, and both the UIM and Vps27/Hrs/STAM (VHS) domains of STAM associate with ubiquitin49, albeit with weaker affinities48,50. Importantly, upon association with membranes, ESCRT-0 exhibits the ability to generate larger complexes, further increasing the number of UBDs presented48,51. Both fluorescence and immunogold electron microscopy-based studies in yeast and animal cells support the idea that ESCRT-0 self-associates at endosomal membranes, creating subdomains that facilitate the retention of ubiquitin-modified cargoes through multiple low-affinity associations52–54. Additionally, ESCRT-0 subdomains may be further stabilized by flat clathrin lattices that are recruited by the carboxyl-terminal clathrin binding motif of Hrs18,53,55. However, a universal requirement for clathrin assembly at MVEs during cargo sorting remains to be demonstrated in all cell types. In the absence of clathrin heavy chain, biosynthetic transport of lysosomal hydrolases into MVEs in yeast proceeds normally56–58. Additionally, subdomains of ESCRT-0 are capable of assembling stably in the absence of flat clathrin lattices in vitro59. One possibility is that clathrin assembly at MVEs increases the efficiency of cargo retention, which may be required in cases where cargo influx becomes elevated in response to developmental and extracellular cues.
Figure 1. The early acting ESCRT machinery harbors multiple ubiquitin binding domains.
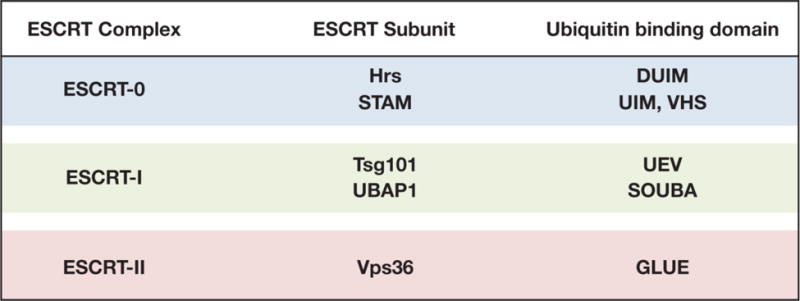
Several components of ESCRT-0, ESCRT-I and ESCRT-II contain domains capable of low affinity association with ubiquitin.
In contrast to ESCRT-0, no evidence exists to suggest that the heterotetrameric ESCRT-I complex multimerizes on membranes51. Nevertheless, based on structural and functional studies, at least two of its components can associate directly with ubiquitin. The core subunit Tsg101 possesses an amino-terminal, catalytically inactive ubiquitin E2 variant (UEV) domain with weak affinity for ubiquitin (Kd of ~510 μM)60. Additionally, a subpopulation of ESCRT-I complexes contain the UBAP1 subunit, which harbors a solenoid of overlapping ubiquitin associated motifs (SOUBA domain) that binds mono- and di-ubiquitin with an affinity of ~70 μM61. Inhibition of UBAP1 leads to cargo-specific trafficking defects, indicating that it (and by extension, ESCRT-I) plays a role in ubiquitin-dependent sorting62. The ESCRT-II complex contains a single subunit (Vps36) capable of binding to ubiquitin via a GRAM-like ubiquitin-binding in Eap45 (GLUE) domain63. Similar to other UBDs found in ESCRT subunits, the affinity of the GLUE domain for ubiquitin is modest (Kd of ~330 μM)63. However, ESCRT-II has been shown to multimerize on membranes in a cholesterol dependent manner, which would elevate its avidity for ubiquitin-modified cargoes64. Additionally, recent evidence in yeast highlighted a mutant form of ESCRT-II that is capable of partially suppressing phenotypes associated with the loss of ESCRT-0 or ESCRT-I, implying a direct role for ESCRT-II in cargo sorting at MVEs65.
Recruitment of ESCRT-III to endosomes is mediated largely by ESCRT-II, via a direct interaction between the ESCRT-II subunit Vps25 and the ESCRT-III subunit Vps2066. However, additional factors have also been identified, which can bridge earlier acting ESCRT complexes with ESCRT-III. In particular, ALIX has been shown to interact with both ESCRT-I (via Tsg101) and Vps32 isoforms67–71. Moreover, ALIX harbors a UBD (UBAN-like), which may contribute to cargo sorting into ILVs72–74. Although defects in cargo trafficking are relatively mild in the absence of ALIX function, they become more pronounced when other components of the ESCRT machinery are compromised75–77, suggesting that ALIX contributes to the overall efficiency of cargo delivery into MVEs.
The ESCRT-III and Vps4 complexes lack the presence of UBDs and their contribution to ubiquitin-dependent cargo sorting has been challenging to reconcile. One compelling hypothesis is that ESCRT-III spiral filaments may encircle cargoes to physically restrict their diffusion on MVEs78,79, although direct evidence for such a model is currently lacking. The ability of ESCRT-III to corral cargoes may be particularly important considering that substrates of the ESCRT pathway are typically subject to deubiquitination prior to internalization into ILVs. In yeast, the ubiquitin peptidase Doa4 has been demonstrated to associate with the ESCRT-associated protein Bro1 (an orthologue of mammalian ALIX), Vps20 and Vps3280–83. In particular, Vps20 binding to Doa4 appears to inhibit its deubiquitinase (DUB) activity, thereby delaying removal of ubiquitin from cargoes, perhaps until ESCRT-III spiral arrays can assemble properly84. In mammalian cells, several ESCRT-III subunits (CHMP1, CHMP2, CHMP3, and Ist1) associate with deubiquitinating enzymes, including AMSH, which specifically cleaves K63-linked ubiquitin chains85–91. However, unlike Doa4, the precise role of AMSH during cargo sorting remains to be clearly defined.
Despite tremendous advances in our understanding of ubiquitin-dependent cargo sorting at MVEs, the key question of how components of the ESCRT machinery function cooperatively to ensure that specific cargoes are properly deposited into forming ILVs remains unanswered. Although ESCRT-0 can bind to ESCRT-I (via a direct association between Hrs and Tsg101), and ESCRT-I can associate with ESCRT-II (via a direct association between the ESCRT-I subunit Vps28 and Vps36), localization studies have failed to demonstrate extensive co-localization between the complexes on endosomes10,92–94. Additionally, there exists little direct evidence to support the idea that these complexes co-assemble to form a higher order assembly in vivo. Instead, each complex may participate independently in cargo binding and due to their low affinities for ubiquitin, rapidly transfer cargoes to one another via transient associations. Based on live cell imaging studies, the majority of ESCRT-0 is found on endosomes, while ESCRT-I and ESCRT-II exhibit a more diffuse cytoplasmic distribution95–98, consistent with biochemical data showing that ESCRT-I and ESCRT-II are soluble99. Thus, ESCRT-0 has been suspected to play the most significant role in ubiquitin-dependent cargo clustering at endosomes. Consistent with this idea, an auto-activated, mutant isoform of the yeast ESCRT-III subunit Vps32 can largely bypass the requirements for ESCRT-I and ESCRT-II function in cargo sorting, but not ESCRT-0100. Additionally, in vitro studies suggest that ESCRT-0, but not ESCRT-I or ESCRT-II, is capable of stably associating with ubiquitin-modified substrates on supported lipid bilayers51. Together, the current evidence suggests a model in which ESCRT-0 functions as the major sorting receptor for ubiquitin-modified substrates at MVEs, with ESCRT-I and ESCRT-II augmenting its actions to create subdomains enriched with select cargoes, and linking the early acting ESCRT machinery to the downstream ESCRT-III complex.
3. Ubiquitin-independent cargo sorting
In recent years, there have been numerous studies indicating that integral membrane proteins lacking ubiquitin modification can still be internalized into MVEs in an ESCRT-dependent fashion. The G protein-coupled protease-activated receptor-1 (PAR1) and the purinergic receptor P2Y1 both contain a YPX3L motif, to which the ESCRT-associated protein ALIX binds19,20. Similarly, the interleukin-2 receptor β (IL-2Rβ) interacts directly with Hrs in a manner that does not require ubiquitin22. The mechanisms by which Hrs and ALIX translocate substrates into ILVs is unclear, but their internalization continues to require the downstream ESCRT machinery (ESCRT-III and the Vps4 complex)19,20.
There have also been reports of soluble cargoes entering ILVs for degradation. In one case, selectivity of cargoes is achieved by the chaperone hsc70, which associates with the limiting membrane of MVEs through a polybasic motif that interacts with phosphatidylserine101. Although unfolding of substrates is unnecessary for deposition into ILVs, both early (ESCRT-I) and late acting (ESCRT-III and Vps4) components of the ESCRT machinery are required. Additionally, association of soluble cargoes with proteins heavily modified by ubiquitin, such as members of the Cos family of tetraspanin-like proteins, can lead to their internalization within ILVs in an ESCRT-dependent manner102.
Beyond the sorting of proteinaceous cargoes, several studies have demonstrated that specific lipid species are preferentially sorted into MVEs. In particular, the phospholipids lysobisphosphatidic acid (LBPA) and phosphatidylinositol 3-phosphate (PI3P) are abundant on ILVs23,103. The ESCRT associated protein ALIX binds directly to LBPA21,104, and depletion studies suggest that ALIX promotes LBPA enrichment specifically at sites of ILV formation21. In vitro, LBPA stimulates membrane deformation on synthetic liposomes21, and it may behave similarly in cells to drive or stabilize the high curvature characteristic of ILVs in mammals (~50–60 nm in diameter). PI3P similarly associates with components of the ESCRT machinery, including ESCRT-0 (via the Hrs FYVE domain)105 and ESCRT-II (via the Vps36 GLUE domain)106. In particular, Hrs associates with PI3P with strong affinity107, and the lipid likely facilitates the formation of ESCRT-0 subdomains on MVEs. Additionally, other ESCRT components, including several ESCRT-III subunits, preferentially interact with acidic phospholipid headgroups like that found on PI3P108. However, given that PI3P becomes enriched in ILVs, it remains unclear how components of the ESCRT machinery ultimately escape the degradative fate of a major interacting lipid partner. In fact, contrary to studies suggesting that ESCRT components are usually recycled10, proteomic studies of exosomes have consistently identified several components of the ESCRT machinery109–111, indicating that recycling of ESCRT subunits may not be as robust as once believed.
In recent years, exosomes have garnered wide interest, based on their involvement in cell-to-cell communication and their roles in immune system regulation, cancer biology, and nervous system development112. The analysis of purified exosomes has revealed the presence of not only proteins and lipids, but several classes of RNAs, including mRNAs and miRNAs113. The mechanisms by which specific RNAs accumulate in ILVs remain unclear. ESCRT-II was shown previously to associate with the 3′ UTR of bicoid mRNA via the Vps36 GLUE domain to direct its localization in Drosophila eggs114. However, since neither ESCRT-I nor ESCRT-III inhibition affected bicoid mRNA distribution, it was believed that the role of ESCRT-II in this process was independent of an endosomal sorting function114. More recent work suggested that the RNA binding protein YBX1 acts as a molecular chaperone to package a subclass of miRNAs into ILVs115. Surprisingly, YBX1 is not membrane bound. Instead, it is largely associated with cytoplasmic RNA granules, which must intersect with MVEs to deliver specific miRNAs into ILVs116. It is possible that YBX1 is ubiquitin modified and/or capable of associating directly with components of the ESCRT machinery or other ubiquitin-modified integral membrane proteins, but additional studies are required to gain a mechanistic understanding of its function.
4. Cargo transfer into ILVs
With several lines of evidence supporting the idea that the early acting ESCRT complexes promote initial cargo sorting and clustering on MVEs and the late acting components restrict cargo diffusion prior to their incorporation into ILVs, a major unresolved issue is the mechanism by which cargoes are efficiently transferred between these ESCRT complexes. Two main models have emerged to explain this phenomenon. In the concentric circle model, cargoes are initially retained within a subdomain established by a combination of ESCRT-0, ESCRT-I and ESCRT-II78. Subsequent nucleation of ESCRT-III complexes by ESCRT-II results in the formation of a flat spiral polymer that surrounds cargoes, which rapidly adopts an energetically unfavorable conformation, leading to mechanical tension on the membrane117,118. Ultimately, the polymer is forced to convert into a three-dimensional helix, deforming the membrane to generate a nascent vesicle, which harbors the cargoes found at its core. To prevent internalization of the early acting ESCRT machinery in this model, each complex must be released from the membrane prior to bud formation (Figure 2). Currently, there exist no data to suggest how displacement of ESCRT-0, ESCRT-I, and ESCRT-II from the limiting MVE membrane is achieved. Nonetheless, there are several testable predictions based on this model. First, the early acting ESCRT machinery should not form stable, long-lived subdomains, but instead generate transient patches on MVEs that turnover with each cycle of ILV formation. Second, the early acting ESCRT complexes should at least transiently co-localize with late acting ESCRT components on MVEs. Finally, ESCRT-III spiral filaments should grow in an outward manner to generate the necessary compression forces on membranes. Although the live cell imaging studies necessary to address the first two predictions have yet to be reported, multiple groups have examined the third in vitro. In both cases, ESCRT-III polymers, consisting largely of Vps32 subunits, were found to exhibit a growing diameter, from ~25 nm at their center to several micrometers at their periphery, consistent with outward polymerization117–119. These data are supported by other studies indicating that Vps32 polymers exhibit a preference for a high radius of curvature120. However, it remains unclear how other ESCRT-III subunits may influence the initial orientation of polymer assembly. If the nucleation process led to the formation of inward spiraling filaments, the ESCRT-III complex could assemble adjacent to a subdomain composed of the early acting ESCRT complexes (Figure 2). In this scenario, it is easy to recognize how ESCRT components would avoid engulfment, but it becomes more challenging to comprehend how cargoes are transferred between the early acting ESCRT machinery and a neighboring ESCRT-III polymer. One possibility is that cargoes are only transiently retained by ESCRT-0, ESCRT-I and ESCRT-II, consistent with their modest affinity for ubiquitin sorting signals. This would enable cargoes to diffuse into juxtaposed subdomains that become encircled by ESCRT-III spiral filaments, targeting them into ILVs. High resolution imaging of native ESCRT complexes will be necessary to resolve these conflicting models.
Figure 2. Two speculative models to describe pathways for ESCRT-III spiral filament assembly.
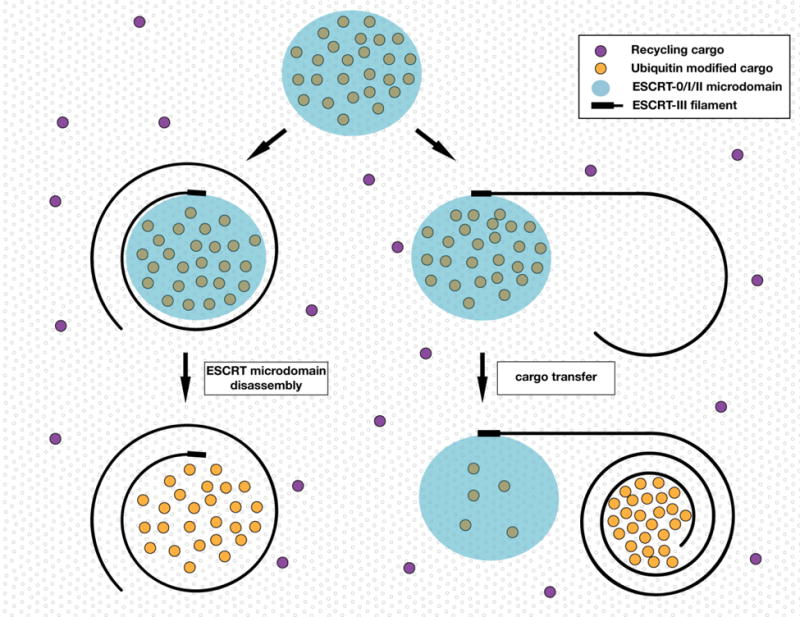
In one model (left), a subdomain formed by the early acting ESCRT machinery nucleates ESCRT-III filaments that spiral outward to surround cargoes destined for incorporation into ILVs. In this model, ESCRT-0, ESCRT-I and ESCRT-II must dissociate from the endosomal membrane to avoid engulfment into ILVs. An alternative model (right) suggests that the early acting ESCRT machinery forms a subdomain adjacent to ESCRT-III filaments, which spiral inwards toward a preferred radius of curvature. In this case, cargoes must be transferred between the early and late acting ESCRT complexes.
5. Concluding perspectives
In the majority of intracellular protein sorting reactions, receptors play an integral function in concentrating or retaining specific cargoes within budding transport carriers. For example, during COPII-mediated transport, members of the Sec24 or Tango1 cargo receptor families play intimate roles in directing specific substrates out of the endoplasmic reticulum121,122. However, the ESCRT machinery acts at the neck of nascent vesicles. While potentially sufficient to restrict cargo escape, this distribution diminishes the ability to prevent the non-specific inclusion of bulk cytoplasmic proteins and nucleic acids within ILVs. Nonetheless, the contents of ILVs appear to be highly selective, suggesting the existence of mechanisms to enrich for specific cargoes. Notably, other regions of the endosomal membrane are also active in cargo sorting. Specifically, retromer activity and other recycling pathways simultaneously regulate the content of endosomal membranes123. Only through the cooperative actions of multiple cargo sorting systems is ILV content appropriately maintained. In the future, continuing improvements to live cell imaging technologies and CRISPR-mediated genome editing approaches to label endogenous ESCRT components will enable us to gain a better understanding of the dynamic actions of the ESCRT machinery at endosomes, which should address many of the outstanding questions that remain in this field.
Acknowledgments
This work was supported in part by a grant from the NIH (GM088151 to AA). We thank Marisa Otegui and members of the Audhya lab for suggestions and critically reading this manuscript.
Abbreviations
- DUB
deubiquitinating enzyme
- DUIM
double ubiquitin-interacting motif
- EGF
epidermal growth factor
- ESCRT
endosomal sorting complex required for transport
- GLUE
GRAM-like ubiquitin-binding in Eap45
- ILV
intralumenal vesicle
- LBPA
lysobisphosphatidic acid
- MVB
multivesicular body
- MVE
multivesicular endosome
- PI3P
phosphatidylinositol 3-phosphate
- SOUBA
solenoid of overlapping ubiquitin associated motifs
- TIRFM
total internal reflection fluorescence microscopy
- UBD
ubiquitin binding domain
- UEV
ubiquitin E2 variant
- UIM
ubiquitin-interacting motif
- VHS
‘Vps27, HRS, STAM’
- Vps
vacuolar protein sorting
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorden P, Carpentier JL, Cohen S, Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci USA. 1978;75:5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haigler HT, McKanna JA, Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. The Journal of Cell Biology. 1979;81:382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felder S, et al. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- 4.van Deurs B, Holm PK, Kayser L, Sandvig K, Hansen SH. Multivesicular bodies in HEp-2 cells are maturing endosomes. European Journal of Cell Biology. 1993;61:208–224. [PubMed] [Google Scholar]
- 5.Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rieder SE, Banta LM, Köhrer K, McCaffery JM, Emr SD. Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol Biol Cell. 1996;7:985–999. doi: 10.1091/mbc.7.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babst M, Sato TK, Banta LM, Emr SD. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. The EMBO Journal. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 9.Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III. Developmental Cell. 2002;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 10.Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Developmental Cell. 2002;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 11.Levkowitz G, et al. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes & Development. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterman H, Levkowitz G, Alroy I, Yarden Y. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. Journal of Biological Chemistry. 1999;274:22151–22154. doi: 10.1074/jbc.274.32.22151. [DOI] [PubMed] [Google Scholar]
- 13.Levkowitz G, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Molecular Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 14.Yokouchi M, et al. Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. Journal of Biological Chemistry. 1999;274:31707–31712. doi: 10.1074/jbc.274.44.31707. [DOI] [PubMed] [Google Scholar]
- 15.Dupré S, Volland C, Haguenauer-Tsapis R. Membrane transport: ubiquitylation in endosomal sorting. CURBIO. 2001;11:R932–4. doi: 10.1016/s0960-9822(01)00558-9. [DOI] [PubMed] [Google Scholar]
- 16.Reggiori F, Pelham HR. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. The EMBO Journal. 2001;20:5176–5186. doi: 10.1093/emboj/20.18.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urbanowski JL, Piper RC. Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole. Traffic. 2001;2:622–630. doi: 10.1034/j.1600-0854.2001.20905.x. [DOI] [PubMed] [Google Scholar]
- 18.Raiborg C, et al. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 19.Dores MR, et al. ALIX binds a YPX(3)L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. The Journal of Cell Biology. 2012;197:407–419. doi: 10.1083/jcb.201110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dores MR, Grimsey NJ, Mendez F, Trejo J. ALIX Regulates the Ubiquitin-Independent Lysosomal Sorting of the P2Y1 Purinergic Receptor via a YPX3L Motif. PLoS ONE. 2016;11:e0157587. doi: 10.1371/journal.pone.0157587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuo H, et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita Y, et al. Ubiquitin-independent binding of Hrs mediates endosomal sorting of the interleukin-2 receptor beta-chain. Journal of Cell Science. 2008;121:1727–1738. doi: 10.1242/jcs.024455. [DOI] [PubMed] [Google Scholar]
- 23.Gillooly DJ, et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. The EMBO Journal. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wurmser AE, Emr SD. Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. The EMBO Journal. 1998;17:4930–4942. doi: 10.1093/emboj/17.17.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of Cell Biology. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. The Journal of Cell Biology. 1987;105:1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driskell OJ, Mironov A, Allan VJ, Woodman PG. Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat Cell Biol. 2007;9:113–120. doi: 10.1038/ncb1525. [DOI] [PubMed] [Google Scholar]
- 28.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 29.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature Reviews Molecular Cell Biology. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 30.Johannes L, Parton RG, Bassereau P, Mayor S. Building endocytic pits without clathrin. Nature Reviews Molecular Cell Biology. 2015;16:311–321. doi: 10.1038/nrm3968. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe S, Boucrot E. Fast and ultrafast endocytosis. Current Opinion in Cell Biology. 2017;47:64–71. doi: 10.1016/j.ceb.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Mayers JR, et al. Regulation of ubiquitin-dependent cargo sorting by multiple endocytic adaptors at the plasma membrane. Proceedings of the National Academy of Sciences. 2013;110:11857–11862. doi: 10.1073/pnas.1302918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimes ML, et al. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. The EMBO Journal. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell. 2006;17:3469–3483. doi: 10.1091/mbc.E05-11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christ L, Raiborg C, Wenzel EM, Campsteijn C, Stenmark H. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem Sci. 2017;42:42–56. doi: 10.1016/j.tibs.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Schuh AL, Audhya A. The ESCRT machinery: From the plasma membrane to endosomes and back again. Crit Rev Biochem Mol Biol. 2014;49:242–261. doi: 10.3109/10409238.2014.881777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Melker AA, van der Horst G, Calafat J, Jansen H, Borst J. c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. Journal of Cell Science. 2001;114:2167–2178. doi: 10.1242/jcs.114.11.2167. [DOI] [PubMed] [Google Scholar]
- 39.Meijer IMJ, van Rotterdam W, van Zoelen EJJ, van Leeuwen JEM. Cbl and Itch binding sites in ERBB4 CYT-1 and CYT-2 mediate K48- and K63-polyubiquitination, respectively. Cell Signal. 2013;25:470–478. doi: 10.1016/j.cellsig.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Grøvdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Experimental Cell Research. 2004;300:388–395. doi: 10.1016/j.yexcr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Stringer DK, Piper RC. A single ubiquitin is sufficient for cargo protein entry into MVBs in the absence of ESCRT ubiquitination. The Journal of Cell Biology. 2011;192:229–242. doi: 10.1083/jcb.201008121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haglund K, et al. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 43.Peschard P, Park M. Escape from Cbl-mediated downregulation: a recurrent theme for oncogenic deregulation of receptor tyrosine kinases. Cancer Cell. 2003;3:519–523. doi: 10.1016/s1535-6108(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 44.Longva KE, et al. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. The Journal of Cell Biology. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piper RC, Dikic I, Lukacs GL. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb Perspect Biol. 2014;6:a016808–a016808. doi: 10.1101/cshperspect.a016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Vendome J, Shapiro L, Ben-Shaul A, Honig B. Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature. 2011;475:510–513. doi: 10.1038/nature10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirano S, et al. Double-sided ubiquitin binding of Hrs-UIM in endosomal protein sorting. Nat Struct Mol Biol. 2006;13:272–277. doi: 10.1038/nsmb1051. [DOI] [PubMed] [Google Scholar]
- 48.Mayers JR, et al. ESCRT-0 assembles as a heterotetrameric complex on membranes and binds multiple ubiquitinylated cargoes simultaneously. J Biol Chem. 2011;286:9636–9645. doi: 10.1074/jbc.M110.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizuno E, Kawahata K, Kato M, Kitamura N, Komada M. STAM proteins bind ubiquitinated proteins on the early endosome via the VHS domain and ubiquitin-interacting motif. Mol Biol Cell. 2003;14:3675–3689. doi: 10.1091/mbc.E02-12-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren X, Hurley JH. VHS domains of ESCRT-0 cooperate in high-avidity binding to polyubiquitinated cargo. The EMBO Journal. 2010;29:1045–1054. doi: 10.1038/emboj.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi H, Mayers JR, Wang L, Edwardson JM, Audhya A. Hrs and STAM function synergistically to bind ubiquitin-modified cargoes in vitro. Biophys J. 2015;108:76–84. doi: 10.1016/j.bpj.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 53.Sachse M, Urbé S, Oorschot V, Strous GJ, Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol Biol Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norris A, et al. SNX-1 and RME-8 oppose the assembly of HGRS-1/ESCRT-0 degradative microdomains on endosomes. Proceedings of the National Academy of Sciences. 2017;114:E307–E316. doi: 10.1073/pnas.1612730114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raiborg C, Bache KG, Mehlum A, Stang E, Stenmark H. Hrs recruits clathrin to early endosomes. The EMBO Journal. 2001;20:5008–5021. doi: 10.1093/emboj/20.17.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. The Journal of Cell Biology. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- 58.Panek HR, et al. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. The EMBO Journal. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garrus JE, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 61.Agromayor M, et al. The UBAP1 subunit of ESCRT-I interacts with ubiquitin via a SOUBA domain. Structure. 2012;20:414–428. doi: 10.1016/j.str.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stefani F, et al. UBAP1 is a component of an endosome-specific ESCRT-I complex that is essential for MVB sorting. Curr Biol. 2011;21:1245–1250. doi: 10.1016/j.cub.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 63.Slagsvold T, et al. Eap45 in mammalian ESCRT-II binds ubiquitin via a phosphoinositide-interacting GLUE domain. Journal of Biological Chemistry. 2005;280:19600–19606. doi: 10.1074/jbc.M501510200. [DOI] [PubMed] [Google Scholar]
- 64.Boura E, Ivanov V, Carlson LA, Mizuuchi K, Hurley JH. Endosomal sorting complex required for transport (ESCRT) complexes induce phase-separated microdomains in supported lipid bilayers. J Biol Chem. 2012;287:28144–28151. doi: 10.1074/jbc.M112.378646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mageswaran SK, Johnson NK, Odorizzi G, Babst M. Constitutively active ESCRT-II suppresses the MVB-sorting phenotype of ESCRT-0 and ESCRT-I mutants. Mol Biol Cell. 2015;26:554–568. doi: 10.1091/mbc.E14-10-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teo H, Perisic O, González B, Williams RL. ESCRT-II, an endosome-associated complex required for protein sorting: crystal structure and interactions with ESCRT-III and membranes. Developmental Cell. 2004;7:559–569. doi: 10.1016/j.devcel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Martin-Serrano J, Zang T, Bieniasz PD. Role of ESCRT-I in retroviral budding. J Virol. 2003;77:4794–4804. doi: 10.1128/JVI.77.8.4794-4804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwedler von UK, et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 69.Katoh K, et al. The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. Journal of Biological Chemistry. 2003;278:39104–39113. doi: 10.1074/jbc.M301604200. [DOI] [PubMed] [Google Scholar]
- 70.McCullough J, Fisher RD, Whitby FG, Sundquist WI, Hill CP. ALIX-CHMP4 interactions in the human ESCRT pathway. Proceedings of the National Academy of Sciences. 2008;105:7687–7691. doi: 10.1073/pnas.0801567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pires R, et al. A Crescent-Shaped ALIX Dimer Targets ESCRT-III CHMP4 Filaments. Structure. 2009;17:843–856. doi: 10.1016/j.str.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joshi A, Munshi U, Ablan SD, Nagashima K, Freed EO. Functional replacement of a retroviral late domain by ubiquitin fusion. Traffic. 2008;9:1972–1983. doi: 10.1111/j.1600-0854.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keren-Kaplan T, et al. Structure-based in silico identification of ubiquitin-binding domains provides insights into the ALIX-V:ubiquitin complex and retrovirus budding. The EMBO Journal. 2013;32:538–551. doi: 10.1038/emboj.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dowlatshahi DP, et al. ALIX is a Lys63-specific polyubiquitin binding protein that functions in retrovirus budding. Developmental Cell. 2012;23:1247–1254. doi: 10.1016/j.devcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bilodeau PS, Winistorfer SC, Kearney WR, Robertson AD, Piper RC. Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. The Journal of Cell Biology. 2003;163:237–243. doi: 10.1083/jcb.200305007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shields SB, et al. ESCRT ubiquitin-binding domains function cooperatively during MVB cargo sorting. The Journal of Cell Biology. 2009;185:213–224. doi: 10.1083/jcb.200811130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pashkova N, et al. The yeast Alix homolog Bro1 functions as a ubiquitin receptor for protein sorting into multivesicular endosomes. Developmental Cell. 2013;25:520–533. doi: 10.1016/j.devcel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nickerson DP, Russell MRG, Odorizzi G. A concentric circle model of multivesicular body cargo sorting. EMBO reports. 2007;8:644–650. doi: 10.1038/sj.embor.7401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teis D, Saksena S, Judson BL, Emr SD. ESCRT-II coordinates the assembly of ESCRT-III filaments for cargo sorting and multivesicular body vesicle formation. The EMBO Journal. 2010;29:871–883. doi: 10.1038/emboj.2009.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bowers K, et al. Protein-protein interactions of ESCRT complexes in the yeast Saccharomyces cerevisiae. Traffic. 2004;5:194–210. doi: 10.1111/j.1600-0854.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 81.Luhtala N, Odorizzi G. Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. The Journal of Cell Biology. 2004;166:717–729. doi: 10.1083/jcb.200403139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Richter CM, West M, Odorizzi G. Doa4 function in ILV budding is restricted through its interaction with the Vps20 subunit of ESCRT-III. Journal of Cell Science. 2013;126:1881–1890. doi: 10.1242/jcs.122499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amerik AY, Nowak J, Swaminathan S, Hochstrasser M. The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol Biol Cell. 2000;11:3365–3380. doi: 10.1091/mbc.11.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson N, West M, Odorizzi G. Regulation of yeast ESCRT-III membrane scission activity by the Doa4 ubiquitin hydrolase. Mol Biol Cell. 2017;28:661–672. doi: 10.1091/mbc.E16-11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agromayor M, Martin-Serrano J. Interaction of AMSH with ESCRT-III and deubiquitination of endosomal cargo. Journal of Biological Chemistry. 2006;281:23083–23091. doi: 10.1074/jbc.M513803200. [DOI] [PubMed] [Google Scholar]
- 86.Tsang HTH, et al. A systematic analysis of human CHMP protein interactions: additional MIT domain-containing proteins bind to multiple components of the human ESCRT III complex. Genomics. 2006;88:333–346. doi: 10.1016/j.ygeno.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Zamborlini A, et al. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc Natl Acad Sci USA. 2006;103:19140–19145. doi: 10.1073/pnas.0603788103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kyuuma M, et al. AMSH, an ESCRT-III associated enzyme, deubiquitinates cargo on MVB/late endosomes. Cell Struct Funct. 2007;31:159–172. doi: 10.1247/csf.06023. [DOI] [PubMed] [Google Scholar]
- 89.Ma YM, et al. Targeting of AMSH to endosomes is required for epidermal growth factor receptor degradation. Journal of Biological Chemistry. 2007;282:9805–9812. doi: 10.1074/jbc.M611635200. [DOI] [PubMed] [Google Scholar]
- 90.McCullough J, et al. Activation of the endosome-associated ubiquitin isopeptidase AMSH by STAM, a component of the multivesicular body-sorting machinery. CURBIO. 2006;16:160–165. doi: 10.1016/j.cub.2005.11.073. [DOI] [PubMed] [Google Scholar]
- 91.Sato Y, et al. Structural basis for specific cleavage of Lys 63-linked polyubiquitin chains. Nature. 2008;455:358–362. doi: 10.1038/nature07254. [DOI] [PubMed] [Google Scholar]
- 92.Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. The Journal of Cell Biology. 2003;162:435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katzmann DJ, Stefan CJ, Babst M, Emr SD. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. The Journal of Cell Biology. 2003;162:413–423. doi: 10.1083/jcb.200302136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kostelansky MS, et al. Molecular architecture and functional model of the complete yeast ESCRT-I heterotetramer. Cell. 2007;129:485–498. doi: 10.1016/j.cell.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xie W, Li L, Cohen SN. Cell cycle-dependent subcellular localization of the TSG101 protein and mitotic and nuclear abnormalities associated with TSG101 deficiency. Proc Natl Acad Sci USA. 1998;95:1595–1600. doi: 10.1073/pnas.95.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lefebvre C, et al. The ESCRT-II proteins are involved in shaping the sarcoplasmic reticulum in C. elegans. Journal of Cell Science. 2016;129:1490–1499. doi: 10.1242/jcs.178467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L, Audhya A. In vivo imaging of C. elegans endocytosis. Methods. 2014;68:518–528. doi: 10.1016/j.ymeth.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proceedings of the National Academy of Sciences. 2011;108:4846–4851. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Current Opinion in Cell Biology. 2008;20:4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang S, et al. ESCRT-III activation by parallel action of ESCRT-I/II and ESCRT-0/Bro1 during MVB biogenesis. eLife. 2016;5:271. doi: 10.7554/eLife.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sahu R, et al. Microautophagy of cytosolic proteins by late endosomes. Developmental Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.MacDonald C, et al. A family of tetraspans organizes cargo for sorting into multivesicular bodies. Developmental Cell. 2015;33:328–342. doi: 10.1016/j.devcel.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kobayashi T, et al. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 104.Bissig C, et al. Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Developmental Cell. 2013;25:364–373. doi: 10.1016/j.devcel.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Komada M, Soriano P. Hrs, a FYVE finger protein localized to early endosomes, is implicated in vesicular traffic and required for ventral folding morphogenesis. Genes & Development. 1999;13:1475–1485. doi: 10.1101/gad.13.11.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teo H, et al. ESCRT-I core and ESCRT-II GLUE domain structures reveal role for GLUE in linking to ESCRT-I and membranes. Cell. 2006;125:99–111. doi: 10.1016/j.cell.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 107.Sankaran VG, Klein DE, Sachdeva MM, Lemmon MA. High-affinity binding of a FYVE domain to phosphatidylinositol 3-phosphate requires intact phospholipid but not FYVE domain oligomerization. Biochemistry. 2001;40:8581–8587. doi: 10.1021/bi010425d. [DOI] [PubMed] [Google Scholar]
- 108.Lin Y, Kimpler LA, Naismith TV, Lauer JM, Hanson PI. Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7-1 with itself, membranes, and the AAA+ ATPase SKD1. Journal of Biological Chemistry. 2005;280:12799–12809. doi: 10.1074/jbc.M413968200. [DOI] [PubMed] [Google Scholar]
- 109.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Théry C, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 111.Krämer-Albers EM, et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl. 2007;1:1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- 112.McGough IJ, Vincent JP. Exosomes in developmental signalling. Development. 2016;143:2482–2493. doi: 10.1242/dev.126516. [DOI] [PubMed] [Google Scholar]
- 113.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 114.Irion U, St Johnston D. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature. 2007;445:554–558. doi: 10.1038/nature05503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife. 2016;5:5003. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang Z, et al. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. Journal of Cell Science. 2004;117:5567–5578. doi: 10.1242/jcs.01477. [DOI] [PubMed] [Google Scholar]
- 117.Shen QT, et al. Structural analysis and modeling reveals new mechanisms governing ESCRT-III spiral filament assembly. The Journal of Cell Biology. 2014;206:763–777. doi: 10.1083/jcb.201403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chiaruttini N, et al. Relaxation of Loaded ESCRT-III Spiral Springs Drives Membrane Deformation. Cell. 2015;163:866–879. doi: 10.1016/j.cell.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee IH, Kai H, Carlson LA, Groves JT, Hurley JH. Negative membrane curvature catalyzes nucleation of endosomal sorting complex required for transport (ESCRT)-III assembly. Proceedings of the National Academy of Sciences. 2015;112:15892–15897. doi: 10.1073/pnas.1518765113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fyfe I, Schuh AL, Edwardson JM, Audhya A. Association of the endosomal sorting complex ESCRT-II with the Vps20 subunit of ESCRT-III generates a curvature-sensitive complex capable of nucleating ESCRT-III filaments. J Biol Chem. 2011;286:34262–34270. doi: 10.1074/jbc.M111.266411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lord C, Ferro-Novick S, Miller EA. The highly conserved COPII coat complex sorts cargo from the endoplasmic reticulum and targets it to the golgi. Cold Spring Harb Perspect Biol. 2013;5:a013367–a013367. doi: 10.1101/cshperspect.a013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma W, Goldberg J. TANGO1/cTAGE5 receptor as a polyvalent template for assembly of large COPII coats. Proceedings of the National Academy of Sciences. 2016;113:10061–10066. doi: 10.1073/pnas.1605916113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Burd C, Cullen PJ. Retromer: a master conductor of endosome sorting. Cold Spring Harb Perspect Biol. 2014;6:a016774–a016774. doi: 10.1101/cshperspect.a016774. [DOI] [PMC free article] [PubMed] [Google Scholar]


