1. Introduction
Early detection and diagnosis of stable coronary artery disease (SCAD) is essential for proactive secondary prevention of myocardial infarction (MI), control of disease progress, and reduction of mortality. Clinical decision-making in modern medicine is increasingly dependent on cardiovascular imaging techniques. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease has been issued by American Heart Association (AHA).[1] European Society of Cardiology (ESC) has issued 2013 ESC guidelines on the management of stable coronary artery disease.[2] Non-invasive imaging examination is recommended as the cornerstone for diagnosis in these guidelines. In China, despite the availability of multiple non-invasive imaging modalities such as electrocardiogram (ECG), echocardiography, radionuclide myocardial perfusion imaging (MPI), cardiovascular magnetic resonance imaging (CMR), and coronary computed tomography angiography (CCTA) in clinical practice, the utilization, clinical indication, and technical operations are not standardized. In the course of diagnosis and treatment, imaging examinations have been focused on “emphasizing structure and disregarding function; simplifying diagnosis and stressing therapy”. In addition, clinicians' training and knowledge of examination techniques are limited and thus they do not master indications and contraindications of each specific modality. Moreover, current Chinese guidelines for the diagnosis and treatment of chronic stable angina pectoris and expert consensus for examination techniques have not provided standard and systematic guidance for non-invasive imaging examination pathways.[3] In order to meet the needs of standardized clinical applications of diagnostic techniques, cardiovascular disease (CVD) experts and imaging experts have jointly written this Chinese expert consensus on the non-invasive imaging examination pathways for stable coronary artery disease (SCAD) on the basis of AHA, and ESC guidelines for SCAD to help Chinese cardiovascular specialists apply non-invasive imaging examination techniques based on patient characteristics and fully realize the clinical values of these examinations in disease diagnosis, risk stratification, and evaluation of therapeutic efficacy.
2. Definition of SCAD
SCAD includes different phases in the development of coronary heart disease, particularly the reversible myocardial oxygen demand and/or oxygen supply mismatch which are related to ischemia or hypoxia and are generally induced by exercise, emotion, or any other cardiac stressors. It may occur repeatedly or spontaneously. The clinical manifestations include exertional angina or silent angina caused by vasospasm or microvascular disease, latent angina, relative stable states after coronary artery stenting or bypass grafting, and ischemic cardiomyopathy.
3. Clinical diagnosis of SCAD
Medical history and physical examinations are the essential first steps for cardiovascular specialists to diagnose patients, and clinicians can make further decisions for patients based on pre-test probabilities (PTP) of SCAD.
3.1. History
It is fundamental to understand the medical history in order to make a diagnosis. Physicians should understand the characteristics of chest pain, including: (1) constricting discomfort in the front of the chest. Paroxysmal attacks may last for several minutes and generally not exceed 10 min. (2) Onset of chronic stable angina is often induced by exertion (such as brisk walking and climbing stairs or slopes) or emotions. (3) Symptoms usually occur with exertion and are relieved by rest. In addition, symptoms may be quickly relieved within 2–5 min after administration of sublingual nitroglycerin. People with typical angina have all the above anginal pain features; people with atypical angina have two of the features; and people with non-anginal chest pain have one or none of the features.[4]
3.2. Physical examination
SCAD patients usually have normal physical examinations. Symptoms of angina often occur concomitantly with tachycardia, elevated blood pressure, anxiety, perspiration, and auscultation findings could include third and the fourth cardiac sounds or gallops, systolic murmur over the cardiac apex, reversed splitting of second heart sound, or occasional rales in lower lungs. Physical examinations may assist to discover non-coronary atherosclerosis findings such as valvular heart disease and cardiomyopathy or risk factors such as hypertension and xanthoma induced by abnormal lipid metabolism, as well as carotid murmur or peripheral vascular disease which may be helpful for the diagnosis of atherosclerosis.[3]
3.3. PTP
PTP of CAD is the probabilities of SCAD determined by clinical findings. The importance of the estimation of PTP in selecting non-invasive imaging technique is gradually increasing and it is a key step for reasonable selection of a non-invasive imaging technique. PTP of CAD is affected by CAD incidence, clinical features, and risk factors (such as smoking, hyperlipidemia, hypertension, diabetes mellitus, obesity, family history of premature CAD, etc). According to SCORE (http://www.heartscore.org/Pages/welcome.aspx) or Framingham risk scoring (http://hp2010.nhlbihin.net/atpiii/calculator.asp) with reference to 2013 ESC guidelines on the management of stable coronary artery disease,[2] calculating PTP in accordance with features of clinical symptoms, sex, and age to stratify patients by values of < 15%, 15–65%, 66–85%, > 85% (Table 1).
Table 1. PTP of CAD in patients with chest pain.[2].
| Ages, yrs | Typical angina |
Atypical angina |
Non-angina pain |
|||
| Male | Female | Male | Female | Male | Female | |
| 30–39 | 59% | 28% | 29% | 10% | 18% | 5% |
| 40–49 | 69% | 37% | 38% | 14% | 25% | 8% |
| 50–59 | 77% | 47% | 49% | 20% | 34% | 12% |
| 60–69 | 84% | 58% | 59% | 28% | 44% | 17% |
| 70–79 | 89% | 68% | 69% | 37% | 54% | 24% |
| > 80 | 93% | 76% | 78% | 47% | 65% | 32% |
Notes: (1) PTP in all groups in white cells is < 15%, indicating very low possibility of CAD. Screen with resting ECG and echocardiogram only. (2) PTP in all groups in blue cells is between 15% and 65%. Patients may select exercise ECG as first examination if applicable. However, if non-invasive imaging examinations are available at the hospital, ischemia can be detected by such examinations. (3) PTP in all groups in light red cells is between 66% and 85% and non-invasive imaging functional tests should be selected for SCAD diagnosis. (4) PTP in all groups in dark red cells is > 85%. SCAD should be assumed and patients only need to receive risk stratification. PTP: pre-test probabilities; SCAD: stable coronary artery disease.
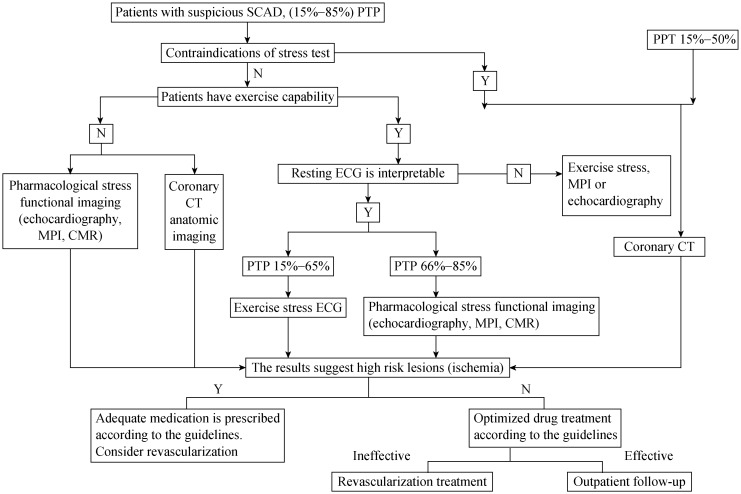
Overall sensitivity and specificity of non-invasive imaging techniques to diagnose CAD is approximately 85%. Therefore, patients with PTP of below 15% for coronary artery stenosis may not need to continue with non-invasive imaging examinations, while patients with PTP above 85% for coronary artery stenosis may directly proceed to invasive imaging examinations. Patients with PTP between 15% and 85% should follow the non-invasive imaging examination pathways to make subsequent decisions (Figure 1).[2]
Figure 1. Clinical pathways for non-invasive imaging technique for patients with suspected SCAD and moderate PTP (15%–85%).
CMR: cardiovascular magnetic resonance imaging; MPI: myocardial perfusion imaging; N: no; PTP: Pre-test probabilities; SCAD: stable coronary artery disease; Y: yes.
4. Non-invasive imaging examinations for SCAD
The non-invasive imaging examinations includes basic exam, cardiac functional stress test, and coronary artery anatomic imaging examination mainly used for the diagnosis of myocardial ischemia, risk stratification, and prognosis evaluation.
4.1. Basic exam
Basic exam is the first choice for all patients with suspected SCAD, mainly including resting ECG, resting echocardiography, and chest X-ray. Resting ECG can provide baseline status, and dynamic ECG alteration associated with symptoms is helpful for diagnosis. Twenty-four hours dynamic ECG can help improve detection rate of myocardial ischemia.[5] Echocardiography provides information of cardiac structure and function. Segmental wall motion abnormality increases the chance of diagnosis of CAD and the overall left ventricular (LV) contractile function is meaningful for prognostic risk assessment of CAD patients. Structural anomaly (such as severe aortic valve stenosis) or cardiomyopathy (such as hypertrophic obstructive cardiomyopathy) should be noted before further stress testing for detecting ischemia. Chest X-ray in patients with heart failure or pulmonary diseases can provide valuable diagnostic information.
4.2. Functional stress test
Functional stress test is to apply exercise or pharmacologic agents to induce myocardial ischemia by increasing myocardial work and oxygen consumption, or through vasodilatation to induce redistribution of coronary blood flow. The main methods to diagnose SCAD for patients with PTP between 15% and 85% are composed of stress ECG, stress echocardiography, stress radionuclide MPI (PET and SPECT), and stress CMR. Exercise stress test is composed of treadmill exercise test (TET), or incumbent or upright exercise bike tests using Bruce exercise protocol. Pharmacological stress test includes positive inotropic drugs (dobutamine) and vasodilators (dipyridamole, adenosine, and regadenoson). Among pharmacological agents used in stress tests in China, adenosine triphosphate (ATP) is more commonly used as an alternative to adenosine but it should be noted that the test results may be influenced by metabolic time.
The appropriate indication of stress tests includes: (1) elucidating the reason for atypical chest pain, shortness of breath reasons in patients with suspected SCAD; (2) in need of assessing the extent and severity of myocardial ischemia; (3) evaluating survival myocardium (pharmacologic test of dobutamine and dipyridamole); (4) risk stratification of noncardiac surgery and post myocardial infarction; and (5) detecting restenosis and prognosis of prior percutaneous coronary artery interventional (PCI) or coronary artery bypass grafting (CABG). All stress tests should be performed with strict adherence to contraindications to reduce clinical risks, which include: (1) unstable disease state after acute MI with ischemic symptoms; (2) high-risk unstable angina; (3) uncontrolled symptomatic arrhythmia with abnormal hemodynamics; (4) symptomatic severe aortic valve stenosis; (5) heart failure with uncontrollable symptoms; (6) acute pulmonary embolism or pulmonary infarction; (7) acute myocarditis or pericarditis; (8) acute aortic dissection; (9) LV thrombi; (10) hypertensive patients with uncontrollable high blood pressure (BP > 200/110 mmHg); (11) inability to tolerate or hypersensitivity to pharmacologic stress agents; (12) obstructive hypertrophic cardiomyopathy; (13) apparent hypotension (dipyridamole); and (14) rejection by patients.
4.2.1. Stress ECG (exercise ECG stress test)
Exercise ECG stress test serves as the cornerstone for the diagnosis of SCAD, and it is the basic examination for suspected SCAD with PTP stratification between 15% and 65%, in patients with exercise capacity.
Treadmill exercise test is the most commonly used test for exercise ECG. In clinical practice, it is usually graded by heart rate (HR) achieved during exercise. Maximal HR = 220-age, sub-maximal HR = 85%–90% maximal HR, or 190 – age (± 10), and low stress HR is 60%–70% of maximal HR. Ischemic ECG is diagnosed with ≥ 1 mm horizontal or down-sloping ST depression in one or more than one lead at peak exercise (at 60–80 ms after J Point).[6] Non-ECG indicators such as drop of blood pressure during exercise stress also indicate severe coronary lesions or left main disease.[7]
Precautions: (1) patients need to be able to perform at least moderate level of physical exercise; (2) in the case of complete left bundle branch block (CLBBB), pacemaker rhythm and Wolff-Parkinson-White syndrome, exercise ECG has no diagnosis value; (3) false positive results are possible in case of LV hypertrophy (LVH), bundle branch block, atrial fibrillation, and use of digoxin for treatment; (4) anti-ischemic treatment may impact HR and reduce myocardial oxygen demand, thus, it may lead to false negative results for diagnosis; however, it may be used to evaluate therapeutic efficacy for ischemia.
The treadmill exercise test is economical, simple and convenient, practical, relatively safe, non-invasive, and repeatable. Its sensitivity and specificity for CAD diagnosis is approximately 23%–100% (average 68%) and 17%–100% (average 77%), respectively. However, it cannot further characterize specific coronary arteries, and the influence of less exercise duration and impaired exercise tolerance should be taken into consideration in analyzing.[8] In addition, the sensitivity and specificity for diagnosis of female patients are lower. Cardiopulmonary exercise testing can significantly improve sensitivity but it is not commonly used in clinical practice.
4.2.2. Stress echocardiography
Stress echocardiography can be used to assess LV shape, size, ventricular segmental wall motion, and systolic function at peak stress. Adding ultrasound contrast agent for myocardial contrast echocardiography allows for the assessment of myocardial perfusion. Ultrasound contrast agent can also be used to improve endocardial definition and improve sensitivity and specificity of stress echocardiography.[9]–[11] It is recommended that when patients undergo stress echocardiography, ultrasound contrast agent be used if two or more continuous segments (in a 17-segment LV model) are poorly visualized.[2]
If ventricular wall motion is normal in both resting and stress states, the test result is negative. Abnormal study findings include those with fixed wall-motion abnormalities or new or worsening abnormalities indicative of ischemia.[12] A segment with resting dysfunction may show either a sustained improvement during stress indicating a non-jeopardized myocardium (stunned) or improve during early stress with subsequent deterioration at peak (biphasic response). The biphasic response is suggestive of viability and ischemia, with jeopardized myocardium fed by a critically coronary stenosis. Resting wall-motion abnormalities, unchanged with stress, are classified as “fixed” and most often represent regions of prior infarction.[13] Stress tests should be terminated in case of significant arrhythmia, hypotension, and intolerable symptoms. Unsynchronized contraction of the right ventricular free wall and reduced downward displacement amplitude of the tricuspid valve can indicate right coronary artery or multi-vessel lesions.
Exercise stress echocardiography may simulate physiological stimuli more accurately, and we should prioritize the selection of exercise stress test in reasonable conditions. In case of significant abnormal resting ventricular wall motion found in the examination (we can use dobutamine for viable myocardium assessment) and/or poor exercise ability of patients, we should prioritize the selection of pharmacologic stress tests. The vasodilator stress echocardiography is more suitable for evaluating myocardial perfusion, but if ventricular wall motion response is desired, we should select dobutamine stress echocardiography.
Stress echocardiography is convenient, non-invasive, operable from the bedside, free from any radioisotope injection and radiation, and iodine containing contrast agents. However, the quality of the examinations can be relatively easily affected by patient acoustic window, such as in cases of morbid obesity and chronic lung disease.
Stress echocardiography combined with strain-rate imaging can quantify movement and displacement of the myocardium in all segments;[14] it may be combined with 3D-echocardiography to capture the real-time 3D images and evaluate movement of each segment or the whole left ventricle.
4.2.3. Radionuclide myocardial perfusion imaging
Radionuclide MPI includes the use of single-photon emission computed tomography (SPECT) or positron emission tomography (PET) techniques to detect and evaluate myocardial perfusion status and myocardial viability to provide functional information of the coronary arteries.[15] MPI has been widely used in the detection of coronary artery disease, including the diagnosis of myocardial ischemia and infarct, evaluation of viability of the myocardium, ECG gated imaging can also provide information of myocardial perfusion, segmental wall motion, mechanical synchronization and cardiac function parameters at the same time.[16] The published literature with SPECT suggests that its average sensitivity for detecting > 50% angiography stenosis is 87% (range: 71%–97%), whereas the average specificity is 73% (range: 36%–100%). With PET perfusion imaging, the reported average sensitivity for detecting > 50% angiography stenosis is 91% (range: 83%–100%), whereas the average specificity is 89% (range: 73%–100%).[17]
The quantity of tracers taken in by the myocardium is related to regional blood flow to the myocardium. When stress MPI is reduced and resting MPI is normal or improved, the diagnosis of myocardial ischemia should be considered. If stress MPI result is negative, then the possibility of myocardial ischemia may be reduced. In case of transient LV enlargement and decreased ejection fraction at the time of stress MPI, it is an important indicator for predicting serious CAD. The premium indication of stress MPI is the patients with intermediate PTP of CAD (PTP ranges: 15%–85%)
The PET MPI has higher spatial resolution and better diagnostic accuracy than SPECT. It can quantify absolute blood flow to detect microvascular diseases and measure myocardial blood flow reserve. It can be used for early detecting CAD, especially for the patients with microvascular disease, balanced multi-vessel CAD and obesities.[18] Although majority of technical advantages have been recognized for a long time, access to PET for routine detection of CAD remains somewhat limited. It has higher cost for new generator to produce 82Rubidium or advanced cyclotron to produce 13N-ammonia, and is not widely used in China. However, Clinical applications of PET/CT are increasing gradually and it is useful for the comprehensive assessment of coronary artery function and myocardial perfusion.
Image quality of MPI may be affected by exercise or artifact and the assessment of coronary arterial vascular wall structures is not possible. The effect dose of radiation exposure for the MPI test is about 5.8 mSv (take 20mCi 99mTc-MIBI as example). SPECT/CT and PET/CT are increasingly applied in clinical practice for detecting coronary structure, calcification and plaque.[19]
4.2.4. Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging (CMR) serves as the gold standard for the evaluation of cardiac structure and function. With stress perfusion, cine CMR, and delay gadolinium enhancement imaging, cardiac structure, function, abnormal ventricular wall motion, myocardial ischemia, infarction, and viability can be evaluated. Additional CMR sequences can also evaluate tissue characteristics, such as edema, fibrosis, and hemorrhage.[20]
The premium indication of stress CMR is the patients with PTP ranges from 66%–85%. CMR perfusion imaging display reduction or deficiency of myocardial perfusion in condition of positive results. For multi-parametric CMR, the sensitivity was 86.5% (95% CI: 81.8%–90.1%), specificity 83.4% (79.5%–86.7%), and negative predictive value 90.5% (87.1%–93.0%). The sensitivity and negative predictive value of CMR are superior than SPECT in CEMARC study.[21] CMR has no ionizing radiation and may image in any orientation. It is not limited by patient body and has high spatial resolution, temporal resolution, and soft tissue contrast. Image quality may be affected by artifact and exercise is not routinely available due to requirement of MRI compatible equipment. Imaging of coronary artery takes longer time than CCTA and the images are not as high resolution as CCTA in clinical practice. It cannot be used in claustrophobic patients and patients implanted with ferromagnetic metals/devices. Some objects with low or non-ferromagnetic object (e.g., stent implantation) may generate artifact.
For past few years, whole-heart synchronized dynamic myocardial perfusion image can be obtained from high-resolution whole-heart 3D CMR perfusion which improves sensitivity of myocardial ischemia diagnosis and accurate quantitative analysis for myocardial ischemia.[22] In addition, the myocardium quantitative imaging (T1 mapping and T2 mapping) is applied to detect diffuse myocardial fibrosis and edema in clinical practice.[23],[24]
4.3. CCTA
CCTA is a non-invasive imaging technique with the best spatial and temporal resolution to assess anatomical structures of coronary artery, and mainly used to diagnose epicardial coronary stenosis. CCTA is characterized by high sensitivity and excellent negative predictive value which is particularly applicable for patients based on PTP stratification of suspected CAD.[25],[26]
CCTA can be used not only to evaluate the narrowing of vessels, but also to quantitatively evaluate plaques to make preliminary diagnosis of plaque vulnerability. Therefore, it has important clinical diagnostic values for suspected CAD.[27],[28] Calcium score scan is used to quantify calcified coronary artery lesions and it may reflect plaque burden which has crucial values for risk stratification.
Suitable population for CCTA: CCTA is mainly used for patients with moderate or low to moderate risk (PTP stratification of 15%–50%). CCTA is suitable for patients with atypical chest pain and clinically suspected CAD but cannot definitely diagnosed by ECG. Some patients who cannot tolerate stress cardiac imaging may also select CCTA as an alternative option.[2],[25] It is widely accepted that CCTA can be used to evaluate graft patency of coronary artery stent > 3 mm, and observe radiological manifestation of complete stenosis, restenosis around the stent, or ruptured stent. Moreover, it has excellent clinical values for pre-operative screening and assessment of non-cardiac surgical patients with suspected CAD.
Contraindication of CCTA include: (1) hypersensitivity to iodinated contrast agents; (2) renal insufficiency [creatinine clearance rate < 60 mL/min per 1.73 m2); (3) severe heart failure; (4) untreated hyperthyroidism; and (5) pregnancy. The above-mentioned contraindications are not absolute. Individual presentation should be considered, and necessary preventative and curative measures should be used to reduce examinations associated complications.
Control of both HR and cardiac rhythm are equally important in CCTA. It is recommended to control HR < 70 beats/min. Only some high-end CT equipment may be used to image in patients with arrhythmia such as atrial fibrillation and premature beats.
CCTA examination is characterized by convenience, rapid acquisition, and high spatial resolution. It can collect data of pulmonary vasculatures, coronary artery, the heart, and ascending and descending aorta.[29] It has the following limitations: (1) ionizing radiation, although radiation dosage of CCTA has significantly reduced. Radiation dosages for most patients may be reduced to < 3 mSv, or even sub-millisievert scanning. The clinical application of CCTA has become safer. (2) CCTA could be impacted by calcification and other blooming artifacts which result in overestimation of vessel narrowing and thus false positive diagnosis. Severe calcification may make it impossible to evaluate the stenosis. (3) Iodinated contrast agent in CCTA may result in hypersensitivity, shock, or adverse reaction such as worsened renal function. However, the dosage of contrast agents has been reduced significantly. It is not recommended to conduct skin tests for iodinated contrast agent.[30] (4) CT evaluation for coronary artery function [CT perfusion and fractional flow reserve (FFR)] needs to be improved.
Recently, evaluation of coronary artery function using cardiac CT has become a hot topic of research. FFRCT are highly accurate in comparison to invasive FFR, and relative technologies are still in research. CT myocardial perfusion may be used to quantitatively evaluate the degree of myocardial ischemia to provide informaiton of both coronary artery anatomy and burden of myocardial ischemia. However, the application of coronary CT functional imaging is still limited due to higher radiation and contrast agent dosage, and the effect of beam-hardening artifact. Thus, it needs further clinical validation.[31],[32]
4.4. Recommendations in clinical practice for non-invasive imaging test
Various non-invasive imaging examination techniques have their own advantages in observing cardiac structure and function. Physicians should choose according to clinical needs to use the best techniques and provide comprehensive assessment. Table 2 summarizes application characteristics of non-invasive imaging examination techniques and recommendations according to clinical needs.
Table 2. Evaluation table of cardiovascular non-invasive imaging examination.
| Coronary artery stenosis | Coronary artery plaque | Cardiac and large blood vessel anatomy | Ventricular wall motion | Ventricular function | Myocardial perfusion | Myocardial viability | Myocardial metabolism | ||
| Echocardiography | - | - | +++* | +++* | +++* | ++ | ++ | - | |
| CCTA | ++++* | +++* | ++++* | +++ | +++ | ++ | + | - | |
| CMR | ++ | + | ++++ | ++++ | ++++ | ++++ | ++++* | + | |
| SPECT | - | - | + | +++ | +++ | ++++* | +++ | ++ | |
| PET | - | + | + | +++ | +++ | ++++ | ++++ | ++++* | |
*Refers to the first recommended in Chinese expert consensus in the current clinical environment; +: diagnosis precision in research phase; ++: diagnosis precision can be used but not commonly used in clinical practice; +++: diagnosis precision had been used in routine; ++++: has highest accuracy of diagnosis; -: cannot evaluate. CCTA: coronary artery CT angiography; CMR: cardiovascular magnetic resonance imaging; SPECT: single-photon emission computed tomography; PET: positron emission tomography.
In terms of evaluating coronary artery characteristics such as stenosis and plaque, CCTA has the highest accuracy in non-invasive imaging examinations and it is highly recommended for clinical application.
For observing anatomical features of the heart and large blood vessel, Echo, CCTA and CMR have high accuracy. Echo should be considered for the serial real-time measurement. For the large blood vessel, the first recommendation is CCTA in the absence of contraindications.
In evaluating the ventricular structure and function, CMR has the highest accuracy with confirmed ventricular wall motion abnormalities, and the first recommendation is echocardiography.
In term of evaluating myocardial perfusion, SPECT, PET, and CMR have higher accuracy, and the first recommendation is SPECT when CMR is not available or contraindicated. CMR and PET have the highest accuracy for detecting viable myocardium, and CMR have priority for detecting fibrosis, the first recommendation is CMR.
In assessment of myocardial metabolism, PET has better advantage and is the first-line examination recommendation in clinical practice.
4.5. Recommendations for clinical pathways of non-invasive imaging modalities for SCAD
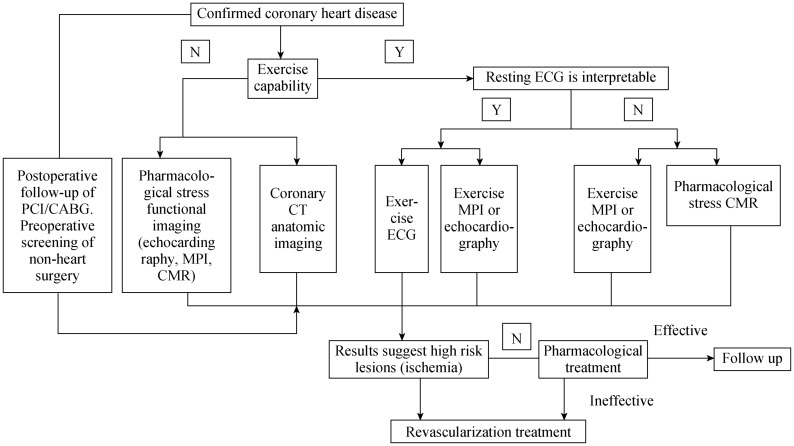
The main purpose is to evaluate the existence and extent of myocardial ischemia. For patients with different stratified PTP, the pathways of all non-invasive imaging modalities are shown in Figure 1 and Figure 2.[2]
Figure 2. Clinical pathways for non-invasive imaging technique for patients with definite diagnosis of CAD to evaluate myocardial ischemia.
CABG: coronary artery bypass grafting; CAD: coronary artery disease; CMR: cardiovascular magnetic resonance imaging; MPI: myocardial perfusion imaging; N: no; PCI: percutaneous coronary artery interventional therapy; Y: yes.
Patients who have contraindications to stress test may use CCTA. Patients with PTP (15%–50%) may also use CCTA.
Patients without any contraindications to stress test and without exercise capability may select pharmacological stress MPI, pharmacological stress echocardiography and pharmacological stress CMR or CCTA.
Patients who have an un-interpretable resting ECG without any contraindications to stress test but with exercise capability should select exercise stress echocardiography or exercise stress MPI.
Patients who have an interpretable resting ECG without contraindications to stress test and with exercise capability should choose according to PTP. Select exercise stress ECG with PTP of 15%–65%. Select stress echocardiography or stress MPI, and the pharmacologic stress CMR with PTP of 66%–85%.
If patients have LVEF < 50% with typical symptoms, they should receive invasive imaging examination. If patients have LVEF < 50% with atypical symptoms, they should receive functional stress imaging examination.
4.6. Patients with definite diagnosis of CAD for evaluating therapy effect (Figure 2).
Patients without exercise capability should select pharmacological stress echocardiography, pharmacological stress MPI, pharmacological stress CMR, or CCTA. Patients who have un-interpretable resting ECG with exercise capability should select exercise stress echocardiography, exercise stress MPI, and pharmacological stress CMR. Patients who underwent resting ECG interpretation with exercise capability should select exercise stress ECG, exercise stress echocardiography, and exercise stress MPI.
4.7. Risk stratification of SCAD with non-invasive imaging examination
Patient event risk can be calculated on the basis of Duke Treadmill Score (DTS), exercise time, degree of ST deviation and chest pain during exercise. DTS is used to detect annual mortality rate > 3% in patients with high risk (http:// www.cardiology.org/tools/medcalc/duke/).[33],[34]
Stress echocardiography: If patients encounter > 3 segments of exercise-induced abnormality in the 17-segment LV model, they should be considered as high risk patients (the corresponding annual mortality rate > 3%) and receive coronary artery angiography.[35],[36]
Stress MPI: semi-quantitative analysis can be used to calculate summed stress score (SSS), summed rest score (SRS) and summed difference score (SDS = SSS-SRS), which was based on 17-segment LV definition and 5-score per segment evaluation system. The proportion of stress-induced reversible perfusion deficiency in LV myocardium less than 10% (1–2 segments, SDS < 7) indicates mild ischemia. The proportion between 10% and 20% (3–4 segments, 7 ≤ SDS < 14) indicates moderate ischemia. The proportion more than 20% (≥ 5 segments, SDS ≥ 14) indicates severe ischemia. Patients with moderate and severe ischemia may benefit from revascularization, and patients with normal or mild ischemia are recommended to receive optimized medical therapy.[37],[38]
Stress CMR: The standard for high risk should be ≥ 3 in 17-segment model, newly set high risk criteria is abnormal ventricular wall motion, or stress induced LV reversible perfusion abnormality of > 10% (≥ 2 segments). For patients of SCAD, stress CMR myocardial perfusion research indicates that 3-year survival rate for patients without any abnormal myocardial perfusion is 99.2%; 3-year survival rate for patients with abnormal myocardial perfusion is 83.5%. Thus, the difference between two groups is statistically significant and incidence of cardiac events for the latter is significantly higher than the former. Abnormal stress myocardial perfusion may serve as an independent predictive factor for the occurrence of adverse cardiac events.
CCTA: Risk stratification of patients can be achieved according to the degree of stenosis in CCTA. Research has indicated that prognosis of patients with significant stenosis is significantly worse than non-significant stenosis.[40] Plaque features can serve for risk stratification of coronary artery lesion. The vulnerable plaque features in CT include low-density plaque (< 30 Hu), positive remodeling, punctate calcification, and “napkin-ring” sign.[41] The new coronary artery disease-reporting and data system (CAD-RADS) has been recently established with updated interpretation and management due to the degree of maximal coronary stenosis.[42]
Table 3 shows risk stratification according to results of all non-invasive examination. Optimize medical therapy for low risk (annual cardiovascular mortality rate < 1%). If symptoms are not improved, patients should receive invasive coronary artery imaging examination, and conduct FFR if necessary. For the moderate risk (annual mortality rate between 1% and 3%), conduct optimized medical therapy and consider invasive coronary artery imaging examination, and conduct FFR if necessary. For high risk (annual CV mortality rate > 3%), patients should receive invasive coronary artery imaging examination, and conduct FFR if necessary.[2]
Table 3. Definition of risks detected in all non-invasive imaging techniques.
| ECG exercise stress test |
High risk | Annual cardiovascular mortality rate > 3% |
| Moderate risk | Annual cardiovascular mortality rate between 1% and 3% | |
| Low risk |
Annual cardiovascular mortality rate < 1% |
|
| Ischemia imaging |
High risk | The ischemic areas > 10% (SPECT > 10%; limited CMR quantitative data - approximately ≥ 2/17 segments of new perfusion abnormality, or the dobutamine-induced malfunction segments ≥ 3; left ventricle segments detected in echocardiography stress test ≥ 3). |
| Moderate risk | The ischemic areas between 1% and 10%, or the ischemic areas lower than CMR or high-risk standard determined by echocardiography stress test. | |
| Low risk |
No ischemia |
|
| Coronaryartery CTA | High risk | Including left main lesion, three-vessel disease, or two blood vessels merged with proximal lesion of anterior descending branch. |
| Moderate risk | Main coronary or significant proximal coronary artery stenosis lesion, but it has not reached the level of high risk | |
| Low risk | Normal coronary artery, or the existence of plaque only, no remarkable stenosis and no features of vulnerable plaque. |
CMR: cardiac magnetic resonance imaging; CTA: computed tomography angiography; SPECT: single-photon emission computed tomography.
5. Conclusions
Fully understanding various non-invasive imaging examination techniques can lead to reasonable selection of the best examination in clinical practice to effectively detect the disease and avoid excessive treatment. The intention for issuing this expert consensus is to standardize clinical pathways and take advantage of multi-modality imaging tech niques for better clinical service. This expert consensus is expected to be validated in clinical practice and improved over time in order to lay the foundation for generating practical and effective clinical guidelines in China.
Acknowledgments
This work was supported by grants from National Key R&D Program of China (2016YFC1300300). The English version was translated by Jing WANG (Chinese PLA General Hospital) and Jun-Jie YANG (from Chinese PLA General Hospital); and edited by Yu-Chi HAN (from University of Pennsylvania, United States).
References
- 1.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 3.Gao Runlin, Huo Yong, Ma Hong, et al. Guidelines on the diagnosis and management of chronic stable angina pectoris. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:195–206. [Article in Chinese] [Google Scholar]
- 4.Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983;1:574–575. doi: 10.1016/s0735-1097(83)80093-x. [DOI] [PubMed] [Google Scholar]
- 5.Dang AM, Ji FS, Li XY, et al. [China expert consensus of diagnosis and treatment of senile coronary heart disease] Zhonghua Lao Nian Yi Xue Za Zhi. 2016;35:683–691. [Article in Chinese] [Google Scholar]
- 6.Lauer M, Froelicher ES, Williams M, et al. Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation. 2005;112:771–776. doi: 10.1161/CIRCULATIONAHA.105.166543. [DOI] [PubMed] [Google Scholar]
- 7.Belardinelli R, Lacalaprice F, Carle F, et al. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur Heart J. 2003;24:1304–1313. doi: 10.1016/s0195-668x(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 8.Shi Yajun, Gao Ling, Wang Jinli, et al. [The analysis of treadmill exercise test for patients over 60 years old] Zhongguo Fen Zi Xin Zhang Bing Xue Za Zhi. 2015;15:1270–1272. [Article in Chinese] [Google Scholar]
- 9.Senior R, Becher H, Monaghan M, et al. Clinical practice of contrast echocardiography: recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur Heart J Cardiovasc Imaging. 2017;18:1205. doi: 10.1093/ehjci/jex182. [DOI] [PubMed] [Google Scholar]
- 10.Xie F, Dodla S, O'Leary E, et al. Detection of subendocardial ischemia in the left anterior descending coronary artery territory with real-time myocardial contrast echocardiography during dobutamine stress echocardiography. JACC Cardiovasc Imaging. 2008;1:271–278. doi: 10.1016/j.jcmg.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Chiou KR, Huang WC, Lin SL, et al. Real-time dobutamine stress myocardial contrast echocardiography for detecting coronary artery disease: correlating abnormal wall motion and disturbed perfusion. Can J Cardiol. 2004;20:1237–1243. [PubMed] [Google Scholar]
- 12.Secknus MA, Marwick TH. Evolution of dobutamine echocardiography protocols and indications: safety and side effects in 3,011 studies over 5 years. J Am Coll Cardiol. 1997;29:1234–1240. doi: 10.1016/s0735-1097(97)00039-9. [DOI] [PubMed] [Google Scholar]
- 13.Pellikka PA, Nagueh SF, Elhendy AA, et al. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Voigt JU, Exner B, Schmiedehausen K, et al. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation. 2003;107:2120–2126. doi: 10.1161/01.CIR.0000065249.69988.AA. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie JL, Bateman TM, Bonow RO, et al. Guidelines for clinical use of cardiac radionuclide imaging. Report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Committee on Radionuclide Imaging), developed in collaboration with the American Society of Nuclear Cardiology. JAm Coll Cardiol. 1995;25:521–547. doi: 10.1016/0735-1097(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 16.Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13:24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Di Carli MF, Dorbala S, Meserve J, et al. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48:783–793. doi: 10.2967/jnumed.106.032789. [DOI] [PubMed] [Google Scholar]
- 18.Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464–1480. doi: 10.1161/CIRCULATIONAHA.106.629808. [DOI] [PubMed] [Google Scholar]
- 19.Kajander S, Joutsiniemi E, Saraste M, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122:603–613. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- 20.Coelho-Filho OR, Rickers C, Kwong RY, et al. MR myocardial perfusion imaging. Radiology. 2013;266:701–715. doi: 10.1148/radiol.12110918. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motwani M, Jogiya R, Kozerke S, et al. Advanced cardiovascular magnetic resonance myocardial perfusion imaging: high-spatial resolution versus 3-dimensional whole-heart coverage. Circ Cardiovasc Imaging. 2013:339–348. doi: 10.1161/CIRCIMAGING.112.000193. [DOI] [PubMed] [Google Scholar]
- 23.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6:806–822. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.François CJ. Current state of the art cardiovascular MR imaging techniques for assessment of ischemic heart disease. Radiol Clin North Am. 2015;53:335–344. doi: 10.1016/j.rcl.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Mark DB, Berman DS, Budoff MJ, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American college of cardiology foundation task force on expert consensus documents. Circulation. 2010;121:2509–2543. doi: 10.1161/CIR.0b013e3181d4b618. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Yang JJ, Yang X, et al. Impact of clinical guideline recommendations on the application of coronary computed tomographic angiography in patients with suspected stable coronary artery disease. Chin Med J (Engl) 2016;129:135–141. doi: 10.4103/0366-6999.173434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007;244:419–428. doi: 10.1148/radiol.2442061218. [DOI] [PubMed] [Google Scholar]
- 28.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 29.Dai RP, Du XK, Gao H, et al. [Expert consensus on cardiac coronary multi-detector computed tomography clinical application] Zhonghua Fang She Xue Za Zhi. 2011;45:9–17. [Article in Chinese] [Google Scholar]
- 30.Chen YD, Chen JY, Fu GH, et al. [Chinese expert consensus on the adverse reaction to iodinated contrast media in angiography] Zhongguo Jie Ru Xin Zang Bing Xue Za Zhi. 2014;22:341–348. [Article in Chinese] [Google Scholar]
- 31.Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) J Am Coll Cardiol. 2014;63:1145–1155. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 32.Rochitte CE, George RT, Chen MY, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J. 2014;35:1120–1130. doi: 10.1093/eurheartj/eht488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mark DB, Shaw L, Harrell FE, Jr., et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325:849–853. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 34.Marwick TH, Case C, Vasey C, et al. Prediction of mortality by exercise echocardiography: a strategy for combination with the duke treadmill score. Circulation. 2001;103:2566–2571. doi: 10.1161/01.cir.103.21.2566. [DOI] [PubMed] [Google Scholar]
- 35.Chelliah R, Anantharam B, Burden L, et al. Independent and incremental value of stress echocardiography over clinical and stress electrocardiographic parameters for the prediction of hard cardiac events in new-onset suspected angina with no history of coronary artery disease. Eur J Echocardiogr. 2010;11:875–882. doi: 10.1093/ejechocard/jeq086. [DOI] [PubMed] [Google Scholar]
- 36.Shaw LJ, Berman DS, Picard MH, et al. Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging. 2014;7:593–604. doi: 10.1016/j.jcmg.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hachamovitch R, Hayes SW, Friedman JD, et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 38.Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 39.Lin FY, Dunning AM, Narula J, et al. Impact of an automated multimodality point-of-order decision support tool on rates of appropriate testing and clinical decision making for individuals with suspected coronary artery disease: a prospective multicenter study. J Am Coll Cardiol. 2013;62:308–316. doi: 10.1016/j.jacc.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 40.Hulten EA, Carbonaro S, Petrillo SP, et al. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;57:1237–1247. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Thomsen C, Abdulla J. Characteristics of high-risk coronary plaques identified by computed tomographic angiography and associated prognosis: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2016;17:120–129. doi: 10.1093/ehjci/jev325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cury RC, Abbara S, Achenbach S, et al. Coronary Artery Disease - Reporting and Data System (CAD-RADS): an expert consensus document of SCCT, ACR and NASCI: Endorsed by the ACC. JACC Cardiovasc Imaging. 2016;9:1099–1113. doi: 10.1016/j.jcmg.2016.05.005. [DOI] [PubMed] [Google Scholar]