1. Introduction
Transcatheter aortic valve implantation (TAVI) constitutes an established treatment in inoperable or high peri-operative risk patients with severe aortic stenosis, demonstrating similar mortality rates (at 30 days and 1 year) with surgical aortic valve replacement (SAVR).[1] Various complications have been reported during TAVI, weeks or months post procedure. The most frequent causes of transcatheter heart valve (THV) failure are paravalvular regurgitation, infective endocarditis (IE), thrombosis and late valve Migration.[1],[2] Post TAVI endocarditis diagnosis can be established according to the valve academic research consortium-2 criteria which include (1) Duke endocarditis criteria; or (2) evidence of abscess, paravalvular leak, pus, or vegetation confirmed by histological or bacteriological studies during a reoperation; or (3) abscess, pus, or vegetation confirmed by an autopsy.[1]
2. Incidence
Prosthetic valve endocarditis (PVE) post ΤΑVI occurs with an incidence of 0.3%–1.2% per patient-year presenting comparable rates with PVE after surgical replacement.[2] However, a much higher incidence of 2.3%–3.4% per patient-year is reported in individual series or registries.[3],[4]
3. Classification
Prosthetic valve IE post TAVI based on the diagnosis time post implantation is classified as: early (occurring in the first 60 days), intermediate (from 60 days to 1 year), and late (> 1 year).[5] The reported incidence rates are estimated up to 18% for the early, 62% for the intermediate and 20% for late post TAVI endocarditis.[5] Notably, the risk of endocarditis is significantly increased in the first year post TAVI.[5]
4. Location of IE in TAVI patients
Infective endocarditis may stem from hematogenous dissemination or contact with infected adherent tissue. The vegetations could be located in valve leaflets (39%), stent frame (17%), and/or in both structures (9.2%).[2],[5],[6] Abscesses and fistulas have been observed in 47% and 9% of TAVI patients, respectively.[5],[6] Satellite endocarditis of the mitral valve as result of the direct contact of the aortic THV with the mitral apparatus has been demonstrated in 24% of PVE cases, while secondary mitral valve involvement is estimated in 10% of patients.[5]
5. Clinical features
The clinical features of post TAVI infective endocarditis are greatly varying from non specific symptoms to acute infection or sepsis with fever or even signs of heart failure or embolic stroke. The symptoms are strongly correlated with the causative microorganism, the presence of previous cardiac disease as well as prior prosthetic valve implantation. Interestingly, atypical signs and symptoms are common in elderly patients.[2],[6] The following symptoms have been reported in post TAVI patients with infective endocarditis: signs of heart failure (> 50%), and non-specific symptoms such as weakness, malaise or weight loss in several cases (20%).[2],[6] Spiked temperature is reported less frequently compared to IE in the general population (75% vs. 90%, respectively) due to relative anergy in older population. The rate of embolic stroke (7.5%) as first manifestation of IE is also reduced compared to previous series (20%–40%).[2],[6] Finally, it should be emphasized that the presence of a new heart murmur consists a less valuable sign in TAVI patients due to respective high rate of residual paravalvular leaks following the procedure or concomitant mitral insufficiency and/or mitral annular calcification. On the other hand, the presence of fever early post TAVI is common, as it might be attributed to an inflammatory foreign body response post implantation, with no bacteraemia or infection. In the meantime, the incidence of in-hospital infection (19.5%) should not be underestimated in post TAVI patients presenting with fever. Specifically, urinary tract infection (43.1%), pneumonia (20.7%), and access-site infection (12.1%) represent common causes of high temperature in TAVI patients. Interestingly, the infection site cannot be determined in 20.7% of TAVI patients, whereas multiple infection sites might be reported (3.4%).[7] The potential causes of prosthetic valve endocarditis are summarized in Table 1.
Table 1. Potential causes of prosthetic valve endocarditis.
| 1. | The non-sterile environment in the majority of cardiac catheterization laboratories. |
| 2. | The high-risk profile of TAVI patients such as diabetes, immuno-suppression (i.e., steroids, myelodysplastic syndromes), and renal failure. |
| 3. | Technical issues, as the frequent requirement to remove and re-implant a malpositioned THV (as leaflet and endothelial injury caused by manipulation) could increase the risk of PVE. |
| 4. | Patient education, especially regarding the importance of post-implantation antibiotic prophylaxis. |
| 5. | Adequate endothelialization of the bioprosthetic valve may require a dual antiplatelet therapy (aspirin and clopidogrel). |
| 6. | Coincident infections. |
| 7. | Low position of aortic THV being in direct contact with the mitral apparatus. |
| 8. | Leaflet compression during transcatheter valve preparation and loading resulting in a degree of leaflet damage. |
| 9. | Higher pre-procedural transaortic gradients in the PVE cases. |
| 10. | Paravalvular leakage as it may be a possible ‘local’ risk factor for endocarditis. |
| 11. | Male sex (2/3 of endocarditis patients) partially explained by the estrogen endothelial protection. |
| 12. | Residual aortic regurgitation may induce endothelial damage (“jet lesions”) serving as a nidus during episodes of transient bacteremia. |
| 13. | Bioprosthesis and the native aortic valve cusp space might be a suitable nidus for pathogen accumulation during transient bacteremia. |
| 14. | Orotracheal intubation and the use of a self-expandable valve system were associated with IE post-TAVI |
| 15. | TAVI in native valve |
| 16. | Vascular complication |
IE: infective endocarditis; PVE: prosthetic valve endocarditis; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve.
6. Microorganisms
The most (81.8%) frequently encountered agents in post-TAVI IE are coagulase-negative staphylococci (24.5%), Staphylococcus aureus (21%), enterococci (21%), and oral (formerly viridans) streptococci.[2],[5],[6],[8] Less common causal agents include gram negative bacteria.[2],[8] Notably, a higher incidence of enterococci-positive blood cultures have been demonstrated in patients undergone transfemoral TAVI compared to the respective patients postsurgical replacement. The use of transfemoral access in TAVI and the proximity of the groin with genitourinary/intestinal system constitute a strong predisposing factor for the frequent isolation of enterococci. Staphyllococci are dominant (29.4%) post transapical TAVI while enterococci represent the most common microorganisms (34.4%) post transfemoral TAVI.[6],[8]
7. Imaging techniques
Transoesophageal echocardiography (TOE) plays a significant role in the diagnosis, management and follow up of IE treated-patients. According to the updated guidelines of European Society of Cardiology in respect to the modified criteria for the diagnosis of IE, the MSCT, MRI, and 18F-fluorodeoxyglucose (FDG) positron emission tomography (18F-FDG PET) should be used as additional imaging techniques.[9]
7.1. Echocardiography
Both TTE and TOE constitute the cornerstone for the diagnosis of IE and further exclusion of local complications. The echocardiographic diagnosis of post TAVI endocarditis is much more challenging in comparison with native or prosthetic valve endocarditis after surgical replacement. The difficulty lies on the technique of the valve implantation, the absence of surgical sewing ring or decalcification area and the remnant of the native calcified aortic valve. The free space between transcatheter and native aortic valve by TEE is also challenging for the diagnosis of vegetation or abscess.[3],[4],[9] Especially, the detection of any small vegetation is limited, as THV, consists of high amounts of metal, further creating a shadowing effect and reflectance.[2]–[4]
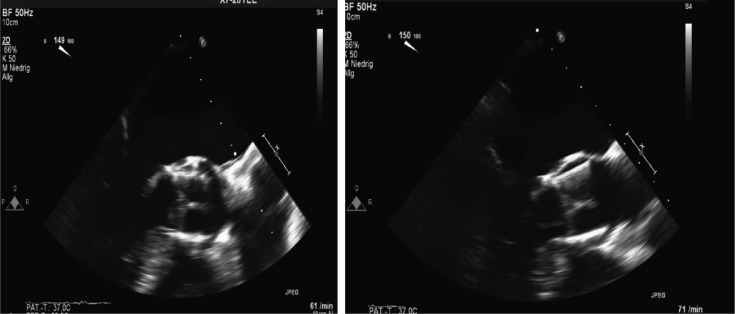
Generally, three of four diagnostic echocardiographic Duke criteria (“abscess”, “new dehiscence of prosthetic valve” and “new valvular regurgitation”) are not easily applicable in the context of TAVI IE, thus complicating the correct diagnosis. The vegetations (50%) constitute the most frequent finding followed by peri-annular complications (Figure 1). Involvement of the mitral, tricuspid valve or pacemaker lead have also been documented.[2],[4],[9] Very small (< 2 mm) vegetations, calcified mitral rings, thickened valves, absent or already embolized vegetations might further lead to false negative results and false rejected PVE.
Figure 1. Late Edwards Sapien-XT prosthesis endocarditis with mobile vegetation.
TOE (long-axis views) demonstrates a floating mobile endocarditic formation (vegetation) attached on the prosthetic aortic leaflet on the posterior aortic wall, 13 months after TAVI with the Edwards Sapien-XT prosthesis (29 mm). TAVI: transcatheter aortic valve implantation; TOE: transoesophageal echocardiography.
In case of remaining suspicion of IE, the TOE should be repeated within 5–7 days.[9] TOE can evaluate the size (larger than 10 mm) or the mobility of the vegetations, and further quantify the risk of systemic embolism as well as the survival rate of post endocarditis patients.
7.2. MSCT and 18F-FDG PET/CT
Cardiac CT is used frequently for bio-prosthetic device evaluation such as peri-valvular structures' visualization. Periannular complications can be detected much more easily by CT techniques compared with TOE, exhibiting similar diagnostic accuracy of vegetation detection with TOE. The 18F-FDG PET/CT constituted another imaging technique to define TAVI IE. Based on the modified ESC diagnostic criteria of IE, an abnormal activity around the site of infected implanted prosthetic valve can be detected by 18F-FDG PET/CT (only if the prosthesis was implanted for > 3 months) or radio-labeled leukocytes SPECT/CT. Radio-labeled WBC SPECT/CT is more specific for the detection of IE and infectious foci compared with 18F-FDG PET/CT.[8],[10],[11] In addition, false-positive results of PET/CT may be obtained in case of severe prosthetic thrombosis, presence of an aortic root graft, active thrombi, soft atherosclerotic plaques, vasculitis, primary cardiac tumors, cardiac metastasis from a non-cardiac tumor.[9]–[11] In a large cohort of patients with presumed PVE, the sensitivity and specificity of this technique is reported to be 73% and 80%, respectively.[10] In case of possible IE or rejected IE despite high suspicion, re-evaluation/re-classification should be performed by using a second round of cardiometabolic imaging.[9]–[11]
8. Approach and management
The presence of an Endocarditis/Heart Team is crucial to decide about the optimal treatment as a multidisciplinary approach can offer the highest benefits in these patients.
8.1. Medical treatment
The antibiotics should be administrated based on the microorganism type and the estimated minimum inhibitory concentration (MIC), and therapy must be prolonged (at least 6 weeks).[9] Rifampicin should be used in foreign body infections, when bacteraemia has been specified. Aminoglycosides demonstrate clinical benefit in enterococci IE but increase renal toxicity. Measures for nephrotoxicity protection in the elderly population with frequent renal impairment include regular assessment of renal function during treatment, with corresponding adjustment of the drug dosage.[9] Enterococci present a highly tolerance to antibiotics due to the intrinsic and acquired resistance and as a consequence of prolonged combined administration (as long as 6 weeks) is required for complete eradication.[2],[9],[12]
8.2. Surgery
The surgical therapy lies on the localization, the concomitant complications, the size and mobility of the vegetation, the isolated microorganism, the hemodynamic status of patient (pulmonary oedema or cardiogenic schock) and finally the individual decision of the heart team. Surgery in these high risk or inoperable patients in general is considered in case of a thromboembolic event, abscess, aneurysm, vegetations, uncontrolled infection and heart failure. TAVI IE has been treated conservatively in most instances due to the high operative risk of these patients (80 ± 7 years of age), frailty and the very high-risk profile (mean logistic Euro-SCORE: 30.4% ± 14.0%).[2],[4],[13],[14] Approximately, 12%–45% of the TAVI-IE patients have received surgical treatment, with valve explantation and aortic valve replacement.[2],[6],[13]
Notably, in case of Corevalve endocarditis, 23% of the patients have been operated in comparison to the much higher percentage of the Εdwards group (57%). Especially, the longer stent frame extending towards the ascending aorta of the CoreValve (Medtronic) may also require further replacement of ascending aorta, rendering the cardiac surgery more challenging.[2],[13] In case of a complicated endocarditis with cardiac abscess, heart wall rupture or fistula, an additional approach is required.
In conclusion, it should be mentioned that there is no established consensus regarding the management of the TAVI IE, although the initial approach is based conservative treatment.
9. Peri-interventional and post-interventional antibiotic prophylaxis
Peri-operative antibiotic prophylaxis should be considered in patients undergoing surgical or transcatheter implantation of a prosthetic valve, or other foreign material with a IIa class of indication (level of recommendation C) according to the recent endocarditis guidelines European Society of Cardiology 2015.[9],[13]
Healthcare-associated IE represents 30% of all cases of endocarditis. The antibiotic prophylaxis is crucial and should be started immediately before the procedure, repeated and terminated 48 h afterwards. Cephalosporins (67%), and less frequently vancomycin (28%) and piperacillin/tazobactam (5%) are often administered.[2],[9],[13]
However, the antibiotic selection must be re-evaluated due to the high prevalence of resistant enterococci in the very early TAVI-PVE. The administration of Glycopeptides and aminoglycosides might be a better option in such cases.
10. Antibiotic prophylaxis
When a high-risk procedure is performed an endocarditis prophylaxis should be considered. According the ESC endocarditis guidelines, an antibiotic prophylaxis should be considered for patients at highest risk for IE with any prosthetic valve, including TAVI with an indication IIa-C. Because of the high endocarditis incidence in the first year, antibiotic prophylaxis is required at least for a year after percutaneous aortic valve implantation.[2],[9],[13]
11. Mortality
The mortality-rate associated with IE post-TAVI [in-hospital (47%) and 1-year (66%)] is the highest ever reported in the endocarditis field in comparison with the respective native or prosthetic valve endocarditis after surgical replacement.[2],[14] The in-hospital mortality rates for early-onset (38%) are higher compared to the late-onset PVE (25%).[2],[13] The more conservative the strategy even in the presence of complication is correlated with poor outcomes. The secondary mitral valve involvement, the health-care associated PVE, and the PVE caused by Staphylococcus aureus are also associated with a poor prognosis.[2],[5],[9],[13]
12. Conclusions
PVE constitutes an uncommon but no rare complication with high mortality. The suspicion and the early diagnosis are crucial for the further course of the disease. The detection of vegetation post TAVI by TOE is challenging. Generally, the optimal therapy remains controversial for prosthetic valve endocarditis after TAVI. The option of surgical or conservative therapy depends on the individual decision of the heart team. An antibiotic prophylaxis is indicated in these patients undergoing high-risk dental or other invasive procedures for IE prevention. Due to the higher incidence of IE during the first year, antibiotic prophylaxis is recommended at least for one year after TAVI.
Footnotes
This article is part of “Transcatheter aortic valve implantation” Special Issue.
Guest Editors: Ioanna Koniari, George Hahalis
References
- 1.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 2.Amat-Santos IJ, Messika-Zeitoun D, Eltchaninoff H, et al. Infective endocarditis following transcatheter aortic valve implantation:results from a large multicenter registry. Circulation. 2015;131:1566–1574. doi: 10.1161/CIRCULATIONAHA.114.014089. [DOI] [PubMed] [Google Scholar]
- 3.Lee HS, Lee SP, Jung JH, et al. Infective endocarditis associated with transcatheter aortic valve replacement: potential importance of local trauma for a deadly nidus. J Cardiovasc Ultrasound. 2014;22:134–138. doi: 10.4250/jcu.2014.22.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puls M, Eiffert H, Hünlich M, et al. Prosthetic valve endocarditis after transcatheter aortic valve implantation: the incidence in a single-centre cohort and reflections on clinical, echocardiographic and prognostic features. Eurointervention. 2013;8:1407–1418. doi: 10.4244/EIJV8I12A214. [DOI] [PubMed] [Google Scholar]
- 5.Mylotte D, Andalib A, Thériault-Lauzier P, et al. Transcatheter heart valve failure review: a systematic review. Eur Heart J. 2015;36:1306–1327. doi: 10.1093/eurheartj/ehu388. [DOI] [PubMed] [Google Scholar]
- 6.Amat-Santos IJ, Ribeiro HB, Urena M, et al. Prosthetic valve endocarditis after transcatheter valve replacement: a systematicreview. JACC Cardiovasc Interv. 2015;8:334–346. doi: 10.1016/j.jcin.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Leshem-Rubinow E, Amit S, Steinvil A, et al. Fever following transcatheter aortic valve implantation: prevalence, pattern and rate attributed to an infectious origin. JACC. 2014;113:1001–1005. doi: 10.1016/j.amjcard.2013.11.063. [DOI] [PubMed] [Google Scholar]
- 8.Regueiro A, Linke A, Latib A, et al. Association between transcatheter aortic valve replacement and subsequent infective endocarditis and in-hospital death. JAMA. 2016;316:1083–10892. doi: 10.1001/jama.2016.12347. [DOI] [PubMed] [Google Scholar]
- 9.Habib G, Lancellotti P, Antunes MJ, et al. ESC guidelines for the management of infective endocarditis the task force for the management of infective endocarditis of the European Society of Cardiology (ESC) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 10.Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374–2382. doi: 10.1016/j.jacc.2013.01.092. [DOI] [PubMed] [Google Scholar]
- 11.Hoey ET, Ganeshan A. Multi-detector CT angiography of the aortic valve—Part 2: disease specific findings. Quant Imaging Med Surg. 2014;4:273–281. doi: 10.3978/j.issn.2223-4292.2014.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollenbeck BL, Rice LB, et al. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence. 2012;3:421–433. doi: 10.4161/viru.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad K, Klaaborg KE, Hjortdal V, et al. Prosthetic valve endocarditis after transcatheter aortic valve implantation-diagnostic and surgical considerations. J Thorac Dis. 2016;8:E1213–E1218. doi: 10.21037/jtd.2016.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Boon RM, Nuis RJ, Benitez LM, et al. Frequency, determinants and prognostic implications of infectious complications after transcatheter aortic valve implantation. Am J Cardiol. 2013;112:104–111. doi: 10.1016/j.amjcard.2013.02.061. [DOI] [PubMed] [Google Scholar]