Abstract
Stem cells and their local microenvironment, or niche, communicate through mechanical, cues to regulate cell fate and cell behaviour, and to guide developmental processes. During embryonic development, mechanical forces are involved in patterning and organogenesis. The physical environment of pluripotent stem cells regulates their differentiation and self-renewal. Mechanical and physical cues are also important in adult tissues, where adult stem cells require physical interactions with the extracellular matrix to maintain their potency. In vitro, synthetic models of the stem cell niche can be used to precisely control and manipulate the biophysical and biochemical properties of the stem cell microenvironment and examine how the mode and magnitude of mechanical cues, such as matrix stiffness or applied forces, direct stem cell differentiation and function. Fundamental insights on the mechanobiology of stem cells also inform the design of artificial niches to support stem cells for regenerative therapies.
1. Introduction
Forces are generated and resisted across many magnitudes and length scales in biology, from a sub-cellular level, for example by actomyosin motors to an organismal level, such as in response to gravity. Similar to intrinsic and extrinsic biochemical factors, mechanical cues resulting from both intracellularly-generated and externally-applied forces have broad impact on stem cell function. Mechanical interactions mediated by adhesion to the extracellular matrix (ECM) and cell-cell junctions play a key part in transmitting forces to and between cells, which regulate intracellular signalling pathways (FIG. 1).
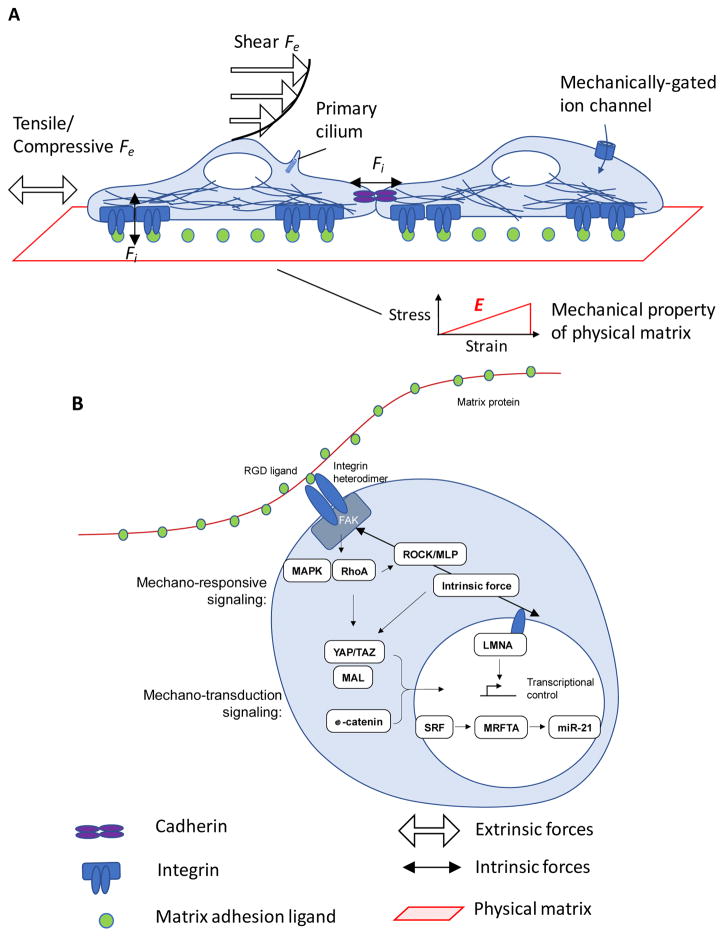
Figure 1.
Stem cells exert forces and are subject to external forces, which regulate their intracellular signaling pathways. A) Intrinsic, or cell-generated forces, (Fi) are generated intracellularly and transferred to other cells through cell-cell junctions, like cadherin receptors, or via traction on extracellular matrix (ECM) adhesion ligands that are bound to integrin receptors. Cells are directly coupled by cell-cell junctions, which link the intrinsic forces of one cell to the cytoskeleton of another. Indirect mechanical coupling between cells occurs by intrinsic forces exerted on an ECM, to which two or more cells are adhered. Physical properties, e.g., elastic modulus, E, of the ECM, govern how mechanical cues are transduced. Extrinsic forces (Fe) are externally applied by shear or tension/compression on cells, and can be sensed by mechanically-gated ion channels, changes in receptor-ligand binding, deformation of the cytoskeleton, and the primary cilium. The cytoskeleton generates and transfers forces from membrane proteins to intracellular structures, like the nucleus. B) ECM and intracellular pathways are biochemically coupled by mechanotransduction pathways. Matrix mechanical resistance to intrinsic forces regulates the stability of focal adhesion complexes that contain focal adhesion kinase (FAK), which phosphorylates and activates mechano-responsive signaling elements, such as mitogen-associated protein kinase (MAPK) and Rho kinase (RhoA). RhoA regulates mechanical feedback by activating ROCK, which phosphorylates myosin light chain (MLP) to generate actomyosin forces. For example, RhoA phosphorylates ROCK, which activates non-muscle myosin II contractility by phosphorylation of myosin light chain (MLP), and upregulates mechano-transduction pathways, such as MAL, a G-actin-binding coactivator of serum response factor (SRF),88.89 Yes-associated protein (YAP), and transcriptional coactivator with PDZ-binding motif (TAZ),90 which induce nuclear transcription via activation and nuclear translocation. Nuclear signaling pathways, such as serum response factor (SRF) and myocardin-related transcription factor-A (MRFTA), regulate transcription of mechanoresponsive genes. For example, mechanics-dependent expression of miR-21 regulates fibrogenic behavior. Wnt activation also promotes activation and nuclear translocation of YAP/TAZ and beta-catenin.160 Mechanical forces are also physically directly coupled to the nucleus via lamina proteins, such as lamin A (LMNA), which can affect chromatin structure and epigenetic regulation of transcription.
Cells exert intrinsic forces on their environment, that is, on the ECM and neighbouring cells, through various mechanisms, including actomyosin contractility and cytoskeletal assembly. Conversely, cell-extrinsic shear, tensile and compressive forces are applied on stem cells from an external load. Whether generated intrinsically or extrinsically, the impact that mechanical forces have on cell behaviour depends on the biological context of a cell, such as biochemical cues and epigenetic state, and on various aspects of its physical environment. The microenvironment may be composed of fluids, solids, gases, or other cells, and provides external resistance or compliance that may store or dissipate forces (Boxes 12). Owing to the complexity of these mechanical interactions, it is important to develop systems in which each type of mechanical cue can be controlled and de-coupled from others to understand how they affect stem cells.
Box 1. Introduction to mechanical terms and concepts.
Various concepts and terms are used to describe and quantify the types and magnitudes of mechanical properties, and the relationship between forces and deformations.
Stress is an indication of the magnitude of force applied to an object, normalized to the area over which the force is applied. Stress is calculated by Force / area, σ = F / A, and is typically reported in Newtons / m2 or Pascals.
Strain is a measure of the deformation resulting from an applied force, as indicated by the normalized change in the length of the object. Strain (ε) = current length / initial length - 1 = Δl / l0 - 1, and is typically reported as a dimensionless fraction or percentage.
The relationship between the force and deformation, or stress and strain, in a system is dependent on the properties of the material that transfers the stress/strain (e.g., the matrix to which cells apply tractional forces). If a material effectively stores energy during the transfer, the material is termed to be Elastic. In linearly elastic materials there is a linear relationship between stress and strain, and energy (e.g., forces) applied to the material are stored (e.g., deformations induced by cells protruding into an elastic material will be stored in the material and “push” back against the cell). For example, rubbers and covalently-crosslinked hydrogels are typically considered to be elastic, and their rigidity or stiffness is determined by the modulus (e.g., elastic modulus, shear modulus), which is closely related to the density of crosslinks between the polymer chains comprising the network. The elasticity, or Young’s modulus, of an elastic material, can be approximated in linearly elastic solids by the slope of the stress versus strain curve at small strains (typically 1–5%).
Many tissues, cells, and extracellular matrices combine mechanical properties of solids and liquids, and these types of materials are termed viscoelastic. They behave as elastic solids and as viscous fluids simultaneously. Viscous fluids demonstrate flow, or permanent deformation, in response to applied forces, and are described by their viscosity, which relates the rate and extent of flow to an applied force. Viscoelastic materials will exhibit stress relaxation (decrease in the stress required over time to maintain a constant level of strain), and creep (increase in strain over time in response to a constant stress). Viscoelastic materials can dissipate applied loads via their permanent deformation, allowing for cells to remodel viscoelastic matrices even in the absence of degradation, and without a build-up in the material of forces that “push” back on the cell.
The term stiffness is typically used in mechanobiology as a metric of the rigidity of a matrix as sensed by cells via application of cell-generated forces. A stiffer matrix will require higher forces to deform the network, whereas a softer matrix can be deformed by lower forces. The stiffness of matrices is often determined under the assumption of linear elastic behavior, making it synonymous with elasticity.
Box 2. Mechanical properties of tissues, cells, and matrix.
Elastic and viscoelastic properties of tissues, cells, and matrix are typically measured by mechanical tests in which a known and defined stress or strain is applied on a sample while the other is measured. Methods of rheology, the study of flow and deformation of matter, are used to characterize both the elastic (G′, storage modulus) and viscous (G″, loss modulus) behavior of viscoelastic materials by dynamically controlling the rate and amount of strain or stress while measuring the other. Bulk compression or tension measurements are used to measure the elastic modulus (E, Young’s modulus), which relates to the density of crosslinks in a hydrogel, by applying a uniaxial strain and measuring the stress. These uniaxial compression measurements or shear compression measurements can also be used to measure the stress relaxation or creep of a material, which measures the time-dependent changes in stress or strain resulting from the application of a constant level of strain or stress. Methods such as atomic force microscopy, nanoindentation, optical tweezers, force traction microscopy, and micro-aspiration, probe the stiffness at the nano- and micro-scale, which allows for mapping of mechanical topographies or gradients in materials. Representative mechanical properties of select tissues and materials are provided in Table 1.
Understanding how mechanical forces regulate stem cell behaviour provides key insights into the understanding of developmental biology, and for the development of regenerative therapies. In this Review, we provide an overview of the types of mechanical cues that affect stem cells and of biomaterial-based systems that can be used to control and manipulate these cues. We focus on how different types of forces regulate stem cell behaviours in early development and organogenesis, control stem cell fate, including differentiation and self-renewal, and can be exploited to promote regeneration.
2. Mechanical cues guide development
The growth, differentiation and morphogenesis of a developing embryo is dependent on intrinsic and extrinsic mechanical forces that drive the assembly of cells and promote growth into higher order structures (Table S1).1 Cell-cell adhesion transmits tensile forces and, as embryonic development progresses, cells become mechanically coupled to matrix proteins in tissues by adhesion molecules (FIG. 2A), which helps drive morphogenesis and maintain stem cells’ position and fate in their niche. This mechanical coupling enables storage of information over time. For example, changes in ECM induced by cells early in development can mechanically trigger changes in interacting cells at a later stage. Moreover, mechanical cues can be more rapidly propagated over long distances during morphogenesis than biochemical cues (for example, the transmission of forces in highly elastic substrates like elastin is almost instantaneous).
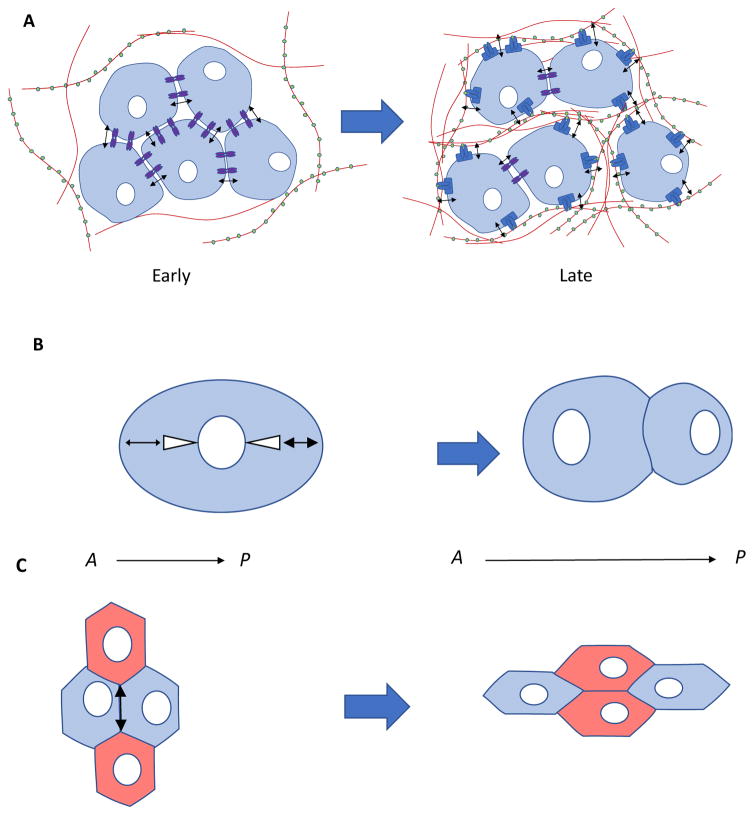
Figure 2.
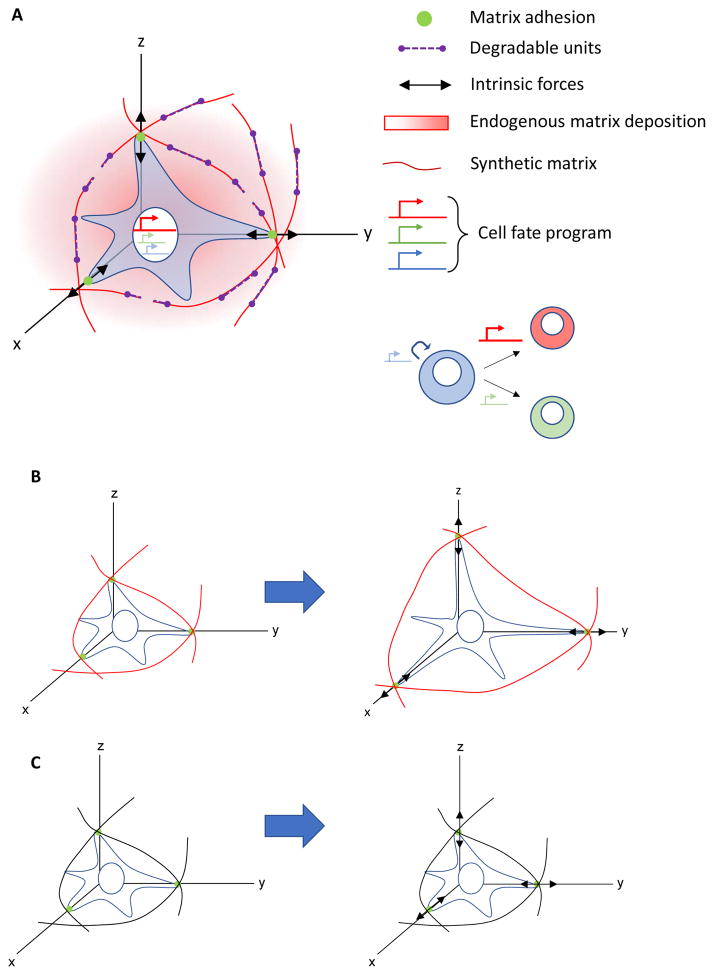
Mechanobiology in the developmental niche. A) While development progresses, intrinsic forces exerted by cells transition from largely cell-cell to more cell-matrix transmission as matrix content in tissues increases. B) Higher astral tension (white triangles) on the posterior axis (right side, bold arrow) of dividing cells is generated by cortical tension and microtubule polymerization, which results in asymmetry in cell size after division.8 C) Cell-cell intrinsic forces in early development modify the pattern of embryonic epithelial adhesion and intercalation, which results in elongation of the anterior- (A) posterior (P) axis.4 D) During epithelial branching morphogenesis of the fetal submandibular salivary gland, cells exert intrinsic actomyosin contractility and traction forces on the extracellular matrix (ECM) (red), which assembles at a clefting region and promotes cell proliferation (pink arrow) in the budding region.33 ECM contains domains of heparan sulfate (HS) that bind FGF growth factors to promote epithelial bud elongation by differentially increasing their local concentration.159 Thus, concerted biochemical and mechanical cues work together to generate proper organ form.
Intrinsic and extrinsic forces guide early embryo development
Prior to implantation, self-organization of the embryo and specification of the germ layers into a blastocyst rely on contractile mechanical cues.2 Tensions are relieved as the embryo transitions from a spherical to elongated body form3 and an intercalated pattern of cell-cell epithelial junctions is generated.4 The presence of intrinsic cell-generated forces in development was demonstrated by observing the deformation of embryonic tissues following their macro-scale dissection at different stages of morphogenesis,5 as well as nano-dissection of the actomyosin network at apical cell junctions. The latter showed that anisotropy of intrinsic forces is sufficient to promote elongation of embryonic epithelia.6
Intrinsic mechanical tensile and compressive forces that regulate multiple aspects of embryonic morphogenesis are generated by non-muscle myosin II in actomyosin complexes6. For example, actomyosin forces promote the establishment of an anterior-posterior axis of development by promoting asymmetric spindle positioning (FIG. 2B)7,8 and the remodelling of intercalated epithelial junctions4 (FIG. 2C). Axis elongation depends on tension generated by multicellular cables of actomyosin9 with both elastic and viscous mechanical behaviour (Box 1), as demonstrated by laser-severing.10 A catch-bond mechanism, in which tensile forces promote the stabilization of cadherin-catenin complexes [G] bound to actin filaments, demonstrates how these cell-cell receptor interactions communicate biochemical signals through mechanical forces.11 These forces also control gastrulation by regulating epithelial invagination12–14 and progenitor cell sorting within the germ layers15, as well as dorsal closure [G].16 Intrinsic forces in embryonic tissues can also be observed when embryonic stem cells (ES cells) are dissociated, as they also display cortical tension [G] generated by actomyosin contractility. This is required to reduce apoptosis, but inhibition of Rho-associated protein kinase (ROCK) [G] relieves this dependence.17
In addition to being subjected to intrinsic mechanical forces, the embryo receives external mechanical input from the fluid surrounding its intercalated cell layers that induce patterning. Fluid shear forces during early development regulate left-versus-right body asymmetry by signalling through primary cilium and by generating morphogen gradients.18,19 Morphogens Sonic hedgehog (SHH) and retinoic acid (RA) are secreted in ‘nodal vesicular parcels’ (NVPs) in a FGF-dependent manner and transported to the left by the nodal flow.19 However, important questions on the mechanobiology of embryonic morphogenesis remain largely unaddressed, such as how mechanical forces interplay with genetic and epigenetic changes, which could inform approaches to manipulate intrinsic forces by control of transcription, and how robust pattern formation results even in the face of natural variations in the magnitude and duration of intrinsic and extrinsic forces.
Cell-ECM interactions guide later stages of development
Early in development cell-cell contacts predominate, but as development progresses, progenitor cells differentiate and begin to deposit ECM to which they adhere20 (FIG. 2A). The number of collagen fibrils and fiber length steadily increase in embryonic development, whereas collagen content remains stable in post-natal growth.21
Cell intrinsic forces are transmitted to matrix proteins such as fibronectin by integrin cell surface receptors, which can form focal complexes that generate forces of 1–3 nN/μm2.22 Increased integrin expression in tissues may alter how cells respond to ECM stiffness, in part by stabilizing matrix-integrin interactions.23 Thus, as the matrix content of tissues increases during development, the coupling increases between ECM mechanical properties and intrinsic mechanical forces, one aspect of mechanosensing.
Cell-ECM interactions enable biological systems to contextualize stimuli because cells respond differently to the same mechanical stimulus depending on their micro-mechanical or biochemical environment. In embryonic avian skin, mechanical and biochemical factors are coupled to the positioning and patterning of hair follicles. Mechanical resistance to dermal cell contraction upregulates β-catenin activation, which drives downstream follicular gene expression programs, including bone morphogenic protein-2 (BMP2).24 Furthermore, mechanics direct multi-scale developmental processes by connecting macro-scale physical inputs with nano-scale molecular signals through chemical cues.25 For example, force transmission through specific receptors enables generic mechanical signals to be transduced into specific cell responses based on the type of integrin receptors that are expressed in the cells or ligands present in the ECM. Logically, mechanical transduction needs to be carefully controlled to maintain homeostasis, because mechanical stimuli are often not specific. Moreover, ECM provides a mechanical rheostat for cells in four-dimensions – time & space, because the matrix can dissipate elastic energy and change over time by stiffening, degradation and matrix deposition.
Mechanical forces regulate cell fate decisions during organogenesis as progenitor cells are directed to diverse specialized functions in fetal organs. The process of germ band extension generates mechanical forces that promote differentiation of the stomodeum [G] and midgut tissues by inducing the expression of Twist, which is one of the earliest expressed embryonic patterning genes.26 Tensile forces, arising from stretching of bronchial epithelium during intrauterine breathing, support development of smooth muscle in the lung.27 Shear forces generated by maternal blood flow promote fetal hematopoiesis and the morphogenesis of cardiac tissues28–30, and signal to epithelia in the developing kidney via the primary cilia, which require polycystin-1 and polycystin-2 proteins to promote kidney morphogenesis.31 In limb development, stress, strain, hydrostatic pressure, and fluid flow precede regional ossification and subsequent bone collar formation.32 The underlying molecular mechanisms are the subject of current studies. These examples highlight how organ development is affected by the physical nature of their microenvironment. Research aiming to recapitulate these developmental processes from stem cells in vitro will require sophisticated systems in which forces can be tightly controlled.
Complex patterning depends on cell-ECM interactions
Biochemical cues initiate morphogenesis, but the formation of cell layers that become organized into defined structures in organs requires physical traction forces [G] on the ECM, the physical properties of which provide a template for organ growth. The concerted action of biochemical signals, cell intrinsic forces, and cell-ECM interactions result in highly organized patterns of development, such as fractal patterns [G] observed in branching morphogenesis.33
In submandibular salivary gland [G] branching morphogenesis, focal adhesions [G] bound to fibronectin promote assembly of fibronectin at the branching cleft through actomyosin contractility34 (FIG. 2D). Traction forces are required for branching, which suggests that the rigidity of the matrix could alter branching by changing actomyosin contractility, but it remains to be directly determined whether matrix mechanical properties can indeed modulate branching in salivary glands.
The study of mechanobiology is complex owing to mechanical stimuli affecting multiple aspects of cell behaviour, including matrix traction forces, membrane curvature, growth factor signalling pathways and cell fate. The physical properties of ECM regulate mammary gland morphogenesis in vitro by affecting cell fate. A two-dimensional (2D) system demonstrated that ECM substrates must be soft and contain laminin to maintain the expression of mammary epithelial differentiation markers, whereas stiffening of the substrate or loss of laminin resulted in reduced expression.35 During endothelium sprouting, increased ECM stiffness and actomyosin contractility can reduce branching as they affect membrane curvature.36 Increased actomyosin contractility in a stiffer environment maintains lower membrane curvature, which impairs cell-scale branching of the endothelial cells.37 It was also shown that matrix stiffness affects biochemical signals during angiogenesis by upregulating expression of vascular endothelial growth factor receptor-2 (VEGFR2).38 Future work should examine the interaction between various effects of altered mechanics. In addition to solid-like properties such as stiffness and composition, further work is required to examine the effects of time-dependent properties of ECM mechanics on organ morphogenesis, such as stress-relaxation, degradation and plasticity. Native embryonic tissues exhibit fluid-like viscoelastic properties, which probably have a role in cell organization and ECM assembly, and thus may affect mechanosensing and biochemical pathways.
Throughout embryonic and fetal development, physical interactions within the stem cell niche play a key part in maintaining stem cell populations and ensuring they persist into adult tissues. Cell-ECM adhesion via integrins maintains stem and progenitor cell pools in germline39,40 and adult epidermal niches.41 Physical stem cell-ECM interactions also regulate the positioning of stem cells within the niche architecture and with respect to their progeny, which affects fate decisions and self-renewal in the perivascular hematopoietic stem cell niche, intestinal crypt and hair follicle. 42 Over time, the ECM helps store biological information by maintaining stem cell positioning and providing a means to transduce transient molecular signals into more permanent architectural features of the niche. Extrinsic forces that result from macro-scale movement of embryonic tissues over time are transmitted to the stem cell niche to help maintain skeletal joint progenitors, which are required for proper joint cavitation and morphogenesis.43 These observations have prompted the development of in vitro physical models of the stem cell niche to improve the maintenance and expansion of pluripotent stem cells.
3. Manipulating mechanobiology
The study of embryonic and fetal development is complicated by the diverse ways in which physical forces and interactions affect stem cells. Engineering systems that act as an interface between materials and stem cells, in vitro, enable the manipulation of physical, chemical and biological parameters of the interaction. Synthetic versions of the stem cell niche have been developed to precisely investigate how mechanical forces regulate stem cell behaviour. Here we introduce the criteria for tuning mechanical stem cell-niche interactions in materials-based systems. We focus on techniques used in studies on mechanoregulation of stem cells and describe how these systems have been exploited to unravel biological mechanisms.
Challenges and criteria for building synthetic niches with tunable mechanics
Various synthetic niches are utilized to study how mechanical cues regulate stem cells in vitro as their properties can be manipulated in a more predictable manner than the niche in vivo.22,44–48 These systems can be used to study how externally applied forces impact individual cells or multi-cellular tissue models. They also enable studies of how cell intrinsic forces regulate cell behaviour, often as a feedback from the resistance to these forces provided by the niche. However, the interpretation of results obtained with these systems is challenging, and it is crucial to achieve independent control of the various physical and chemical properties of the synthetic niche.
Polystyrene is a classic cell culture model, but it is a very rigid plastic surface that adsorbs serum and cell-secreted proteins in a non-specific manner, and thus cannot be used to control physical or biochemical cues. Purified ECM proteins, such as collagen,49 laminin33 and recombinant basement membrane (Matrigel)38 are used as synthetic niches to provide a more defined and physiologic microenvironment for in vitro studies. Alternatively, decellularization of tissues yields native extracellular matrices that may have more representative physicochemical properties of the native niche.50,51 However, decellularized tissues and native ECM often have poorly defined composition, and may contain many different biochemical and physical cues, making it difficult to test specific hypotheses. Raising the concentration of ECM proteins in hydrogels is often used to increase the stiffness to a limited range, but it also increases the density of adhesion ligands available for cellular receptors. (FIG. 3A)
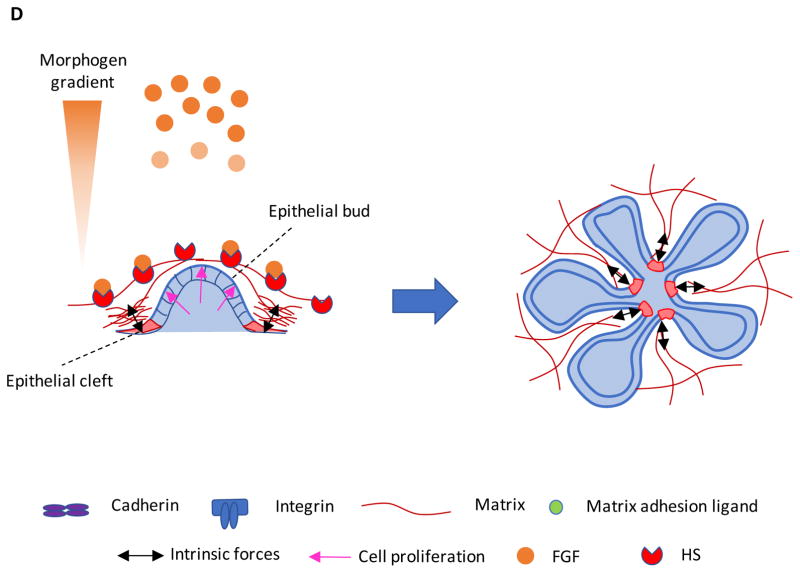
Figure 3.
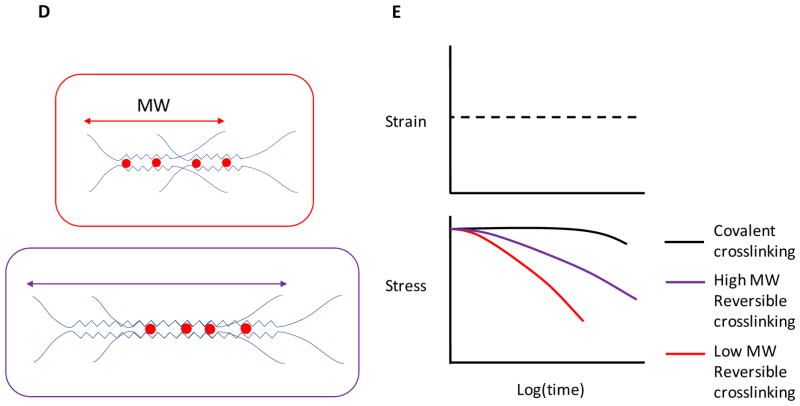
Material systems to study stem cell mechanobiology. When engineering a synthetic niche, alterations in the overall polymer concentration may change the density of adhesion ligands, while changing crosslinking without altering the polymer content may vary the network mesh size (spacing between crosslinks), which can affect how molecules diffuse through the network. A) Artificial niches fabricated from naturally-derived ECM typically manipulate stiffness by altering the concentration of the matrix proteins, which increases ligand density and decreases mesh size in parallel. B) Synthetic polymer systems can offer independent control of stiffness and ligand density, by maintaining a constant polymer concentration while altering the crosslink density. However, the mesh size is altered in parallel. C) Matrices formed from alginate polymers can be crosslinked to various extents while maintaining constant ligand density and mesh size, and thus enable one to independently examine how matrix stiffness affects stem cells. (Inset) Crosslinking in this system occurs via cooperative sharing of divalent cations (red) in blocks of one type of sugar residue (G-block) on the chains, and increases in the number of crosslink sites occupied in the aligned blocks do not alter the architecture of the chains. D) Alginate polymer molecular weight (MW) can be used to control the viscoelasticity of an ionically-crosslinked alginate network. Low MW alginate (red arrow and box) forms into a network with less physical entanglement and overlap of the alginate chains. High MW alginate (purple arrow and box) has higher chain entanglement and overlap (shaded blue region), which decreases the ability of the polymer network dissipate stress. E) The low MW network (red line) is more viscous, shown by its rapid relaxation of stress while a constant strain is applied. The high MW network (purple line) dissipates stress more slowly due to more physical entanglement and overlap. The covalently-crosslinked network (black line) is more elastic than the viscoelastic reversibly-crosslinked alginate, and does not significantly dissipate stress over time.
Niches fabricated using synthetic or non-mammalian ECM-derived polymers enable one to decouple cell adhesion properties from gel mechanical properties. The mechanical properties of synthetic gels can be modified by altering the density of cross-links between the polymers in the system, while independently altering the density of cell adhesion ligands (FIG. 3B), which are required for cellular mechanosensing.52,53 Many studies of 2D cell culture have been performed using polyacrylamide materials coated with ECM proteins.54 For example, poly(2-hydroxyethyl methacrylate) and polyacrylamide substrates coated with recombinant basement membrane were used to tune the elasticity of the ECM to regulate mammary gland morphogenesis in vitro.35 Covalent coupling of peptides that bind integrin or other cell adhesion receptors can also be used to independently regulate adhesion potential and mechanics and control cell-cell and cell-matrix signals. The fibronectin ligand arginine-glycine-aspartate (RGD) mimics cell-matrix contacts, whereas N-cadherin ligands mimic cell-cell junctions of mesenchymal cells.22 ECM-coated polyacrylamide materials are thus suitable for the control mechanical stiffness; however, they are limited in their ability to mimic other important aspects of in vivo stem cell niches, such as their 3D organization and their ability to be remodelled by cells.
A key goal has been the engineering of defined, synthetic niche systems with complete control of mechanical, matrix and soluble cues as an alternative to animal-derived matrices for the long-term culture of functional stem cell organoids. 55 Although in the past decade stiffness was regarded as a key metric for how the matrix resists cellular traction forces to regulate stem cell fate, these studies are typically based on an often-unstated assumption that the native niche is purely elastic. However, natural matrices, such as those comprised of self-assembled collagen,56 and tissues and organs in the body, such as brain, liver, adipose tissues, coagulated bone marrow, bone fracture hematoma, and cranial sutures,57 have viscoelastic properties over various time-scales47 (Box 2). Viscoelastic materials dissipate applied forces, which can dramatically alter how the niche responds and stores cell-generated traction forces and how external mechanical forces are conveyed to cells (Box 1). Therefore, the study of stem cell mechanobiology should consider both the elastic and viscoelastic mechanical properties of synthetic niches.
The interactions of stem cells with their in vivo niche can be more accurately mimicked by 3D than 2D systems. 3D systems differ in the way cells physically interact with their immediate environment in a geometrically-confined manner and in how molecules (e.g., growth factors) diffuse and are available to cells, which can regulate autocrine and paracrine stem cell functions.58 It is challenging to control mechanical properties and changes in diffusion separately in 3D matrices, because, in a chemically-crosslinked network such as poly(ethylene glycol) (PEG) (Box 2), altering the density of cross-links also alters the material’s mesh size (FIG. 3A and B), thus affecting the diffusion of macromolecules and complicating interpretation of studies. Hydrogels fabricated from alginate enable one to decouple changes in crosslink density, and resulting stiffness, from mesh size, ligand density and diffusion properties.59 Alginate is a polysaccharide that is crosslinked by cooperative binding of divalent cations by blocks of sugar residues in adjacent polymer chains, leading to no change in the arrangement of the polymer chains as crosslinking is increased (FIG. 3C).60 Moreover, the viscoelasticity of alginate systems can be tuned by the polymer molecular weight, independent of ligand density, stiffness, degradability and transport (FIG. 3D–E).47,61–63 Thus, alginate systems are suitable to analyse how these physical parameters influence stem cell mechanobiology, whereas many other systems have limitations as these parameters cannot be regulated independently.
Lastly, it is important to recognize that mechanotransduction is not a one-way path, where mechanical cues impact cell behaviour, as ECM remodelling through the degradation and synthesis of matrix components has important roles in mechanotransduction45 (FIG. 4A). In response to increased stiffness or loading, cells can secrete matrix components or proteases that enhance or diminish adhesive interactions, stiffen or soften the ECM and activate or inactivate downstream signalling pathways.64 Synthetic and non-mammalian derived ECM materials can be engineered to either resist degradation or to be degraded in a controlled manner, by building in proteolytically labile crosslinks45,65 and/or using hydrolytically labile gels,66 in which the impact of degradation can be probed. Moreover, stem cells can remodel the surrounding artificial matrix by applying traction forces and protruding actin structures that plastically deform a viscoelastic matrix, independently of degradation (FIG. 4B–C),67 as it occurs during organ morphogenesis.
Figure 4.
Three-dimensional synthetic niches physically confine stem cells and present mechanical cues that impact cell behavior and fate through forces. A) The synthetic extracellular matrix (ECM) provides resistance to cell-generated forces, and in response, stem cells can actively remodel the niche by traction-mediated deformations, degradation of cleavable domains by proteases (purple segments), and production of additional ECM. The stiffness and other mechanical cues from the synthetic matrix network can trigger a self-renewal program (blue arrow), or programs defining distinct lineages of daughter cells (red and green arrows). B) Viscoelastic synthetic niche allows cells to remodel their surrounding matrix network (red), by applying traction forces (black arrows) on matrix ligands (green circles) that allow the cell to spread and change shape by plastically, or permanently, deforming the polymer chains. C) Conversely, a purely elastic non-degradable synthetic niche (black) does not permit cells to plastically deform the polymer network and prevents cell spreading.
Several key criteria should be taken into account when engineering synthetic niches for modelling and manipulating the mechanical microenvironment of stem cells in order for these systems to provide meaningful data. Components of the synthetic niche should interact with specific cell receptors to act on well-defined mechanotransduction pathways. At the same time, it should be possible to independently control mechanical properties (such as stiffness, viscoelasticity, degradation, transport and swelling) to study their effects. Not discussed in this Review, the architecture of the ECM and topology of a material system may also impact how stem cells respond to mechanical cues.68,69
Applying extrinsic forces to directly probe mechanoresponses
Extrinsic forces can be applied to stem cells in bulk matrices that contain large numbers of cells, or at the microscale on individual or small numbers of cells. The forces can be applied in a dynamic or static manner. In many in vivo tissues, cells are subject to mechanical strain cyclically, owing to biological rhythms such as breathing, movement and blood flow, which provides mechanical cues that regulate development. Static forces often result in loading of the axial skeleton or isometric muscle contraction [G]. In synthetic systems, it is easier to apply cyclic or static strain to cells on 2D substrates.70 Compressive or tensile loads can also be applied to bulk 3D material systems using mechanical loading devices or hydrostatic pressure,71 but loading can induce convective flow [G] in the system, potentially altering nutrient availability and thus affecting cell viability or function. External loads can also be applied by acoustic waves, mechanical vibrations and microbubble cavitation, among others (Box S1).72–74
Elucidation of cellular mechanotransduction pathways may require precise control of extrinsic mechanical cues at the interface with individual or small collections of cells, which can be achieved using micro-scale probes such as micropipette aspiration,75 or magnetic devices such as twisting cytometry (Box S1).76 Magnetic probes can generate localized and repeatable extrinsic forces on the cortex of individual cells in a high-throughput and scalable manner.77 Microfluidics [G] can be used to deform single cells by extensional flow to measure their mechanical properties in a cytometer.78 Micro-scale technologies can also apply cyclic stretches on cells to mimic biological rhythms such as movement, breathing and blood flow. For example, a micro-fabricated device has been developed to mimic lung alveolar form and function by culturing a layer of epithelial cells in contact with air on one side of a porous elastic material and fluid on the other and applying controlled cyclic strain on the cells. This system can model lung disease and drug toxicity.79
External forces can also be combined with micro-scale measurement and/or control of cell-generated forces. For example, polymer micropillars on a planar substrate80 can be used to measure cytoskeletal tension and focal adhesion dynamics under equiaxial static stretch.81 Mechanical tension in cells can be stimulated by photo-actuation with thermally-responsive micropillars.82 Furthermore, micro-scale technologies can tune spatial cues to show how cell shape and spreading area is linked to how they respond to mechanics.83,84 These technologies offer the possibility to control multiple modes of external mechanical loading on stem cells. Further understanding of stem cell behaviour could be achieved by combining these technologies with sophisticated biological read-outs (e.g., single-cell and next generation sequencing, highly multiplexed mass spectrometry), advances in synthetic biology and gene editing with CRISPR to precisely control gene expression in mammalian cells, as well as advances in soft robotic systems that closely mimic the physical properties of the native ECM.85
4. Stem cells respond to forces
Stem cells respond to mechanical cues and properties of their physical niche through mechanosensing and mechanotransduction, which affects proliferation, self-renewal and differentiation into specific cell fates, as well as their self-assembly and organization (Table S2).
Principles of mechanosensing and mechanotransduction in stem cells
Stem cells sense their mechanical environment through cell-cell and cell-ECM adhesion, mechanosensitive ion channels and their primary cilium (FIG. 1A).81,86,87 Several classical pathways transduce physical cues to biochemical signals, although it is important to note that mechanotransduction can function differently depending on context and cell type. Integrin receptors bind ECM ligands such as RGD, which activates focal adhesion kinase (FAK, FIG. 1B), an important regulator of cell adhesion. In response to mechanical perturbations, mechanical homeostasis in stem cells is maintained by modifying focal adhesion ligand affinity, by regulating focal adhesion assembly and disassembly, as well as by regulating the underlying cytoskeleton and actomyosin contractility. 81 These adhesion complexes and interacting cytoskeleton activate mechano-responsive signaling, such as Rho kinase (RhoA) and mitogen-association protein kinase (MAPK), and downstream mechano-transduction pathways, such as MAL, a G-actin-binding coactivator of serum response factor (SRF),88,89 Yes-associated protein (YAP), and transcriptional coactivator with PDZ-binding motif (TAZ)90 (FIG. 1B). Moreover, the cytoskeleton is associated with nuclear structures, linking the physical properties of cells to gene expression. Cytoskeletal forces are transmitted to the nucleus by the lamin A component of the nuclear lamina (LMNA, FIG. 1B), altering chromatin structure, for example inducing stretching and opening, which regulates ttranscription factor accessibility.76 LMNA expression and assembly of the nuclear lamina increases with tissue stiffness.75 Extrinsic forces may also directly upregulate transcription by deforming the nucleus.91 Neural stem cells respond to cell membrane tension through a mechanically-gated Piezo1 calcium ion channel (Piezo1).86 Moreover, primary cilia have important roles in mechanoregulation in adult tissues by engaging the cytoskeleton to activate signalling pathways that promote the differentiation of stem cells in response to mechanical cues.87,92
Mechanoregulation of self-renewal and proliferation
The mechanical properties of cell adhesion substrates regulate stem cell self-renewal in culture, which is consistent with observations in developmental studies. While developmental studies have primarily examined the role of adhesion receptors, synthetic niche systems are also used to examine how the mechanical properties of the environment regulate stem cell behaviour. For example, increased stiffness of tropoelastin substrates enhanced hematopoietic stem and progenitor cell expansion, which, interestingly, was dependent on the structure and contractility of the cytoskeleton but was independent of integrin signaling.93 Similarly, the stiffness of electrospun substrates controlled iPSC proliferation.94 In adult and neonatal cardiomyocytes, compliant elastic matrices enhanced clonal expansion by suppressing maturation and promoting de-differentiation, modifying the sarcomere network, and promoting cell division.95
Synthetic niche systems have been used to examine how ECM proteins in fibrillar structures, as opposed to homogeneous and nonfibrillar matrices, can differentially regulate stem cells. Decreasing fibrillar stiffness enhanced stem cell proliferation by enhancing traction forces and ligand clustering, independent from the overall substrate mechanical properties.96 Extrinsic forces also regulate stem cell proliferation and self-renewal. Mechanical strain applied to ES cells on 2D elastomeric substrates coated with recombinant basement membrane increased their self-renewal and reduced differentiation.97 However, these 2D system do not mimic the environment of the developing embryo.
Bone marrow-derived mesenchymal stem cells (MSCs) are a useful model to study stem cell mechanobiology, as they are post-natal cells that can be isolated from adult tissues. MSCs can differentiate into multiple lineages, giving rise to tissues with a wide range of mechanical properties, including rigid bone and cartilage, and soft fat and marrow stroma.54 Uniaxial stretch of MSCs upregulated the Wnt/β-Catenin pathway, suggesting that mechanical strain promotes proliferation.98 However, further work is required to determine the magnitude and mode of strain that supports MSC proliferation. Exploiting mechanical cues to maintain and expand stem cell populations in culture is potentially important for scaling up the production of cells for therapeutic applications such as transplantation.
Mechanoregulation of stem cell fate in synthetic niches
Intrinsic mechanical signals play an important part in the generation of microtissues from ES and iPS. The differentiation state of microtissues derived from ES cells increases with the stiffness and viscosity of the cell aggregate, which is regulated by actomyosin contractility.99 Soft collagen-coated polyacrylamide 2D substrates that matched the intrinsic softness of ES cells maintained their pluripotency,100 whereas localized stresses externally applied to the surface of ES cells by RGD ligand-coated magnetic beads induced ES cell spreading and differentiation.101 The responses were correlated with the intrinsic softness of the cell,101 probably by regulating actomyosin contractility.
The spatial orientation of ES cell microtissues guides the formation of embryonic germ layers, which is mediated by cell-cell contact and cortex contractility that transmits forces in the cell layers.102 3D scaffolds that encapsulate ES cell-derived embryoid structures tune cell fate and germ layer specification by enabling the manipulation of cell-generated forces.103 By mimicking the soft biophysical architecture of embryoid bodies early in development, artificial niches were used to guide iPSC self-organization to promote amniogenesis in a BMP-SMAD-dependent manner.104 Moreover, a soft micropillar substrate system was used to regulate the Hippo pathway in iPS cells to promote neural induction, similar to what is observed in embryonic development during neural plate specification and A-P axis formation during neurulation.105
Defined mechanical and biochemical cues of synthetic niches can also facilitate the reprogramming of somatic cells into iPS cells, as indicated by the ability of soft ECM with a particular composition to regulate mesenchymal-to-epithelial transition and epigenetic remodeling to enhance reprogramming.106 It remains to be determined specifically how viscoelastic properties regulate in vitro generation of microtissues from stem cells, although these are likely to play a prominent role. Recent work showed expansion of stem cells and development of structures in a synthetic PEG system with reversible crosslinks that are expected to impart viscoelasticity, although the mechanics were not yet characterized.107
Mechanical cues regulate the fate of stem and progenitor cells isolated from adult tissues
Many studies demonstrate that the differentiation of MSCs derived from adipose or bone marrow tissue is regulated by the elasticity of their matrix,44,54 and MSCs appear to switch to the fate of cells whose native ECM is most closely matched to the elasticity of the substrate or matrix, i.e., stiffer substrates promote osteogenic differentiation, while softer substrates induce fat or neuronal differentiation.54 Hydrogel macroscale mechanics directly contribute to differentiation fate, which is jointly regulated by the specific ECM proteins or immobilized ligands utilized for adhesion.52 N-cadherin ligands that mimic cell-cell adhesive junctions of MSCs increased the mechanical threshold for YAP/TAZ signaling (FIG. 1B) and reduced actomyosin contractility compared to only RGD ligands that mimic cell-matrix adhesion.22 In other cell types, substrates that match the soft mechanics of bone marrow promoted the differentiation of megakaryocytes and generation of proplatelets.108 Neural stem cells were directed towards neuronal differentiation in soft conditions, and glial cells in stiffer conditions.109 These findings have been extended to 3D systems, and the use of alginate-based systems demonstrated that MSC fate in 3D is controlled independently by stiffness, while diffusion and overall gel architecture were held constant.44 The development of artificial 3D ECMs has also revealed that mechanical and matrix factors contribute to the programming of adult stem cells in organoids.55 Highly stiff synthetic hydrogels enhanced the expansion of adult intestinal stem cells in a YAP-dependent manner, while low matrix stiffness promoted cell differentiation and intestinal organoid formation. The spatial properties and heterogeneities of the stem cell niche affect how stem cells transduce mechanical cues into changes of cell fate, as more random, less-ordered mechanical cues suppressed differentiation markers of MSCs and maintained stem cell lineage markers compared to a more ordered structure.110 Furthermore, MSCs may be more responsive to mechanical gradients of stiffness, which can be established by varying the level of crosslinking along a spatial axis, as compared to stiffness alone.111–113
The impact of mechanics on cell fate appears to be regulated in a cell-intrinsic manner by YAP/TAZ signaling, which allows cells to store information from the past physical environments to influence cell fate and suggests they possess a mechanical memory.114 Retention of mechanical history in MSCs is regulated by mi-R21 levels (FIG. 1B), which gradually increased during priming on stiff substrates to promote their fibrogenic program.115 MSCs may also integrate the mechanical properties over time, as earlier stiffening induced more osteogenic differentiation compared to later stiffening in a dynamic system.116
In addition to stiffness, or elasticity, the ability of the ECM to flow and dissipate stress, or viscoelasticity (Box 1), can regulate the spreading behaviour and differentiation of MSCs through traction forces in a cell intrinsic manner.47,117 Matrices that rapidly relax an applied stress can enhance osteogenic differentiation and matrix production by MSCs. Additionally, substrate creep [G] and stress-stiffening [G] (Box 2) affect MSCs differentiation.48,118 Unlike purely elastic systems, physical environments that exhibit viscoelastic behaviour allow cells to reversibly change their shape and volume in response to cues (FIG. 4B–C). Changes in cell volume may be partially responsible for the impact of mechanics on stem cell fate. These findings are consistent with the role of mechanics in regulating signaling over space and time, and cells’ dependence on re-organization and/or dissipation of energy in the matrix.
Externally applied forces also can regulate the fate of adult stem cells, although the precise mechanotransduction pathways are often unclear. Articular and vertebral cartilage are subject to loading from movement and forces transmitted through the axial skeleton, and synthetic niche systems are used to investigate how external forces, like hydrostatic pressure, promote chondrogenesis and matrix production of stem cells in vitro. 119 Mechanoregulation of chondrogenesis also appears to be coupled to the rate- and time-dependence of loading. Dynamic compression of MSCs by cyclic compressive strain (10%, 1 Hz) increased the activity of downstream TGF-β1 signaling involving the Smad-2/3 pathways, and promoted chondrogenesis.120 The axial skeleton is also subject to external loading, and mechanical stimulation by cyclic stretching, which induces Notch signaling, has been found to promote osteogenic differentiation of MSCs and to suppress histone deacetylase expression, providing an additional potential mechanism for regulation of mechanotransduction in MSCs.121 Furthermore, fluid shear stress enhanced the osteogenic potential of MSCs.122,123 However, it is important to note that hydrogels may dissipate stress, so less force may be transmitted to encapsulated cells than believed, and cytoskeletal changes in response to external stimuli may change how mechanical input is perceived or felt by the cell. Further studies are required to determine how these external cues are sensed by stem cells and transduced into mechanoresponses. These challenges could possibly be addressed by integrating traction force microscopy with macroscale mechanical stimuli, allowing one to observe cytoskeletal dynamics in response to external loading, or the use of mechanically-sensitive molecular probes to measure ligand-receptor interactions in response to macroscale loading.
5. Regenerative medicine applications
Regenerative medicine aims to treat injuries or disease with therapies that repair or replace organ or tissue function. Many approaches focus on the use of endogenous or transplanted stem cells, and controlling cell fate stability is likely key to successful regeneration. Following systemic or local transplantation, stem cells often lose viability or regenerative potential, or are cleared in the lungs, liver and spleen. Safety concerns are paramount with the use of pluripotent cells, highlighting the importance of controlling their localization and fate in vivo.
Exploiting cell intrinsic forces to enhance tissue regeneration
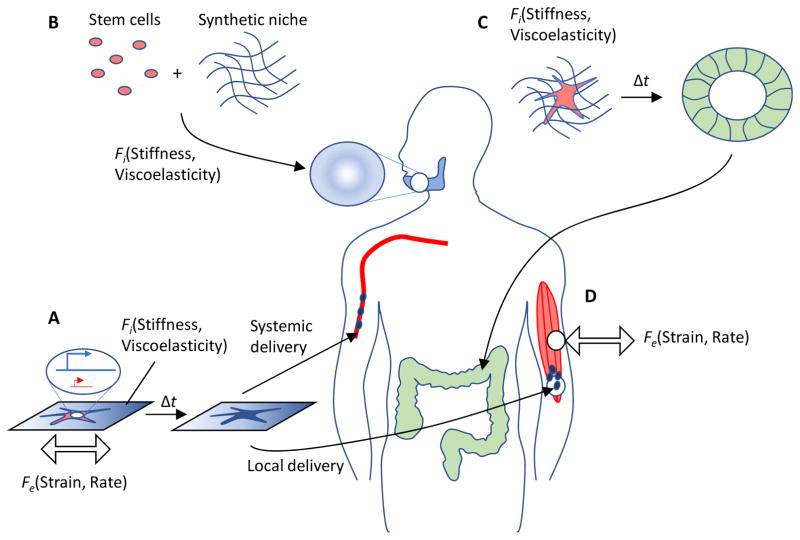
Synthetic matrices with defined mechanical properties and biophysical properties are used to prime stem cells ex vivo prior to transplantation to improve their capacity for regeneration (FIG. 5A), as well as control their behavior in vivo following transplantation (FIG. 5B). Priming hematopoietic stem and progenitor cells with materials of specific mechanical properties can increase their yield following transplantation and enhance reconstitution.93,124 Hydrogel substrates (2D PEG substrate coated with laminin) with a stiffness value that mimicked that of muscle promoted muscle stem cell self-renewal and increased their regenerative capacity when transplanted. 125 Substrate stiffness may also affect how stem cells regulate immune responses, for example by modifying the secretory profile of MSCs. 126 Soft elastic materials in 2D, composed of fibronectin-coated polyacrylamide, induced reactive oxygen species (ROS) signaling in human bone marrow MSCs, whose conditioned media was shown to improve wound healing in vivo. 127 In vivo, soft alginate-based scaffolds with matrix metalloprotease (MMP)-cleavable domains improved MSC invasion into host tissue in mouse models compared to a stiffer material, and matrix production at the delivery site was significantly enhanced. 65 In engineering 3D human cardiac tissues, enhanced sarcomere [G] organization and contractile function resulted from the use of a chemically-crosslinked gelatin-PEG material with stiffness similar to native cardiac tissue that facilitated the ability of cells to establish cell-cell adhesions. 128 Gelatin degradation over time was likely important to these effects, because chemical crosslinking of PEG and gelatin initially leads to a purely elastic material that would not otherwise allow cells to self-organize or remodel matrix. Synthetic niches can improve MSC transplantation for bone tissue repair by tuning stiffness to direct cell fate, as well as programming porosity to facilitate host integration, using a viscoelastic alginate system129 (FIG. 5B). Microfluidic platforms can form synthetic niches on the single-cell level with specified stiffness to improve the distribution and paracrine function of MSCs after transplantation into the systemic circulation of animals. 130 An alginate matrix with rapid relaxation has been shown to enhance bone regeneration compared to more slowly relaxing hydrogels, even in the absence of stem cell delivery, presumably by promoting osteoblast differentiation and matrix remodeling. 131 Similarly, an appropriate combination of a material’s chemical and physical properties can regulate stem cell migration into a site of injury, and be used to support rapid cutaneous tissue regeneration. 132 The challenge of controlling stem cell fate and morphogenesis after transplantation may be addressed by harnessing the potential for organogenesis in vitro. Mechanical cues have been shown to promote organogenesis,55 so synthetic niches may be able to generate organoid tissues in vitro for subsequent repair or replacement of damaged organs in vivo (FIG. 5C).
Figure 5.
Tissue regeneration can be enhanced by exploiting stem cell mechanobiology. A) Stem cells can be pre-conditioned with mechanical cues, either by culturing on or in matrices with specific stiffness or viscoelasticity, or by applying external forces of a desired strain and rate to the substrate, prior to collection of cells for localized (e.g., skeletal muscle site) or systemic delivery. B) Stem cells can instead be transplanted on or in synthetic matrices with defined mechanical properties, such as stiffness and viscoelasticity, that promote proliferation and/or a particular stem cell fate; in this example, stem cells are programmed by the matrix to enhance bone repair in the mandible. C) Mechanical cues of stiffness and viscoelasticity can also be used in vitro to mimic embryonic development by driving stem cells to undergo self-organization, differentiation, and morphogenesis into organoid tissues, which could then be subsequently transplanted for organ repair or replacement; in this example, repair of the colon. D) Direct application of externally applied forces can be utilized to enhance regeneration by endogenous stem cells; in this example, stem cells in injured skeletal muscle tissue. Both the absolute magnitude of strain and rate of application may regulate the regenerative process.
Mechanically loading cells and tissues to enhance tissue regeneration
It has long been known that external mechanical forces regulate responses to injury. Mechanical unloading of bone in microgravity favors maintenance and expansion of mesenchymal and hematopoietic stem cells in bone marrow, while limiting their differentiation,133 and disuse atrophy is observed in skeletal muscle. Current clinical practices exploit active mechanical cues on a regular basis to drive tissue remodeling and regeneration. Distraction osteogenesis, which applies external strain on segments of bone, has been widely used clinically to promote bone formation. 134 Orthopaedic implants have been designed to minimize strain-shielding at a fracture site, in order to promote strain-mediated effects on bone remodeling and regeneration. 135 A recent clinical trial showed low-magnitude, high-frequency mechanical stimulation in pediatric cancer survivors with low bone mineral density was safe and efficacious in enhancing peak bone mass during youth. 136 In dentistry, orthodontic tooth movement is achieved by stressing the periodontal ligament to induce tissue remodeling. 137
Success to date in the clinic with selected tissues generates the question of whether mechanical therapies can be extended to other disease and tissue contexts, such as muscle, and motivates the development of new therapies that exploit stem cell mechanobiology with actuating rigid or soft materials. Cyclic external strain has been used to pre-condition muscle stem cells in 3D animal-derived matrices prior to implantation, and improved the function of subsequently regenerated muscle in mouse models138,139 (FIG. 5A). However, it is not clear precisely how strain impacts cells in these systems, because the mechanics and composition of these materials are variable and not completely controlled. Alternatively, muscle can also be directly stimulated in vivo with externally applied forces to promote healing and reduce inflammatory injury (FIG. 5D). Applying forces to muscle during massage reduced inflammatory injury caused by exercise by activating FAK and extracellular signal-regulated kinase 1/2 (ERK1/2) pathways (FIG. 1B), which increase mitochondrial biogenesis and inhibit nuclear translocation of nuclear factor κβ (p65).140 Repeated forces applied via soft robotic devices were found to promote skeletal muscle regeneration in animals following severe injury, and reduced fibrosis and inflammation.141 However, it remains to be shown clinically whether and how external stimulation impacts stem cells in muscle. An important goal of future work is to connect clinical data with pre-clinical findings relating mechanical stimuli and cell responses.
6. Summary, Conclusions and Perspectives
Biological tissues are composed of materials, which transmit forces and exhibit diverse mechanical properties, and cells that generate and are subject to physical forces. These mechanical properties and forces regulate stem cell function and guide developmental processes, and tissue repair. As one can independently manipulate specific biophysical properties of synthetic matrices, these systems are advancing our understanding of mechanotransduction mechanisms in stem cells, and leading to technologies to guide stem cell fate. However, care must be taken when comparing results from studies as parameters such as dimensionality, chemistry, viscoelastic properties, degradability, and diffusional transport may vary substantially. Moreover, reported values of mechanical parameters can be biased by the measurement technique (Box 2). Lastly, many research groups may not have access to these synthetic systems owing to their limited commercial availability; therefore a need exists for standardized and more easily accessible model systems.
Stem cell transplantation has previously been limited by lack of control over distribution and loss of cell viability, and while biomaterials can address certain of these challenges,130 future efforts should exploit mechanics to control post-transplant cell fate. Beyond regeneration, materials systems that control the physical and biochemical microenvironment of individual cells open up exciting opportunities in stem cell research. For example, they may be valuable in building human-based in vitro models of organs and disease. New chemistries are being discovered to expand the limited repertoire of materials for stem cell culture by using screening techniques adapted from the microfabrication industry. 142 Finally, additive manufacturing can fabricate devices and material systems from computer-aided designs that mimic organ-level physiology and disease. 143
Mechanical cues likely play key roles in many other processes in biology and disease, and the advances to date in the stem cell field may find broad application in these areas as well. For example, fibrosis and cancer feature changes in the physical interactions of cells and their matrix. Fibrosis is involved in many disease processes, but it is unclear how mechanical interactions of putative cells and their niche regulate initiation and development of disease. While mechanics has been implicated in the initiation and progression of malignant lesions, 144 studies are just now demonstrating how intrinsic or extrinsic forces and the tumor microenvironment regulate tumor rejection or resistance to therapies,145 and how mechanical forces are involved in oncogenic signalling pathways. 146 Future work will bridge the gap between basic research in mechanobiology and improved therapies for patients.
Supplementary Material
Table 1.
Representative mechanical properties of tissue and materials
| Tissue Type | Tissue or Material | Typical Applications | E (kPa) | t1/2 (s) |
|---|---|---|---|---|
| Interstitial and connective | Fat | - | 0.02147 | 10047 |
| Tendon | - | 310,000147 | 1149 | |
| Skin | - | 4.5–8148 | - | |
| Vascular | Carotid artery | - | 90147 | - |
| Nervous | Spinal cord | - | 27–89147 | - |
| Brain | - | 0.2–1.0147,150 | 10047 | |
| Viscera | Lung | - | 5147 | - |
| Kidney | - | 2.5147 | - | |
| Liver | - | 0.64147 | 10047 | |
| Lymph node | - | 0.12147 | - | |
| Mammary gland | - | 0.16147 | - | |
| Musculoskeletal | Cardiac muscle | - | 20–150147 | - |
| Skeletal muscle | - | 10–100147,150 | - | |
| Pre-calcified bone | - | 30150 | - | |
| Bone marrow | - | 0.3–24.7151 | 1047 | |
| Cartilage | - | 20150 | - | |
| Articular cartilage | - | 950147 | - | |
| Bone - Cancellous | - | 350,000152 | - | |
| Bone – Compact | - | 11,500,000152 | - | |
| Tooth dentin | - | >10,000,000153 | - | |
| Tooth enamel | - | ~100,000,000153 | - | |
| Embryonic | Gastrulation | - | 0.01154 | - |
| Natural ECM | Collagen hydrogels | - | 0.01–6155,158 | 1155 |
| Fibrin hydrogels | - | 0.01–0.5155 | 1155 | |
| rBM (Matrigel) | - | 0.01–0.5155 | 50155 | |
| Gelatin – covalently crosslinked | - | 0.6–13156 | n/a | |
| Hyaluronic acid hydrogels | - | 4–9545 | n/a | |
| Synthetic matrix | Alginate hydrogels | 2D and 3D matrices, programmable viscoelastic properties, coupled with cell adhesive ligands or interpenetrating network with natural ECM, option for degradability | 0.1–11044,47 | 44–330047 |
| Polyacrylamide | 2D elastic substrates coated with ECM | 0.1–4054 | n/a | |
| Agarose hydrogels | 2D elastic substrates coated with ECM | 5–10044,155 | >1000155 | |
| Poly(ethylene glycol) hydrogels | Covalently-crosslinked 2D and 3D matrices, coupled with cell adhesive ligands, incorporation of degradable elements | 0.1–16044,110 | n/a | |
| Polystyrene | Traditional tissue culture material (plasma-treated for promoting cell adhesion) | 3,000,000125 | n/a | |
| PDMS | 2D elastomer for applying extrinsic strain | 5–100115 | n/a | |
| PDMS micropillars | 2D studies to measure and/or manipulate cell intrinsic forces | 2.8–60157* | n/a |
Values = XReference, E = Elastic modulus (assume E = 2G′ (1+ ν), G′ storage modulus, ν = 0.5), t1/2 = time to reach half of initial normalized stress during constant applied strain (Box 1), rBM = recombinant basement membrane, PDMS = polydimethylsiloxane; - indicates value not reported
Effective elastic modulus, Eeff
Key points.
Stem cells are regulated by cell intrinsic and extrinsic forces in development, homeostasis, and regeneration.
Mechanical tension regulates early embryogenesis ex vivo in embryoid self-organization, germ band elongation, invagination and dorsal closure, and sorting of the germ layers.
During development, mechanical forces regulate the generation of organ systems by directing the specification and expansion of stem cells, as well as re-organizing the extracellular matrix that begins to accumulate in embryonic tissues.
Synthetic matrices enable one to control biophysical properties of the stem cell niche in order to test specific hypotheses on how mechanical cues regulate stem cells.
Synthetic matrices have been used to demonstrate how mechanical cues, such as stiffness and viscoelasticity, as well as externally-applied mechanical loads, control stem cell self-renewal and proliferation, differentiation and organoid formation.
Externally applied mechanical forces can stimulate stem cells to promote tissue regeneration.
Acknowledgments
We’d like to thank Dr. Jianyu Li for assistance with revising this manuscript and David Zhang for input on the figures. Funding was provided by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Numbers 5R01DE013033 (DM) and K08DE025292 (KV). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- cadherin-catenin complexes
Complexes of cellular receptors termed cadherins, which bind to other cells, with β-catenin, an intracellular molecule, that connect to the actin cytoskeleton in epithelial tissues to convey forces between cells.
- dorsal closure
Closure of a dorsal epidermal opening that is initially formed naturally during embryonic development of Drosophila melanogaster; this process has similarity to wound healing in mammals.
- cortical tension
Actomyosin-generated forces cause tension in the cytoskeleton of cells, which contributes to their shape and mechanical properties.
- Rho-associated protein kinase
A serine-threonine kinase that can regulate actomyosin contractility and is downstream of RhoA and other pathways.
- Stomodeum
A frontal opening in the developing embryo that forms a primordial mouth, separated from the pharynx by an oropharyngeal membrane.
- traction forces
Forces on ECM or other cells generated by receptor-binding and actomyosin contractility.
- fractal patterns
Highly branched geometry that is formed from repeated symmetric branching, often across multiple length scales.
- submandibular salivary gland
One of the major salivary glands. Features a branched ductal structure that opens into the oral cavity, with secretory end pieces, called acini, that produce saliva by secretion of water, salts, proteins, and other macromolecules.
- focal adhesions
Complexes of matrix receptors, actin cytoskeleton, and other cytoskeletal and signaling molecules that link the cytoskeleton to ECM ligands.
- isometric muscle contraction
Forces generated by muscle while maintaining constant muscle length and joint angle.
- convective flow
Fluid flow that transfers mass and/or heat down a fluid pressure gradient.
- Microfluidics
Precise control of fluid shear forces and flow rates in micro-scale geometries, such as micro-channels.
- substrate creep
Deformation, or flow, of a material during a constant application of stress.
- stress-stiffening
Mechanical stiffening of a polymer network with increasing strain.
- Sarcomere
Fundamental active unit in skeletal muscle that generates force from overlapping striations of actin and myosin
Biographies
Prof. Mooney is Robert P. Pinkas Family Professor of Bioengineering and Wyss Institute Core Faculty Member. His laboratory studies how environmental signals are sensed by and impact cells, in order to design and synthesize new biomaterials for a variety of applications, including mechanotransduction studies, regenerative medicine, and cancer therapies.
Dr. Vining is a Bioengineering Fellow and Ph.D. candidate. His research aims to determine how the physical microenvironment of cells can regulate immune responses in regeneration and cancer. He previously completed his D.D.S. at the University Minnesota, as well as a fellowship in the NIH Medical Research Scholars Program.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
AUTHORS CONTRIBUTION:
D. J. M. and K. H. V. researched data for the article, contributed to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
References
- 1.Steinberg MS. Reconstruction of Tissues by Dissociated Cells. Science. 1963;141:401–408. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- 2.Maître JL, et al. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature. 2016;536:344–348. doi: 10.1038/nature18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ninomiya H, Winklbauer R. Epithelial coating controls mesenchymal shape change through tissue-positioning effects and reduction of surface-minimizing tension. Nature Cell Biology. 2008;10:61–69. doi: 10.1038/ncb1669. [DOI] [PubMed] [Google Scholar]
- 4.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. Planar remodeling of cell-cell junctions in embryonic tissue is driven by intrinsic local forces, which are required for germ-band elongation during embryonic development. [DOI] [PubMed] [Google Scholar]
- 5.Beloussov LV, Dorfman JG, Cherdantzev VG. MECHANICAL STRESSES AND MORPHOLOGICAL PATTERNS IN AMPHIBIAN EMBRYOS. Journal of Embryology and Experimental Morphology. 1975;34:559–574. [PubMed] [Google Scholar]
- 6.Rauzi M, Verant P, Lecuit T, Lenne PF. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nature Cell Biology. 2008;10:1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- 7.Grill SW, Gonczy P, Stelzer EHK, Hyman AA. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature. 2001;409:630–633. doi: 10.1038/35054572. [DOI] [PubMed] [Google Scholar]
- 8.Colombo K, et al. Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science. 2003;300:1957–1961. doi: 10.1126/science.1084146. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Gonzalez R, Simoes SD, Roper JC, Eaton S, Zallen JA. Myosin II Dynamics Are Regulated by Tension in Intercalating Cells. Developmental Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophysical Journal. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckley CD, et al. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346:1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of myosin-II-driven cell ingression in Drosophila epithelia. Developmental Cell. 2007;13:730–742. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical Signals Trigger Myosin II Redistribution and Mesoderm Invagination in Drosophila Embryos. Science Signaling. 2009;2:8. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- 15.Krieg M, et al. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biology. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 16.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed Forces Timed by a Ratchet-like Mechanism Drive Directed Tissue Movement during Dorsal Closure. Cell. 2009;137:1331–1342. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 18.Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 20.Cosgrove BD, et al. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat Mater. 2016;15:1297–1306. doi: 10.1038/nmat4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalson NS, et al. A structure-based extracellular matrix expansion mechanism of fibrous tissue growth. Elife. 2015;4:1–22. doi: 10.7554/eLife.05958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. Journal of Cell Biology. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elosegui-Artola A, et al. Rigidity sensing and adaptation through regulation of integrin types. Nat Mater. 2014;13:631–637. doi: 10.1038/nmat3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shyer AE, et al. Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science. 2017 doi: 10.1126/science.aai7868. Published Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petridou NI, Spiro Z, Heisenberg CP. Multiscale force sensing in development. Nat Cell Biol. 2017;19:581–588. doi: 10.1038/ncb3524. [DOI] [PubMed] [Google Scholar]
- 26.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Developmental Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Beqaj S, Kemp P, Ariel I, Schuger L. Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. J Clin Invest. 2000;106:1321–1330. doi: 10.1172/JCI8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hove JR, et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. In this work, the intracardiac high-shear flow in zebrafish embryos was characterized, demonstrating that perturbations in fluid flow can result in developmental anomalies that are similar to defects observed in patients with congenital heart disease. [DOI] [PubMed] [Google Scholar]
- 29.Adamo L, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. This study established the role of pulsatile fluid shear stress in hematopoietic development, by mimicking forces exerted on embryonic vasculature and demonstrating enhanced expression of Runx1, which is a master regulator of hematopoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North TE, et al. Hematopoietic Stem Cell Development Is Dependent on Blood Flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genetics. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 32.Nowlan NC, Murphy P, Prendergast PJ. A dynamic pattern of mechanical stimulation promotes ossification in avian embryonic long bones. J Biomech. 2008;41:249–258. doi: 10.1016/j.jbiomech.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 33.Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Developmental Biology. 2009;336:169–182. doi: 10.1016/j.ydbio.2009.09.037. This study established the role of mechanical forces in regulating the initiation and propagation of clefting during epithelial branching morphogenesis, mediated through actomyosin contractility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daley WP, Kohn JM, Larsen M. A Focal Adhesion Protein-Based Mechanochemical Checkpoint Regulates Cleft Progression During Branching Morphogenesis. Dev Dyn. 2011;240:2069–2083. doi: 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alcaraz J, et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. Embo Journal. 2008;27:2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer RS, Gardel M, Ma XF, Adelstein RS, Waterman CM. Local Cortical Tension by Myosin II Guides 3D Endothelial Cell Branching. Current Biology. 2009;19:260–265. doi: 10.1016/j.cub.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott H, et al. Myosin II controls cellular branching morphogenesis and migration in three dimensions by minimizing cell-surface curvature. Nat Cell Biol. 2015;17:137–147. doi: 10.1038/ncb3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mammoto A, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song XQ, Zhu CH, Doan C, Xie T. Germline, stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 40.Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nature Cell Biology. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu AJ, Haase I, Watt FM. Signaling via beta 1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6728–6733. doi: 10.1073/pnas.96.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahn J, et al. Muscle Contraction Is Necessary to Maintain Joint Progenitor Cell Fate. Developmental Cell. 2009;16:734–743. doi: 10.1016/j.devcel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Materials. 2010;9:518–526. doi: 10.1038/nmat2732. Using a well-controlled 3D physical environment, this study showed that matrix stiffness can direct stem cell fate in 3D culture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khetan S, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rape AD, Zibinsky M, Murthy N, Kumar S. A synthetic hydrogel for the high-throughput study of cell-ECM interactions. Nat Commun. 2015;6:8129. doi: 10.1038/ncomms9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaudhuri O, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15:326–334. doi: 10.1038/nmat4489. By manipulating the stress relaxation behavior of synthetic matrices, this study demonstrated that stem cell fate is regulated by time-dependent, or viscoelastic, properties of their physical environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das RK, Gocheva V, Hammink R, Zouani OF, Rowan AE. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat Mater. 2016;15:318–325. doi: 10.1038/nmat4483. [DOI] [PubMed] [Google Scholar]
- 49.Grinnell F, Ho CH, Tamariz E, Lee DJ, Skuta G. Dendritic Fibroblasts in Three-dimensional Collagen Matrices. Molecular Biology of the Cell. 2003;14:384–395. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prewitz MC, et al. Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat Methods. 2013;10:788–794. doi: 10.1038/nmeth.2523. [DOI] [PubMed] [Google Scholar]
- 51.Beachley VZ, et al. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Meth. 2015;12:1197–1204. doi: 10.1038/nmeth.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen JH, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nature Materials. 2014;13:979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nature Materials. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 54.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. This study showed that the lineage of MSCs could be controlled by the elasticity of the substrate to which they were adhered. [DOI] [PubMed] [Google Scholar]
- 55.Gjorevski N, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. This study described a synthetic replacement of Matrigel, which enhanced intestinal stem cell expansion through mechanotransduction, and could control organoid formation. [DOI] [PubMed] [Google Scholar]
- 56.Mohammadi H, Arora PD, Simmons CA, Janmey PA, McCulloch CA. Inelastic behaviour of collagen networks in cell–matrix interactions and mechanosensation. J R Soc Interface. 2015;12:20141074. doi: 10.1098/rsif.2014.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jasinoski SC, Reddy BD. Mechanics of cranial sutures during simulated cyclic loading. J Biomech. 2012;45:2050–2054. doi: 10.1016/j.jbiomech.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Mahadik BP, Bharadwaj NAK, Ewoldt RH, Harley BAC. Regulating dynamic signaling between hematopoietic stem cells and niche cells via a hydrogel matrix. Biomaterials. 2017;125:54–64. doi: 10.1016/j.biomaterials.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 60.Kong HJ, Lee KY, Mooney DJ. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymer. 2002;43:6239–6246. [Google Scholar]
- 61.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nature Materials. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gobaa S, et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 63.Zhao X, Huebsch N, Mooney DJ, Suo Z. Stress-relaxation behavior in gels with ionic and covalent crosslinks. Journal of Applied Physics. 2010;107:063509–063505. doi: 10.1063/1.3343265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasper G, et al. Matrix metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells. 2007;25:1985–1994. doi: 10.1634/stemcells.2006-0676. [DOI] [PubMed] [Google Scholar]
- 65.Fonseca KB, et al. Injectable MMP-Sensitive Alginate Hydrogels as hMSC Delivery Systems. Biomacromolecules. 2013;15:380–390. doi: 10.1021/bm4016495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 67.Ho FC, Zhang W, Li YY, Chan BP. Mechanoresponsive, omni-directional and local matrix-degrading actin protrusions in human mesenchymal stem cells microencapsulated in a 3D collagen matrix. Biomaterials. 2015;53:392–405. doi: 10.1016/j.biomaterials.2015.02.102. [DOI] [PubMed] [Google Scholar]
- 68.Ye K, et al. Matrix Stiffness and Nanoscale Spatial Organization of Cell-Adhesive Ligands Direct Stem Cell Fate. Nano Letters. 2015;15:4720–4729. doi: 10.1021/acs.nanolett.5b01619. [DOI] [PubMed] [Google Scholar]
- 69.McMurray RJ, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nature Materials. 2011;10:637–644. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 70.Park JS, et al. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnology and Bioengineering. 2004;88:359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 71.Mauck RL, Byers BA, Yuan X, Tuan RS. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomechanics and Modeling in Mechanobiology. 2007;6:113–125. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 72.Zablotskii V, et al. Down-regulation of adipogenesis of mesenchymal stem cells by oscillating high-gradient magnetic fields and mechanical vibration. Appl Phys Lett. 2014;105:5. [Google Scholar]
- 73.Guo F, et al. Controlling cell-cell interactions using surface acoustic waves. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:43–48. doi: 10.1073/pnas.1422068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Compton JL, Luo JC, Ma H, Botvinick E, Venugopalan V. High-throughput optical screening of cellular mechanotransduction. Nat Photonics. 2014;8:710–715. doi: 10.1038/nphoton.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swift J, et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tajik A, et al. Transcription upregulation via force-induced direct stretching of chromatin. Nature Materials. 2016;15:1287–1296. doi: 10.1038/nmat4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tseng P, Judy JW, Di Carlo D. Magnetic nanoparticle-mediated massively parallel mechanical modulation of single-cell behavior. Nat Methods. 2012;9:1113–1119. doi: 10.1038/nmeth.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gossett DR, et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7630–7635. doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huh D, et al. Reconstituting Organ-Level Lung Functions on a Chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu JP, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weng S, Shao Y, Chen W, Fu J. Mechanosensitive subcellular rheostasis drives emergent single-cell mechanical homeostasis. Nat Mater. 2016;15:961–967. doi: 10.1038/nmat4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sutton A, et al. Photothermally triggered actuation of hybrid materials as a new platform for in vitro cell manipulation. Nature Communications. 2017;8:14700. doi: 10.1038/ncomms14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 85.Rus D, Tolley MT. Design, fabrication and control of soft robots. Nature. 2015;521:467–475. doi: 10.1038/nature14543. [DOI] [PubMed] [Google Scholar]
- 86.Pathak MM, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoey DA, Tormey S, Ramcharan S, O’Brien FJ, Jacobs CR. Primary Cilia-Mediated Mechanotransduction in Human Mesenchymal Stem Cells. Stem Cells. 2012;30:2561–2570. doi: 10.1002/stem.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 89.Connelly JT, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nature Cell Biology. 2010;12:711–U177. doi: 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- 90.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 91.Pagliara S, et al. Auxetic nuclei in embryonic stem cells exiting pluripotency. Nat Mater. 2014;13:638–644. doi: 10.1038/nmat3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen JC, Hoey DA, Chua M, Bellon R, Jacobs CR. Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism. Faseb Journal. 2016;30:1504–1511. doi: 10.1096/fj.15-276402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holst J, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28:1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 94.Maldonado M, et al. The effects of electrospun substrate-mediated cell colony morphology on the self-renewal of human induced pluripotent stem cells. Biomaterials. 2015;50:10–19. doi: 10.1016/j.biomaterials.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 95.Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. eLife. 2015;4:e07455. doi: 10.7554/eLife.07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baker BM, et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater. 2015;14:1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saha S, Lin J, De Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. Journal of Cellular Physiology. 2006;206:126–137. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- 98.Zhao C, et al. The Effect of Uniaxial Mechanical Stretch on Wnt/beta-Catenin Pathway in Bone Mesenchymal Stem Cells. J Craniofac Surg. 2017;28:113–117. doi: 10.1097/SCS.0000000000003252. [DOI] [PubMed] [Google Scholar]
- 99.Kinney MA, Saeed R, McDevitt TC. Mesenchymal morphogenesis of embryonic stem cells dynamically modulates the biophysical microtissue niche. Sci Rep. 2014;4:4290. doi: 10.1038/srep04290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lü D, Luo C, Zhang C, Li Z, Long M. Differential regulation of morphology and stemness of mouse embryonic stem cells by substrate stiffness and topography. Biomaterials. 2014;35:3945–3955. doi: 10.1016/j.biomaterials.2014.01.066. [DOI] [PubMed] [Google Scholar]
- 101.Chowdhury F, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nature Materials. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vrij E, et al. Directed Assembly and Development of Material-Free Tissues with Complex Architectures. Advanced Materials. 2016;28:4032–4039. doi: 10.1002/adma.201505723. [DOI] [PubMed] [Google Scholar]
- 103.Zoldan J, et al. The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials. 2011;32:9612–9621. doi: 10.1016/j.biomaterials.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shao Y, et al. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat Mater. 2017;16:419–425. doi: 10.1038/nmat4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun Y, et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater. 2014;13:599–604. doi: 10.1038/nmat3945. In this study, mechanically-tuned soft substrates accelerated the differentiation of human pluripotent stem cells into functional motors neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caiazzo M, et al. Defined three-dimensional microenvironments boost induction of pluripotency. Nat Mater. 2016;15:344–352. doi: 10.1038/nmat4536. In this study, biophysical factors in 3D microenvironments were shown to act in parallel with transcriptional factors to support the plasticity of somatic cells and enhance reprogramming through facilitating mesenchymal-to-epithelial transition. [DOI] [PubMed] [Google Scholar]
- 107.Nguyen EH, et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat Biomed Eng. 2017;1:0096. doi: 10.1038/s41551-017-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aguilar A, et al. Importance of environmental stiffness for megakaryocyte differentiation and proplatelet formation. Blood. 2016;128:2022–2032. doi: 10.1182/blood-2016-02-699959. [DOI] [PubMed] [Google Scholar]
- 109.Saha K, et al. Substrate Modulus Directs Neural Stem Cell Behavior. Biophysical Journal. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang C, et al. Spatially patterned matrix elasticity directs stem cell fate. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E4439–E4445. doi: 10.1073/pnas.1609731113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tse JR, Engler AJ. Stiffness Gradients Mimicking In Vivo Tissue Variation Regulate Mesenchymal Stem Cell Fate. Plos One. 2011;6:9. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sunyer R, et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science. 2016;353:1157–1161. doi: 10.1126/science.aaf7119. [DOI] [PubMed] [Google Scholar]
- 113.Raab M, et al. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. The Journal of Cell Biology. 2012;199:669–683. doi: 10.1083/jcb.201205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li CX, et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater. 2017;16:379–389. doi: 10.1038/nmat4780. [DOI] [PubMed] [Google Scholar]
- 116.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nature Communications. 2012;3:9. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 117.Chaudhuri O, et al. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015;6:6364. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32:5979–5993. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Angele P, et al. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21:451–457. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 120.Mouw JK, Connelly JT, Wilson CG, Michael KE, Levenston ME. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells. 2007;25:655–663. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
- 121.Wang J, et al. Mechanical stimulation orchestrates the osteogenic differentiation of human bone marrow stromal cells by regulating HDAC1. Cell Death Dis. 2016;7:12. doi: 10.1038/cddis.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Datta N, et al. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36:1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 124.Shin JW, et al. Contractile Forces Sustain and Polarize Hematopoiesis from Stem and Progenitor Cells. Cell Stem Cell. 2014;14:81–93. doi: 10.1016/j.stem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gilbert PM, et al. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. In this study, substrate elasticity was shown to regulate self-renewal of skeletal muscle stem cells in vitro, which were capable of regenerating muscle tissue when transplanted in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seib FP, Prewitz M, Werner C, Bornhäuser M. Matrix elasticity regulates the secretory profile of human bone marrow-derived multipotent mesenchymal stromal cells (MSCs) Biochemical and Biophysical Research Communications. 2009;389:663–667. doi: 10.1016/j.bbrc.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 127.Yang HB, Nguyen KT, Leong DT, Tan NS, Tay CY. Soft Material Approach to Induce Oxidative Stress in Mesenchymal Stem Cells for Functional Tissue Repair. Acs Applied Materials & Interfaces. 2016;8:26591–26599. doi: 10.1021/acsami.6b09222. [DOI] [PubMed] [Google Scholar]
- 128.Lee S, et al. Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials. 2017;131:111–120. doi: 10.1016/j.biomaterials.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huebsch N, et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14:1269–1277. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mao AS, et al. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat Mater. 2017;16:236–243. doi: 10.1038/nmat4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Darnell M, et al. Substrate Stress-Relaxation Regulates Scaffold Remodeling and Bone Formation In Vivo. Advanced Healthcare Materials. 2017;6:1601185. doi: 10.1002/adhm.201601185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater. 2015;14:737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blaber EA, et al. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 2014;13:181–201. doi: 10.1016/j.scr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 134.Kessler P, Neukam FW, Wiltfang J. Effects of distraction forces and frequency of distraction on bony regeneration. Br J Oral Maxillofac Surg. 2005;43:392–398. doi: 10.1016/j.bjoms.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 135.Cilla M, Checa S, Duda GN. Strain shielding inspired re-design of proximal femoral stems for total hip arthroplasty. J Orthop Res. 2017 doi: 10.1002/jor.23540. [DOI] [PubMed] [Google Scholar]
- 136.Mogil RJ, Kaste SC, Ferry RJ, Jr, et al. Effect of low-magnitude, high-frequency mechanical stimulation on bmd among young childhood cancer survivors: A randomized clinical trial. JAMA Oncology. 2016;2:908–914. doi: 10.1001/jamaoncol.2015.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappa B ligand up-regulation via prostaglandin E-2 synthesis. Journal of Bone and Mineral Research. 2002;17:210–220. doi: 10.1359/jbmr.2002.17.2.210. [DOI] [PubMed] [Google Scholar]
- 138.Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. American Journal of Physiology - Cell Physiology. 2002;283:C1557–C1565. doi: 10.1152/ajpcell.00595.2001. This work highlighted the important role of externally applied mechanical forces in promoting the generation of tissue-engineered muscle fibers in vitro from human cells. [DOI] [PubMed] [Google Scholar]
- 139.Moon DG, Christ G, Stitzel JD, Atala A, Yoo JJ. Cyclic Mechanical Preconditioning Improves Engineered Muscle Contraction. Tissue Engineering Part A. 2008;14:473–482. doi: 10.1089/tea.2007.0104. [DOI] [PubMed] [Google Scholar]
- 140.Crane JD, et al. Massage Therapy Attenuates Inflammatory Signaling After Exercise-Induced Muscle Damage. Science Translational Medicine. 2012;4:119ra113. doi: 10.1126/scitranslmed.3002882. [DOI] [PubMed] [Google Scholar]
- 141.Cezar CA, et al. Biologic-free mechanically induced muscle regeneration. Proc Natl Acad Sci U S A. 2016;113:1534–1539. doi: 10.1073/pnas.1517517113. In this study, externally-applied forces alone, without growth factors or drugs, reduced fibrosis and inflammation in severally injured muscle, and enhanced muscle regeneration and improved muscle function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Celiz AD, et al. Discovery of a Novel Polymer for Human Pluripotent Stem Cell Expansion and Multilineage Differentiation. Advanced Materials. 2015;27:4006–4012. doi: 10.1002/adma.201501351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proceedings of the National Academy of Sciences. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chaudhuri O, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 145.Shin JW, Mooney DJ. Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proceedings of the National Academy of Sciences. 2016;113:12126–12131. doi: 10.1073/pnas.1611338113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saxena M, et al. EGFR and HER2 activate rigidity sensing only on rigid matrices. Nat Mater. 2017 doi: 10.1038/nmat4893. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 148.Pailler-Mattei C, Bec S, Zahouani H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys. 2008;30:599–606. doi: 10.1016/j.medengphy.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 149.McDonald SJ, et al. Early Fracture Callus Displays Smooth Muscle-Like Viscoelastic Properties Ex Vivo: Implications for Fracture Healing. J Orthop Res. 2009;27:1508–1513. doi: 10.1002/jor.20923. [DOI] [PubMed] [Google Scholar]
- 150.Discher DE, Mooney DJ, Zandstra PW. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jansen LE, Birch NP, Schiffman JD, Crosby AJ, Peyton SR. Mechanics of intact bone marrow. Journal of the Mechanical Behavior of Biomedical Materials. 2015;50:299–307. doi: 10.1016/j.jmbbm.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Follet H, Boivin G, Rumelhart C, Meunier PJ. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone. 2004;34:783–789. doi: 10.1016/j.bone.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 153.Zhang YR, Du W, Zhou XD, Yu HY. Review of research on the mechanical properties of the human tooth. In J Oral Sci. 2014;6:61–69. doi: 10.1038/ijos.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nature Reviews Molecular Cell Biology. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nam S, Hu KH, Butte MJ, Chaudhuri O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proceedings of the National Academy of Sciences. 2016;113:5492–5497. doi: 10.1073/pnas.1523906113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu M, et al. A biomimetic gelatin-based platform elicits a pro-differentiation effect on podocytes through mechanotransduction. Scientific Reports. 2017;7:43934. doi: 10.1038/srep43934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Trichet L, et al. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6933–6938. doi: 10.1073/pnas.1117810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Loy C, Laine A, Mantovani D. Rotation-based technique for the rapid densification of tubular collagen gel scaffolds. Biotechnology Journal. 2016;11:1673–1679. doi: 10.1002/biot.201600268. [DOI] [PubMed] [Google Scholar]
- 159.Makarenkova HP, et al. Differential Interactions of FGFs with Heparan Sulfate Control Gradient Formation and Branching Morphogenesis. Science Signaling. 2009;2:ra55. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Azzolin L, et al. YAP/TAZ Incorporation in the beta-Catenin Destruction Complex Orchestrates the Wnt Response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.