Abstract
AIM
To describe the frequency and clinical characteristics of patients with undifferentiated periodic fever (UPF) and to investigate whether a clinical classification of UPF based on the PRINTO-Eurofever score can help predicting the response to treatment and the outcome at follow-up.
METHODS
Clinical and therapeutic information of patients with recurrent fever who presented at a single pediatric rheumatology center from January 2006 through April 2016 were retrospectively collected. Patients with a clinical suspicion of hereditary periodic fever (HPF) syndrome and patients with clinical picture of periodic fever, aphthae, pharingitis, adenitis (PFAPA) who were refractory to tonsillectomy underwent molecular analysis of five HPF-related genes: MEFV (NM_000243.2), MVK (NM_000431.3), TNFRSF1A (NM_001065.3), NLRP3 (NM_001079821.2), NLRP12 (NM_001277126.1). All patients who had a negative genetic result were defined as UPF and further investigated. PRINTO-Eurofever score for clinical diagnosis of HPF was calculated in all cases.
RESULTS
Of the 221 patients evaluated for periodic fever, twelve subjects with a clinical picture of PFAPA who were refractory to tonsillectomy and 22 subjects with a clinical suspicion of HPF underwent genetic analysis. Twenty-three patients (10.4%) resulted negative and were classified as UPF. The median age at presentation of patients with UPF was 9.5 mo (IQR 4-24). Patients with UPF had a higher frequency of aphthae (52.2% vs 0%, P = 0.0026) and musculoskeletal pain (65.2% vs 18.2%, P = 0.0255) than patients with genetic confirmed HPF. Also, patients with UPF had a higher frequency of aphthous stomatitis (52.2% vs 10.7%, P < 0.0001), musculoskeletal pain (65.2% vs 8,0%, P < 0.0001), and abdominal pain (52.2% vs 4.8%, P < 0.0001) and a lower frequency of pharyngitis (56.6% vs 81.3%, P = 0.0127) compared with typical PFAPA in the same cohort. Twenty-one of 23 patients with UPF (91.3%) received steroids, being effective in 16; 13 (56.2%) were given colchicine, which was effective in 6. Symptoms resolution occurred in 2 patients with UPF at last follow-up. Classification according to the PRINTO-Eurofever score did not correlate with treatment response and prognosis.
CONCLUSION
UPF is not a rare diagnosis among patients with periodic fever. Clinical presentation place UPF half way on a clinical spectrum between PFAPA and HPF. The PRINTO-Eurofever score is not useful to predict clinical outcome and treatment response in these patients.
Keywords: Hereditary periodic fever syndromes, Therapy, Genetics, Autoinflammatory diseases, Undifferentiated periodic fever
Core tip: Children with non-infectious recurrent fever more often fall into two diagnostic categories. The first and most common is periodic fever, aphthae, pharingitis, adenitis (PFAPA), the second, far more rare, are hereditary periodic fevers. Very recently a third category has been increasingly recognized, and is that of undifferentiated periodic fevers or undifferentiated periodic fever (UPF). UPF include patients who do not meet the diagnostic criteria for PFAFA or for a monogenic disease. The clinical presentation and management of patients with UPF are poorly defined. In this study, the authors describe a cohort of patients with UPF showing that: (1) The clinical manifestations are on a half way of clinical spectrum between PFAPA and hereditary periodic fever; (2) PRINTO-Eurofever score is not useful to guide treatment choices and does not predict disease course; and (3) Both steroids and colchicine are useful to control symptoms in most cases. The authors conclude that further studies are needed to better define UPF and guide their management in clinical practice.
INTRODUCTION
Periodic fever is defined as recurrences of seemingly unprovoked episodes of fever that last from a few days to a few weeks, separated by symptom-free intervals of variable duration[1].
The most common cause of periodic fever syndrome in children is PFAPA (periodic fever, aphthae, pharingitis, adenitis), an autoinflammatory condition[2] characterized by recurrence of fever associated with aphthous stomatitis, pharyngitis, and/or cervical adenopathy. The diagnosis of PFAPA is based on Thomas criteria: recurring fevers that begin before age 5 and are accompanied by at least one of the clinical signs aphthous stomatitis, pharyngitis or adenitis, without upper respiratory infection[3]. The pathogenesis of PFAPA is likely multifactorial, but evidence of familial recurrence of this syndrome may support a genetic predisposition. Patient with PFAPA respond to steroids[2] and heal spontaneously, usually in few years, or after tonsillectomy without sequelae[4,5]. Lack of response to tonsillectomy in PFAPA is uncommon and should raise the suspicion of a monogenic condition[6,7].
Other rarer periodic fever syndromes are due to definite monogenic defects involving mechanisms of inflammation (hereditary periodic fevers - HPF). The first described was familial mediterranean fever (FMF) which has been linked to MEFV gene in late nighties. Afterwards, a number of HPF syndromes have been described, and their genetic bases has been provided. This is the case for example of: tumor necrosis factor receptor-associated periodic syndrome (TRAPS), associated with TNFRSF1A gene defects, hyperimmunoglobulinemia D with periodic fever syndrome (HIDS), related to MVK gene, and cryopyrin-associated periodic syndromes (CAPS) for which defects on either NLRP3 or NLRP12 have been identified[8,9].
Timely diagnosis of HPF is essential since effective therapies are now available, and complications, such as amyloidosis and sensor neural impairment, can be avoided[10].
The term undifferentiated periodic fever (UPF) has been increasingly used to define those patients presenting with a complaint of periodic fever in whom the diagnostic criteria for PFAPA are not satisfied and genetic work-up for periodic fever gives a negative result.
On a practical ground, subjects with PFAPA who are refractory to tonsillectomy and/or to steroids may be as well included in the group of UPF.
Even though patients with UPF are increasingly recognized in clinical practice, the only epidemiologic data available comes from the Eurofever Registry were, in 2014, patients with an “undefined periodic fever” accounted for almost 9% of cases[11].
The management of such patients is challenging, as clinical manifestations are poorly described and the prognosis and response to treatment are unknown. Federici et al[12] recently validated a score for clinical classification of patients with periodic fever into the main four phenotypes of HPF (PRINTO-Eurofever score), and proposed that it could be used to classify patients with periodic fever in daily practice. The score considers age at disease onset, ethnicity and the presence or absence of specific clinical symptoms.
With the present study, we aimed to describe the frequency and clinical characteristics of patients with a diagnosis of UPF. Moreover, we calculated the PRINTO-Eurofever score in subjects with UPF to investigate if a clinical classification in HPF-like phenotypes (clinical score of HPF in the absence of genetic confirmation) could help predicting the response to treatment and prognosis.
MATERIALS AND METHODS
Patients
This was a retrospective cohort study conducted at a single Rheumatology Center caring for pediatric patients with rheumatologic and autoinflammatory conditions. The Study was approved by the local Ethics Committee. Clinical charts of patients who received a diagnosis of periodic fever during a ten-year period, from January 2006 through April 2016, were reviewed. Patients older than 18 years at the time of data collection were not included as they were no more referred to our Institute.
Criteria
All the patients with periodic fever were included in the study. Subjects with a clinical suspicion of HPF and subjects with a suspicion of PFAPA (Thomas’s criteria) who didn’t respond to steroid treatment or had a recurrence of symptoms after tonsillectomy underwent genetic analysis for five genes mostly involved in HPF, namely: MEFV (NM_000243.2) for familial mediterranean fever (FMF), MVK (NM_000431.3) for mevalonate kinase deficiency (MKD), TNFRSF1A (NM_001065.3) for TNFα receptor associated periodic syndrome (TRAPS), NLRP3 (NM_001079821.2) and NLRP12 (NM_001277126.1) for familial cold urticaria syndrome. Patients with known causative mutation were diagnosed with the corresponding disorder.
Subjects with negative results or polymorphisms of unknown significance were considered as UPF[13], irrespective of whether they came from the group of PFAPA who were refractory to tonsillectomy or patients suspected with HPF. This choice was based on the consideration that both subjects with a clinical suspicion of HPF and subjects with a suspicion of PFAPA, refractory to tonsillectomy or to steroids, share the same problems as concerns the lack of a definite prognosis and of a therapeutic approach.
Thus, three groups of subjects were finally identified based on clinical and genetical features: Typical PFAPA, genetically confirmed HPF, UPF.
Data collection
Data collected included information on: gender, family history, ethnicity, duration of fever episodes, presence of pharyngitis, adenitis or aphthae, presence of musculoskeletal, chest or abdominal pain, diarrhea, vomiting, skin rash, conjunctivitis, and sensory neural hearing loss. Data were collected at disease onset and during follow-up. PRINTO-Eurofever score was calculated retrospectively at last follow-up[12]. Based on PRINTO-Eurofever score, patients who scored positive were assigned to a specific HPF phenotype according to the same scoring system. A possible relationship between the clinical diagnosis based on PRINTO-Eurofever criteria and response to treatment at follow up was assessed.
Statistical analysis
Statistical analysis were made using GraphPad Prism 5 software. Categorical variables were summarized as frequency and percentage and were compared across independent groups by the Fisher’s exact test (two tailed, confidence interval 95%). Numerical variables with asymmetrical distribution were summarized by median and interquartile range (IQR) and were compared by the Kruskal-Wallis test. A P value < 0.05 was considered for significance.
RESULTS
During a ten-year period, 221 patients were evaluated for periodic fever. Twenty-two patients had a suspicion of HPF and a definite diagnosis could be genetically confirmed in 11 of them (5 MKD, 3 TRAPS, 2 FMF, 1 FCU). The other 11 subjects resulted negative and were considered as UPF.
PFAPA was suspected in 199 subjects, 12 of whom did not respond to steroids or persisted after tonsillectomy and underwent genetic analysis. None of these subjects had causative mutations of HPF genes. In 6 patients, variants of unknown significance were found. Four patients had heterozygous mutations of which two were in the MEFV gene (P369S-R408Q and V726A), one in the MVK gene (V377I) and one in NLRP12 (variation H304Y). Two patients had a homozygous R202Q variant in MEFV. Thus, all 12 subjects were considered as UPF.
Overall, a total of 23 patients (10%) met the diagnosis of UPF and where thus included in the analysis. UPF Cohort selection is reported in Figure 1.
Figure 1.
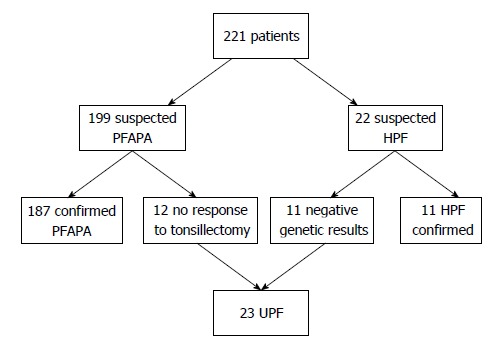
Selection of undifferentiated periodic fever cohort. PFAPA: Periodic fever, aphthae, pharingitis, adenitis; HPF: Hereditary periodic fever; UPF: Undifferentiated periodic fever.
Baseline characteristics of patients with UPF and comparison with HPF and PFAPA
Baseline demographic and clinical characteristics of patients are described in Table 1.
Table 1.
Baseline characteristics and symptoms distribution of patients with periodic fever in our cohort
| Undifferentiated periodic fever (n = 23) | PFAPA (n = 187) | HPF (n = 11) | |
| Females, n (%) | 18 (70%) | 74 (38%) | 7 (63%) |
| Ethnicity, n | |||
| EU | 20 | 183 | 11 |
| Arabian | 2 | 2 | 0 |
| Mix | 1 | 2 | 0 |
| Median age at onset, mo (IQR) | 9.5 (4-24) | 12.5 (2.5-96) | 9 (1-174) |
| Median age at first visit, mo (IQR) | 51 (33-113) | 42.15 (8-120) | 48 (12-216) |
| Symptoms, n (%) | |||
| Pharyngitis | 13 (56.6%) | 152 (81.3%) | 4 (36.3%) |
| Aphtae | 12 (52.2%) | 20 (10.7%) | 0 (0%) |
| Chest pain | 0 (0%) | 0 (0%) | 1 (9%) |
| Abdominal pain | 12 (52.2%) | 9 (4.8%) | 6 (54.5%) |
| Musculoskeletal pain | 15 (65.2%) | 15 (8.0 %) | 2 (18.2%) |
The most frequent complaints at first visit in UPF were exudative pharyngitis (13 patients, 56.6%), musculoskeletal pain (15 pt, 65.2%), aphthous stomatitis (12 pt, 52.2%) and abdominal pain (12 pt, 52.2%). No patient suffered from chest pain. Abdominal pain in the UPF group was reported as mild to moderate and never led to an evaluation for acute abdomen.
Median duration of disease at last follow-up was 6.3 years (IQR 0.9-17.8 year). Median follow-up since inclusion in the study was 3.3 years (IQR 0.3-9.4 year).
Subjects with UPF had a similar age at disease onset compared with HPH (P = 0.9), while they had an earlier onset compared with PFAPA (P = 0.008).
Compared with HPF, patients with UPF were more likely to have aphthae (52.2% vs 0%, P = 0.0026) and musculoskeletal pain (65.2% vs 18.2%, P = 0.0255), while compared with PFAPA they were more likely to have aphthous stomatitis (52.2% vs 10.7%, P < 0.0001), musculoskeletal pain (65.2% vs 8.0%, P < 0.0001), abdominal pain (52.2% vs 4.8%, P < 0.0001) but were less likely to have pharyngitis (56.6% vs 81.3%, P = 0.0127).
Therapeutic strategies received at any time and response to therapy in UPF
An oral glucocorticoid (betamethasone) was the first therapeutic choice in 21/23 patients. In 16 patients (69.5%), oral administration of betamethasone determined immediate resolution of the acute episode; in 11 patients, a standard dose of 0.1 mg/kg of betamethasone was effective, in 5 higher doses of 0.2-0.3 mg/kg were required. In 8 patients, who came from the group with initial suspicion of PFAPA, acute episodes became more frequent after glucocorticoid treatment. Tonsillectomy was performed in 12 patients being ineffective in all of them.
Thirteen patients were treated with daily oral colchicine (0.5-1.5 mg/die), which reduced or abated symptoms recurrence in 6 patients (46%). All patients receiving colchicine had been previously treated with steroids. The reason for switching to colchicine was steroids being ineffective in 3 patients, and need for too high or too frequent corticosteroid doses in 10 patients.
At last follow-up, 21/23 (91%) were still receiving medical treatment for the management of acute symptoms: 10 patients received corticosteroids during acute episodes; 7 patients were on daily colchicine and 4 patients were receiving non-steroidal antinflammatory drugs (NSAID); 2 patients healed spontaneously.
Classification of patients based on PRINTO-Eurofever score at baseline and response to therapy
At baseline, 11 subjects could be classified as HPF-like: 3 patients had a positive score for FMF, 5 for MKD, 3 for TRAPS, 1 for CAPS (1 patient was positive for FMF and MKD). Twelve patients had negative scores for HPF.
We didn’t observe a clear relationship between PRINTO-Eurofever score at baseline and response to treatment received at any time. In particular, no patient who responded to colchicine had a clinical score supportive of FMF. As opposite, 2 out of 7 patients who did not respond to colchicine had a positive score for FMF.
DISCUSSION
In a single pediatric rheumatology center, 10% of patients presenting with a complaint of periodic fever could be classified as UPF. Even if PFAPA was by far the most frequent diagnosis, UPF was a more common diagnosis compared to HPF.
Age at symptoms presentation patients with UPF was quite varied, ranging from 2 to 111 mo. Interestingly, the median age at disease onset in of UPF was closer to that of HPF than that of PFAPA, similarly to what has been reported in the literature[11], suggesting the possibility of a stronger contribution of genetic factors in UPF compared with PFAPA.
Musculoskeletal pain and recurrent pharyngitis were the most frequent complaints followed by recurrence of aphthous stomatitis and abdominal pain. The high frequency of pharyngitis and stomatitis reflects the fact that the majority of patients classified as UPF had been initially diagnosed as PFAPA. It should be noted, however, that all these patients had a more complex phenotype compared to patients with a definitive diagnosis of PFAPA, as most of them complained of musculoskeletal or abdominal pain, in addition to pharyngitis.
The frequency of abdominal pain alone was similar in UPF and HPF, and in both cases it was significantly higher than in PFAPA. Notably, chest pain was never reported in patients with UPF.
According to these observations, UPF seem to be half way on a clinical spectrum of disease severity ranging from PFAPA to HPF. Patients with UPF in fact had more frequent “extrapharingeal” symptoms, namely musculoskeletal and abdominal pain, compared to PFAPA but despite their frequency, these symptoms were never as severe as those reported in HPF.
Overall, steroids were an effective strategy to control symptoms in a high proportion of patients.
For patients who needed steroids at high doses or frequency, daily oral colchicine was a useful alternative strategy to control symptom recurrence.
Tonsillectomy did not resolve fever recurrence in UPF patients, but this observation is biased, as this was one of the criteria that we chose for patients’ inclusion in the UPF group.
We evaluated if a clinical classification of our cases could have been used to predict the response to therapy. So far, the only suitable classification proposed for subjects with periodic fever syndromes is the PRINTO-Eurofever score, which was developed to help experts classifying patients with suspected autoinflammatory fever syndromes without relying on genetic analyses. The score was calculated based on clinical features selected on a multivariate analysis on a large group of patients with different periodic fevers[12].
We showed that PRINTO-Eurofever classification at baseline could not predict response to any treatment.
Thus, even though the short time to last follow up for some patients, PRINTO-Eurofever score at baseline seems not to be predictive of symptoms persistence over time.
Several limitations of the study are acknowledged. Due to its retrospective design, clinical data were not systematically collected. The sample size is small, and recruitment occurred at one Tertiary-referral Clinic thus limiting the generalizability of our observation. Moreover, the median time to follow-up was too short to estimate long-term prognosis. In fact, considering that a significant proportion of UPF showed both recurrent aphthous ulcers and abdominal or musculoskeletal pain, it is still possible that part of them will develop a Behcet disease (BD) on a longer follow-up. Indeed, Cantarini et al[14] recently showed that many subjects with adult BD complained of periodic fever and aphthae during childhood. Moreover, we cannot exclude that some patient with UPF could have a different monogenic disorder, but this seem unlikely at present, given that increasing the number of candidate genes in genetic panels did not result in increased diagnoses[15].
In conclusion, according to our observation patients with UPF seem not to be rare in clinical practice. Clinical presentation places them half way on a clinical spectrum between PFAPA and HPF. Classification of patients with UPF into a specific HPF phenotype according PRINTO-Eurofever criteria is not useful to guide treatment choice and does not predict disease prognosis. Given the above-mentioned limitation, what we observed represent and initial attempt to describe a poorly defined subset of patients with periodic fever commonly encountered in clinical practice. Further studies are needed to better define patients with UPF and guide their management in clinical practice.
ARTICLE HIGHLIGHTS
Research background
Undifferentiated periodic fevers (UPF) include periodic fever not meeting the diagnostic criteria for typical PFAPA of for hereditary periodic fever syndromes. Even if UPF are increasingly recognized, there is currently no recommendation to guide the management of children with this condition.
Research motivation
Clinical criteria to classify periodic fever without the help of genetic analyses have been proposed by PRINTO Eurofever, and might be particularly useful for subjects with UPF. Thus, we studied the clinical features of our patients in relation with their scores in the PRINTO-Eurofever classification.
Research objectives
Our study aims at improving knowledge on UPF and at evaluating if the application of the PRINTO-Eurofever classification can help the clinical management of these patients.
Research methods
A data base was filled in by retrospective review of clinical records, follow-up visits and phone calls. A structured questionnaire was used to classify all the subjects with the PRINTO-Eurofever score. The response to therapies and the prognosis at follow-up was compared with the clinical diagnosis obtained with the PRINTO-Eurofever score.
Research results
The clinical manifestations are on a half way of clinical spectrum between PFAPA and hereditary periodic fever. PRINTO-Eurofever score is not useful to guide treatment choices and does not predict disease course. Both steroids and colchicine are useful to control symptoms in most cases.
Research conclusions
UPF are as common as hereditary periodic fever, however knowledge on prognosis and response to therapies in these patients is lacking.
Research perspectives
Multicenter studies and experts’ agreement are needed to develop recommendations for the management of UPF.
Footnotes
Supported by the Institute for Maternal and Child Health IRCCS Burlo Garofolo, No. RC36/11.
Institutional review board statement: The study was approved by the IRB at the IRCCS Burlo Garofolo.
Informed consent statement: The written informed consent was obtained by all the subjects recruited in the study or by their parents/guardians.
Conflict-of-interest statement: The authors declare no conflict of interest.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Peer-review started: September 24, 2017
First decision: November 27, 2017
Article in press: December 14, 2017
Specialty type: Pediatrics
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P- Reviewer: Al-Biltagi M, Fretzayas A, Kute VBB, Sergi C, Watanabe T, Wang R, Yellanthoor RB S- Editor: Kong JX L- Editor: A E- Editor: Li RF
Contributor Information
Silvia De Pauli, Department of Medicine, Surgery and Health, University of Trieste, Trieste 34142, Italy.
Sara Lega, Department of Medicine, Surgery and Health, University of Trieste, Trieste 34142, Italy; Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste 34137, Italy.
Serena Pastore, Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste 34137, Italy.
Domenico Leonardo Grasso, Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste 34137, Italy.
Anna Monica Rosaria Bianco, Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste 34137, Italy.
Giovanni Maria Severini, Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste 34137, Italy.
Alberto Tommasini, Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste 34137, Italy.
Andrea Taddio, Department of Medicine, Surgery and Health, University of Trieste, Trieste 34142, Italy; Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste 34137, Italy.
References
- 1.McDermott MF, Frenkel J. Hereditary periodic fever syndromes. Neth J Med. 2001;59:118–125. doi: 10.1016/s0300-2977(01)00149-8. [DOI] [PubMed] [Google Scholar]
- 2.Stojanov S, Lapidus S, Chitkara P, Feder H, Salazar JC, Fleisher TA, Brown MR, Edwards KM, Ward MM, Colbert RA, et al. Periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) is a disorder of innate immunity and Th1 activation responsive to IL-1 blockade. Proc Natl Acad Sci U S A. 2011;108:7148–7153. doi: 10.1073/pnas.1103681108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas KT, Feder HM Jr, Lawton AR, Edwards KM. Periodic fever syndrome in children. J Pediatr. 1999;135:15–21. doi: 10.1016/s0022-3476(99)70321-5. [DOI] [PubMed] [Google Scholar]
- 4.Manthiram K, Nesbitt E, Morgan T, Edwards KM. Family History in Periodic Fever, Aphthous Stomatitis, Pharyngitis, Adenitis (PFAPA) Syndrome. Pediatrics. 2016;138:pii e20154572. doi: 10.1542/peds.2015-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peridis S, Koudoumnakis E, Theodoridis A, Stefanaki K, Helmis G, Houlakis M. Surgical outcomes and histology findings after tonsillectomy in children with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis syndrome. Am J Otolaryngol. 2010;31:472–475. doi: 10.1016/j.amjoto.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Lantto U, Koivunen P, Tapiainen T, Renko M. Long-Term Outcome of Classic and Incomplete PFAPA (Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Adenitis) Syndrome after Tonsillectomy. J Pediatr. 2016;179:172–177.e1. doi: 10.1016/j.jpeds.2016.08.097. [DOI] [PubMed] [Google Scholar]
- 7.Pehlivan E, Adrovic A, Sahin S, Barut K, Kul Cınar O, Kasapcopur O. PFAPA Syndrome in a Population with Endemic Familial Mediterranean Fever. J Pediatr. 2017;192:253–255. doi: 10.1016/j.jpeds.2017.08.078. [DOI] [PubMed] [Google Scholar]
- 8.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, Stein L, Russo R, Goldsmith D, Dent P, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinon F, Aksentijevich I. New players driving inflammation in monogenic autoinflammatory diseases. Nat Rev Rheumatol. 2015;11:11–20. doi: 10.1038/nrrheum.2014.158. [DOI] [PubMed] [Google Scholar]
- 10.Touitou I, Galeotti C, Rossi-Semerano L, Hentgen V, Piram M, Koné-Paut I; CeRéMAI, French reference center for autoinflammatory diseases. The expanding spectrum of rare monogenic autoinflammatory diseases. Orphanet J Rare Dis. 2013;8:162. doi: 10.1186/1750-1172-8-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toplak N, Frenkel J, Ozen S, Lachmann HJ, Woo P, Koné-Paut I, De Benedetti F, Neven B, Hofer M, Dolezalova P, Kümmerle-Deschner J, Touitou I, Hentgen V, Simon A, Girschick H, Rose C, Wouters C, Vesely R, Arostegui J, Stojanov S, Ozgodan H, Martini A, Ruperto N, Gattorno M; Paediatric Rheumatology International Trials Organisation (PRINTO), Eurotraps and Eurofever Projects. An international registry on autoinflammatory diseases: the Eurofever experience. Ann Rheum Dis. 2012;71:1177–1182. doi: 10.1136/annrheumdis-2011-200549. [DOI] [PubMed] [Google Scholar]
- 12.Federici S, Sormani MP, Ozen S, Lachmann HJ, Amaryan G, Woo P, Koné-Paut I, Dewarrat N, Cantarini L, Insalaco A, Uziel Y, Rigante D, Quartier P, Demirkaya E, Herlin T, Meini A, Fabio G, Kallinich T, Martino S, Butbul AY, Olivieri A, Kuemmerle-Deschner J, Neven B, Simon A, Ozdogan H, Touitou I, Frenkel J, Hofer M, Martini A, Ruperto N, Gattorno M; Paediatric Rheumatology International Trials Organisation (PRINTO) and Eurofever Project. Evidence-based provisional clinical classification criteria for autoinflammatory periodic fevers. Ann Rheum Dis. 2015;74:799–805. doi: 10.1136/annrheumdis-2014-206580. [DOI] [PubMed] [Google Scholar]
- 13.Shinar Y, Obici L, Aksentijevich I, Bennetts B, Austrup F, Ceccherini I, Costa JM, De Leener A, Gattorno M, Kania U, et al. Guidelines for the genetic diagnosis of hereditary recurrent fevers. Ann Rheum Dis. 2012;71:1599–1605. doi: 10.1136/annrheumdis-2011-201271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantarini L, Vitale A, Bersani G, Nieves LM, Cattalini M, Lopalco G, Caso F, Costa L, Iannone F, Lapadula G, et al. PFAPA syndrome and Behçet’s disease: a comparison of two medical entities based on the clinical interviews performed by three different specialists. Clin Rheumatol. 2016;35:501–505. doi: 10.1007/s10067-015-2890-5. [DOI] [PubMed] [Google Scholar]
- 15.Rusmini M, Federici S, Caroli F, Grossi A, Baldi M, Obici L, Insalaco A, Tommasini A, Caorsi R, Gallo E, et al. Next-generation sequencing and its initial applications for molecular diagnosis of systemic auto-inflammatory diseases. Ann Rheum Dis. 2016;75:1550–1557. doi: 10.1136/annrheumdis-2015-207701. [DOI] [PubMed] [Google Scholar]


