Abstract
While genetic diversity within species is influenced by both geographical distance and environmental gradients, it is unclear what other factors are likely to promote population genetic structure. Using a machine learning framework and georeferenced DNA sequences from more than 8000 species, we demonstrate that geographical attributes of the species range, including total size, latitude and elevation, are the most important predictors of which species are likely to contain structured genetic variation. While latitude is well known as an important predictor of biodiversity, our work suggests that it also plays a key role in shaping diversity within species.
Keywords: GBIF, genetic structure, machine learning, latitude, elevation
1. Background
Intraspecific genetic variation is a key component of evolution. Population genetic theory predicts that the physical separation of individuals limits the exchange of alleles, producing genetic variation that is geographically structured [1]. Within a species, genetic distance should be positively correlated with geographical distance under an isolation-by-distance (IBD) model, and might enable local adaptation along environmental gradients [2]. For example, a meta-analysis of 70 studies by [3], found that isolation-by-environment (IBE) plays a strong role in structuring populations. Whether correlated with geographical or environmental distance, genetic structure has been detected in a variety of species with vastly different distribution patterns [4–6].
While thousands of phylogeographic investigations have been published [7], the discipline has not addressed questions on the broadest scales. Several meta-analyses have examined IBD, IBE or both [3,8,9], and while informative, are limited in scope due to the nature of meta-analyses and often contain conflicting results [3,9,10], which stem from differences in study design, search criteria and publication bias [8] that are difficult to circumvent. Rather than attempt such a meta-analysis, we repurpose existing georeferenced genetic data from online repositories: GenBank and Global Biodiversity Information Facility (GBIF). Because the collection of these data was motivated by a variety of reasons, repurposing enabled us to assess IBD and IBE in an unbiased manner on a larger scale. We compare both geographical and environmental distance matrices to a matrix of genetic distance for over 8000 species and apply a machine learning approach to identify intrinsic and extrinsic characteristics that best explain variation in population genetic structure among species.
2. Material and methods
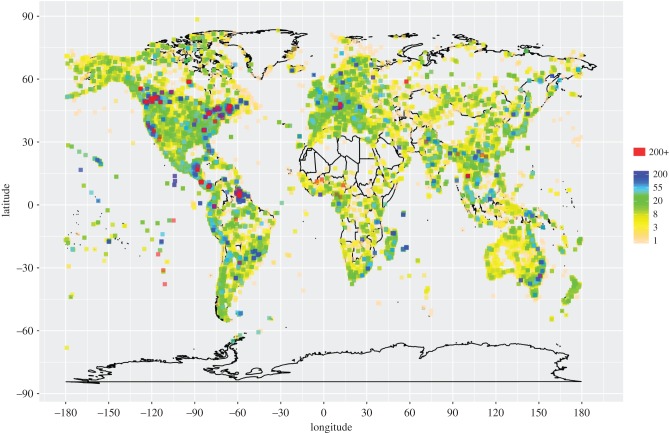
We downloaded all occurrence data from GBIF and identified records that included GenBank accessions, retrieved these sequences from GenBank and conducted multiple sequence alignment on a gene-by-gene basis for each species. All statistical analyses were conducted using R v. 3.2.3 [11]. See electronic supplementary material for more details. The distribution of georeferenced data was mapped by calculating the frequency of localities associated with each GPS coordinate (figure 1; electronic supplementary material, table S1). We calculated genetic, geographical and environmental distance matrices for each dataset. In order to characterize the environmental conditions experienced by each species, we followed [12]. Given that geography and environment are often correlated, as we observe in our data (mean r = 0.77), we conducted a multiple matrix regression with randomization (MMRR) [12] to examine the effects of two different distance matrices (geographical (IBD) and environmental (IBE)) on the response variable (genetic distance), while controlling for the other matrix.
Figure 1.
Global sampling. Collection localities from 561,534 georeferenced sequences. Colours correspond to the numbers of individuals sampled from that locality.
A data table was developed to identify the strongest predictors of population genetic structure: habit (terrestrial, aquatic, volant, parasitic), metabolism (ectotherm, endotherm, photosynthetic), gene type (nDNA, mtDNA, cpDNA), number of individuals (n) in the dataset, total area of species' range, minimum distance from the equator, mid-point of latitude, the extent of latitude and elevation mean and standard deviation. Taxonomy was included to assess the role that phylogenetic relatedness plays in structuring populations, and served as a proxy for organismal traits common to particular clades. Finally, the proportion of GPS coordinates within each of the 23 land cover classes described by the European Space Agency GlobCover Portal [13] was included to evaluate environmentally dependent organismal traits [14].
Random forest analysis was used to determine which of the above variables were the most important predictors of IBD or IBE [15,16]. This is a machine learning approach that uses multiple decision trees (a forest) to predict the response based on many potential predictor variables, and is designed to deal with large correlated datasets. The importance of each variable is determined by measuring the mean decrease in accuracy (MDA) of the prediction after the removal of each variable from the predictive function. We categorized datasets as either being significant for IBD or IBE (p-value < 0.05), or not. We conducted a series of random forest analyses with different cut-offs for n, and used several downsampling schemes to assess biases in the data, such as uneven response variables, and uneven geographical sampling. Classification error rates were calculated to assess the accuracy of the models.
3. Results
After filtering data that did not contain sufficient sample sizes, we analysed 9730 datasets from 8955 species. A total of 19% of the datasets were significant for IBD and 15% were significant for IBE (table 1). In most taxonomic groups, there were more datasets with population genetic structure than expected by chance (p < 0.05) (table 1; figure 2; electronic supplementary material, figure S1). Out of the datasets that were significant for either IBD or IDE, 57% were significant for both, 27% were significant for IBD only, and 15% were significant for IBE only (electronic supplementary material, figure S2).
Table 1.
The proportion of datasets significant for geography (Geo) and the environment (Env). p-value of a binomial test (p = 0.05) of whether the proportion of significant datasets was greater than expected by chance.
| Group | n datasets | prop.sig Geo. | p-value Geo. | prop.sig Env. | p-value Env. |
|---|---|---|---|---|---|
| fungi | 23 | 0.04 | 0.69 | 0.04 | 0.69 |
| mosses | 10 | 0 | 1 | 0 | 1 |
| ferns | 7 | 0 | 1 | 0 | 1 |
| Gymnosperms | 111 | 0.07 | 0.19 | 0.06 | 0.32 |
| angiosperms | 870 | 0.1 | <0.01 | 0.1 | <0.01 |
| arthropods | 6014 | 0.15 | <0.01 | 0.13 | <0.01 |
| vertebrates | 2577 | 0.29 | <0.01 | 0.21 | <0.01 |
| Annelida | 33 | 0.21 | 0 | 0.15 | 0.02 |
| Cnidaria | 6 | 0.5 | 0 | 0 | 1 |
| Echinodermata | 14 | 0.21 | 0.03 | 0.21 | 0.03 |
| Mollusca | 44 | 0.18 | 0.01 | 0.16 | 0.01 |
| Nematoda | 6 | 0.33 | 0.03 | 0.33 | 0.03 |
| Platyhelminthes | 15 | 0 | 1 | 0.2 | 0.04 |
| total | 9730 | 0.19 | <0.01 | 0.15 | <0.01 |
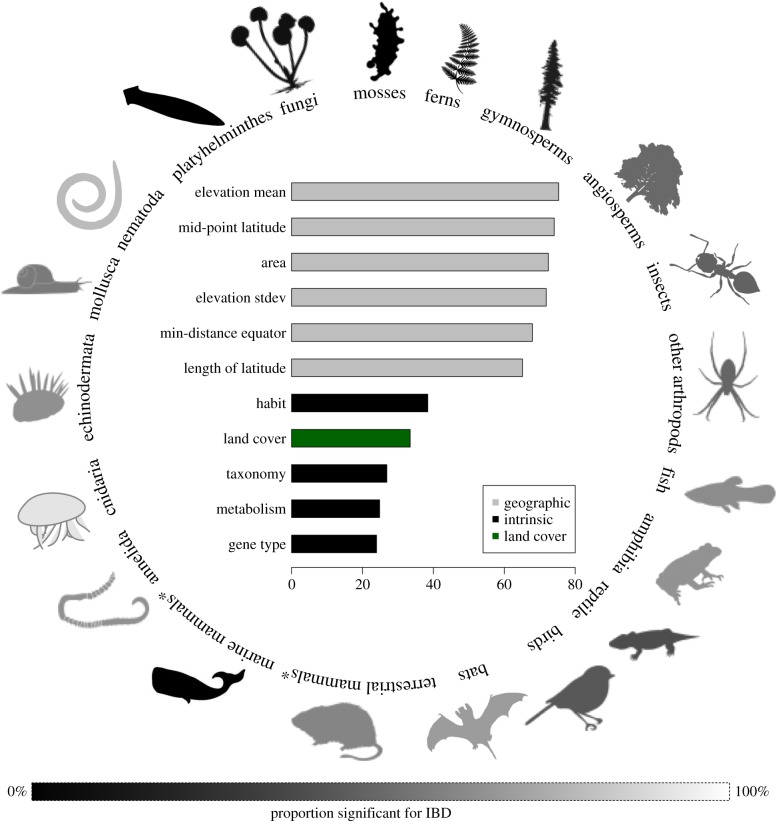
Figure 2.
Predictor variables and the proportion of IBD by group. (Inset) Mean decrease in accuracy of predictor variables. Taxonomic rank and land cover variables were averaged for clarity of presentation. (Outer) Proportion of species that exhibit significant population genetic structure (see scale on the lower portion of the figure). Groups marked with an asterisk (*) are not monophyletic clades, but are grouped together due to small sample sizes and similarity of life-history traits. (Online version in colour.)
The variable with the most predictive ability was sample size (n; electronic supplementary material, table S4), which is indicative of a bias introduced by low sample sizes. To address this, we plotted a rarefaction curve to see where the proportion of datasets that are significant for IBD levels off as a function of sample size (electronic supplementary material, figure S3). There was a large jump from n > 3 to n > 10, and at n > 20 the proportion of datasets that are significant levels off. We therefore repeated the analysis with n > 10 and n > 20 (electronic supplementary material, tables S5–S10 and S13–S14; n > 10 datasets = 4,304). The accuracy of the random forest model improved when the response variable was even, and slightly improved when geographical sampling was even (electronic supplementary material, tables S15–S17). The top predictor variables in all analyses were related to the geographical range: latitude, area and elevation (figure 2; electronic supplementary material, tables S4–S14). This suggests that regardless of potential sampling size and/or biases, the importance of the geographical variables in predicting IBD and IBE is a strong signal in the data. Results were similar when using p < 0.01 as significant for IBD or IBE (electronic supplementary material, tables S18–S19).
Whether the most important variables were significantly different in species with or without IBD was examined using t-tests (table 2). The mean size of the geographical range of a species with IBD was almost twice that of a species without population genetic structure, while the total latitudinal length is 1.5× longer, and datasets with IBD were significantly further from the equator for both mid-point latitude and minimum distance. The standard deviation of elevation for those with IBD was significantly larger than those without, while the mean elevation was higher, but not significantly different.
Table 2.
Comparison of geographical range characteristics for species with and without IBD.
| variable | mean with IBD | mean without IBD | t-test p-value |
|---|---|---|---|
| area (km2) | 6.24 × 106 | 3.50 × 106 | 1.93 × 10−7 |
| minimum distance from equator | 29.6712 | 32.1637 | 7.12 × 10−10 |
| mid-point latitude of range | 31.4947 | 34.1037 | 6.92 × 10−8 |
| length of latitude° | 14.5924 | 10.4653 | 2.20 × 10−16 |
| mean elevation | 113.9991 | 112.9753 | 0.4563 |
| standard deviation elevation | 63.5219 | 59.7870 | 3.92 × 10−7 |
4. Discussion
There is a considerable amount of population genetic structure within species that can be explained by geographical and environmental differences. Geographical distance had a slightly stronger signal, although neither is substantially more responsible for genetic structure across all taxonomic groups (table 1; electronic supplementary material, figures S2–S5). Our random forest analyses identified several predictor variables related to the geographical range of species, such as area and measurements related to latitude (figure 2; electronic supplementary material, tables S4–S12), as important in predicting population genetic structure, similar to findings by [17]. These variables are likely important because they are related to both organismal dispersal ability and physiological adaptations to conditions in the abiotic environment [18,19], and hold true even after controlling for latitudinal sampling bias. Attributes of the geographical range were significantly different in species with and without IBD (table 2). As observed in other studies (e.g. [20]), IBD and IBE are identified along elevation gradients. We suspect that the reason why elevation mean is an important predictor in the random forest, but not significantly different between species with and without IBD, is because while important, the elevation at which it influences IBD depends on geographical location. The complex relationship between these variables should be considered in future studies.
While mapping genetic diversity on a global scale provides important information [21], identifying factors that influence genetic diversity within species will improve our ability to protect biodiversity [22]. This structure is important as species adapt across their geographical ranges and their life-history traits evolve in response to environmental pressures. Furthermore, we are likely underestimating global genetic structure given limitations of available data. This supposition is supported by the difference in rate of IBD estimated from the full dataset (approx. 15%) as opposed to that estimated from species where more than 100 samples are available (approx. 40%; electronic supplementary material, figure S3). While our analyses suggest that we have detected IBD and IBE in a greater number of species than expected by chance (electronic supplementary material, figure S1), it is very likely that we lack sufficient genetic data for most species and thus are underestimating the proportion of species that are structured by geography, the environment, or both.
Geographical variation in intraspecific genetic structure likely results from variation in speciation, migration and extinction rates. Lower rates of speciation in temperate regions of the world [23–25] might explain the difference in IBD due to latitude because as species remain intact, there is more time for genetic differentiation to accumulate across geographical space. We suspect that area is an important predictor of IBD and IBE due to both the intrinsic dispersal ability of species and the larger amounts of landscape variability that are likely to be found in large ranges.
Our findings were made possible by repurposing existing georeferenced genetic data that contain immense potential for insight [7,26,27]. Unfortunately, most available sequence data are not linked to geographical coordinates [28]. This disassociation of genetic and geographical accessions limits the utility of open source databases and must be addressed if biodiversity scientists are to leverage the information contained within existing data to meet the challenges associated with conservation of species on a global scale.
Supplementary Material
Acknowledgements
We thank A. Morales, S. Hird, A. Espindola, G. Wheeler for comments on earlier drafts of this manuscript. Computational resources provided by Ohio Supercomputer Center.
Data accessibility
Scripts, data table (electronic supplementary material, appendix S1) and GenBank accessions (electronic supplementary material, appendix S2) are available on Dryad: DOI: http://dx.doi.org/10.5061/dryad.q1j20 [29].
Authors' contributions
All authors conceived the idea, wrote and approved the final manuscript. T.A.P. collected and analysed the data.
Competing interests
We have no competing interests.
Funding
This study was funded by Division of Environmental Biology (NSF DEB-60046038).
References
- 1.Wright S. 1943. Isolation by distance. Genetics 28, 114–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felsenstein J. 1976. The theoretical population genetics of variable selection and migration. Annu. Rev. Genet. 10, 253–280. ( 10.1146/annurev.ge.10.120176.001345) [DOI] [PubMed] [Google Scholar]
- 3.Sexton JP, Hangartner SB, Hoffmann AA. 2013. Genetic isolation by environment or distance: which pattern of gene flow is most common? Evolution 68, 1–15. ( 10.1111/evo.12258) [DOI] [PubMed] [Google Scholar]
- 4.Kuchta SR, Tan A-M. 2005. Isolation by distance and post-glacial range expansion in the rough-skinned newt, Taricha granulosa. Mol. Ecol. 14, 225–244. ( 10.1111/j.1365-294X.2004.02388.x) [DOI] [PubMed] [Google Scholar]
- 5.Frantz AC, Pope LC, Etherington TR, Wilson GJ, Burke T. 2010. Using isolation-by-distance-based approaches to assess the barrier effect of linear landscape elements on badger (Meles meles) dispersal. Mol. Ecol. 19, 1663–1674. ( 10.1111/j.1365-294X.2010.04605.x) [DOI] [PubMed] [Google Scholar]
- 6.Relethford J. 2004. Global patterns of isolation by distance based on genetic and morphological data. Hum. Biol. 76, 499–513. ( 10.1353/hub.2004.0060) [DOI] [PubMed] [Google Scholar]
- 7.Garrick RC, et al. 2015. The evolution of phylogeographic datasets. Mol. Ecol. 24, 1164–1171. ( 10.1111/mec.13108) [DOI] [PubMed] [Google Scholar]
- 8.Jenkins DG, et al. 2010. A meta-analysis of isolation by distance: relic or reference standard for landscape genetics? Ecography 33, 215–320. ( 10.1111/j.1600-0587.2010.06285.x) [DOI] [Google Scholar]
- 9.Shafer ABA, Wolf JBW. 2013. Widespread evidence for incipient ecological speciation: a meta-analysis of isolation-by-ecology. Ecol. Lett. 16, 940–950. ( 10.1111/ele.12120) [DOI] [PubMed] [Google Scholar]
- 10.Wang IJ, Glor RE, Losos JB. 2013. Quantifying the roles of ecology and geography in spatial genetic divergence . Ecol. Lett. 16, 175–182. ( 10.1111/ele.12025) [DOI] [PubMed] [Google Scholar]
- 11.R Development Core Team 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 12.Wang IJ. 2013. Examining the full effects of landscape heterogeneity on spatial genetic variation: a multiple matrix regression approach for quantifying geographic and ecological isolation. Evolution 67, 3403–3411. ( 10.1111/evo.12134) [DOI] [PubMed] [Google Scholar]
- 13.Louvain & ESA. GLOBCOVER. 2009. Université Catholique de Louvain & European Space Agency. http://due.esrin.esa.int/page_globcover.php.
- 14.Van Oudtshoorn KVR, Van Rooyen MW. 2013. Dispersal biology of desert plants. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 15.Breiman L. 2001. Random forests. Mach. Learn. 45, 5 ( 10.1023/A:1010933404324) [DOI] [Google Scholar]
- 16.Biau G. 2012. Analysis of a random forests model. J. Mach. Learn. Res. 13, 1063–1095. [Google Scholar]
- 17.Martin PR, McKay JK. 2003. Latitudinal variation in genetic divergence of populations and the potential for future speciation. Evolution 58, 938–945. ( 10.1111/j.0014-3820.2004.tb00428.x) [DOI] [PubMed] [Google Scholar]
- 18.Mori GM, Zucchi MI, Souza AP. 2015. Multiple-geographic-scale genetic structure of two mangrove tree species: the roles of mating system, hybridization, limited dispersal and extrinsic factors. PLoS ONE 10, e0118710 ( 10.1371/journal.pone.0118710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDevitt AD, Oliver MK, Piertney SB, Szafrańska PA, Konarzewski M, Zub K. 2013. Individual variation in dispersal associated with phenotype influences fine-scale genetic structure in weasels. Conserv. Genet. 14, 499–509. ( 10.1007/s10592-012-0376-4) [DOI] [Google Scholar]
- 20.Guarnizo CE, Amézqutia A, Bermingham E. 2009. The relative roles of vicariance versus elevational gradients in the genetic differentiation of the high Andean tree frog, Dendroposophus labialis. Mol. Phylogenet. Evol. 50, 84–92. ( 10.1016/j.ympev.2008.10.005) [DOI] [PubMed] [Google Scholar]
- 21.Miraldo A, et al. 2016. An anthropocene map of genetic diversity. Science 353, 1532–1535. ( 10.1126/science.aaf4381) [DOI] [PubMed] [Google Scholar]
- 22.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 23.Willig MR, Kaufman DM, Stevens RD. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309. ( 10.1146/annurev.ecolsys.34.012103.144032) [DOI] [Google Scholar]
- 24.Jablonski D, Roy K, Valentine JW. 2006. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106. ( 10.1126/science.1130880) [DOI] [PubMed] [Google Scholar]
- 25.Leffer EM, Bullaughey K, Matute DR, Meyer WK, Ségurel L, Venkat A, Andolfatto P, Przeworski M. 2012. Revisiting an old riddle: what determines genetic diversity levels within species? PLoS Biol. 10, e1001388 ( 10.1371/journal.pbio.1001388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson MN. 2014. Natural experiments and meta-analyses in comparative phylogeography. J. Biogeogr. 41, 52–65. ( 10.1111/jbi.12190) [DOI] [Google Scholar]
- 27.Soltis DE, Soltis PS. 2016. Mobilizing and integrating big data in studies of spatial and phylogenetic patterns of biodiversity. Plant Divers. 38, 264–270. ( 10.1016/j.pld.2016.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marques AC, Maronna MM, Collins AG. 2013. Putting GenBank data on the map. Science 341, 1341 ( 10.1126/science.341.6152.1341-a) [DOI] [PubMed] [Google Scholar]
- 29.Pelletier TA, Carstens BC. 2017. Data from: Geographic range size and latitude predict population genetic structure in a global survey Dryad Digital Repository. ( 10.5061/dryad.q1j20) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pelletier TA, Carstens BC. 2017. Data from: Geographic range size and latitude predict population genetic structure in a global survey Dryad Digital Repository. ( 10.5061/dryad.q1j20) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Scripts, data table (electronic supplementary material, appendix S1) and GenBank accessions (electronic supplementary material, appendix S2) are available on Dryad: DOI: http://dx.doi.org/10.5061/dryad.q1j20 [29].