Abstract
The last large marsupial carnivores—the Tasmanian devil (Sarcophilis harrisii) and thylacine (Thylacinus cynocephalus)—went extinct on mainland Australia during the mid-Holocene. Based on the youngest fossil dates (approx. 3500 years before present, BP), these extinctions are often considered synchronous and driven by a common cause. However, many published devil dates have recently been rejected as unreliable, shifting the youngest mainland fossil age to 25 500 years BP and challenging the synchronous-extinction hypothesis. Here we provide 24 and 20 new ages for devils and thylacines, respectively, and collate existing, reliable radiocarbon dates by quality-filtering available records. We use this new dataset to estimate an extinction time for both species by applying the Gaussian-resampled, inverse-weighted McInerney (GRIWM) method. Our new data and analysis definitively support the synchronous-extinction hypothesis, estimating that the mainland devil and thylacine extinctions occurred between 3179 and 3227 years BP.
Keywords: extinction, AMS dating, thylacine, devil, Holocene
1. Background
During the Late Pleistocene, Tasmanian devils (Sarcophilus harrisii) and thylacines (Tasmanian tiger or wolf, Thylacinus cynocephalus) were widespread over the Australian continent [1,2]. Both species subsequently became extinct on mainland Australia, only surviving into historical times on the island of Tasmania. The thylacine was hunted to extinction after European arrival [3], while devils have suffered declines of more than 80% in less than 20 years, largely due to a transmissible cancer [4]. Based on the youngest dated fossils available, both species are assumed to have become extinct on mainland Australia during the mid-Holocene (approx. 3500 years before present, BP) [5].
The cause of these extinctions is the subject of debate, with introduced dingoes, human intensification and climate change being the three main competing, but not necessarily mutually exclusive, hypotheses [3]. Debate around extinction drivers has almost always assumed that both extinctions were roughly synchronous and therefore, potentially attributable to a common cause (or set of causes). However, the reliability of many fossil ages for devils across Australia has recently been questioned [6], shifting the mainland devil's youngest reliable fossil age back to 25 500 years BP and challenging the synchronous-extinction hypothesis.
The youngest fossil age of an extinct taxon is nearly always an inaccurate proxy for the final extinction date. These two dates will inevitably diverge due to incomplete sampling, taphonomic bias and uncertainty in radiometric dating [7,8]. Many statistical models have been developed to estimate extinction time (and the associated uncertainty) using the time series of fossil ages, but their accuracy varies with the mode of extinction and sampling density over time [5].
To address these issues, we combined 44 new, high-quality ages for mainland devils and thylacines with existing data that met stringent quality requirements. We applied the Gaussian-resampled, inverse-weighted McInerney (GRIWM [9]) method, which incorporates both sampling density and dating errors to calculate the confidence bounds of the mainland extinction dates for both species.
2. Material and methods
We collected less than 1 g of bone or tooth from previously undated thylacine (n = 20) and devil (n = 24) fossils from southern mainland Australia (electronic supplementary material, figure S1 and table S1) in existing museum palaeontology and mammal collections. Samples were pre-treated using ultrafiltration [10] and the collagen fraction radiocarbon-dated at the Australian National University, the University of Waikato or the Oxford Radiocarbon Accelerator Unit. We added these new ages to 129 existing mainland devil and 104 mainland thylacine museum records extracted from the FosSahul database (doi:10.4227/05/564E6209C4FE8) [11]. We removed all unreliable ages using a set of objective criteria based on the reliability of the dating procedure used, followed by an evaluation of the confidence in the stratigraphic relationship of the dated material to the target taxon (for full details, see [6]). We calibrated all dates to calendar years (BP) using the Southern Hemisphere Calibration curve (ShCal13) from the OxCal radiocarbon calibration tool v. 4.2 (https://c14.arch.ox.ac.uk). As there is uncertainty about whether Sarcophilus laniarius was a separate, co-occurring species to Sarcophilus harrisii, or the same lineage that experienced dwarfism during the Pleistocene [12], we repeated our analyses excluding S. laniarius ages.
To estimate the mainland extinction date for these species, we followed a general guide (model-selection key in Saltré et al. [5]) for choosing the most appropriate model among the five most commonly used frequentist approaches (i.e. Solow's [7], Marshall's [13], McCarthy's [14], McInerny's [15] and GRIWM [9]) for a given series of dated fossils. Identifying the appropriateness of the models depends largely on how they treat both the probability of record occurrence and the uncertainties in record dates. GRIWM was the most appropriate method as a function of the statistical characteristics of the dataset [5] (see full analysis in electronic supplementary information and table S2). In addition to GRIWM [9], and following the same approach as Lima-Ribeiro & Diniz-Filho [16], we used the four other, well-evaluated methods regardless of the nature of sampling effort over time [17]. As these methods make different assumptions about the type of extinction (e.g. sudden versus gradual), they estimate a complete range of potential true extinction dates (see both full description and results of these approaches in the electronic supplementary material).
3. Results and discussion
The 44 new radiocarbon ages for mainland devils and thylacines (electronic supplementary material, table S1) include the youngest, reliably dated samples for each species (devil: 3245 ± 62 years BP and thylacine: 3290 ± 49 years BP). All our new ages passed quality filtering, but only 31 of the 129 previous devil (24.0%) and 27 of 104 thylacine (26.0%) ages in the FosSahul database [11] met the minimum reliability criteria (A*- or A-rated only) [11]. Adding these records to the new dates produced final, high-quality datasets of 56 devil and 48 thylacine ages (figure 1). Excluding S. laniarius from the devil dataset left 45 reliable ages.
Figure 1.
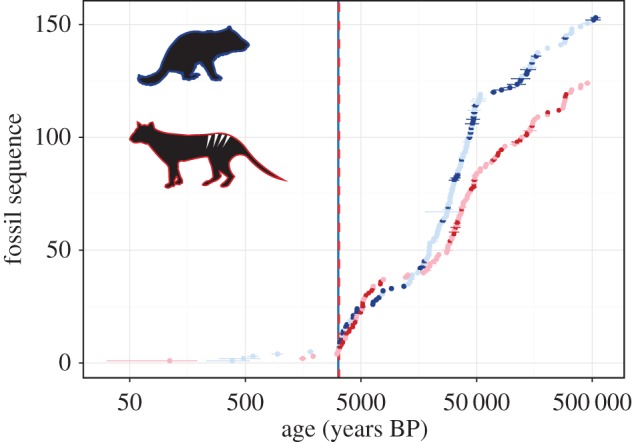
Time series of new and previously published ages of Tasmanian devil (genus Sarcophilus: blue) and thylacine (genus Thylacinus: red) fossils from mainland Australia, showing the temporal sequence of fossils from youngest to oldest (y-axis) against logarithmic ages ±1 s.d. (x-axis). Ages that passed quality filtering and were used to estimate extinction time are shown in dark colours, while ages that did not pass quality filtering are shown in light colours. Vertical bars represent the estimated extinction time for each species (devils: blue/solid, thylacines: red/dashed) as produced using the GRIWM approach.
The continent-wide GRIWM approach produced mainland extinction estimates (Text) of 3179 years BP (95% CI: 3131–3224) for devils, and 3227 years BP (CI: 3170–3281) for thylacines (figure 1). Removing S. laniarius barely modified the estimate for devils (ΔText = 6 years). These results are supported by the outputs of the other statistical methods (electronic supplementary material, table S3). Our youngest reliable age for mainland devils contrasts the most recent estimate based on high-quality ages at 25 500 years BP [6]. As such, the addition of 24 new dates changes the reliable persistence timeline for this species by approximately 22 000 years.
Younger dates assessed by Rodríguez-Rey et al. [6] were mostly rejected based on inappropriate pre-treatment protocols and/or unsuitable materials used, highlighting the importance of choice in dating method. For example, all ages of bone and dentin collagen processed using ultrafiltration, ninhydrin or XAD-2 protocols or ages on individual amino acids are considered highly reliable, whereas decalcified bone or tooth dated with no information about collagen presence or those presenting collagen purification issues were discarded (see the detailed application of dating criteria for radiocarbon ages of vertebrate fossils in electronic supplementary material, table S4). Regarding ages of fossils that were not dated directly, but based on association, the strength of this association (i.e. the stratigraphic relationship between the fossil of a target species and the dated remains) was tested. Dates with no stratigraphic control or any lack of stratigraphic integrity that affects the depositional context of the target species led to rejection of that date (for more detail, see [6]).
Our results constrain the reliable dates of mainland devil and thylacine extinction to within a short (less than 50 years) period, between 3179 and 3227 years BP, which is consistent with a scenario of synchronous extinction. Synchronous extinctions have been proposed on other continents and at different time points as evidence for large-scale, common extinction drivers [18–20]. For example, analysis of the extinction chronology in North America's Pleistocene mammals suggested that a single event wiped out up to 35 genera across the continent over a 2000-year period [21]. Extending this concept to derive the most likely cause of extinctions, Cooper et al. [19] examined multiple waves of synchronous extinctions and biotic transitions across the Holarctic and found them coincident with climate warming events that likely exacerbated declines arising from human hunting. Conversely, the concurrent extinction of Australian megafauna during the Pleistocene seems to be independent of continental-scale climate change, potentially indicating a dominant human role [18,22].
Under the assumption that the mainland devil and thylacine extinctions were coincident, several studies have explored possible causes. For example, as the dingo arrived in Australia approximately 4000 years BP and never reached Tasmania, dingoes have been suspected of driving the mainland extinctions of devils and thylacines [23]. Johnson & Wroe [24] suggested that human innovation in hunting technology and more intensive use of resources could also have led to the mainland extinctions. Prowse et al. [3] used a modelling approach to conclude that human intensification, followed by climate change, were the most likley candidate determinants.
Other studies have avoided the assumption of synchronous extinctions by focusing on devils or thylacines separately. Letnic et al. [25] used morphological analyses to conclude that direct killing of thylacines by dingoes was biologically feasible and could therefore have contributed to their demise. Similarly, Brown [26] argued that climate instability associated with the onset of El Niño–Southern Oscillation (ENSO) events could have affected mainland devils, but not thylacines.
Our estimated extinction window for mainland devils and thylacines is similar to the assumed, but until now unvalidated, extinction time in most previous studies, and therefore does not challenge any aforementioned arguments, nor do our results exclude the possibility of separate or multiple causes of these extinctions. However, by supporting the assumption of synchronous extinctions with reliably dated fossil specimens, and taking into account the notion that the youngest fossil age is an inaccurate proxy for the true extinction time, our analyses provide a strong and defendable basis on which further research can build. Our understanding of these extinctions will become more complete as more palaeoclimatic, palaeoecological and archaeological data are used to uncover the biogeographic histories of these species.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Western Australian Museum, Museum Victoria, the Australian National Wildlife Collection (CSIRO) and M. Bunce (Curtin University) for granting permission to sample subfossil devil and thylacine specimens. We are grateful to E. Bunney for providing the quality-rating information in electronic supplementary material, table S1. We also thank members of the Australian Centre for Ancient DNA for constructive comments on earlier drafts of this manuscript.
Data accessibility
All new radiocarbon dates are available from the AEKOS Data Portal (doi:10.4227/05/57BA9BE3ABC2A) and have been added to the FosSahul database (doi:10.4227/05/564E6209C4FE8).
Authors' contributions
L.C.W. helped design the study, acquired and analysed the data and drafted the manuscript; F.S. helped design the study, analysed the data and drafted the manuscript; C.J.A.B. helped design the study, interpreted the results and helped revise the manuscript; J.J.A. helped design the study, collected the samples, interpreted the results and helped revise the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This research was supported by Australian Research Council (ARC) Discovery Projects DP130104055 and DP130103842, and ARC Future Fellowships FT100100108 and FT110100306 to J.J.A. and C.J.A.B., respectively.
References
- 1.Dixon JM. 1989. Thylacinidae. In Fauna of Australia (eds Walton DW, Richardson BJ), pp. 549–559. Canberra, Australia: Australian Government Publishing Service. [Google Scholar]
- 2.Morton SR, Dickman CR, Fletcher TP. 1989. Dasyuridae. In Fauna of Australia (eds Walton DW, Richardson BJ), pp. 560–582. Canberra, Australia: Australian Government Publishing Service. [Google Scholar]
- 3.Prowse TAA, Johnson CN, Bradshaw CJA, Brook BW. 2014. An ecological regime shift resulting from disrupted predator–prey interactions in holocene Australia. Ecology 95, 693–702. ( 10.1890/13-0746.1) [DOI] [PubMed] [Google Scholar]
- 4.Epstein B, et al. 2016. Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nat. Commun. 7, 12684 ( 10.1038/ncomms12684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saltré F, Brook BW, Rodriguez-Rey M, Cooper A, Johnson CN, Turney CSM, Bradshaw CJA. 2015. Uncertainties in dating constrain model choice for inferring extinction time from fossil records. Quat. Sci. Rev. 112, 128–137. ( 10.1016/j.quascirev.2015.01.022) [DOI] [Google Scholar]
- 6.Rodríguez-Rey M, et al. 2015. Criteria for assessing the quality of Middle Pleistocene to Holocene vertebrate fossil ages. Quat. Geochronol. 30, 69–79. ( 10.1016/j.quageo.2015.08.002) [DOI] [Google Scholar]
- 7.Solow AR, Roberts DL, Robbirt KM. 2006. On the Pleistocene extinctions of Alaskan mammoths and horses. Proc. Natl Acad. Sci. USA 103, 7351–7353. ( 10.1073/pnas.0509480103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Signor PM, Lipps JH. 1982. Sampling bias, gradual extinction patterns, and catastrophes in the fossil record. In Geological implications of impacts of large asteroids and comets on the earth (eds Silver LT, Schultz PH), pp. 291–296. Boulder, CO: Geological Society of America. [Google Scholar]
- 9.Bradshaw CJA, Cooper A, Turney CSM, Brook CSM. 2012. Robust estimates of extinction time in the geological record. Quat. Sci. Rev. 33, 14–19. ( 10.1016/j.quascirev.2011.11.021) [DOI] [Google Scholar]
- 10.Brock F, Higham T, Ditchfield P, Ramsey C. 2010. Current pretreatment methods for AMS radiocarbon dating at the Oxford radiocarbon accelerator unit (Orau). Radiocarbon 52, 103–112. ( 10.1017/S0033822200045069) [DOI] [Google Scholar]
- 11.Rodríguez-Rey M, et al. 2016. A comprehensive database of quality-rated fossil ages for Sahul's Quaternary vertebrates. Sci. Data 3, 160053 ( 10.1038/sdata.2016.53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiler ER. 1992. The Tasmanian devil. Hobart, Australia: St. David's Park Publishing. [Google Scholar]
- 13.Marshall CR. 1997. Confidence intervals on stratigraphic ranges with nonrandom distributions of fossil horizons. Paleobiology 23, 165–173. ( 10.2307/2401050) [DOI] [Google Scholar]
- 14.McCarthy MA. 1998. Identifying declining and threatened species with museum data. Biol. Conserv. 83, 9–17. ( 10.1016/S0006-3207(97)00048-7) [DOI] [Google Scholar]
- 15.McInerny GJ, Roberts DL, Davy AJ, Cribb PJ. 2006. Significance of sighting rate in inferring extinction and threat. Conserv. Biol. 20, 562–567. ( 10.1111/j.1523-1739.2006.00377.x) [DOI] [PubMed] [Google Scholar]
- 16.Lima-Ribeiro MS, Diniz-Filho JAF. 2014. Obstinate overkill in Tasmania? The closest gaps do not probabilistically support human involvement in megafaunal extinctions. Earth-Sci. Rev. 135, 59–64. ( 10.1016/j.earscirev.2014.04.005) [DOI] [Google Scholar]
- 17.Rivadeneira MM, Hunt G, Roy K. 2009. The use of sighting records to infer species extinctions: an evaluation of different methods. Ecology 90, 1291–1300. ( 10.1890/08-0316.1) [DOI] [PubMed] [Google Scholar]
- 18.Saltré F, et al. 2016. Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat. Commun. 7, 10511 ( 10.1038/ncomms10511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper A, Turney C, Hughen KA, Brook BW, McDonald HG, Bradshaw CJA. 2015. Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science 349, 602–606. ( 10.1126/science.aac4315) [DOI] [PubMed] [Google Scholar]
- 20.Metcalf JL, et al. 2016. Synergistic roles of climate warming and human occupation in Patagonian megafaunal extinctions during the last deglaciation. Sci. Adv. 2, e1501682 ( 10.1126/sciadv.1501682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faith JT, Surovell TA. 2009. Synchronous extinction of North America's Pleistocene mammals. Proc. Natl Acad. Sci. USA 106, 20 641–20 645. ( 10.1073/pnas.0908153106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson CN, et al. 2016. What caused extinction of the Pleistocene megafauna of Sahul? Proc. R. Soc. B 283, 52399 ( 10.1098/rspb.2015.2399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbett L. 1995. The dingo in Australia and Asia. Sydney, Australia: University of New South Wales Press. [Google Scholar]
- 24.Johnson CN, Wroe S. 2003. Causes of extinction of vertebrates during the Holocene of mainland Australia: arrival of the dingo, or human impact? Holocene 13, 941–948. ( 10.1191/0959683603hl682fa) [DOI] [Google Scholar]
- 25.Letnic M, Fillios M, Crowther MS. 2012. Could direct killing by larger dingoes have caused the extinction of the thylacine from mainland Australia? PLoS ONE 7, e34877 ( 10.1371/journal.pone.0034877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown O.J.F. 2005. Tasmanian devil (Sarcophilus harrisii) extinction on the Australian mainland in the mid-Holocene: multicausality and ENSO intensification. Alcheringa 30, 49–57. ( 10.1080/03115510609506855) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All new radiocarbon dates are available from the AEKOS Data Portal (doi:10.4227/05/57BA9BE3ABC2A) and have been added to the FosSahul database (doi:10.4227/05/564E6209C4FE8).


