Abstract
Persistent phenotypic changes due to early-life stressors are widely acknowledged, but their relevance for wild, free-living animals is poorly understood. We evaluated effects of two natural stressors experienced when young (maltreatment by adults and nutritional stress) on stress physiology in wild Nazca boobies (Sula granti) 6–8 years later, an exceptionally long interval for such studies. Maltreatment as a nestling, but not nutritional stress, was associated years later with depressed baseline corticosterone in females and elevated stress-induced corticosterone concentration [CORT] in males. These results provide rare evidence of long-term hormonal effects of natural early-life stress, which may be adaptive mechanisms for dealing with future stressors.
Keywords: organizational effects, growth rate, abuse
1. Introduction
Early-life stress during critical periods of development can have important and persistent ‘programming effects' on the neural and endocrine systems [1], often through the actions of glucocorticoid hormones released by the hypothalamic–pituitary–adrenal (HPA) axis [1]. Captive animal and human studies generally suggest that early-life stress programmes a hyperactive response, depending on the timing/type of stressor [1,2]. However, the persistence of stress-induced programming effects for wild species remains largely unknown. Some degree of persistence is required for either adaptive (e.g. forecasting future environment) or non-adaptive explanations (e.g. based on constraint) of observed programming effects [3]. While captive animal studies have revealed stress-induced programming effects in controlled environments, the few long-term studies conducted in the wild have produced mixed results [4].
A seabird, the Nazca booby (Sula granti), offers a promising system to evaluate persistence of programming effects in the wild because their nestlings experience early-life stress in the form of maltreatment, which correlates with later-life maltreatment behaviour as adults [5]. Adult non-breeding Nazca boobies often approach unguarded nestlings and engage in aggressive, sexual and ‘affiliative’ maltreatment ([6]; electronic supplementary material, Video S1). Typical maltreatment episodes involve one non-parental adult visitor (NAV) ([6]; electronic supplementary material, figure S1). Most (84%) nestlings are victims of at least one NAV event [6], during which nestling corticosterone concentration [CORT] is upregulated approximately fivefold, remaining elevated for up to 23 h [7].
Nazca booby nestlings also vary substantially in growth rate due to nutritional stress, typical of single-chick pelagic seabirds [8]. Low food intake slows growth, maintains lower mass throughout the nestling period [8] and is a strong negative predictor of juvenile survival in Nazca boobies [9]. Nutritional stress raises circulating glucocorticoids in most vertebrates and other seabirds [10,11], thus providing opportunity for programming effects to occur.
Here, we ask whether natural early-life stress is correlated with long-term changes in circulating glucocorticoids in free-living Nazca boobies into adulthood (6–8 years), a duration of life experience rarely examined in this context [4]. We monitored nestling exposure to two early-life stressors: maltreatment (NAV victimization) and nutritional stress (growth rate). Years later, we located these individuals to measure the CORT stress response and evaluate the relative and combined explanatory ability of these stressors.
2. Material and methods
Nazca boobies tolerate human proximity on Isla Española, Galápagos (1.39° S, 89.62° W) [8]. Adults (life expectancy 14 years; [12]) were identified by numbered metal leg bands. Age at which only 1% of downy plumage remains (a proxy for growth period; [8]) and NAV interactions were recorded for all nestlings during three breeding seasons (2001–2003; [5]). Nests were patrolled by one to two observers from 13.00 to 17.00 h, when the majority of NAV interactions occur [6], from January to March, when nestlings are at ages attractive to NAVs [6]. NAV interactions were observed easily in the open colony (electronic supplementary material, video S1).
In March 2009, we conducted standardized capture–restraint tests [13] on 87 of these birds as adults (6–8 years old; median = 6). We obtained blood within 3 min of initial disturbance (1 ml), then at 10, 25 and 40 min post-capture (400 µl), between 02.30 and 06.00 h, when circulating [CORT] is least affected by external stimuli [14]. Mass and ulna length were measured at the end of the test. Total [CORT] was measured by quantitative competitive enzyme immunoassay (see electronic supplementary material, S1 for additional methods).
We evaluated [CORT] via linear mixed modelling with a continuous autoregressive covariance structure and a random intercept for each bird ID in R v. 3.3.1 (package ‘nlme’; [15]). Growth rate was calculated as days from hatching to 1% down plumage, and NAV victimization was the total count of NAV events experienced during the observation period. time between disturbance and the first blood sample (disturbance–sample time) was strongly correlated with NAV victimization (r = −0.34, p < 0.001); thus, we used the residuals of disturbance–sample time regressed on NAV victimization thereafter (residuals disturbance–sample time, RDST). [CORT] was natural-log-transformed to correct minor deviations from normality. NAV victimization, growth rate, and sampling date were z-scored. The global model included the predictors NAV victimization, growth rate, sex, scaled mass index (Mi, a measure of body condition; [16]), sampling date, start time (24 h time at initial sample), RDST, time series (one to four; sequence of samples) and (time Series)2. Second- and third-order interactions were allowed, except those involving sampling date and (time series)2. Models were evaluated by Akaike's Information Criterion (AICc) model comparison (R package ‘MuMIn’; [17]).
3. Results
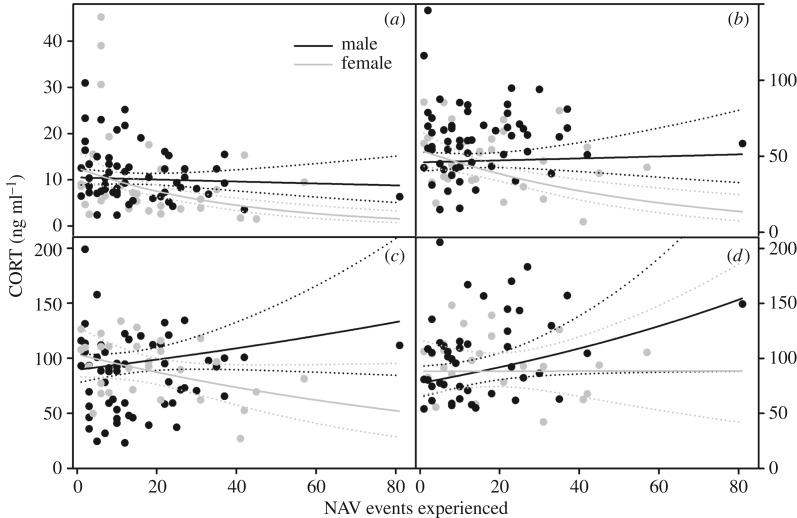
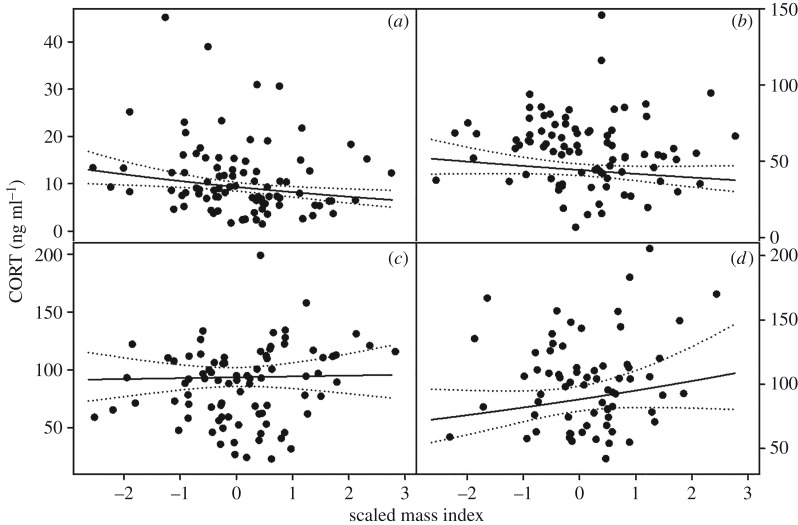
Time series, sex and RDST moderated the effect of NAV victimization on [CORT] (the interaction between sex, time series, and NAV victimization was included in our top model by AICc selection). [CORT] decreased with NAV victimization early in tests (table 1; electronic supplementary material, S2), especially for females (figure 1a). However, 40 min after capture, this effect reversed, such that stress-induced [CORT] increased with NAV victimization, especially for males (figure 1d). Females had lower [CORT] than males at all time points (figure 1). [CORT] decreased with scaled mass index initially, and this effect reversed across time series (figure 2). RDST had a slight moderating effect on the relationship between NAV victimization and [CORT] (electronic supplementary material, S2). Growth rate was not in the top model, although it was within 4 AICc from the top model. Age did not predict [CORT] (ΔAICc = 10.09) (see electronic supplementary material, S2 for model rankings, model-averaged coefficients and [CORT] time courses).
Table 1.
β-coefficients, associated 95% confidence intervals (95% CI), t-values and p-values of variables in the top model predicting [CORT] over a 40-min period (marginal r2 = 0.81, n = 329, 87 individuals). ‘×’ indicates an interaction.
| predictor | β | ±95% CI | t-value | p-value |
|---|---|---|---|---|
| time series | 2.748 | 0.211 | 25.467 | <0.001 |
| (time series)2 | −0.405 | 0.042 | −18.910 | <0.001 |
| scaled mass index | −0.177 | 0.123 | −2.829 | 0.006 |
| RDST | 0.832 | 0.604 | 2.701 | 0.009 |
| NAV victimization | −0.124 | 0.156 | −1.562 | 0.122 |
| sex (female) | −0.260 | 0.262 | −1.939 | 0.056 |
| sampling date | 0.079 | 0.075 | 2.064 | 0.042 |
| scaled mass index × time series | 0.063 | 0.042 | 2.902 | 0.004 |
| RDST × time series | −0.261 | 0.207 | −2.467 | 0.014 |
| sex × time series | 0.061 | 0.087 | 1.380 | 0.169 |
| NAV victimization × sex | −0.409 | 0.279 | −2.872 | 0.005 |
| NAV victimization × time series | 0.064 | 0.050 | 2.484 | 0.014 |
| NAV victimization × RDST | −1.370 | 0.730 | −3.680 | <0.001 |
| NAV victimization × time series × sex | 0.071 | 0.091 | 1.533 | 0.127 |
| NAV victimization × RDST × time series | 0.426 | 0.247 | 3.381 | 0.001 |
Figure 1.
Sex-specific relationship between NAV victimization and [CORT] (n = 57 males, 30 females) at (a) 0–3, (b) 10, (c) 25 and (d) 40 min post-disturbance. Dots represent individuals, solid lines are regression lines and dotted lines are 95% confidence intervals. Removal of the male with the highest NAV events experienced did not change our results.
Figure 2.
Relationship between Scaled Mass Index and [CORT] (n = 87) at (a) 0–3, (b) 10, (c) 25 and (d) 40 min post-disturbance. Dots represent individuals, solid lines are regression lines and dotted lines are 95% confidence intervals.
4. Discussion
Wild Nazca boobies exhibited sex-specific correlations between adult [CORT] and post-natal stress exposure, providing rare evidence of long-term hormonal effects of natural early-life stress in a free-living vertebrate. Maltreatment experience was correlated with depressed baseline [CORT] (especially for females) and elevated stress-induced [CORT] (especially for males), with females exhibiting lower [CORT] than males. The effect of early-life stress on later-life baseline [CORT] in other vertebrates is highly variable, both between and within species, and depends on timing and type of stressor [18,19]. However, our results regarding stress-induced [CORT] correspond to an emerging, but not universal, vertebrate trend of HPA-axis hypersensitivity following post-natal stress [18,19]. Sex-dependence of these long-term effects is also observed (e.g. [20]) and suggests interaction between the HPA and hypothalamic–pituitary–gonadal (HPG) axes. Effects of [CORT] dynamics on fitness depend on ecological, social and individual conditions [19,21], and further research would determine if our observed correlations are adaptive in this species.
Growth rate by itself or interacting with maltreatment was not strongly correlated with [CORT] dynamics. However, we did find correlations between current body condition and [CORT] in adulthood, suggesting that current nutritional stress affects the Nazca booby stress response more strongly than early-life nutritional stress. Boobies with low body condition had increased baseline [CORT], corresponding to findings for other seabirds during nutritional stress [22]. We also detected a positive relationship between body condition and stress-induced [CORT], perhaps due to energetic constraints of mounting a [CORT] stress response.
Our findings regarding early-life nutritional stress contrast with results from other altricial birds [19], but not precocial Japanese quail (Coturnix japonica) [23]. In most altricial species, perinatal food deprivation is associated with sibling competition, whereas brood reduction to one nestling occurs before food is limiting in obligately siblicidal Nazca boobies [24]. Within this context, sibling competition (and involvement of the HPG axis) may be the stronger cue for long-term HPA-axis programming than early-life nutritional stress alone [25]. Alternatively, early-life nutritional stress may not be a stressor capable of inducing organizational effects in boobies, or we may be unable to detect effects due to reduced juvenile survival following nutritional stress [9]. Regardless, our results indicate that maltreatment affects adult CORT stress physiology more strongly than early-life nutritional stress.
The unexpected strong negative correlation between NAV victimization and time between disturbance and sampling suggest that early-life maltreatment also affects behaviour, a hypothesis supported by correlations between early-life maltreatment experience and later-life maltreatment behaviour in this species [5]. Longer times to sample resulted from stronger resistance to handling, which is highly repeatable in other species [26,27]. Maltreatment thus appears to be correlated with both hormonal and behavioural response to later-life stress, although further research is needed.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Galápagos National Park, Charles Darwin Research Station and TAME Airline; M. Müller and E. Porter for NAV histories; four anonymous reviewers, E. Tompkins, T. Maness, F. Estela, N. Huffeldt and M. Fuxjager for comments.
Ethics
This study was permitted under Wake Forest University IACUC (protocol A08-029) and Galápagos National Park, and adheres to NIH and the Ornithological Council's standards for animal use in research.
Data accessibility
Raw data available via OAKTrust (http://hdl.handle.net/1969.1/165171) [28].
Author contributions
J.K.G. designed the project, collected data on adult birds and conducted analyses. D.J.A. secured funding and maintained long-term datasets. J.K.G. wrote and D.J.A. edited the manuscript. Both gave their final approval and are accountable for its content.
Competing interests
The authors have no competing interests.
Funding
This work was supported by a Wake Forest University Science Research Fund award and NSF grants DEB 98-06606, DEB 0235818 and DEB 0842199 to D.J.A. and an NSF GRF to J.K.G.
References
- 1.Seckl JR. 2004. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 151, U49–U62. ( 10.1530/eje.0.151U049) [DOI] [PubMed] [Google Scholar]
- 2.De Bellis MD. 2001. Developmental traumatology: the psychobiological development of maltreated children and its implications for research, treatment, and policy. Dev. Psychopathol. 13, 539–564. ( 10.1017/S0954579401003078) [DOI] [PubMed] [Google Scholar]
- 3.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645. ( 10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond H, Ancona S. 2015. Observational field studies reveal wild birds responding to early-life stresses with resilience, plasticity, and intergenerational effects. Auk 132, 563–576. ( 10.1642/AUK-14-244.1) [DOI] [Google Scholar]
- 5.Müller MS, Porter ET, Grace JK, Awkerman JA, Birchler KT, Gunderson AR, Schneider EG, Westbrock MA, Anderson DJ. 2011. Maltreated nestlings exhibit correlated maltreament as adults: evidence of a ‘cycle of violence’ in Nazca boobies (Sula granti). Auk 128, 1–5. ( 10.1525/auk.2011.11008) [DOI] [Google Scholar]
- 6.Anderson DJ, Porter ET, Ferree ED. 2004. Non-breeding Nazca boobies (Sula granti) show social and sexual interest in chicks: behavioural and ecological aspects. Behaviour 141, 959–977. ( 10.1163/1568539042360134) [DOI] [Google Scholar]
- 7.Grace JK, Dean K, Ottinger MA, Anderson DJ. 2011. Hormonal effects of maltreatment in Nazca booby nestlings: implications for the ‘cycle of violence’. Horm. Behav. 60, 78–85. ( 10.1016/j.yhbeh.2011.03.007) [DOI] [PubMed] [Google Scholar]
- 8.Apanius V, Westbrock MA, Anderson DJ. 2008. Reproduction and immune homeostasis in a long-lived seabird, the Nazca booby (Sula granti). Ornithol. Monogr. 65, 1–46. ( 10.1525/om.2008.65.1.1) [DOI] [Google Scholar]
- 9.Maness TJ, Anderson DJ. 2013. Predictors of juvenile survival in birds. Ornithol. Monogr. 2013, 1–55. ( 10.1525/om.2013.78.1.1.1) [DOI] [Google Scholar]
- 10.Kitaysky AS, Piatt JF, Wingfield JC, Romano M. 1999. The adrenocortical stress-response of Black-legged Kittiwake chicks in relation to dietary restrictions. J. Comp. Physiol. B 169, 303–310. ( 10.1007/s003600050225) [DOI] [Google Scholar]
- 11.Pravosudov VV, Kitaysky AS. 2006. Effects of nutritional restrictions during post-hatching development on adrenocortical function in western scrub-jays (Aphelocoma californica). Gen. Comp. Endocrinol. 145, 25–31. ( 10.1016/j.ygcen.2005.06.011) [DOI] [PubMed] [Google Scholar]
- 12.Tompkins EM, Townsend HM, Anderson DJ. 2017. Decadal-scale variation in diet forecasts persistently poor breeding under ocean warming in a tropical seabird. PLoS ONE 12, e0182545 ( 10.1371/journal.pone.0182545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grace JK, Anderson DJ. 2014. Corticosterone stress response shows long-term repeatability and links to personality in free-living Nazca boobies. Gen. Comp. Endocrinol. 208, 39–48. ( 10.1016/j.ygcen.2014.08.020) [DOI] [PubMed] [Google Scholar]
- 14.Tarlow EM, Hau M, Anderson DJ, Wikelski M. 2003. Diel changes in plasma melatonin and corticosterone concentrations in tropical Nazca boobies (Sula granti) in relation to moon phase and age. Gen. Comp. Endocrinol. 133, 297–304. ( 10.1016/S0016-6480(03)00192-8) [DOI] [PubMed] [Google Scholar]
- 15.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team 2017. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-131. https://CRAN.R-project.org/package=nlme
- 16.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 17.Barton K.2015. MuMIn: Multi-Model Inference. See http://cran.r-project.org/package=MuMIn .
- 18.Welberg LAM, Seckl JR. 2001. Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 13, 113–128. ( 10.1111/j.1365-2826.2001.00601.x) [DOI] [PubMed] [Google Scholar]
- 19.Schoech SJ, Rensel MA, Heiss RS. 2011. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr. Zool. 57, 514–530. ( 10.1093/czoolo/57.4.514) [DOI] [Google Scholar]
- 20.Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KCF. 2006. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender-dependent effects. Brain Res. 1097, 123–132. ( 10.1016/j.brainres.2006.04.066) [DOI] [PubMed] [Google Scholar]
- 21.Bonier F, Martin PR, Moore IT, Wingfield JC. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642. ( 10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 22.Kitaysky A, Piatt J, Wingfield J. 2007. Stress hormones link food availability and population processes in seabirds. Mar. Ecol. Prog. Ser. 352, 245–258. ( 10.3354/meps07074) [DOI] [Google Scholar]
- 23.Zimmer C, Boogert NJ, Spencer KA. 2013. Developmental programming: cumulative effects of increased pre-hatching corticosterone levels and post-hatching unpredictable food availability on physiology and behaviour in adulthood. Horm. Behav. 64, 494–500. ( 10.1016/j.yhbeh.2013.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson DJ. 1989. The role of hatching asynchrony in siblicidal brood reduction of two booby species. Behav. Ecol. Sociobiol. 25, 363–368. ( 10.1007/BF00302994) [DOI] [Google Scholar]
- 25.Carere C, Drent P, Koolhaas J, Groothuis T. 2005. Epigenetic effects on personality traits: early food provisioning and sibling competition. Behaviour 142, 1329–1355. ( 10.1163/156853905774539328) [DOI] [Google Scholar]
- 26.Kralj-Fiser S, Scheiber IBR, Blejec A, Moestl E, Kotrschal K. 2007. Individualities in a flock of free-roaming greylag geese: behavioral and physiological consistency over time and across situations. Horm. Behav. 51, 239–248. ( 10.1016/j.yhbeh.2006.10.006) [DOI] [PubMed] [Google Scholar]
- 27.Réale D, Gallant B, Leblanc M, Festa-Bianchet M. 2000. Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim. Behav. 60, 589–597. ( 10.1006/anbe.2000.1530) [DOI] [PubMed] [Google Scholar]
- 28.Grace JK, Anderson DJ.2018. Data for ‘Early-life maltreatment predicts adult stress response in a long-lived wild bird’ (Biology Letters) OAKTrust. see http://hdl.handle.net/1969.1/165171 . [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Grace JK, Anderson DJ.2018. Data for ‘Early-life maltreatment predicts adult stress response in a long-lived wild bird’ (Biology Letters) OAKTrust. see http://hdl.handle.net/1969.1/165171 . [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data available via OAKTrust (http://hdl.handle.net/1969.1/165171) [28].