Abstract
Background
Cachectic patients with chronic obstructive pulmonary disease (COPD) may benefit from nutritional support. This double‐blind, randomized, controlled trial evaluated the safety and efficacy of targeted medical nutrition (TMN) vs. an isocaloric comparator in pre‐cachectic and cachectic patients with COPD.
Methods
Patients aged ≥50 years with moderate‐to‐severe COPD and involuntary weight loss or low body mass index (16–18 kg/m2) were randomized 1:1 to receive TMN (~230 kcal; 2 g omega‐3 fatty acids; 10 μg 25‐hydroxy‐vitamin D3) or isocaloric comparator twice daily for 12 weeks (ClinicalTrials.gov Identifier: NCT02442908). Primary safety endpoints comprised adverse events and changes in vital signs, laboratory parameters, and concomitant medications. Secondary efficacy endpoints included changes in weight, body composition, exercise tolerance, metabolic biomarkers, and systemic inflammation.
Results
Forty‐five patients were randomized to receive TMN (n = 22; mean 69.2 years) or isocaloric comparator (n = 23; mean 69.7 years). TMN was well tolerated. Adverse events were similar in number and type in both groups. Compliance to both products was good (TMN, 79%; comparator, 77%). Both groups gained weight, but the TMN group gained comparatively more fat mass (P = 0.0013). Reductions in systolic blood pressure (P = 0.0418) and secondary endpoints of triglycerides (P = 0.0217) and exercise‐induced fatigue (P = 0.0223) and dyspnoea (P = 0.0382), and increases in high‐density lipoprotein cholesterol (P = 0.0254), were observed in the TMN vs. the comparator group by week 12.
Conclusions
Targeted medical nutrition containing high‐dose omega‐3 fatty acids, vitamin D, and high‐quality protein is well tolerated with a good safety profile and has positive effects on blood pressure and blood lipids and on exercise‐induced fatigue and dyspnoea. Therefore, this TMN could be clinically beneficial in the nutritional and metabolic support of pre‐cachectic and cachectic patients with COPD.
Keywords: Cachexia, Chronic obstructive pulmonary disease, Nutrition, Omega‐3 fatty acids, Pre‐cachexia
Introduction
Cachexia is a complex syndrome frequently present in patients with chronic obstructive pulmonary disease (COPD) and other chronic illnesses including cancer1 and congestive heart failure.2 Cachexia is associated with increased mortality,3 impairments in health‐related quality of life (HRQoL), and muscle weakness.4, 5 The clinical phenotype of cachexia ranges from minimal or no weight loss, with signs of muscle wasting (e.g. anorexia, inflammation) to severe weight loss, muscle depletion, fatigue, and reduced mobility.
In patients with COPD, early multimodal intervention, including nutritional support, has clinical benefits, both in patients with overt cachexia and in those with minimal or no weight loss (‘pre‐cachexia’).6 Meta‐analyses of randomized controlled trials (RCTs) have demonstrated the benefits of nutritional interventions on body weight, fat‐free mass index, exercise tolerance, and respiratory and non‐respiratory muscle strength in patients with COPD.7, 8 Limited evidence also suggests that body composition and exercise capacity may be improved, and inflammatory activity reduced, by nutritional interventions containing high doses of omega‐3 polyunsaturated fatty acids (PUFAs). In an 8‐week RCT in 80 patients with COPD, daily supplementation with a PUFA blend including eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha‐linolenic acid significantly improved exercise capacity compared with placebo.9 In another RCT, daily supplementation with omega‐3 PUFAs alongside low‐intensity exercise for 12 weeks produced clear benefits compared with standard care in 32 clinically stable, malnourished patients with moderate‐to‐severe COPD10; the intervention significantly increased energy intake, improved body composition, and reduced levels of the inflammatory biomarkers, C‐reactive protein, interleukin (IL)‐6, IL‐8, and tumour necrosis factor. The results from these RCTs are noteworthy given that in a separate study, non‐responders to a high‐calorie nutritional supplement had higher inflammatory biomarker levels than those who gained weight.11
There is also an evidence‐based rationale for patients with COPD taking other nutrients including vitamin D12 and some dietary antioxidants including plant polyphenols.13 Furthermore, high‐quality protein is acknowledged to be important for optimal skeletal muscle protein synthesis.14
To date, few RCTs of nutritional support for patients with COPD have included appropriate controls. This trial is the first RCT of its kind to compare the safety and efficacy of supplementation with a complex targeted medical nutrition (TMN) formulation containing high‐dose omega‐3 PUFAs, 25‐hydroxy‐vitamin D3, plant antioxidants, and high‐quality whey protein with an isocaloric comparator matched for energy content, in pre‐cachectic and cachectic patients with COPD. This trial primarily aimed to assess safety and tolerability; secondary objectives were to evaluate the efficacy of TMN in improving measures of clinical relevance in COPD, including body composition, inflammation, muscle function, and HRQoL.
Methods
Study design
This 12‐week randomized, parallel group, double‐blind, placebo‐controlled, multicentre trial assessed the safety and tolerability of TMN (Nutrifriend Cachexia; Smartfish, Oslo, Norway) in patients with COPD (ClinicalTrials.gov Identifier: NCT02442908). Patients at five sites in Sweden (Ladulaas Kliniska Studier, Borås; Pharmasite, Helsingborg; Pharmasite, Malmö; A+ Science City Site, Stockholm; Probare, Lund) were randomized 1:1 to receive TMN or isocaloric comparator. It was determined a priori that 25 evaluable patients per group would be sufficient to meet the primary objective; thus, no formal power calculation was made.
The study was conducted in accordance with good clinical practice and the Declaration of Helsinki. All patients provided written informed consent. The study protocol and amendment were reviewed and approved by the Regional Ethics Committee in Gothenburg, Sweden.
Participants
Eligible participants were patients aged ≥50 years with moderate‐to‐severe COPD [based on forced expiratory volume in 1 s (FEV1) 30–60% of predicted value, post‐bronchodilator] and involuntary weight loss in the 12 months before randomization [≤10% for body mass index (BMI) of 18–32 kg/m2] or a low BMI (16–18 kg/m2) without weight loss, and adequate hepatic and renal function. A 5% weight loss threshold was used to distinguish between overt vs. pre‐cachexia, as per the European Society for Clinical Nutrition and Metabolism's consensus definitions15; cachexia was defined as 5–10% weight loss and pre‐cachexia as low BMI or ≤5% weight loss over the 12 months before randomization. Classification according to European Respiratory Society's definition(s) of pre‐cachexia (unintentional weight loss of >5%) and cachexia [unintentional weight loss of >5% with fat‐free mass index of <17 kg/m2 (for men) or <15 kg/m2 (for women), as determined by dual‐energy X‐ray absorptiometry] was also assessed.16 Exclusion criteria included having received >5 mg/day oral corticosteroids, anabolic steroids, or nutritional supplements containing EPA/DHA within 3 months prior to screening. Patients experiencing COPD exacerbations or major changes in COPD maintenance treatment within 3 months prior to screening were also excluded (refer to Table S1 for full inclusion/exclusion criteria). During the study, products containing omega‐3 PUFAs, oral steroids >5 mg/day (unless as treatment for ongoing COPD exacerbation), and nutritional supplements that the investigator considered could influence safety and efficacy outcomes were restricted.
Nutritional intervention
Patients were randomized at baseline to receive TMN (approximately 230 kcal; 10 g whey protein concentrate, minimum 2.0 g DHA + EPA, and 10 μg 25‐hydroxy‐vitamin D3 per 200 mL) or a milk‐based comparator that contained no 25‐hydroxy‐vitamin D3, milk protein instead of pure whey protein, and sunflower oil in place of omega‐3 PUFA‐containing fish oil (approximately 200 kcal per 200 mL). Doses of TMN components were chosen based on published data on omega‐3 PUFA‐containing supplements and other medical nutrition products from Smartfish.17, 18, 19, 20 Trial Form Support, AB (Lund, Sweden) implemented randomization by assigning patients a three‐digit number allocating them to a treatment group; patients at each site were assigned numbers sequentially. Access to randomization details was restricted until study end.
Patients were instructed to drink two 200 mL study product containers daily for 12 weeks. Labelling conformed to local regulations and ensured that the contents were not identifiable. Patients received dietary and exercise advice at screening and were required to report changes in smoking habits or diet, with the exception of increasing caloric intake if appetite increased. They were assessed at screening (days −14 to −1), baseline (day 1), and every 3 weeks thereafter (weeks 3–12). Patients were followed up by telephone on weeks 3 and 9; all other assessments were face‐to‐face. No COPD inhalation treatment was permitted on the mornings of screening, baseline, and final assessment; patients fasted after 22:00 h the night before face‐to‐face assessments after screening.
Outcome measures
Table 1 gives details of all study endpoints and how they were measured.21, 22, 23, 24, 25, 26 Primary safety endpoints comprised number and type of adverse events (AEs) over 12 weeks and change from baseline to week 12 in vital signs, laboratory safety parameters, and concomitant medications.
Table 1.
Measurement of primary, secondary, and exploratory study endpoints
| Endpoint | Measurement | Time point |
|---|---|---|
| Primary safety endpoints | ||
| Adverse events |
|
|
| Physical examination |
|
|
| Vital signs |
|
|
| Laboratory safety measures |
|
|
| Other safety measures |
|
|
| Secondary efficacy endpoints | ||
| Weight and body measurements |
|
|
| Exercise tolerance and muscle function |
|
|
| Inflammation |
|
|
| Appetite |
|
|
| Glucose metabolism |
|
|
| Lipid metabolism |
|
|
| Daily activity |
|
|
| Fat mass |
|
|
| Lean body mass |
|
|
| Lung function |
|
|
| HRQoL |
|
|
| Compliance |
|
|
| Exploratory efficacy endpoints | ||
| COPD progression |
|
|
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; FEV1, forced expiratory volume in 1 s; HDL, high‐density lipoprotein; HRQoL, health‐related quality of life; LDL, low‐density lipoprotein; TNF, tumour necrosis factor.
Secondary endpoints included change from baseline to weeks 6 and 12 in BMI, weight and waist and calf circumference, and inflammatory biomarkers. Changes in exercise tolerance and muscle function, appetite, and glucose and lipid metabolism were evaluated at the same time points. Changes in fat mass (FM), lean body mass (LBM), lung function, and HRQoL from baseline to week 12 were also assessed. Plasma EPA and DHA levels were also measured. Compliance was assessed based on drink consumption diaries and changes in omega‐3 to omega‐6 (O3:O6) ratio and vitamin D3 levels from baseline to week 12. Numbers of patients experiencing exacerbations and signs of COPD progression (defined as an AE related to COPD, addition of a new COPD‐related medication, or an increase in dose in existing COPD medication) were examined as exploratory efficacy endpoints.
Statistical analysis
Safety and efficacy analyses were performed for the safety and full analysis sets, respectively. Safety endpoints were summarized using descriptive statistics: differences reported are numerical only, unless otherwise specified. For continuous efficacy endpoints, differences between treatment groups from baseline to weeks 6 or 12 were assessed using analyses of covariance (ANCOVA) with baseline value as the covariate.
Greater than expected reductions in the safety endpoint of blood pressure (BP) were observed between baseline and week 12, so a post hoc ANCOVA was conducted using treatment as the fixed factor and baseline value as the covariate for this endpoint; because of observed differences in inflammation between groups, EPA and DHA levels were also assessed in the same type of post hoc analysis.
Significant differences between groups in disease severity [assessed by Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria] and FEV1 were observed at baseline. Therefore, post hoc ANCOVAs were conducted with stage of COPD [moderate vs. severe, assessed by GOLD criteria (Table 1)] as a factor, for efficacy endpoints in which treatment effects of P < 0.1 were observed in one or more variables in an efficacy outcome category in the primary analysis; treatment and stage of COPD were fixed factors, and baseline value was a covariate.
All tests were two‐sided, and P ≤ 0.05 was considered significant. No adjustments were made for multiple comparisons owing to the exploratory nature of the analysis. Data were analysed using Statistical Analysis System (v9.3) software. Unless otherwise specified, data in parentheses show mean change from baseline to week 12 in the TMN group vs. the isocaloric comparator group, alongside baseline‐adjusted P values. Disease severity‐adjusted and baseline‐adjusted P values are provided where specified.
Results
Between 7 May 2015 and 5 April 2016, 48 patients were screened, and 45 were randomized to receive TMN (n = 22) or comparator (n = 23). Figure 1 shows patient flow. Baseline characteristics, including age, weight loss, and proportion of patients with cachexia, did not differ between groups (Table 2). The proportion of women was slightly, but not significantly, higher in the TMN group than the comparator group (P > 0.1). A greater proportion of patients in the TMN vs. the comparator group had severe disease based on GOLD criteria at baseline; this was accounted for in post hoc analyses.
Figure 1.
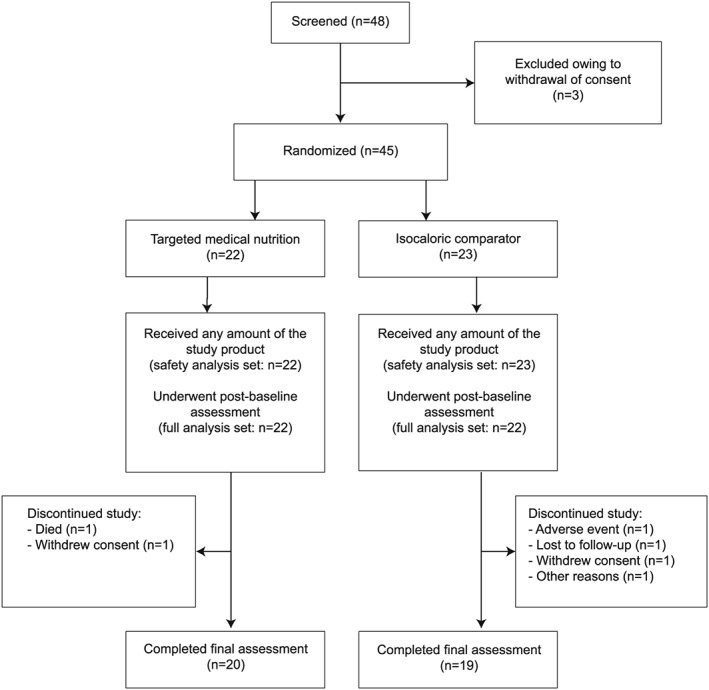
Patient flow.
Table 2.
Baseline patient demographics and characteristics
| Variable | TMN (n = 22) | Comparator (n = 23) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 69.2 | 6.3 | 69.7 | 8.2 |
| Weight (kg) | 64.1 | 10.8 | 69.4 | 15.2 |
| Weight loss (%) | 4.1 | 2.5 | 4.0 | 2.6 |
| Height (cm) | 168.9 | 9.0 | 171.3 | 8.8 |
| BMI (kg/m2) | 22.5 | 3.7 | 23.5 | 4.0 |
| FEV1 (% of FVC) | 45.0a | 10.0 | 52.4 | 8.9 |
| n | % | n | % | |
| Women | 12 | 54.5 | 10 | 43.5 |
| Severe COPD based on GOLD criteriab | 15a | 68.2 | 8 | 34.8 |
| Weight loss | ||||
| No weight history | 2 | 9.1 | 2 | 8.7 |
| No weight lossc and BMI 16–18 kg/m2 | 1 | 4.5 | 1 | 4.3 |
| ≤5% weight lossc | 11 | 50.0 | 12 | 52.2 |
| 5–10% weight lossc | 8 | 36.4 | 8 | 34.8 |
| Stage of cachexia according to the ERS statement16 | ||||
| Pre‐cachexiad | 1 | 4.5 | 2 | 8.7 |
| Cachexiae | 7 | 31.8 | 6 | 26.0 |
| Smoking status | n = 21 | n = 22 | ||
| Current | 13 | 61.9 | 11 | 50.0 |
| Former | 8 | 38.1 | 11 | 50.0 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; ERS, European Respiratory Society; FEV1, forced expiratory volume in 1 s; FFMI, fat‐free mass index; FVC, forced expiratory vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SD, standard deviation; TMN, targeted medical nutrition.
P < 0.05 for the TMN vs. isocaloric comparator group.
FEV1 30–50% of predicted value.
Over the 12 months before randomization.
Unintentional weight loss of >5%.
Unintentional weight loss of >5% and FFMI <17 kg/m2 (for men) or <15 kg/m2 (for women).
Safety
Targeted medical nutrition was well tolerated, and AEs did not differ in number or type between the TMN and comparator groups (36 AEs in 16 patients vs. 36 AEs in 17 patients, respectively), with the exception of diarrhoea, which occurred in a greater number of patients in the comparator group (1 vs. 5 patients, respectively). Approximately one‐third of AEs were considered treatment‐related (Table 3 (a)). Four serious AEs occurred (TMN: death and inguinal hernia; comparator: arteriosclerosis event and pulmonary embolism), but none was considered related to treatment by the investigator.
Table 3.
Safety parameters in the targeted medical nutrition and isocaloric comparator groups: (a) treatment‐related adverse events deemed to be related to study products by the investigator, (b) vital signs, (c) proportion of patients with abnormal outcomes in physical examinations, and (d) laboratory safety parameters
| (a) | Adverse events | ||||
|---|---|---|---|---|---|
| Severity | Mild n (%) | Moderate n (%) | |||
| Eventa | TMN (n = 22) | Comparator (n = 23) | TMN (n = 22) | Comparator (n = 23) | |
| Gastrointestinal disorders | |||||
| Constipation | 2 (9.1) | 0 | 0 | 0 | |
| Diarrhoea | 1 (4.5) | 2 (8.7) | 0 | 2 (8.7) | |
| Flatulence | 0 | 1 (4.3) | 0 | 0 | |
| Gastro‐oesophageal reflux disease | 1 (4.5) | 0 | 0 | 0 | |
| Nausea | 2 (9.1) | 2 (8.7) | 0 | 0 | |
| Vomiting | 1 (4.5) | 1 (4.3) | 1 (4.5) | 1 (4.3) | |
| Metabolism and nutrition disorders | |||||
| Decreased appetite | 1 (4.5) | 0 | 0 | 1 (4.3) | |
| Nervous system disorders | |||||
| Headache | 1 (4.5) | 0 | 0 | 0 | |
| Respiratory, thoracic, and mediastinal disorders | |||||
| Increased viscosity of bronchial secretion | 0 | 0 | 1 (4.5) | 0 | |
| (b) | Vital signs | ||||
|
Baseline Mean (SD) |
Week 12 Mean (SD) |
||||
| TMN (n = 22) | Comparator (n = 23) | TMN (n = 22) | Comparator (n = 23) | ||
| Systolic BP (mmHg) | 144.7 (22.7) | 141.6 (17.8) | 129.4 (20.0) | 142.7 (19.3) | |
| Diastolic BP (mmHg) | 81.6 (10.1) | 78.0 (10.2) | 75.8 (10.5) | 78.6 (10.7) | |
| Heart rate (bpm) | 75.1 (11.9) | 75.74 (9.7) | 76.9 (14.8) | 77.1 (10.9) | |
| (c) | Physical examination | ||||
| Baseline n (%) | Week 12 n (%) | ||||
| Abnormal observations | TMN (n = 22) | Comparator (n = 23) | TMN (n = 20) | Comparator (n = 19) | |
| Abdomen | 0 | 0 | 0 | 0 | |
| Cardiovascular system | 0 | 0 | 0 | 0 | |
| Skin | 1 (4.5) | 0 | 0 | 0 | |
| Nervous system | 0 | 0 | 0 | 0 | |
| Respiratory system | 0 | 0 | 1 (5.0) | 1 (5.3) | |
| (d) | Laboratory parameters | ||||
|
Baseline Mean (SD) |
Week 12 Mean (SD) |
||||
| TMN | Comparator | TMN | Comparator | ||
| Clinical chemistry parameters | (n = 22) | (n = 23) | (n = 19) | (n = 19) | |
| ALP (μkat/L) | 1.16 (0.35) | 1.22 (0.27) | 1.17 (0.34) | 1.25 (0.31) | |
| ALT (μkat/L) | 0.37 (0.20) | 0.37 (0.12) | 0.43 (0.20) | 0.37 (0.12) | |
| AST (μkat/L) | 0.40 (0.14) | 0.39 (0.12) | 0.43 (0.16) | 0.38 (0.09) | |
| Bilirubin (μmol/L) | 6.55 (2.34) | 7.00 (3.71) | 5.63 (2.09) | 6.74 (4.53) | |
| Creatinine (μmol/L) | 74.82 (20.12) | 78.13 (21.48) | 71.74 (24.40) | 78.58 (18.57) | |
| Potassium (mmol/L) | 4.32 (0.41) | 4.10 (0.34) | 4.14 (0.31) | 4.13 (0.26) | |
| Sodium (mmol/L) | 140.23 (2.47) | 139.52 (2.61) | 138.74 (2.68) | 139.68 (1.73) | |
| Haematological parameters | n = 21 | n = 22 | n = 20 | n = 19 | |
| Haematocrit | 0.46 (0.04) | 0.44 (0.03) | 0.47 (0.04) | 0.44 (0.04) | |
| Haemoglobin (g/L) | 146.10 (11.30) | 140.32 (12.32) | 146.70 (10.07) | 139.74 (12.08) | |
| Platelet count (×109/L) | 303.24 (97.21) | 295.23 (85.97) | 283.30 (89.91) | 295.63 (78.31) | |
| Red blood cell count (×1012/L) | 4.82 (0.56) | 4.65 (0.44) | 4.86 (0.47) | 4.62 (0.47) | |
| White blood cell differential count (×109/L) | 7.71 (2.15) | 7.14 (2.33) | 8.08 (2.35) | 7.15 (2.45) | |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure; bpm, beats per minute; SD, standard deviation; TMN, targeted medical nutrition.
Common chronic obstructive pulmonary disease complications including breathlessness, wheezing, coughing, and sputum production were not reported as adverse events unless they were considered to be serious.
Changes in mean BP from baseline to week 12 were greater than expected in the TMN group, but no patient experienced hypotension; heart rate was numerically similar between groups at both time points (Table 3). Mean systolic BP decreased in the TMN but not the comparator group (−12.7 vs. +0.2 mmHg; P = 0.0118); this difference remained significant after correction for disease severity (P = 0.0418). Mean diastolic BP decreased from >90 to ≤90 mmHg in 20.0 and 5.3% of patients in the TMN and comparator groups, respectively (Table S2); the between‐group difference in mean diastolic BP was not significant (−3.9 vs. +0.8 mmHg; P = 0.2445). Physical examination outcomes, laboratory safety assessment results (Table 3), and concomitant medication use initiated post‐baseline (Table S3) did not differ numerically between groups.
Body composition and weight
Patients in TMN and comparator groups gained similar amounts of weight, and thus, BMI rose similarly (Table S4). There were no between‐group differences in changes in waist and calf circumference, appendicular LBM, or skeletal muscle index; however, the TMN group gained significantly more FM than the comparator group (+1.87 vs. +0.60 kg; P = 0.0013), and a difference was evident regardless of baseline weight loss (Table S5). This difference remained after adjustment for COPD severity (P = 0.0013).
Exercise tolerance and muscle function
Borg scale‐assessed fatigue after the 6 min walk test was significantly reduced in the TMN vs. comparator group by week 12; a similar pattern was observed for dyspnoea, although the difference missed statistical significance (Figure 2A). When accounting for COPD severity, reductions in fatigue (P = 0.0223) and dyspnoea (P = 0.0382) in the TMN group were both significant. Changes in exercise tolerance were similar regardless of weight loss at baseline (Table S5). Change in 6 min walk distance from baseline to week 12 did not differ significantly between groups (Figure 2B), and neither did hand grip strength or amount of self‐reported exercise.
Figure 2.
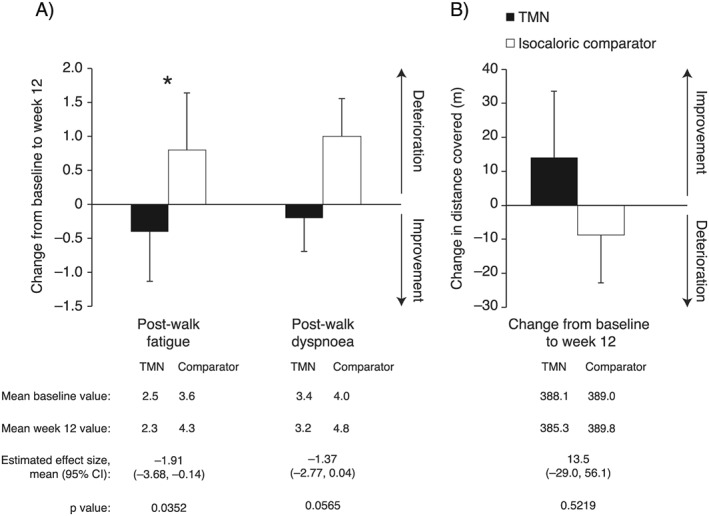
Change from baseline to week 12 in results of the 6 min walk test in the TMN group and the isocaloric comparator group. (A) Borg scale measured post‐walk fatigue and dyspnoea. (B) Distance covered. Data on figures show mean ± SEM change from baseline to week 12, calculated as week 12 value minus baseline value for each patient, divided by the n number; n = 19 for both groups. Data below figures show mean baseline and week 12 values, calculated as the mean of all values recorded. Effect sizes and P values were estimated by analysis of covariance adjusted for baseline value. *P < 0.05 for the TMN vs. isocaloric comparator group. CI, confidence interval; SEM, standard error of the mean; TMN, targeted medical nutrition.
Inflammation
Fasting plasma concentrations of all four biomarkers of inflammation were numerically lower in the TMN vs. comparator group at weeks 6 and 12. Concentrations of IL‐6, IL‐8, and tumour necrosis factor decreased in the TMN group and increased in the comparator group over 12 weeks, whereas C‐reactive protein concentrations increased in both groups (Figure 3). These differences were not significant, before or after taking disease severity into account, but were similar in patients with >5 and ≤5% weight loss at baseline (Table S5).
Figure 3.
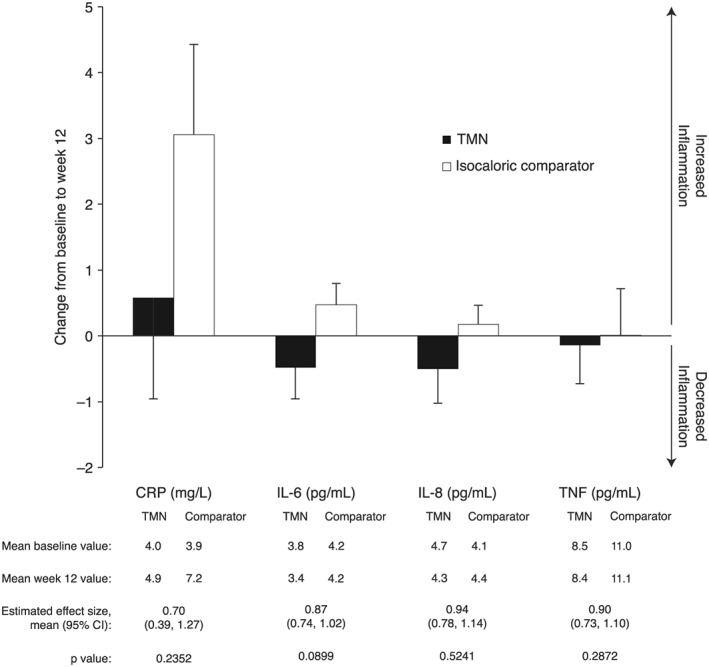
Change in levels of markers of inflammation from baseline to week 12 in the TMN group and the isocaloric comparator group. Data on figures show mean ± SEM change from baseline to week 12, calculated as week 12 value minus baseline value for each patient, divided by the n number; n = 22 for both groups. Data below figures show mean baseline and week 12 values, calculated as the mean of all values recorded. Effect sizes and P values were estimated by analysis of covariance adjusted for baseline value. CI, confidence interval; CRP, C‐reactive protein; IL, interleukin; SEM, standard error of the mean; TMN, targeted medical nutrition; TNF, tumour necrosis factor.
Metabolic biomarkers
Fasting plasma low‐density lipoprotein [(LDL): +0.29 vs. −0.21 mmol/L] and high‐density lipoprotein [(HDL) +0.13 vs. −0.04 mmol/L] cholesterol levels increased over the study period in the TMN but not the comparator group, while the opposite was true for triglyceride levels (−0.21 vs. +0.06 mmol/L). Differences in LDL and triglycerides were significant before (LDL: P = 0.0226; triglycerides: P = 0.0286) and after correcting for COPD severity (LDL: P = 0.0325; triglycerides: P = 0.0217), whereas differences in HDL levels were significant only after correction (P = 0.0254). These changes were evident regardless of weight loss at baseline. Total cholesterol, HDL:LDL ratio, and fasting blood glucose and serum insulin level changes did not differ between groups.
Health‐related quality of life
Overall scores on the St. George's Respiratory Questionnaire (SGRQ) did not differ between groups, but scores on the activity subdomain significantly improved in the TMN vs. comparator group (Figure 4). This difference was not significant when accounting for disease severity (P = 0.0592). There were no between‐group differences in changes in COPD Assessment Test and COPD Clinical Questionnaire scores.
Figure 4.
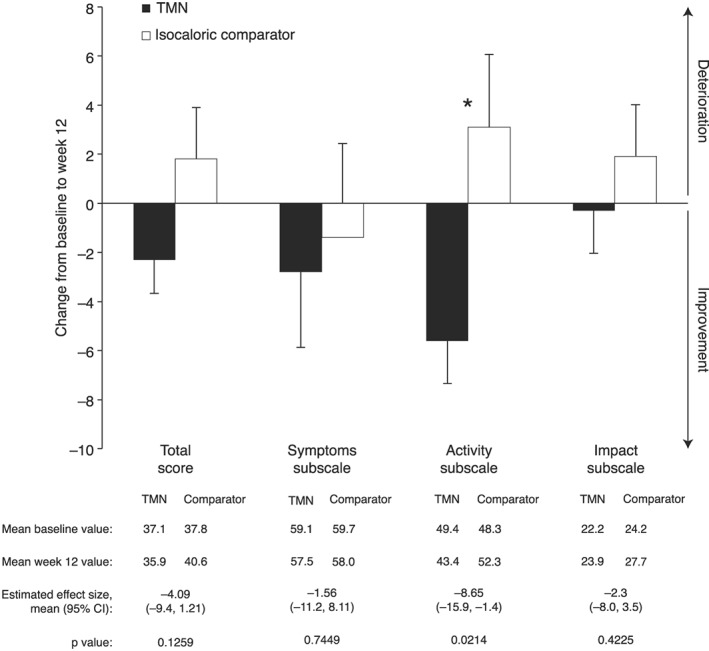
Change from baseline to week 12 in scores on the St. George's Respiratory Questionnaire in the TMN group and the isocaloric comparator group. Data on figures show mean ± SEM change from baseline to week 12, calculated as week 12 value minus baseline value for each patient, divided by the n number; n = 22 for both groups. Data below figures show mean baseline and week 12 values, calculated as the mean of all values recorded. Effect sizes and P values were estimated by analysis of covariance adjusted for baseline value. CI, confidence interval; SEM, standard error of the mean; TMN, targeted medical nutrition.
Compliance
Compliance was good in the TMN (79%) and comparator (77%) groups based on drink consumption diaries. Furthermore, O3:O6 ratio and vitamin D3 levels increased significantly in the TMN vs. comparator group (Figure 5), and between‐group differences remained when accounting for disease severity (O3:O6 ratio: P < 0.0001; vitamin D3: P < 0.0001). At baseline, EPA and DHA comprised a small proportion of the total plasma fatty acids in both groups [mean ± standard error of the mean (SEM), EPA: 1.30 ± 0.17 vs. 1.14 ± 0.09%; DHA: 2.43 ± 0.14 vs. 2.25 ± 0.14%], while by week 12, the proportion of these PUFAs had increased significantly in the TMN vs. the comparator group (Figure 5). These changes were apparent after correcting for disease severity (EPA: P < 0.0001; DHA: P < 0.0001).
Figure 5.
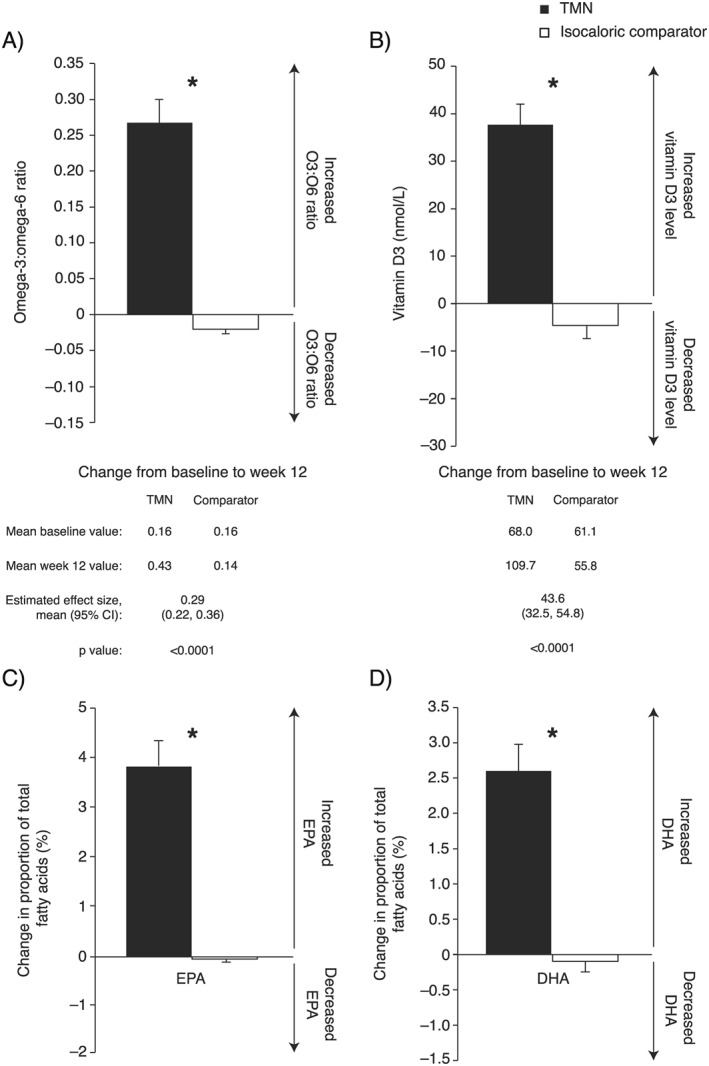
Change from baseline to week 12 in the TMN group and the isocaloric comparator group. (A) Plasma omega‐3 to omega‐6 ratio. (B) Plasma vitamin D3 level. (C) EPA as a proportion of total plasma fatty acids. (D) DHA as a proportion of total plasma fatty acids. Data on figures show mean ± SEM change from baseline to week 12, calculated as week 12 value minus baseline value for each patient, divided by the n number; TMN group, n = 22; comparator group; n = 22–23. Data below figures show mean baseline and week 12 values, calculated as the mean of all values recorded. Effect sizes and P values were estimated by analysis of covariance adjusted for baseline value. *P < 0.0001 for the TMN group vs. the isocaloric comparator group. CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; SEM, standard error of the mean; TMN, targeted medical nutrition.
Appetite and calorie intake
Changes in scores on the Functional Assessment of Anorexia/Cachexia Therapy questionnaire, Council on Nutrition Appetite Questionnaire, and palatability questionnaire were minor and did not differ between groups. Changes in self‐reported calorie intake were minor and did not differ significantly between the groups by week 12 (P = 0.8381).
Chronic obstructive pulmonary disease progression
In total, 40.9% (n = 9) of patients in the TMN group vs. 50% (n = 11) of those in the comparator group experienced COPD progression, as defined by the criteria used in this study. COPD exacerbations were experienced by numerically fewer individuals in the TMN than the comparator group [4.5% (n = 1) vs. 13.6% (n = 3), respectively]. There was no between‐group difference in the change in mean FEV1 over time (P = 0.3689).
Discussion
Targeted nutritional supplementation was well tolerated and had clinical benefits in pre‐cachectic and cachectic patients with COPD, in this first of its kind study evaluating the safety and efficacy of a complex, targeted nutritional supplement relative to an isocaloric comparator.
No safety issues were observed, while compliance with both products was high. AE profiles were similar in both groups; diarrhoea was more common in the comparator group, possibly because the comparator was milk‐based, as are most currently available nutritional supplements. Safety parameters were comparable between the two groups, with the exception that patients receiving TMN benefited from reduced systolic BP. Patients with a high baseline BP seemed to benefit the most, as expected based on previous studies of high‐dose omega‐3 PUFAs,27 which is noteworthy given that hypertension is commonly comorbid with COPD.28
Targeted medical nutrition was associated with a consistent trend towards reduced inflammation relative to the isocaloric comparator. This effect was apparent even though elevated baseline inflammatory biomarker levels were not a requirement for inclusion in this trial. Whether the anti‐inflammatory effects were due to increases in plasma DHA or EPA levels is debatable,29 but both omega‐3 PUFAs are known anti‐inflammatory agents, thought to act by several mechanisms including altering cell membrane phospholipid fatty acid composition and inhibiting pro‐inflammatory and activating anti‐inflammatory transcription factors.30
Targeted medical nutrition had positive effects on measures of exercise tolerance. Exercise‐induced dyspnoea and fatigue were better by a clinically relevant degree (>1 point)31 in patients receiving TMN vs. isocaloric comparator by week 12, and the mean difference between the groups in the change in 6 min walk distance was near the level of improvement that has been reported as clinically important (≥25 m).32 However, this study may not have been adequately powered to detect a difference in the latter statistically. Although it might be expected that exercise‐induced fatigue and exercise capacity improve concurrently, the two may not be directly linked, because fatigue has several precipitating factors.33 Greater improvements in 6 min walk test results might have been expected had the trial been longer, or had an exercise programme been included, as demonstrated previously in malnourished patients with COPD.10 A clinically relevant improvement in activity‐related quality of life, as measured by the SGRQ, was also notable in the TMN group.34 Although nutrition is only one of many variables affecting HRQoL, improved exercise tolerance has previously been linked with better HRQoL in COPD.4, 35
A major goal for improving the prognosis of patients with COPD is reversal of unintentional weight loss.36 Weight increased in both groups in this trial and an increase in FM was associated with TMN. Furthermore, this benefit was evident both in patients with pre‐cachexia (≤5% weight loss) and those with cachexia (>5% weight loss), despite the broad definitions of these syndromes used in this study. As the calorie intake derived from study products was similar in both groups, this change in body composition is likely a result of the unique combination of ingredients in the TMN. Although an increase in LBM would have been preferable, this might have been achieved had an exercise programme been specified, as was recently demonstrated in an RCT of a nutritional intervention combined with high‐intensity exercise in patients with COPD and low muscle mass.37 Moreover, any weight gain in this patient group could be beneficial in terms of survival.36
The impact of TMN on lipid profiles was generally favourable, as expected based on studies of high‐dose omega‐3 PUFAs in healthy adults.38 Increases in fasting plasma HDL and decreases in triglycerides were promising, and as the HDL:LDL ratio did not change, no safety risk was indicated. Increases in LDL were presumably due to increases in particle size, from smaller, more atherogenic particles, to larger, less damaging particles.39 Insulin and glucose levels remained unchanged, most likely because patients were normoglycaemic at baseline.
This trial has several strengths compared with those reported previously. Firstly, interventions were well matched for energy content, allowing evaluation of the combination of ingredients in the TMN. Secondly, the patient withdrawal rate was low, and treatment compliance was high, despite the fish oil content of the TMN, as verified by significant enrichment of blood vitamin D3, EPA, and DHA levels. Finally, patients were well characterized. The main limitation of the study was its small sample size.
Conclusions
This randomized, double‐blind, controlled trial demonstrated that this TMN containing high‐dose omega‐3 PUFAs, vitamin D, and high‐quality protein is well tolerated, with a good safety profile. Furthermore, TMN has positive effects on BP and blood lipids, and on exercise‐induced fatigue and dyspnoea, and therefore could be clinically beneficial in the nutritional and metabolic support of cachectic and pre‐cachectic patients with COPD.
Conflict of interest
P.C.C. has received a research grant and personal fees from Smartfish. A.L. and M.M. have received personal fees from Smartfish. F.L. is a medical advisor to and has received personal fees from Smartfish. M.Ö. is an employee of Smartfish. A.S. has received fees from Smartfish and Nutricia to her institution.
Supporting information
Table S1 Inclusion and exclusion criteria
Table S2 Numbers of patients with changes in BP from baseline to week 12 in the TMN and isocaloric comparator groups
Table S3 Concomitant medications initiated post‐baseline in patients in the TMN and isocaloric comparator groups
Table S4 Weight and body composition of patients in the TMN and isocaloric comparator groups
Table S5 Changes in body composition, exercise tolerance, inflammation and metabolic markers from baseline to week 12 in patients in the TMN and isocaloric comparator group, by weight loss group
Supplementary Material 6
Acknowledgements
The study was conducted in accordance with good clinical practice and the Declaration of Helsinki. All patients provided written informed consent. The study protocol and amendment were reviewed and approved by the Regional Ethics Committee in Gothenburg, Sweden.
All authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle.40
The authors thank Harriet Crofts, PhD, and Katie Pillidge, PhD, of PharmaGenesis London, UK for medical writing support and editorial assistance in the preparation of this manuscript and Tommy Schyman for assistance with statistical analyses of the data, funded by Smartfish AB, Stockholm, Sweden.
This study was funded by Smartfish AB, Stockholm, Sweden.
Calder, P. C. , Laviano, A. , Lonnqvist, F. , Muscaritoli, M. , Öhlander, M. , and Schols, A. (2018) Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: a randomized, controlled trial. Journal of Cachexia, Sarcopenia and Muscle, 9: 28–40. doi: 10.1002/jcsm.12228.
References
- 1. Muscaritoli M, Molfino A, Lucia S, Rossi Fanelli F. Cachexia: a preventable comorbidity of cancer. A T.A.R.G.E.T. approach. Crit Rev Oncol Hematol 2015;94:251–259. [DOI] [PubMed] [Google Scholar]
- 2. Onwuamaegbu ME, Henein M, Coats AJ. Cachexia in malaria and heart failure: therapeutic considerations in clinical practice. Postgrad Med J 2004;80:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schols AM, Broekhuizen R, Weling‐Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005;82:53–59. [DOI] [PubMed] [Google Scholar]
- 4. Mostert R, Goris A, Weling‐Scheepers C, Wouters EF, Schols AM. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med 2000;94:859–867. [DOI] [PubMed] [Google Scholar]
- 5. Bernard S, LeBlanc P, Whittom F, Carrier G, Jobin J, Belleau R, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:629–634. [DOI] [PubMed] [Google Scholar]
- 6. Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo‐controlled randomized trial. Am J Respir Crit Care Med 1995;152:1268–1274. [DOI] [PubMed] [Google Scholar]
- 7. Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;12: CD000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collins PF, Elia M, Stratton RJ. Nutritional support and functional capacity in chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Respirology 2013;18:616–629. [DOI] [PubMed] [Google Scholar]
- 9. Broekhuizen R, Wouters EF, Creutzberg EC, Weling‐Scheepers CA, Schols AM. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 2005;60:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugawara K, Takahashi H, Kasai C, Kiyokawa N, Watanabe T, Fujii S, et al. Effects of nutritional supplementation combined with low‐intensity exercise in malnourished patients with COPD. Respir Med 2010;104:1883–1889. [DOI] [PubMed] [Google Scholar]
- 11. Creutzberg EC, Schols AM, Weling‐Scheepers CA, Buurman WA, Wouters EF. Characterization of nonresponse to high caloric oral nutritional therapy in depleted patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:745–752. [DOI] [PubMed] [Google Scholar]
- 12. Malinovschi A, Masoero M, Bellocchia M, Ciuffreda A, Solidoro P, Mattei A, et al. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respir Res 2014;15:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biswas S, Hwang JW, Kirkham PA, Rahman I. Pharmacological and dietary antioxidant therapies for chronic obstructive pulmonary disease. Curr Med Chem 2013;20:1496–1530. [DOI] [PubMed] [Google Scholar]
- 14. Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci 2015;80:A8–A15. [DOI] [PubMed] [Google Scholar]
- 15. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 16. Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscaritoli M, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J 2014;44:1504–1520. [DOI] [PubMed] [Google Scholar]
- 17. Colomer R, Moreno‐Nogueira JM, Garcia‐Luna PP, Garcia‐Peris P, Garcia‐de‐Lorenzo A, Zarazaga A, et al. N‐3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr 2007;97:823–831. [DOI] [PubMed] [Google Scholar]
- 18. van der Meij BS, Langius JA, Spreeuwenberg MD, Slootmaker SM, Paul MA, Smit EF, et al. Oral nutritional supplements containing n‐3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT. Eur J Clin Nutr 2012;66:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weed HG, Ferguson ML, Gaff RL, Hustead DS, Nelson JL, Voss AC. Lean body mass gain in patients with head and neck squamous cell cancer treated perioperatively with a protein‐ and energy‐dense nutritional supplement containing eicosapentaenoic acid. Head Neck 2011;33:1027–1033. [DOI] [PubMed] [Google Scholar]
- 20. Wigmore SJ, Ross JA, Falconer JS, Plester CE, Tisdale MJ, Carter DC, et al. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition 1996;12:S27–S30. [DOI] [PubMed] [Google Scholar]
- 21. Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD‐specific version of the St. George Respiratory Questionnaire. Chest 2007;132:456–463. [DOI] [PubMed] [Google Scholar]
- 22. Stallberg B, Nokela M, Ehrs PO, Hjemdal P, Jonsson EW. Validation of the clinical COPD Questionnaire (CCQ) in primary care. Health Qual Life Outcomes 2009;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline LN. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648–654. [DOI] [PubMed] [Google Scholar]
- 24. Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community‐dwelling adults and nursing home residents. Am J Clin Nutr 2005;82:1074–1081. [DOI] [PubMed] [Google Scholar]
- 25. Ribaudo JM, Cella D, Hahn EA, Lloyd SR, Tchekmedyian NS, Von Roenn J, et al. Re‐validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res 2000;9:1137–1146. [DOI] [PubMed] [Google Scholar]
- 26. ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 27. Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens 2002;20:1493–1499. [DOI] [PubMed] [Google Scholar]
- 28. Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allaire J, Couture P, Leclerc M, Charest A, Marin J, Lepine MC, et al. Randomized, crossover, head‐to‐head comparison of EPA and DHA supplementation to reduce inflammation markers in men and women: the Comparing EPA to DHA Study. Am J Clin Nutr 2016. [DOI] [PubMed] [Google Scholar]
- 30. Calder PC. Omega‐3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 2013;75:645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD 2005;2:105–110. [DOI] [PubMed] [Google Scholar]
- 32. Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six‐minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010;91:221–225. [DOI] [PubMed] [Google Scholar]
- 33. Spruit MA, Vercoulen JH, Sprangers MAG, Wouters EFM. FATIGUE consortium. Fatigue in COPD: an important yet ignored symptom. Lancet Respir Med 2017; https://doi.org/10.1016/S2213‐2600(17)30158‐3. [DOI] [PubMed] [Google Scholar]
- 34. Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005;2:75–79. [DOI] [PubMed] [Google Scholar]
- 35. Curtis JR, Deyo RA, Hudson LD. Pulmonary rehabilitation in chronic respiratory insufficiency. 7. Health‐related quality of life among patients with chronic obstructive pulmonary disease. Thorax 1994;49:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1791–1797. [DOI] [PubMed] [Google Scholar]
- 37. van de Bool C, Rutten EPA, van Helvoort A, Franssen FME, Wouters EFM, Schols AMWJ. A randomised clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J Cachexia Sarcopenia Muscle Accepted April 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega‐3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 2006;189:19–30. [DOI] [PubMed] [Google Scholar]
- 39. Abeywardena MY, Patten GS. Role of omega3 long‐chain polyunsaturated fatty acids in reducing cardio‐metabolic risk factors. Endocr Metab Immune Disord Drug Targets 2011;11:232–246. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Inclusion and exclusion criteria
Table S2 Numbers of patients with changes in BP from baseline to week 12 in the TMN and isocaloric comparator groups
Table S3 Concomitant medications initiated post‐baseline in patients in the TMN and isocaloric comparator groups
Table S4 Weight and body composition of patients in the TMN and isocaloric comparator groups
Table S5 Changes in body composition, exercise tolerance, inflammation and metabolic markers from baseline to week 12 in patients in the TMN and isocaloric comparator group, by weight loss group
Supplementary Material 6


