Abstract
Background
Physical frailty and loss of mobility in elderly individuals lead to reduced independence, quality of life, and increased mortality. Vitamin B12 deficiency has been linked to several age‐related chronic diseases, including in the musculo‐skeletal system, where vitamin B12 deficiency is generally believed to be linked to poor nutritional intake. In the present study, we asked whether aging and frailty associate with altered vitamin B12 homeostasis in humans and investigated the underlying molecular mechanisms using preclinical models.
Methods
We analysed a subset of the Singapore Longitudinal Aging Study and stratified 238 participants based on age and Fried frailty criteria. Levels of methyl‐malonic acid (MMA), a marker for vitamin B12 deficiency, and amnionless, the vitamin B12 co‐receptor that anchors the vitamin B12 transport complex to the membrane of epithelial cells, were measured in plasma. In addition, vitamin B12 levels and the molecular mechanisms of vitamin B12 uptake and excretion were analysed in ileum, kidney, liver, and blood using a rat model of natural aging where nutritional intake is fully controlled.
Results
We demonstrate that aging and frailty are associated with a higher prevalence of functional vitamin B12 deficiency that can be detected by increased levels of MMA in blood (ρ = 0.25; P = 0.00013). The decline in circulating vitamin B12 levels is recapitulated in a rat model of natural aging where food composition and intake are stable. At the molecular level, these perturbations involve altered expression of amnionless in the ileum and kidney. Interestingly, we demonstrate that amnionless can be detected in serum where its levels increase during aging in both rodents and human (P = 3.3e‐07 and 9.2e‐07, respectively). Blood amnionless levels negatively correlate with vitamin B12 in rats (r2 = 0.305; P = 0.0042) and positively correlate with the vitamin B12 deficiency marker MMA in humans (ρ = 0.22; P = 0.00068).
Conclusions
Our results demonstrate that aging and frailty cause intrinsic vitamin B12 deficiencies, which can occur independently of nutritional intake. Mechanistically, vitamin B12 deficiency involves the physio‐pathological decline of both the intestinal uptake and the renal reabsorption system for vitamin B12. Finally, amnionless is a novel biomarker which can detect perturbed vitamin B12 bioavailability during aging and physical frailty.
Keywords: Aging, Frailty, Sarcopenia, Vitamin B12, Cobalamin, Amnionless, Methylmalonic acid
Introduction
The worldwide increase in life expectancy during the 20th century ranks as one of the greatest achievements of public health and society. Nevertheless, longer lifespan has raised other challenges as the world population is aging at an unprecedented pace such that the number of aged people is projected to be more than doubled by 2050.1 In particular, the prevalence of age‐related chronic diseases such as cardiac, cognitive, metabolic, or musculo‐skeletal disorders has dramatically increased and puts elderly people at risk of disability, loss of independence, and early mortality.2 In particular, walking speed is one of the best predictors of disability and mortality in the elderly.3 Consequently, physical frailty has emerged as a medical condition of high relevance to manage the autonomy and quality of life of geriatric patients.4, 5 Sarcopenia, which captures both the loss of physical function and the loss of skeletal muscle mass with age, has recently been recognized as a disease through an ICD‐10 code.6 Sarcopenia and frailty are believed to partially overlap and to affect 5–20% of the elderly population where physical decline reaches advanced disability.7, 8, 9, 10 Importantly, loss of muscle mass and function with age is a gradual process that begins as early as 30 years of age and affects all individuals, albeit to different extents. Loss of mobility during aging therefore affects quality of life before reaching a debilitating diseased state, and both preventive and therapeutic approaches are needed to manage physical decline, frailty, and sarcopenia.
Poor nutritional status is recognized as a risk factor for many age‐related conditions and, more recently, the role of vitamin B12 deficiency in the development of several age‐related chronic diseases has emerged.11 Vitamin B12 or cobalamin is a class of cobalt‐containing hydrosoluble vitamins which cannot be synthetized by the human body and therefore have to be taken up from food or synthesized by the gut microbiota.11 After unloading from food matrices at low pH in the stomach, dietary vitamin B12 binds to the intrinsic factor (IF) in the small intestine where it is absorbed through a specific heterodimeric transmembrane receptor termed cubam, composed of the cubilin (CBN) and amnionless (AMN) proteins.12 CBN specifically binds the IF‐B12 complex while AMN allows internalization of the receptor within the enterocytes, where the CBN‐AMN‐IF‐B12 complex is degraded in lysosomes.11, 13 Vitamin B12 is subsequently transported across the cytoplasm and the basolateral membrane of the enterocyte to enter the bloodstream. Vitamin B12 transport is also important in the kidney where vitamin B12 is not filtered by the glomeruli and requires to be reuptaken in proximal tubules to prevent urinary loss. The mechanisms of renal reabsorption of vitamin B12 are not fully elucidated but involve a complex interplay between AMN, CBN, and megalin/LRP2.14, 15 Megalin is specifically expressed in the kidney where it binds transcobalamin, the blood transporter for vitamin B12. However, CBN, and AMN are also expressed in kidney where they cooperate with megalin for the urinary reabsorption of a variety of proteins and micro‐nutrients including vitamin B12.
Vitamin B12 deficiencies can have either inherited or acquired origins. Congenital inherited deficiencies in vitamin B12 result from mutations in the intrinsic factor causing hereditary intrinsic factor deficiency or from mutations in cubam causing hereditary megalobastic anaemia.16 In contrast to inherited vitamin B12 deficiencies which manifest clinically within the first years of childhood, acquired vitamin B12 deficiencies manifest much later in life and are thought to be caused by inappropriate nutritional intake or altered intestinal absorption. Indeed during aging, the gastrointestinal function deteriorates, mainly as a result of atrophic gastritis, a chronic inflammation of the stomach affecting up to 30% of people over 60 years.17 Atrophic gastritis is characterized by an atrophy of the mucosa leading to reduced gastric acid secretion and therefore diminished B12 absorption as hydrochloric acid and pepsin are essential for the release of vitamin B12 from food proteins.18 In addition, there is emerging evidence for moderate vitamin B12 deficiencies associating with several chronic diseases, especially those such as stroke,19, 20 cognitive impairment,21 osteoporosis,22 or physical dysfunction,23, 24 which have increased prevalence in elderly populations. In particular, a recent study demonstrated that sarcopenic patients have lower levels of circulating vitamin B12 compared with age‐matched elderly controls,25 suggesting that vitamin B12 deficiency could impact skeletal muscle function.
In this study, we demonstrate that aging and frailty lead to vitamin B12 decline that manifests by increased blood levels of methyl‐malonic acid (MMA) in an Asian elderly population. Using preclinical models of aging, our work establishes that vitamin B12 deficiency during aging can occur independently of nutritional intake through age‐related impairments in intestinal and renal (re)absorption. We find that AMN can be detected in serum where circulating levels increase with age and inversely correlate with vitamin B12 levels while positively correlating with MMA, establishing AMN as a new early circulating marker of vitamin B12 deficiency during aging.
Materials and methods
Clinical study
Participants were selected from the Singapore Longitudinal Aging Studies, which is an ongoing population‐based cohort study of aging and health among Chinese older adults aged 55 and above.26, 27 Participants underwent interview sessions, performance‐based testing, and blood collection by trained research nurses for an extensive measurement of demographic, neurocognitive, medical, psychosocial, and biological variables. The study excluded those who were physically or mentally unable to give informed consent or participate. Adult controls were aged between 23–35 years (n = 30) and elderly individuals (aged between 65–86 years) were enrolled according to Fried criteria for frailty and classified in robust (n = 62), pre‐frail (n = 86), and frail groups (n = 60). The Fried physical frailty status was assessed based on the five syndrome components proposed and validated in the Cardiovascular Health Study.4 Involuntary or unintentional weight loss was defined as a body mass index below 18.5 kg/m2 and/or unintentional weight loss of more than 10 lb (4.5 kg) in the past 6 months. Slowness was classified as the lowest quintile values (stratified for gender and height) in the average of two measurements of the 6‐m fast gait speed test. Weakness was measured by dominant knee extension, and participants in the lowest quintile of a gender‐adjusted and body mass index‐adjusted average value from three trials were defined as weak. Exhaustion was denoted as a score of <10/15 on the vitality domain in the SF‐12. Physical activities were assessed using self‐reported time (in hours) spent daily doing light, moderate, and vigorous activities. Participants were defined as low activity if the total amount of time they spent on performing moderate to vigorous activities per week was in the gender‐specific lowest quintile. A participant with three or more components was grouped as frail, one to two components as pre‐frail, and none of the components as robust. All participants were of Chinese ethnicity and both male and female participants were included. All participants were home‐dwelling and patients with recent cancer, heart failure, or dialysis were excluded. All elderly participants lived in a very similar environment (nutritional, physical activity, and socio‐economic status). The participants were also screened for cognitive functions, and individuals with a Mini‐Mental State Examination <23 (classified as cognitively impaired) were excluded. The study has been approved by the National University of Singapore‐Institutional Review Board 04–140, and all participants gave written, informed consent. Blood from overnight fasting participants was drawn in CPT tubes (BD Biosciences, San Jose, CA, USA), and after centrifugation at 1650 rpm for 20 min at room temperature, plasma was collected and stored at −80°C.
Animal studies
All experimental procedures on animals were in agreement with Swiss and European Union ethical guidelines and approved by the internal Nestlé Animal Study Committee and the local animal experimentation committee of the Canton of Vaud (licence number VD2630). Male Wistar rats aged 8 to 24 months were obtained from Janvier Labs (Le Genest‐Saint‐Isle, France). Upon arrival, all animals were housed by two in standard type 4 cages in a specific pathogen free environment, with ad libitum access to food and water on a 12 h light/dark cycle at a temperature between 20–24°C and a relative humidity between 50–60%. All rats labelled by age were grouped by date of birth under the following categories: adult (8–10 months of age), middle‐aged (16–18 months of age), and aged (22–24 months of age) and kept for 2–4 weeks in our facilities before sacrifice. Rats were fed with a complete diet (Sniff S8189‐S085 derived from R/M‐H Ered‐I, see Figure S1 ), containing a full vitamin supplement including vitamin B12, both before and after transfer to our facility. All physiological measurements were performed after at least 2 weeks of acclimatization in our facility. Food intake was evaluated by weighing food pellets twice a week. Rats were sacrificed by exsanguination followed by diaphragm puncture under isoflurane anaesthesia, and organs of interest were dissected free of fat. Liver, kidney, and phosphate buffer‐rinsed ileum were dry frozen in liquid nitrogen. Blood was collected in specific tubes to obtain serum, which was subsequently frozen in liquid nitrogen.
Blood measurements
Total vitamin B12 levels in serum were measured using the competitive binding assay from Beckman (Beckman Coulter, Nyon, Switzerland). MMA levels were determined in plasma by GC‐MS/MS at BeVital laboratories (Bergen, Norway), as described previously.28 The reported lower limit of detection was 0.03 μmol/L and within‐day and between‐day coefficients of variation were 1–4% and 3–8%, respectively. AMN measurements in rat serum were performed using DNA aptamer‐based recognition (SomaLogic, Boulder, CO, USA), as previously described.29 Median normalized relative fluorescence units were log2‐transformed before applying principal component analysis and linear models. Statistical analyses were performed in R 3.1.3 (R Foundation for Statistical Computing). AMN measurements in human serum were performed by sandwich Enzyme‐Linked Immunosorbent Assay (Cloud‐Clone Corp. #SEC295Hu).
Urine and liver measurements
For 24 h urine collection, rats were placed for 24 h in individual cages equipped with grids and a collection disposal containing 1 mL of 1 M HCl to prevent bacterial growth. Urine volume was then measured and samples were snap‐frozen in liquid nitrogen. Total vitamin B12 and creatinine levels in urine were measured using the competitive binding assay from Abbott (Abbott AG, Switzerland). For AMN measurement, a customized sandwich‐ELISA test was developed using anti‐AMN antibodies (RD‐Systems #AF7139 and Novus Biologicals #NBP1–91203) (RD‐Biotech, Besançon, France).
For hepatic vitamin B12 measurements, a small piece of approximately 60 mg of liver was mechanically lysed using a Polytron homogenizer in a buffer with 50 mM Tris (pH 7.5), 1 mM EDTA, 1 mM EGTA, 0.27 M sucrose, 1% Triton X‐100. Total vitamin B12 levels were measured on liver extracts using the competitive binding assay from Abbott (Abbott AG, Switzerland) and were normalized to the amount of tissue analysed.
Western blot
Kidney and ileum frozen samples were mechanically lysed using a Polytron homogenizer, and the proteins were extracted in 50 mM Tris (pH 7.5), 1 mM EDTA, 1 mM EGTA, 0.27 M sucrose, 1% Triton X‐100, 20 mM glycerol‐2‐phosphate disodium, 50 mM NaF, 5 mM Na4P2O7, 0.5 mM PMSF, 1 mM benzamidine, 1 mg/mL leupeptin, 1 mg/mL pepstatin A, 1 mM DTT, 1 mM microcystin, 1 mM Na3VO4. Protein concentration was determined by a bicinchoninic acid assay (Pierce), and samples were diluted at 2.6 mg/mL and boiled 5 min in Laemmli buffer. Human serum samples diluted 1/10, and human recombinant AMN (R&D systems #1860‐AM‐050) were also boiled 5 min in Laemmli buffer. AMN levels were resolved by standard western blot procedures. For megalin and CBN, samples were run on 3–8% Tris‐acetate gradient gels (Novex #EA03755) and then transferred using the wet system from Life Technologies. Antibodies used were Lrp2/Megalin (Santa Cruz #16478, 1/200), CBN (Santa Cruz #20609, 1/200), AMN (Abcam #170893, 1/1000 for rat samples; R&D systems #MAB1860‐SP, 1/1000 for human samples), HSC70 (Santa Cruz #7298, 1/500 000).
Reverse transcription and quantitative polymerase chain reaction
Total RNA was extracted from ileum and kidney using a commercial kit (miRNeasy Mini Kit, Qiagen) according to the manufacturer's instruction, and the concentration was measured using a NanoDrop device. Total RNA extracts were diluted at 50 ng/mL and reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative polymerase chain reaction reactions were performed using LightCycler DNA Green Master (Roche) on the LightCycler 384 Real‐Time PCR System thermocycler (Roche). The amplification curves were analysed by the LightCycler 480 SW 1.5 software. Specific SYBR Green primers used for each gene are AMN Fwd 5′‐GTTCCCGGCGGACAAGATGG‐3′ Rev 5′‐GACGAACTCCCCGTTCAGCG‐3′; EEF2 (reference gene) Fwd 5′‐TGGTGGGCGGAATCTATGGT‐3′ Rev 5′‐CGGCAGTGAAGCCAAAGGAT‐3′.
Statistical analysis
All the statistical analyses of preclinical data were performed using the GraphPad Prism software, and statistical significance was assessed by t‐test for two sample comparisons. For comparison of more than two groups, one‐way ANOVA followed by Bonferroni's multiple comparison test was used. All data are expressed as mean value +/− SEM.
All the statistical analyses of clinical data were performed using the R 3.3.2 and figures were generated using the R library ggplot2_2.2.0. Associations between two continuous variables or between a continuous variable and an ordinal variable were estimated using Spearman's rank correlation. Two‐sample Wilcoxon test (also known as Mann–Whitney test) was performed to compare continuous data between two groups, and Kruskal‐Wallis test was used to compare more than two groups. Fisher's exact test for count data was used to analyse tabular data. All tests were two‐sided.
Results
Verlaan et al. recently demonstrated that sarcopenic patients have lower levels of circulating vitamin B12 compared with age‐matched elderly controls.25 This observation suggested that vitamin B12 levels could be linked to mobility and physical function in the elderly, although measuring total vitamin B12 levels may detect variations that are not clinically meaningful as they may not be sufficient to impact vitamin B12 enzymes throughout the body. In this study, we used blood measurements of MMA as a proxy for functional vitamin B12 deficiency. MMA is an intracellular metabolite upstream of methyl‐malonyl‐CoA mutase, one of the two vitamin B12‐dependent enzymes in the body. MMA is cell permeable and accumulates in blood when MMA increases intracellularly as a result of low methyl‐malonyl‐CoA mutase activity caused by vitamin B12 deficiency. MMA levels were measured in the blood of 238 participants to the Singapore Longitudinal Aging Study and analysed according to age and frailty status (Table 1). Elderly participants had significantly higher levels of blood MMA than young controls (Figure 1A, Kruskal‐Wallis chi‐squared = 19.9, df = 3, P‐value = 1.8e‐04). We then stratified the elderly population according to the Fried criteria for physical frailty,4 resulting in a gradual decline of gait speed and strength in pre‐frail and frail groups (Table 2). MMA levels increased in frail elderly compared with pre‐frail and robust elderly (Figure 1A). Robust elderly had a 4.8‐fold higher prevalence of vitamin B12 deficiency compared with young adult controls (prevalence of 3.3% in young adult controls vs 16.1% in robust elderly; Figure 1B), and the prevalence of vitamin B12 deficiency in frail elderly was two‐fold higher than in robust elderly and 1.7‐fold higher than in pre‐frail elderly (prevalence of 16.1% in robust elderly, 18.6% in pre‐frail, and 31.7% in frail; Figure 1B). Altogether, our results confirm that aging leads to vitamin B12 deficiency in an Asian population and demonstrate that age‐related vitamin B12 deficiency is aggravated in people showing loss of physical function and mobility detected by the Fried frailty criteria.
Table 1.
Cohort characteristics
| Young Adults | Elderly | |
|---|---|---|
| Number participants | 30 | 208 |
| Number men (%) | 11 (37) | 80 (38) |
| Number women (%) | 19 (63) | 128 (62) |
| Age | 28.0 ± 3.6 | 67.6 ± 15.9 |
| BMI | 22.8 ± 3.5 | 23.6 ± 3.6 |
BMI, body mass index
Figure 1.
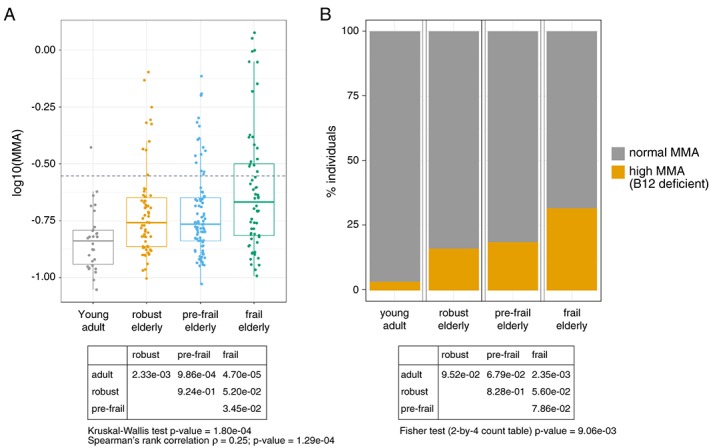
Aging and frailty associate with elevated methyl‐malonic acid (MMA) and a higher prevalence of vitamin B12 deficiency. (A) Levels of MMA were measured in the serum of 23–35 years young adults and aged (≥65 years) participants to the Singapore longitudinal aging study and plotted according to the Fried frailty score (robust = no functional impairment; pre‐frail = impairment of 1–2 domains; frail = impairment of 3 or more domains). The dotted line represents the threshold for the clinical diagnosis of vitamin B12 deficiency based on the MMA cut‐off of 0.28 μM. The overall statistical significance was tested using a Kruskal‐Wallis test, and two group comparisons were analysed using Wilcoxon tests. MMA status was also analysed using frail status as an ordinal categorical variable using Spearman's rank correlation. (B) Percentage of individuals per group with vitamin B12 deficiency (high MMA) as measured by a MMA level above the cut‐off value of 0.28 μM. The overall statistical significance was tested using a Fisher's exact test on a 2‐by‐4 table with count data, and two group comparisons were tested by Fisher's exact tests on 2‐by‐2 tables. Detailed statistics are provided in Table S1 .
Table 2.
Stratification of the elderly participants according to physical frailty. Statistics were performed using a Wilcoxon test compared to the robust group
| Robust | Pre‐Frail | Frail | |
|---|---|---|---|
| Number participants | 62 | 86 | 60 |
| Number men (%) | 27 (43) | 32 (37) | 21 (35) |
| Number women (%) | 35 (57) | 54 (63) | 39 (65) |
| Age (years) | 72.1 ± 4.9 | 73.0 ± 5.1 | 75.2 ± 5.5 |
| BMI (kg/m2) | 24.0 ± 3.0 | 24.1 ± 3.7 | 22.9 ± 3.9 |
| Gait speed (m/s) | 1.21 ± 0.26 | 1.04 ± 0.23*** | 0.91 ± 0.29*** |
| Hand‐grip strength (kg) | 21.7 ± 10.0 | 16.3 ± 5.4* | 15.6 ± 6.2* |
| Knee extension strength (kg) | 16.3 ± 5.0 | 13.8 ± 4.5** | 9.7 ± 3.9*** |
P < 0.05
P < 0.01
P < 0.001
Aging associates with physiological perturbations of several organs, including the gut, liver, and kidney that are important for vitamin B12 homeostasis.11, 15 We therefore speculated that aging could potentially alter vitamin B12 metabolism independently of dietary intake. In order to test this possibility, we evaluated how circulating vitamin B12 levels change with age in a rat model of natural aging bred in a controlled environment and fed during their entire life with the same controlled diet containing the recommended amounts of vitamin B12 for rodents (Figure S1 ; food reference S8189‐S085, SNNIFF). Under these conditions, aged rats naturally develop sarcopenia and frailty starting at 18 months of age, and the phenotype worsens until 24 months of age when they start to die.30, 31 When comparing circulating levels of total vitamin B12 in adult and aging animals, we observed a progressive decrease that became significant at 22–24 months (referred to as ‘aged’) (Figure 2A). Liver is one of the major reservoirs for storage of vitamin B12 in the body.11 Measurement of vitamin B12 levels in liver extracts revealed that aging also leads to a reduction of endogenous pools as hepatic vitamin B12 levels were lower in aged than adult rats (Figure 2B). Importantly, we confirmed that vitamin B12 deficiency during aging did not arise from poor dietary intake as food intake was similar in adult and aged animals (Figure 2C), and rats consumed a controlled diet rich in vitamin B12 (150 μg/kg cobalamin). In addition, all groups of rats had similar body weights (data not shown), excluding the possibility that body size could confound vitamin B12 metabolism. These results therefore demonstrate that aging causes specific physiological perturbations that cause vitamin B12 deficiency independent of dietary intake.
Figure 2.
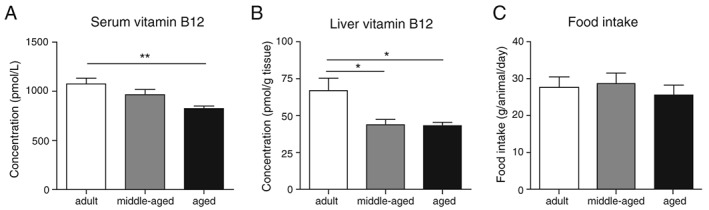
Serum vitamin B12 levels are decreased with age independently of food intake in rats. (A) Circulating levels of total vitamin B12 were measured in adult (8–10 months old), middle‐aged (16–18 months old), and aged (22–24 months old) Wistar rats (n = 9–10 rats per group). (B) Hepatic levels of total vitamin B12 were measured in adult, middle‐aged, and aged rats (n = 9–10 rats per group). (C) Average food intake was measured in adult, middle‐aged, and aged rats (n = 5 cages per group). Data are mean +/− SEM. **P < 0.01 with a one‐way ANOVA test.
To further investigate the causes of decreased circulating vitamin B12 in aging rats, we next evaluated whether aging would impact the intestinal absorption and/or the renal reabsorption systems. In intestine, vitamin B12 is absorbed in the ileum where the IF‐B12‐CBN complex requires AMN for internalization.12 Western blot analysis for AMN in rat ileum revealed that AMN is strongly reduced at old age (Figure 3), suggesting that active intestinal absorption of vitamin B12 is impaired in aged animals. In adults, AMN is expressed specifically in the small intestine as well as in kidney (Fig. S2). Renal reabsorption after glomerular filtration is an important process to regulate the urinary loss of small nutrients. Although biliary excretion is the major route of vitamin B12 elimination,32 urinary loss of vitamin B12 is receiving increasing attention15, 33, 34 and has been reported to reach up to 30% of total vitamin B12 excretion.32 Vitamin B12 tubular reabsorption relies on the CBN/AMN receptor together with a third player called megalin/LRP2.15, 35 Western blot analysis for AMN, CBN, and LRP2 in kidney revealed that both CBN and LRP2 levels are stable with age while AMN is increased at old age (Figure 4A and 4B). To evaluate how this regulation of AMN could impact renal function, we measured the urinary levels of vitamin B12 over 24 h and found a significant increase in vitamin B12 excretion in aged rats (Figure 4C). In parallel, levels of creatinine were stable over the age range analysed (Figure 4D), demonstrating that global renal function is not impaired. Together, these results suggest that impaired vitamin B12 renal reabsorption during aging is a specific phenomenon rather than the consequence of a global renal failure and that AMN plays a major role in this process.
Figure 3.
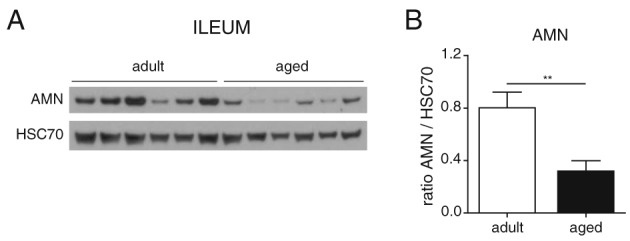
Aging impairs the intestinal vitamin B12 uptake system through down‐regulation of Amnionless. (A) Ileum protein levels of amnionless (AMN) in adult and aged rats quantified by western blot (n = 6 per group). (B) Graphs showing the protein quantification of the six replicates. Data are represented as mean +/− SEM. ** P < 0.01 with an unpaired t‐test.
Figure 4.
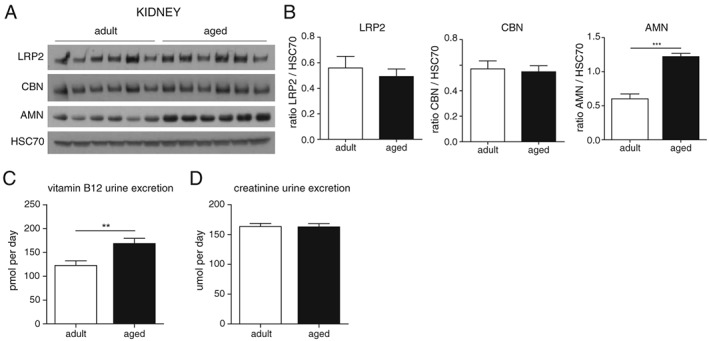
Renal vitamin B12 reabsorption is impaired with age. (A) Kidney protein level of amnionless (AMN), cubilin (CBN), and megalin (LRP2) in adult and aged rats quantified by western blot (n = 6 per group). (B) Graphs showing the protein quantification of the six replicates. (C,D) vitamin B12 (C) and creatinine (D) levels in urine of adult and aged rats over 24 h (n = 14 per group). Data are represented as mean +/− SEM. For all graphs *P < 0.05 **P < 0.01 ***P < 0.001 with an unpaired t‐test.
The alterations of AMN levels with age in the intestine and kidney raised the possibility of using AMN as a biomarker of vitamin B12 bioavailability provided that it could be detected in matrices that are accessible in humans. AMN has previously been detected in urine,12 but to our knowledge, has never been measured in blood. Our initial tests demonstrated that AMN is detectable in human serum by ELISA (quantitative results will be presented in Figure 6 further down). In order to confirm this observation and understand the nature of the circulating AMN protein, we used a western blotting assay to detect AMN in human serum and compare it to recombinant AMN (Figure 5A). AMN was robustly detected in serum both as a full length 48 kDa protein (Figure 5A black arrows) and two shorter fragments of approximately 28 and 30 kDa which are more abundant. AMN levels in urine were not affected by age in rats (Figure 5C). In contrast, AMN levels in serum increased significantly by ~30% with age (Figure 5B). The observation that AMN levels are increased with age in kidney while decreased in ileum (Figures 3 and 4) suggests that the increased AMN level in serum originate from kidney. In support of this hypothesis, we found that basal levels of AMN are higher in kidney than ileum (Figure 5D). In addition, AMN is expressed as a 48 kDa full length protein in kidney without any shorter degradation fragment (Fig. S3). Together with the fact that full‐length AMN is detected in serum (Figure 5A), this result suggests that AMN is released in the circulation as a full length protein. Interestingly, the increase of AMN in serum during aging precedes the drop of vitamin B12 levels, suggesting that AMN could be used as an early, specific, and sensitive biomarker of vitamin B12 bioavailability during aging. Indeed, we found a negative correlation between serum AMN and vitamin B12 levels in rats (Figure 5E). Finally, we confirmed our preclinical observations using the human Singapore Longitudinal Aging Studies cohort where circulating AMN levels increased significantly by 21% in elderly individuals compared with 23–35 year old young adults (Figure 6A; P‐value = 9.2e‐07). In this cohort, AMN levels did not correlate with frailty (Table 3) and physical function (data not shown). We then evaluated whether AMN levels are related to vitamin B12 deficiency in humans. AMN levels were positively correlated with MMA in blood (Figure 6B; r = 0.22, P‐value = 6.8e‐04), suggesting that AMN correlates with functional vitamin B12 deficiency marked by high MMA levels. To confirm this possibility, we stratified the population according to MMA levels below or above 0.28 μM, the threshold indicative of vitamin B12 deficiency.36, 37 Vitamin B12 deficient participants with high blood MMA levels showed significantly higher AMN levels (Figure 6C; P‐value = 0.017). These data suggest that elevated AMN levels could be indicative of vitamin B12 deficiency in the elderly.
Figure 6.
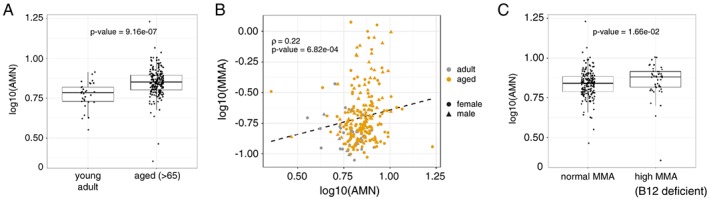
Serum amnionless levels increase with age and positively correlate with MMA levels in humans. (A) Levels of amnionless (AMN) were measured in the serum of young adult controls (n = 30) and aged participants of the Singapore longitudinal aging study (n = 238). P‐value was calculated with a Wilcoxon rank sum test with continuity correction. (B) Scatter plot of AMN levels and methyl‐malonic acid (MMA) levels in serum. Spearman rank correlation (ρ) and its P‐value are reported. Dashed line: linear regression. (C) Levels of AMN in individuals with normal MMA levels or presenting a vitamin B12 deficiency (high MMA, i.e. above the 0.28 μM threshold for deficiency). P‐value was calculated with a Wilcoxon rank sum test with continuity correction. Detailed statistics are provided in Table S1 and graphs presented in linear scale are provided in Fig. S4.
Figure 5.
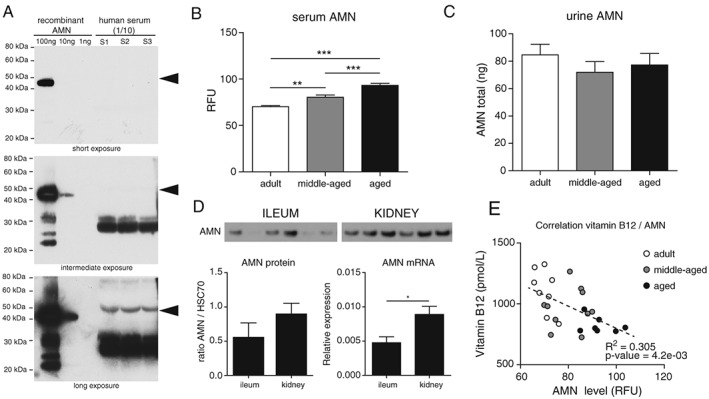
Amnionless is detected in serum and increases with age while inversely correlating with vitamin B12 levels in rats. (A) Amnionless (AMN) protein was detected by western blot using a specific AMN antibody on three independent human sera diluted 1/10, and compared with 1, 10, and 100 ng of human recombinant AMN. Black arrows indicate the size of full‐length AMN at 48 kDa. (B‐C) levels of AMN protein were measured in the serum (B) and urine (C) of adult, middle‐aged, and aged rats (n = 9–10 per group). (D) Comparative protein and mRNA levels of AMN in ileum and kidney from adult rats. (E) Association between circulating levels of AMN and vitamin B12 in the same animals as in (B). Dashed line: linear regression. Data are represented as mean +/− SEM. *P < 0.05 **P < 0.01 ***P < 0.001 with a one‐way ANOVA test.
Table 3.
Plasma amnionless levels stratified by age and frailty status. The overall statistical significance was tested using a Kruskal‐Wallis test, and two group comparisons were analysed using Wilcoxon tests
| AMN (ng/mL) median ± i.q.r | P‐value vs. young adult | P‐value vs. robust elderly | |
|---|---|---|---|
| Young adult | 6.08 ± 1.23 | ‐ | ‐ |
| Robust elderly | 7.25 ± 1.59 | 1.26e‐05 | ‐ |
| Pre‐Frail elderly | 6.99 ± 1.39 | 1.16e‐05 | 0.511 |
| Frail elderly | 7.29 ± 1.56 | 1.26e‐05 | 0.910 |
Kruskal‐Wallis chi‐squared = 24.76, df = 3, P‐value = 1.73e‐05
AMN, amnionless
Taken together, we have demonstrated that aging and physical frailty associate with increased prevalence of vitamin B12 deficiency. Low blood vitamin B12 levels can have causes independent of dietary intake which are linked to poor intestinal transport and altered renal reabsorption. In addition, our results establish AMN as a novel biomarker with the potential to detect perturbed vitamin B12 bioavailability during aging.
Discussion
Acquired vitamin B12 deficiency is often linked to poor nutritional intake, in particular in vegetarians and vegans where the absence of meat consumption creates a risk for deficient vitamin B12 intake as vitamin B12 is primarily found in animal tissues such as liver or muscle.38, 39 In addition, a significant proportion of the elderly population suffers from chronic malnutrition and appetite loss which create variability when trying to understand how aging impacts micro‐nutrient metabolism in the body. In this study, we combined preclinical and clinical investigation of vitamin B12 physiopathology to overcome these limitations and study molecular mechanisms directly in the relevant organs. Overall, our work confirms that aging is an important risk factor for vitamin B12 deficiency in humans.11, 40 We demonstrate that both aging and frailty associate with higher levels of MMA indicative of a higher prevalence of vitamin B12 deficiency in the Singapore Longitudinal Aging Study. A similar association was also recently demonstrated in a different human population with mobility deficits where sarcopenic patients had lower levels of circulating vitamin B12 compared with age‐matched elderly controls.25 Taken together, these observations strongly suggest that vitamin B12 deficiency could directly impact muscle performance and physical function in the elderly.
Importantly, we bypassed the inter‐individual variability in nutritional intake that occurs in humans by using a preclinical model of aging rats fed their entire life the same controlled diet containing high amounts of vitamins and micro‐nutrients. Our results demonstrate that pathophysiological cues associated with aging can directly alter blood vitamin B12 levels independently of nutritional intake through perturbed absorption and elimination. Intestinal vitamin B12 intake is an active process where IF‐bound vitamin B12 binds to its receptor CBN and is then internalized into the enterocyte via AMN. Our data show that AMN protein is massively reduced in aged ileum. The role of AMN in regulating vitamin B12 intake has been demonstrated through the identification of monogenic associations of AMN mutations with recessive hereditary megaloblastic anaemia, an inherited condition of severe vitamin B12 deficiency.41 In addition, the identification of breeds of dogs with AMN mutations has revealed that altered AMN function dysregulates CBN localization at the brush‐border and thereby impairs vitamin B12 absorption.42 The reduction of intestinal AMN expression which we observe during aging, therefore, most likely contributes to poor vitamin B12 absorption and blood vitamin B12 decline with age.
Interestingly, we also observed perturbations in the aged kidney which lead to a urinary loss of vitamin B12 during renal reabsorption. Another potential contributor to age‐related loss of vitamin B12 which could not be tested experimentally is the biliary excretion of vitamin B12 as this is the primary mechanism for vitamin B12 elimination.32 Nevertheless, amnionless and CBN are not expressed in the liver and can therefore not contribute to biliary loss of vitamin B12. In contrast, CBN and AMN are highly expressed in the kidney, together with megalin / LRP2 which can bind urinary ligands and mediate endocytosis in nephritic cells to promote their reuptake in the blood.15 AMN levels were significantly elevated in the kidney of aged rats in direct association with the urinary loss of vitamin B12. It is unlikely that urinary loss of vitamin B12 with age results from the higher expression of AMN as the majority of vitamin B12 entering the renal tubules is bound to blood carriers such as haptocorrin or transcobalamin, which bind to LRP2.43 LRP2 does not need AMN for internalization, but close interactions exist between LRP2, CBN, and AMN.44, 45 LRP2‐deficient mice display high levels of vitamin B12 in the urine, supporting the fact that LRP2 is the major ligand for vitamin B12 renal reabsorption.43 However a small proportion of IF‐bound vitamin B12 is present in the blood and can be reabsorbed in the kidney through AMN/CBN as IF has been identified in urine.46 It was recently shown in drosophila that AMN is required for CBN‐mediated protein reabsorption.47 Most of the renal CBN ligands can, however, also bind to megalin,15 suggesting that defects in AMN would only lead to partial defects in CBN‐mediated absorption. Consistently, the stable levels of creatinine in urine indicated that global renal function was not altered with age in our experiments and that urinary loss is specific for vitamin B12 and not a consequence of the entire reuptake machinery in kidney. It is therefore likely that the increased levels of AMN in kidney could be due to a compensatory mechanism for the dysfunction of a specific renal transporter that could be mislocalized or impaired posttranslationally.
Our study also reports for the first time the detection of a circulating form of AMN in serum using independent detection by ELISA and western blot. Consistently, AMN has been detected in the global human plasma proteome measured by non‐targeted mass spectrometry,48 as well as in urine using antibody‐based detection.12 The concept of a soluble version of a membrane‐bound vitamin B12 transporter has previously been described for CD320,49, 50, 51 the receptor which binds the transcobalamin/vitamin B12 complex in blood and mediates its import in target tissues.52 Although soluble CD320 (sCD320) expression correlates with vitamin B12 levels and changes with physio‐pathological situations such as pregnancy or dementia,49, 50, 51 the biological function of soluble CD320 remains unclear. Since sCD320 is in contact with holo‐transcobalamin in blood and contains the active binding domain, one possibility is that the soluble receptor acts as a decoy which sequesters holo‐transcobalamin away from target tissues and limits cellular bioavailability of vitamin B12. Soluble AMN is not biologically active alone as it requires CBN or megalin to bind its ligands. According to plasma proteome databases (http://plasmaproteomedatabase.org/), CBN does not seem to be present in blood while megalin and AMN peptides are detected. Given that megalin is a very large 500 kDa protein, it remains unclear whether megalin circulates as a full length functional protein. Another difference is that soluble CD320 contains only the extracellular domain of the CD320 receptor while circulating AMN is present, at least in part, as a full length protein. This difference may arise from the fact that AMN is expressed exclusively in polarized epithelial cells of the intestine and kidney where AMN mediates the transport from the apical to the basolateral side of the epithelium. Interestingly, epithelial cells are an important source of exosomes and micro‐vesicles which travel in the circulation and can be used as tissue surrogates for biomarker detection.53 AMN is a protein which mediates the transport of its ligands through endocytosis and therefore enriches in cellular vesicles. Given that part of the circulating AMN was detected as full length protein and that smaller degradation fragments are not detected in the tissues expressing AMN, it is therefore possible that AMN is secreted as a membrane‐bound protein in exosomes. In addition, we also detected lower molecular weights of AMN in serum, similar to what has been observed in urine,12 which could correspond to proteolytic cleavage of the full length protein given that they are not expressed in AMN‐producing tissues.
Acquired vitamin B12 deficiency in human is most commonly due to impaired gastrointestinal function. Early studies have, however, failed to demonstrate a link between low vitamin B12 levels and atrophic gastritis or simply age.54 Our data suggest an important role for the small intestine and kidney in mediating the circulating vitamin B12 pool. We demonstrate major perturbations of intestinal and renal AMN expression which most likely contribute to impaired epithelial cell transport during aging. Consistently, urinary vitamin B12 excretion has been reported to increase with age in both rodents55 and humans.33 Together, these data highlight the importance of renal function in vitamin B12 and micronutrient metabolism and demonstrate that aging is a risk factor for loss of vitamin B12 in the urine.
Poor vitamin B12 status has been correlated to several age‐related chronic conditions.11, 40 However, limited knowledge exists on vitamin B12 interventions in the elderly despite the fact that vitamin B12 is widely recommended to prevent age‐related conditions. Placebo controlled randomized clinical trials have shown that vitamin B12 supplementation can restore circulating vitamin B12 levels and direct biomarkers of vitamin B12 deficiency in some but not all elderly.56, 57 There is only limited evidence for the efficacy of vitamin B12 on frailty parameters, and studies evaluating vitamin B12 in the elderly are often performed in combination with other micronutrients. One study evaluating the effect of a combination of vitamin B12 and folate on endpoints of physical function failed to detect significant improvements in parameters linked to frailty.58 Our results have demonstrated that the patho‐physiological decline of intestinal and renal function of vitamin B12 transport during aging directly contributes to poor vitamin B12 status. It is therefore likely that a proportion of individuals in the elderly population are poorly responsive to vitamin B12 supplementation. We have shown that AMN can be detected in serum, and its levels are progressively increasing during aging. In addition, blood AMN levels negatively correlate with vitamin B12 and positively correlates with MMA. As such, AMN could be used as a novel biomarker to detect early stages of vitamin B12 deficiency and stratify the elderly population according to perturbations of endogenous vitamin B12 processing and potentially predict responsiveness to vitamin B12 intervention.
Acknowledgements
We thank the NIHS Preclinical Investigation team for help in performing animal experimentation.
Alice Pannérec, Joris Michaud, Sonia Karaz, and Laurence Goulet performed experiments. Alice Pannérec, Eugenia Migliavacca, Antonio De Castro, Laurence Goulet and Serge Rezzi analysed data. Alice Pannérec, Tze Pin Ng, Nabil Bosco, Anis Larbi and Jerome N. Feige designed and interpreted experiments. Alice Pannérec and Jerome N. Feige wrote the manuscript. All authors read and edited the manuscript.
Alice Pannérec, Eugenia Migliavacca, Joris Michaud, Sonia Karaz, Laurence Goulet, Serge Rezzi, and Jerome N. Feige are full‐time employees the Nestlé Institute of Health Sciences and Antonio De Castro and Nabil Bosco are full‐time employees of Nestlé.
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.59
Conflict of interest
Tze Pin Ng and Anis Larbi declare that they have no conflict of interest.
Supporting information
Supplementary Table S1. Summary statistics of the clinical data in figures 1 and 6.
Supplementary Figure S1. Composition of the diet used for rodent studies.
Supplementary Figure S2. Expression profile of Amnionless mRNA in mouse tissues. Data was recovered from www.biogps.org.
Supplementary Figure S3. Unprocessed images from the western blot data in rat kidney presented in Figure 4A. Western blots with anti‐AMN and anti‐GAPDH antibodies as well an amido black staining of total protein loading on the membrane are shown together with the molecular weight ladder.
Supplementary Figure S4. Graphs of the clinical data in figures 1 and 6 presented in linear scale.
Pannérec, A. , Migliavacca, E. , De Castro, A. , Michaud, J. , Karaz, S. , Goulet, L. , Rezzi, S. , Ng, T. P. , Bosco, N. , Larbi, A. , and Feige, J. N. (2018) Vitamin B12 deficiency and impaired expression of amnionless during aging. Journal of Cachexia, Sarcopenia and Muscle, 9: 41–52. doi: 10.1002/jcsm.12260.
References
- 1. United Nations . World population ageing. 2015. http://www.un.org/en/development/desa/ population/publications/pdf/ageing/WPA2015_Report.pdf. 2016.
- 2. Tian Q, An Y, Resnick SM, Studenski S. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: results from the Baltimore Longitudinal Study of Aging. Age Ageing 2016;46:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 5. Williams SC. Frailty research strengthens with biomarker and treatment leads. Nat Med 2013;19:517. [DOI] [PubMed] [Google Scholar]
- 6. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landi F, Calvani R, Cesari M, Tosato M, Martone AM, Bernabei R, et al. Sarcopenia as the biological substrate of physical frailty. Clin Geriatr Med 2015;31:367–374. [DOI] [PubMed] [Google Scholar]
- 9. Choi J, Ahn A, Kim S, Won CW. Global prevalence of physical frailty by Fried's criteria in community‐dwelling elderly with national population‐based surveys. J Am Med Dir Assoc 2015;16:548–550. [DOI] [PubMed] [Google Scholar]
- 10. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community‐dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–1492. [DOI] [PubMed] [Google Scholar]
- 11. Hughes CF, Ward M, Hoey L, McNulty H. Vitamin B12 and ageing: current issues and interaction with folate. Ann Clin Biochem 2013;50:315–329. [DOI] [PubMed] [Google Scholar]
- 12. Fyfe JC, Madsen M, Hojrup P, Christensen EI, Tanner SM, de la Chapelle A, et al. The functional cobalamin (vitamin B12)‐intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood 2004;103:1573–1579. [DOI] [PubMed] [Google Scholar]
- 13. Seetharam B, Yammani RR. Cobalamin transport proteins and their cell‐surface receptors. Expert Rev Mol Med 2003;5:1–18. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen MJ, Rasmussen MR, Andersen CB, Nexo E, Moestrup SK. Vitamin B12 transport from food to the body's cells—a sophisticated, multistep pathway. Nat Rev Gastroenterol Hepatol 2012;9:345–354. [DOI] [PubMed] [Google Scholar]
- 15. Birn H. The kidney in vitamin B12 and folate homeostasis: characterization of receptors for tubular uptake of vitamins and carrier proteins. Am J Physiol Renal Physiol 2006;291:F22–F36. [DOI] [PubMed] [Google Scholar]
- 16. Aminoff M, Carter JE, Chadwick RB, Johnson C, Grasbeck R, Abdelaal MA, et al. Mutations in CUBN, encoding the intrinsic factor‐vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nat Genet 1999;21:309–313. [DOI] [PubMed] [Google Scholar]
- 17. van Asselt DZ, de Groot LC, van Staveren WA, Blom HJ, Wevers RA, Biemond I, et al. Role of cobalamin intake and atrophic gastritis in mild cobalamin deficiency in older Dutch subjects. Am J Clin Nutr 1998;68:328–334. [DOI] [PubMed] [Google Scholar]
- 18. Carmel R. Cobalamin, the stomach, and aging. Am J Clin Nutr 1997;66:750–759. [DOI] [PubMed] [Google Scholar]
- 19. Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin intervention for stroke prevention trial: an efficacy analysis. Stroke 2005;36:2404–2409. [DOI] [PubMed] [Google Scholar]
- 20. Rafnsson SB, Saravanan P, Bhopal RS, Yajnik CS. Is a low blood level of vitamin B12 a cardiovascular and diabetes risk factor? A systematic review of cohort studies. Eur J Nutr 2011;50:97–106. [DOI] [PubMed] [Google Scholar]
- 21. Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, Scott J, et al. Low vitamin B‐12 status and risk of cognitive decline in older adults. Am J Clin Nutr 2007;86:1384–1391. [DOI] [PubMed] [Google Scholar]
- 22. Dhonukshe‐Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res 2005;20:921–929. [DOI] [PubMed] [Google Scholar]
- 23. Mithal A, Bonjour JP, Boonen S, Burckhardt P, Degens H, El Hajj FG, et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos intJ established as result of coop between the Eur Found Osteoporos National Osteoporos Found USA 2013;24:1555–1566. [DOI] [PubMed] [Google Scholar]
- 24. van Schoor NM, Swart KM, Pluijm SM, Visser M, Simsek S, Smulders Y, et al. Cross‐sectional and longitudinal association between homocysteine, vitamin B12 and physical performance in older persons. Eur J Clin Nutr 2012;66:174–181. [DOI] [PubMed] [Google Scholar]
- 25. Verlaan S, Aspray TJ, Bauer JM, Cederholm T, Hemsworth J, Hill TR, et al. Nutritional status, body composition, and quality of life in community‐dwelling sarcopenic and non‐sarcopenic older adults: a case‐control study. Clin Nutr 2017;36:267–274. [DOI] [PubMed] [Google Scholar]
- 26. Feng L, Zin Nyunt MS, Gao Q, Feng L, Yap KB, Ng TP. Cognitive frailty and adverse health outcomes: findings from the Singapore Longitudinal Ageing Studies (SLAS). J Am Med Dir Assoc 2017;18:252–258. [DOI] [PubMed] [Google Scholar]
- 27. Lu Y, Tan CT, Nyunt MS, Mok EW, Camous X, Kared H, et al. Inflammatory and immune markers associated with physical frailty syndrome: findings from Singapore longitudinal aging studies. Oncotarget 2016;7:28783–28795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Windelberg A, Arseth O, Kvalheim G, Ueland PM. Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography‐mass spectrometry. Clin Chem 2005;51:2103–2109. [DOI] [PubMed] [Google Scholar]
- 29. Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer‐based multiplexed proteomic technology for biomarker discovery. PLoS One 2010;5: e15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pannerec A, Springer M, Migliavacca E, Ireland A, Piasecki M, Karaz S, et al. A robust neuromuscular system protects rat and human skeletal muscle from sarcopenia. Aging (Albany NY) 2016;8:712–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol 2013;33:194–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okuda K, Grasbeck R, Chow BF. Bile and vitamin B12 absorption. J Lab Clin Med 1958;51:17–23. [PubMed] [Google Scholar]
- 33. Fukuwatari T, Sugimoto E, Tsuji T, Hirose J, Fukui T, Shibata K. Urinary excretion of vitamin B12 depends on urine volume in Japanese female university students and elderly. Nutr Res 2009;29:839–845. [DOI] [PubMed] [Google Scholar]
- 34. Birn H, Nexo E, Christensen EI, Nielsen R. Diversity in rat tissue accumulation of vitamin B12 supports a distinct role for the kidney in vitamin B12 homeostasis. Nephrol dialysis, Transplant : official Publ Eur Dialysis and Transplant Assoc Eur Ren Assoc 2003;18:1095–1100. [DOI] [PubMed] [Google Scholar]
- 35. Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol 2001;280:F562–F573. [DOI] [PubMed] [Google Scholar]
- 36. Vogiatzoglou A, Oulhaj A, Smith AD, Nurk E, Drevon CA, Ueland PM, et al. Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B12 status. Clin Chem 2009;55:2198–2206. [DOI] [PubMed] [Google Scholar]
- 37. Hvas AM, Nexo E. Diagnosis and treatment of vitamin B12 deficiency‐‐an update. Haematologica 2006;91:1506–1512. [PubMed] [Google Scholar]
- 38. Watanabe F. Vitamin B12 sources and bioavailability. Exp Biol Med (Maywood) 2007;232:1266–1274. [DOI] [PubMed] [Google Scholar]
- 39. Rizzo G, Lagana AS, Rapisarda AM, La Ferrera GM, Buscema M, Rossetti P, et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Forum Nutr 2016;8:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Groot LC. Nutritional issues for older adults: addressing degenerative ageing with long‐term studies. Proc Nutr Soc 2016;75:169–173. [DOI] [PubMed] [Google Scholar]
- 41. Tanner SM, Aminoff M, Wright FA, Liyanarachchi S, Kuronen M, Saarinen A, et al. Amnionless, essential for mouse gastrulation, is mutated in recessive hereditary megaloblastic anemia. Nat Genet 2003;33:426–429. [DOI] [PubMed] [Google Scholar]
- 42. He Q, Madsen M, Kilkenney A, Gregory B, Christensen EI, Vorum H, et al. Amnionless function is required for cubilin brush‐border expression and intrinsic factor‐cobalamin (vitamin B12) absorption in vivo. Blood 2005;106:1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Birn H, Willnow TE, Nielsen R, Norden AG, Bonsch C, Moestrup SK, et al. Megalin is essential for renal proximal tubule reabsorption and accumulation of transcobalamin‐B(12). Am J Physiol Renal Physiol 2002;282:F408–F416. [DOI] [PubMed] [Google Scholar]
- 44. De S, Kuwahara S, Saito A. The endocytic receptor megalin and its associated proteins in proximal tubule epithelial cells. Membranes (Basel) 2014;4:333–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahuja R, Yammani R, Bauer JA, Kalra S, Seetharam S, Seetharam B. Interactions of cubilin with megalin and the product of the amnionless gene (AMN): effect on its stability. Biochem J 2008;410:301–308. [DOI] [PubMed] [Google Scholar]
- 46. Ramanujam KS, Seetharam S, Ramasamy M, Seetharam B. Renal brush border membrane bound intrinsic factor. Biochim Biophys Acta 1990;1030:157–164. [DOI] [PubMed] [Google Scholar]
- 47. Zhang F, Zhao Y, Chao Y, Muir K, Han Z. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J Am Soc Nephrol JASN 2013;24:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu X, Valentine SJ, Plasencia MD, Trimpin S, Naylor S, Clemmer DE. Mapping the human plasma proteome by SCX‐LC‐IMS‐MS. J Am Soc Mass Spectrom 2007;18:1249–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abuyaman O, Andreasen BH, Kronborg C, Vittinghus E, Nexo E. The soluble receptor for vitamin B12 uptake (sCD320) increases during pregnancy and occurs in higher concentration in urine than in serum. PLoS One 2013;8: e73110. doi:https://doi.org/10.1371/journal.pone.0073110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abuyaman O, Nexo E. The soluble transcobalamin receptor (sCD320) is present in cerebrospinal fluid and correlates to dementia‐related biomarkers tau proteins and amyloid‐beta. Scand J Clin Lab Invest 2015;75:514–518. [DOI] [PubMed] [Google Scholar]
- 51. Arendt JF, Quadros EV, Nexo E. Soluble transcobalamin receptor, sCD320, is present in human serum and relates to serum cobalamin—establishment and validation of an ELISA. Clin Chem Lab Med 2011;50:515–519. [DOI] [PubMed] [Google Scholar]
- 52. Quadros EV, Nakayama Y, Sequeira JM. The protein and the gene encoding the receptor for the cellular uptake of transcobalamin‐bound cobalamin. Blood 2009;113:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karpman D, Stahl AL, Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol 2017;13:545–562. [DOI] [PubMed] [Google Scholar]
- 54. van Asselt DZ, van den Broek WJ, Lamers CB, Corstens FH, Hoefnagels WH. Free and protein‐bound cobalamin absorption in healthy middle‐aged and older subjects. J Am Geriatr Soc 1996;44:949–953. [DOI] [PubMed] [Google Scholar]
- 55. Fukuwatari T, Wada H, Shibata K. Age‐related alterations of B‐group vitamin contents in urine, blood and liver from rats. J Nutr Sci Vitaminol (Tokyo) 2008;54:357–362. [DOI] [PubMed] [Google Scholar]
- 56. Hill MH, Flatley JE, Barker ME, Garner CM, Manning NJ, Olpin SE, et al. A vitamin B‐12 supplement of 500 mug/d for eight weeks does not normalize urinary methylmalonic acid or other biomarkers of vitamin B‐12 status in elderly people with moderately poor vitamin B‐12 status. J Nutr 2013;143:142–147. [DOI] [PubMed] [Google Scholar]
- 57. Dullemeijer C, Souverein OW, Doets EL, van der Voet H, van Wijngaarden JP, de Boer WJ, et al. Systematic review with dose‐response meta‐analyses between vitamin B‐12 intake and European Micronutrient Recommendations Aligned's prioritized biomarkers of vitamin B‐12 including randomized controlled trials and observational studies in adults and elderly persons. Am J Clin Nutr 2013;97:390–402. [DOI] [PubMed] [Google Scholar]
- 58. Swart KM, Ham AC, van Wijngaarden JP, Enneman AW, van Dijk SC, Sohl E, et al. A Randomized controlled trial to examine the effect of 2‐Year vitamin B12 and folic acid supplementation on physical performance, strength, and falling: Additional Findings from the B‐PROOF Study. Calcif Tissue Int 2016;98:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Summary statistics of the clinical data in figures 1 and 6.
Supplementary Figure S1. Composition of the diet used for rodent studies.
Supplementary Figure S2. Expression profile of Amnionless mRNA in mouse tissues. Data was recovered from www.biogps.org.
Supplementary Figure S3. Unprocessed images from the western blot data in rat kidney presented in Figure 4A. Western blots with anti‐AMN and anti‐GAPDH antibodies as well an amido black staining of total protein loading on the membrane are shown together with the molecular weight ladder.
Supplementary Figure S4. Graphs of the clinical data in figures 1 and 6 presented in linear scale.


