Abstract
Background
Sarcopenia is defined as the age‐related loss of skeletal muscle mass and function. While all humans lose muscle with age, 2–5% of elderly adults develop functional consequences (disabilities). The aim of this study was to investigate muscle myogenesis in healthy elderly adults, with or without sarcopenia, compared with middle‐aged controls using both in vivo and in vitro approaches to explore potential biomarker or causative molecular pathways associated with sarcopenic versus non‐sarcopenic skeletal muscle phenotypes during ageing.
Methods
Biomarkers of multiple molecular pathways associated with muscle regeneration were analysed using quantitative polymerase chain reaction in quadriceps muscle samples obtained from healthy elderly sarcopenic (HSE, n = 7) or non‐sarcopenic (HENS, n = 21) and healthy middle‐aged control (HMC, n = 22) groups. An in vitro system of myogenesis (using myoblasts from human donors aged 17–83 years) was used to mimic the environmental challenges of muscle regeneration over time.
Results
The muscle biopsies showed evidence of satellite cell activation in HENS (Pax3, P < 0.01, Pax7, P < 0.0001) compared with HMC. Early myogenesis markers Myogenic Differentiation 1 (MyoD1) and Myogenic factor 5 (Myf5) (P < 0.0001) and the late myogenesis marker myogenin (MyoG) (P < 0.01) were increased in HENS. In addition, there was a 30‐fold upregulation of TNF‐α in HENS compared with HMC (P < 0.0001). The in vitro system demonstrated age‐related upregulation of pro‐inflammatory cytokines (2‐fold upregulation of interleukin (IL)‐6, IL‐8 mRNA, increased secretion of tumor necrosis factor‐α (TNF‐α) and IL‐6, all P < 0.05) associated with impaired kinetics of myotube differentiation.
The HSE biopsy samples showed satellite cell activation (Pax7, P < 0.05) compared with HMC. However, no significant upregulation of the early myogenesis (MyoD and Myf5) markers was evident; only the late myogenesis marker myogenin was upregulated (P < 0.05).
Higher activation of the oxidative stress pathway was found in HENS compared with the HSE group. In contrast, there was 10‐fold higher upregulation of HSPA1A a stress‐induced chaperone acting upon misfolded proteins in HSE compared with the HENS group.
Conclusions
Both pathological and adaptive processes are active in skeletal muscle during healthy ageing. Muscle regeneration pathways are activated during healthy ageing, but there is evidence of dysregulation in sarcopenia. In addition, increased cellular stress, with an impaired oxidative‐stress and mis‐folded protein response (HSPA1A), may be associated with the development of sarcopenia. The in vitro system of young and old myoblasts replicated some of the differences between young and old muscle.
Keywords: Ageing, Satellite cells, Sarcopenia, Muscle regeneration, Cellular stress
Introduction
Sarcopenia is defined as an age‐related loss of skeletal muscle mass and function,2, 3 which may develop in the absence of overt disease. Age‐related muscle loss is accelerated by reduced physical activity, as well as by acute or chronic disease. Age‐related sarcopenia and other muscle atrophy conditions share common functional consequences, including a loss of muscle strength and power, and are major causes of mobility disability.4 The severity and functional consequences of sarcopenia vary widely in older adults of the same chronological age, and the molecular processes underpinning healthy ageing without sarcopenia are still not well understood. Muscle regeneration,5, 6, 7 senescence,8 inflammation,9, 10 oxidative stress,11 and apoptosis12, 13 are all cellular processes that influence muscle mass and therefore may play a role in the development or protection from sarcopenia during ageing.14, 15 Accumulating evidence suggests that these complex cellular processes, alongside increased cytokine activity, play a role in satellite cell activation and differentiation during muscle fibre repair and remodelling in humans.16, 17 There have been prior studies comparing skeletal muscle in healthy elderly adults with those with pathologies including cancer,18 but studies in healthy elderly adults without sarcopenia, which assess multiple biomarkers of muscle regeneration and underlying cellular processes in tandem in the same human muscle biopsies are lacking.
To study sarcopenia in vivo, researchers use animal models of different ages e.g., Ibebunjo et al., 2013.19 Here, we focus on the investigation of the regeneration process in healthy ageing human muscle and the underlying multiple underlying cellular processes including senescence, apoptosis, and chronic inflammation in the muscle microenvironment utilizing both in vitro and in vivo methods. We asked whether the development of sarcopenia in elderly adults might be associated with dysregulation of muscle regeneration and whether the latter might be due to impaired defence mechanisms against increasing oxidative stress. The aim of this study was first, to establish whether myogenesis is altered in healthy elderly adults with or without sarcopenia, compared with healthy middle‐aged controls (HMCs). Secondly, we explored molecular pathways underpinning differences in muscle phenotypes during healthy ageing.
Methods
Participants
All participants gave written informed consent prior to entry into the study. All procedures were approved by the NHS Lothian and Maastricht University Hospital local research Ethics Committees. The study conformed to the standards set by the Declaration of Helsinki.
Healthy Elderly [Healthy Elderly Sarcopenic (HSE); Healthy Elderly Non‐Sarcopenic (HENS)]: 30 participants aged 79 years or over were recruited via advertisements. They were defined as healthy on the basis of their responses to previously published health selection criteria.20 None were engaged in any form of physical training. All participants were screened using body composition analysis by Dual‐energy X‐ray absorptiometry scanning and were classified as either HSE or HENS according to established cut‐offs based on the relative skeletal muscle index.21 Muscle biopsies of 28 volunteers were available for analyses (HSE; n = 7 and HENS; n = 21).
HMC: 22 participants were recruited via advertisements. Participants had no recent weight loss or any common disease associated with cachexia (cancer, severe COPD, congestive heart failure, active infectious disease). Individuals taking hormones or continual oral steroids were excluded.
Measurement of C‐reactive protein
C‐reactive protein was measured in serum using an automated blood chemistry analyser technique (Abbott TDX).
Dual‐energy X‐ray absorptiometry body composition analysis (Healthy Elderly Non‐Sarcopenic, Healthy Elderly Sarcopenic, and Healthy Middle‐aged Control)
Dual‐energy X‐ray absorptiometry [DPX‐L; Lunar Radiation Corp (HMC) and Hologic QDR4500A (HSE; HENS)] was used to measure different body compartments (i.e., fat mass, lean mass, and bone mineral content). Muscle biopsy.
A Bergstrom needle muscle biopsy was obtained from the lateral mass of the quadriceps under local anaesthetic, after a 12‐h overnight fast. The biopsy was cleaned of gross blood contamination and snap frozen in liquid nitrogen and stored at −80 °C.
Total RNA isolation
Tissue was homogenized in Qiazol (Qiagen, UK) reagent using a Polytron PT1200E (Kinematica AG, Switzerland). Total RNA was extracted using miRNEasy columns (Qiagen, UK) as directed by the manufacturer, with an on‐column DNAse digestion step. RNA was quantified using the Nanodrop instrument (Labtech Intl, UK). Quality and purity of RNA was examined using 260/280 and 260/230 ratios. The Agilent bioanalyzer (Agilent, UK) was used to assess RNA integrity using previously published protocols.22 All samples had 260/280 ratios above 1.8, and RNA integrity number scores above 7.5.
Quantitative polymerase chain reaction
The High Capacity RNA‐to‐cDNA kit (Applied Biosystems, UK) was used to convert 1 μg of RNA to cDNA following the manufacturer's directions. The quantitative validation of the expression of selected genes was performed using quantitative polymerase chain reaction (QPCR) (Applied Biosystems StepOne Real‐Time PCR Systems) using custom PrimerDesign primers and applying the Sybr Green PCR master mix (Applied Biosystems, Foster City, CA, USA), following the manufacturer's protocol. Reactions were run in triplicate on a StepOne Plus instrument (Applied Biosystems, UK). Running conditions were 95 °C 10 min followed by 40 cycles of 95 °C 15 s and 60 °C 60 s. Amplification was performed for each cDNA (20 ng) sample in triplicate. The fold change in expression of the target gene relative to the internal control gene (SDHA, CYC1, and GAPDH) was assessed. QPCR data were presented as the fold‐change in gene expression normalized to the average value of two common endogenous reference genes and relative to the control (HMC muscle samples). See supplied supporting information Table 1 for QPCR primers.
Identification of suitable reference genes
We utilized the geNorm Housekeeping Gene Selection Kit (PrimerDesign) to evaluate 12 commonly used housekeeping genes in HENS, HSE, and HMC muscle. Reference genes tested were 18S (18S ribosomal RNA subunit), β‐Actin (beta‐actin), ATP5b (ATP synthase subunit 5b), B2M (beta‐2 microglobulin), TOP1 (topoisomerase 1), CYC1 (cyclin D1), EiIF4a2 (eukaryotic initiation factor 4a2), GAPDH (glyceraldehyde‐3‐phosphate dehydrogenase), RPL13a (ribosomal protein L13a), SDHA (succinate dehydrogenase complex, subunit A), UBC (ubiquitin C), and YHWAZ (phospholipase A2). The geNorm output ranked the candidate reference gene according to their expression stability (M). Using this approach, we identified 3 housekeeping genes (CYC1, SDHA, and GAPDH) as being the most stably expressed in, HSE, HENS, and HMC groups, respectively (see Supporting Information). These three reference genes were used in subsequent analyses.
Cultivation of human skeletal muscle cells
To study the age dependent phenotype in an in vitro system we ordered primary human skeletal muscle cells (hsKMC; Cook Myosite), obtained from donors with different ages (aged 83 years; P101042‐83 M, 73 years; P101043‐73 M, 69 years; P101064‐69 M, 51 years; P101061‐51 M, 50 years; P301014‐50 M, 20 years; P101040‐20 M, and 17 years; P201052‐17 M: See supplied supporting information Table 2 for further information). The hsKMC were cultured according to the manufacturer's protocol (Cook Myosite PA 15238, USA). For the analysis, the results obtained with hsKMC are represented in two groups younger (donor age 17–51 years) and older (69–83 years), a categorisation that is consistent with literature reporting an increased prevalence of sarcopenia in adults older than 70 years.23
Fusion assay
For the fusion assays, 10 000 hsKMC cells/well were seeded onto collagen in 96‐well plates in GM supplemented with 20% FBS, 1% Gentamicin at 37 °C, 5% CO2, and 95% humidity and grown at 37 °C for 1 day. Next day, the cells were washed once with serum‐free differentiation medium and then cultivated in differentiation medium supplemented with 2% heat inactivated HES, 1% FBS, and 1% Gentamicin for 96 h at 37 ° C, 5% CO2, and 95% humidity. The fusion index was calculated as the percentage of nuclei in myotubes (myosin heavy chain positive stained cells) compared with the total number of nuclei.
Determination of secreted proteins in the supernatant of primary human skeletal muscle cells.
Supernatants of the primary skeletal muscle cells from donors of different age (17 years, 20 years, 50 years, 51 years, 69 years, 73 years, and 83 years) were taken at 48 h during differentiation and measured using the RayBio human cytokine array (AAH‐CYT‐G4000; RayBiotech Inc, Norcross GA, USA) according to the manufacturer's protocol. Cytokine arrays were measured using the GenePix 4000B (Molecular Devices, CA, USA).
Preparation for protein extraction
Proteins were extracted from pulverized human skeletal muscle cells by homogenizing the samples in the Precellys 24 system. Briefly, 300 μl of PhosphoSafe Extraction Reagent (Millipore) was added to minced and ground human skeletal muscle tissue (8 mg) in Precellys 24 lysing kit tubes. Tissue was further homogenized using the high‐throughput homogenizer Precellys 24, for 10s. After incubation on ice for 5 min, the lysates were spun at 800xg for 5 min at 4 °C. Supernatants were transferred into new tubes and spun for another 12 min at 1600xg at 4 °C. Pellets (insoluble fraction) were stored at −80 °C until further us. Supernatants were collected and protein concentrations measured using the BCA Protein Assay Kit (Pierce) with BSA as a standard. Afterwards, phosphatase inhibitor cocktail (Roche) was added and the samples were stored at −80 °C until further use.
Western blots
Ten microgram of human skeletal muscle cell protein extracts (17‐ and 83‐year‐old donor) in reducing Laemmli SDS sample buffer were boiled for 5 min at 95 °C and then separated by SDS‐PAGE on 4–20% gradient gels (Bio‐Rad, Cressier, Switzerland), blotted to Nitrocellulose membranes (Bio‐Rad) using the Trans‐Blot Turbo Transfer System (Bio‐Rad), blocked for 1 h in blocking buffer (5% non‐fat milk in Tris‐buffered saline 0.05% Tween‐20), incubated overnight with primary antibody, rinsed, and incubated for 1 h with peroxidase‐conjugated goat anti‐rabbit IgG at room temperature. Blots were developed using ECL (Roche, Rotkreuz, Switzerland) or SuperSignal West Femto substrate (Thermo Scientific, Wohlen, Switzerland) and exposed to Kodak film (Kodak, Rochester, NY, USA). Antibodies: phospho‐p38 (THR180, Tyr182) (Novus Biologicals, Littleton, CO, USA); p38 (Cell Signalling Technologies, Danvers, MA, USA), Troponin I skeletal fast (abcam, Cambridge, UK). Western blots were analysed densitometrically using ImageJ software version 1.45 (NIH, Bethesda, MD, USA; http://rsbweb.nih.gov/ij). Band intensity of each sample was normalized to coomassie blue stained gels.
Statistical analysis
The Graphpad prism6 software package (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. Statistical significance of the quantitative polymerase chain reaction results was assessed using non‐parametric Kruskal‐Wallis tests. Statistical significance of the Western blot data was assessed using the Mann Whitney Test as described by Eaton et al., 2013.24 Results were considered significant at P < 0.05.
Results
Participant characteristics
The characteristics of the HENS, HSE, and HMC groups are presented in Table 1. The healthy elderly participants were balanced in their sex distribution and had a mean age of 79 years. HENS participants had a normal BMI and had normal serum C‐reactive protein. Twenty five percent (25%; n = 7) of the elderly participants were classified as HSE. The HMC group were, on average, nearly two decades younger than the HENS. There was a predominance of men and average BMI was normal.
Table 1.
Characteristics of all the recruited subjects. The majority of the sarcopenic participants were male (4M: 3F)
| Participants | Healthy elderly (n = 28) | Healthy middle age (n = 22) | |
|---|---|---|---|
| HENS (n = 21) | HSEa (n = 7) | ||
| M:F | 11:10 | 5:2 | 12:8 e |
| Age | 79 (3.4) | 79.3 (3.5) | 61 (7) e |
| Weight (kg) | 69 (14) | 65 (8) | 73 (12) e |
| Height (cm) | 1.65 (0.09) | 1.67 (0.09) | 1.73 (0.10) e |
| BMI | 25.4 (3.9) | 23.3 (2.3) | 24.2 (3.3) e |
| LBM (kg) | 48.1 (10.5) | 45.9 (8.4) | 53.3 (9.8) e |
| FM (kg) | 19.1 (6.7) | 16.8 (4.1) | 17.6 (2.1) e |
| ASM (kg/m2) | 7.3 (1.3) | 6.4 (0.9) | 7.7 (1.0) e |
| CRP (mg/L) | 3 (3) | 3 (3) | — |
ASM, Appendicular skeletal muscle mass; CRP, C‐reactive protein; e, estimated; HENS, Healthy Elderly Non‐Sarcopenic; HSE, Healthy Elderly Sarcopenic. Healthy elderly participants, 21 (11M:10F) were non‐sarcopenic and had a mean age (±SD) of 79 ± 3.6years and a BMI (±SD) of 25.4 ± 3.8, all were weight‐stable and none had an elevated serum C‐reactive protein. Seven (4M:3F) of the healthy elderly were sarcopenic and had a mean age (±SD) of 80.3 ± 3.9 years and an average BMI (±SD) of 22.8 ± 2.6, and none had an elevated serum C‐reactive protein. The healthy middle‐aged controls had a mean age (±SD) of 61 ± 7 years. There was a predominance of male participants, average BMI (±SD) was 24.2 ± 3.3, and all were weight‐stable. (Values are mean and SD,
Defined by >10% WL,
Baumgartner's criteria).
Differences between groups in early and late myogenesis
To explore whether healthy ageing without sarcopenia was underpinned by differences in myogenesis or muscle generation, we measured multiple transcriptional markers compared with middle‐aged controls. The expression of markers indicating satellite cell activation, i.e., the muscle transcription factor Pax3 showed 4‐fold (P < 0.01), and the muscle transcription factor Pax7 a 9‐fold expression (P < 0.0001) in HENS compared with HMC (Figure 1a). The expression of markers of early differentiation [MyoD and Myogenic factor 5 (Myf5)] was increased (7‐fold, P < 0.0001), as well as a 6‐fold (P < 0.05) elevated expression of myogenin (MyoG), a marker of terminal differentiation, detected in HENS compared with HMC (Figure 1b and 1c). However, the muscle remodelling factor myocyte enhancer factor‐2 (Mef2) was significantly downregulated in HENS compared with HMC (Figure 1d).
Figure 1.
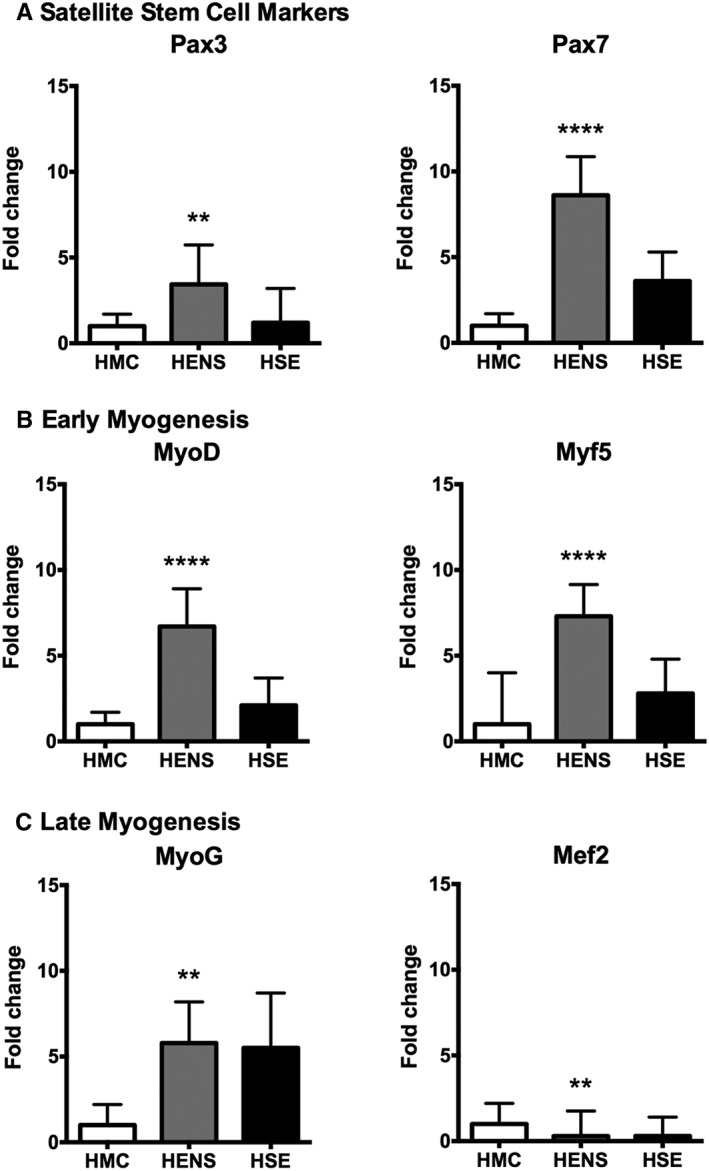
Fold change in mRNA expression (±SD) of genes related to a.) satellite cell activation, b.) myoblast proliferation/early differentiation, c.) late myocyte/myotube differentiation, and d.) myotube remodelling in the quadriceps muscle of Healthy Elderly Sarcopenic (HSE), Healthy Elderly Non‐Sarcopenic (HENS), and healthy middle‐aged control (HMC) groups: HMC (n = 22), HENS (n = 20), HSE (n = 7). Fold‐change is relative to HMC participants. Quantitative polymerase chain reaction results show upregulation of mRNA levels of Pax3 (** P < 0.01 in HENS) and Pax7 (**** P < 0.0001 in HENS; * P < 0.05 in HSE). Also, expression of early myogenesis markers was increased: MyoD and Myogenic factor 5 (Myf5) (**** P < 0.0001 in HENS). mRNA of myogenin (MyoG) is upregulated in HENS (** P < 0.01) and in HSE (* P < 0.05). mRNA of remodelling muscle marker myocyte enhancer factor‐2 (Mef2) is downregulated in HENS (** P < 0.01) and in HSE (* P < 0.05).
To explore whether sarcopenia during ageing was underpinned by differences in myogenesis or muscle generation, we measured multiple transcriptional markers compared with middle‐aged controls. The expression of markers indicating satellite cell activation, i.e., the muscle transcription factor Pax7 was higher in HSE (4‐fold, P < 0.05) compared with HMC (Figure 1a). The expression of markers of early differentiation (MyoD and Myf5) were not significantly increased in the HSE group when compared with HMC (Figure 1b). However, in the HSE group elevated expression of MyoG, a marker of terminal differentiation, was found (6‐fold, P < 0.01) (Figure 1c). In addition, in the HSE group, the muscle remodelling factor Mef2 was significantly downregulated (Figure 1d) when compared with HMC. In the series of human muscle biopsies, the expression of the pro‐apoptotic gene Bax (Figure 5a) was increased in HENS compared with HMC (3‐fold, P < 0.01). Bcl2 (Figure 5a) was increased in HENS (6‐fold, P < 0.0001) and HSE (4‐fold, P < 0.05) compared with HMC.
Figure 5.
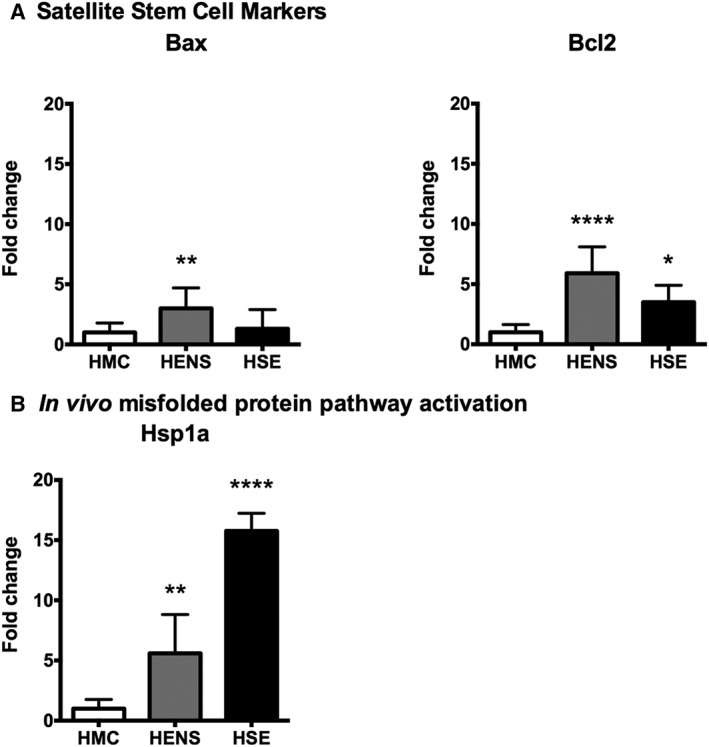
Fold change in mRNA expression (±SD) of a) mitochondrial factors Bax and Bcl2, b) misfolded proteins (Hsp1) pathways in the quadriceps muscle of Healthy Elderly Sarcopenic (HSE), Healthy Elderly Non‐Sarcopenic (HENS), and healthy middle‐aged control (HMC) groups: HMC (n = 22), HENS (n = 17), HSE (n = 6). Fold‐change of mRNA expression is relative to HMC. Quantitative polymerase chain reaction results show upregulation of mitochondrial factors Bax (** P < 0.01) in HENS participants and significantly higher upregulation of Bcl2 (**** P < 0.0001) in HENS and (* P < 0.05) in HES. b) mRNA of Hsp1 is upregulated in HSE (*** P < 0.001) and in HENS (** P < 0.01).
With the in vitro system of myogenesis using human skeletal muscle cells from donors of increasing age (17–83 years), there was a reduction of the fusion index calculated from myoblast cultures of younger (17–51 years) and older (69–83 years) donors after 72 h of myogenic differentiation (Figure 3a). In the older group, there was a lower expression of both the differentiation marker MyoD (P < 0.01) (Figure 3b) and the remodelling factor Mef2C (P < 0.0001) (Figure 3c) when compared with the younger group. In contrast, the expression of the differentiation marker Myf5 was not altered. The expression of the late myogenesis markers (MyoG, Troponin T1 (TNNT1)) were reduced (P < 0.0001) in the older donor group compared with the younger donor group (Figure 3b).
Figure 3.
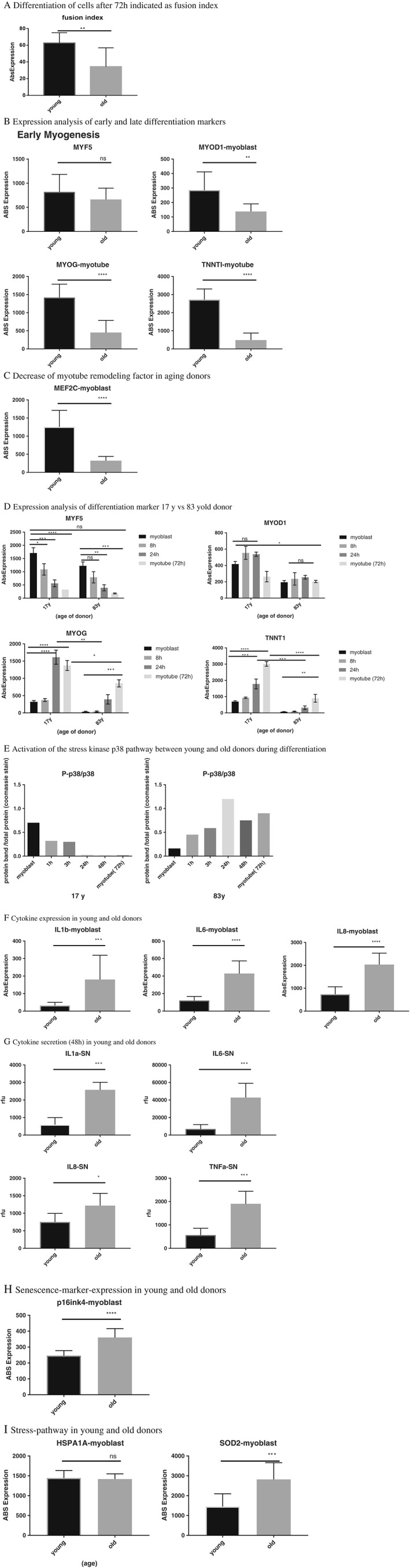
In vitro system analysis of a.) fusion index presented as the percentage of nuclei in myotubes [myosin heavy chain positive (MHC) positive] compared with the total number of nuclei of young and old donors after 72 h of myogenic differentiation b.) differences of early and late myogenesis markers expression in young and old donors, c.) expression of myocyte enhancer factor 2 (Mef2) d.) expression analysis time course of differentiation marker from a 17‐ and 83‐year‐old donor e.) semi‐quantitative western blot analysis of activation of the p38 pathway in human skeletal muscle cells from 17‐ and 83‐year‐old donor f.) cytokine expression analysis of young and old donors (myoblast) g.) level of cytokines secreted in the supernatant of young and old donors after 48 h of differentiation h.) expression analysis of the senescence marker p16INK4a (Cdkn2) between young and old donors i.) analysis of stress‐pathway activation in young vs old donors. Myf5, Myogenic factor 5; MyoG, myogenin; IL, interleukin.
Detailed comparison of the myogenic process in a young (17 years) and old (83 years) myoblast donor
The early differentiation marker Myf5 showed the same expression pattern (significant downregulation over time) in both donors. However, MyoD expression was higher (P < 0.01) in the young donor and not modulated over the period of myogenesis. The most marked differences were detected in the late differentiation markers MyoG and TNNT1. In the young donor, 3‐fold upregulation of TNNT1 (P < 0.0001) and a 0.5‐fold upregulation of MyoG (P < 0.0001) was seen at 72 h compared with the old donor (Figure 3d). The higher expression and secretion of cytokines in hsKMC of the old donor was associated with an increased activation of the p38 pathway throughout the myogenesis process. Initial activation of the p38 pathway (first 3 h) was observed only in the young donor (Figure 3e). However, the p38 pathway was activated in the old donor and was detected through the whole differentiation process starting at 1 h until myotube formation at 72 h (Figure 3e).
Influence of the muscle environment on ageing and sarcopenic muscle
When compared with HMC, TNF‐α was highly expressed in the muscle of HENS (30‐fold, P < 0.0001) but interleukin (IL)‐6 expression was not significantly different (Figure 2). In line with this finding, in the in vitro system, significant increases of IL‐6, IL‐8, and IL‐1b were observed in the older donor group (Figure 3f). Furthermore, an increase in expression of the senescence marker p16INK4a (Cdkn2) in the older donor group was observed (Figure 3h) and was consistent with the increased upregulation of cytokine expression. The higher expression of the senescence marker p16INK4a could not be confirmed in the muscle biopsies (Figure 4).
Figure 2.
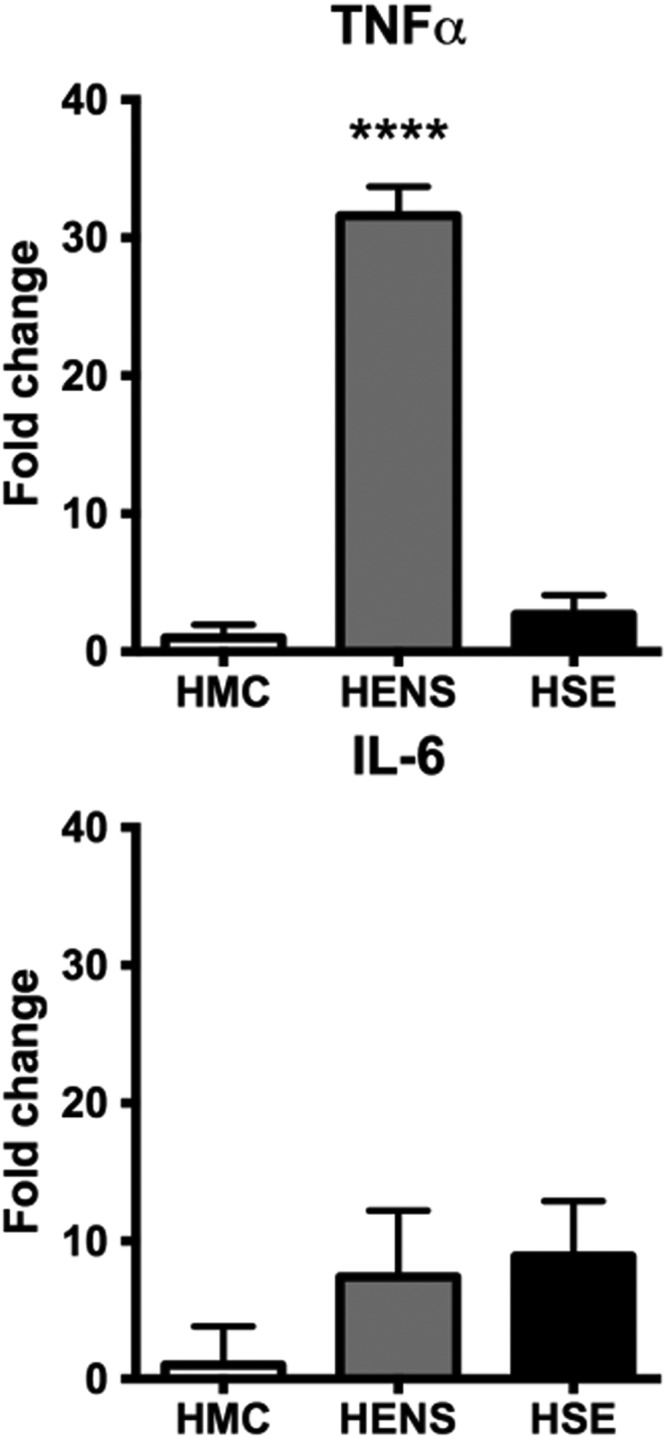
Fold change in mRNA expression (±SD) of genes of pro‐inflammatory cytokines in the quadriceps muscle of Healthy Elderly Sarcopenic (HSE), Healthy Elderly Non‐Sarcopenic (HENS), and healthy middle‐aged control (HMC) groups: HMC (n = 22), HENS (n = 17), HSE (n = 7). Fold‐change of mRNA expression is relative to HMC participants. Quantitative polymerase chain reaction show mRNA upregulation of TNF‐α in HENS (**** P < 0.0001). Also, expression of interleukin (IL)‐6 in HENS and HSE was markedly increased.
Figure 4.
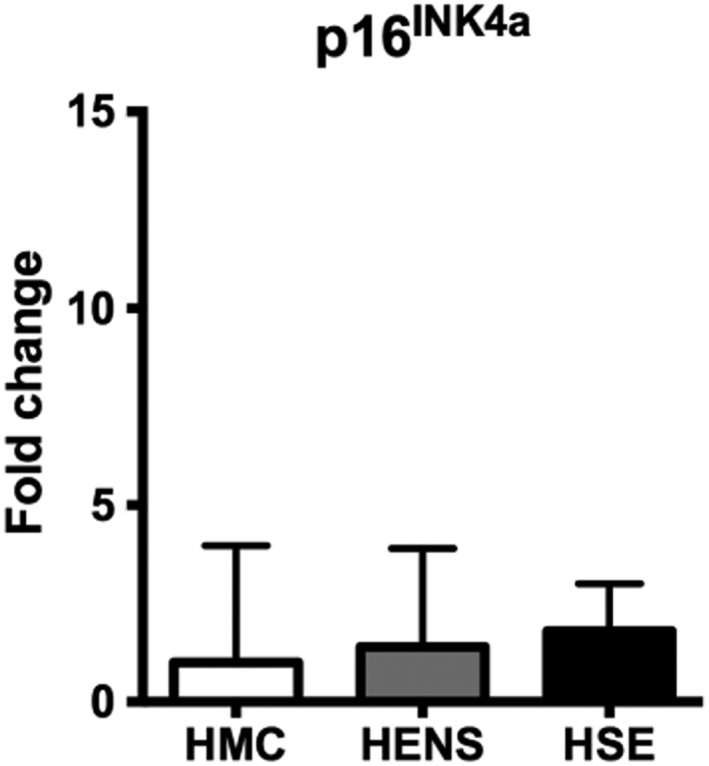
Fold change in mRNA expression (±SD) of senescence marker in the quadriceps muscle of Healthy Elderly Sarcopenic (HSE), Healthy Elderly Non‐Sarcopenic (HENS), and healthy middle‐aged control (HMC) groups: HMC (n = 22), HENS (n = 15), HSE (n = 6). Quantitative polymerase chain reaction show increasing trend of the master regulator of senescence p16INK4a (Cdkn2) mRNA upregulation in HENS and HSE muscles with no statistical significance.
The detection of elevated expression of cytokines in vitro raised the question of whether this would translate to a higher secretion of cytokines in the culture media. Analysis of the cell supernatant at 48 h showed increases in the secretion of the cytokines IL1a (P < 0.001), IL‐6 (P < 0.001), IL‐8 (P < 0.05), and TNF‐α (P < 0.001) in the older donor group compared with the younger donor group (Figure 3g).
HSPA1A a response marker for misfolded proteins, was upregulated 15‐fold (P < 0.001) in HSE (Figure 5b) and 5‐fold (P < 0.01) in HENS compared with HMC. Oxidative stress defence markers were also upregulated to a greater extent in HENS compared with HSE. Nrf2 (Nuclear Factor, Erythroid 2 like2) was upregulated 25‐fold (P < 0.001) in HENS and 12‐fold (P < 0.01) in HSE when compared with HMC (Figure 6). Expression of Sod2 (superoxide Dismutase 2) was similar in all groups. Note that the in vitro data differed from the in vivo data, i.e., no significant old–young differences in HSPA1A (Figure 3i) and the oxidative stress genes Nrf2 and GCLM (Glutamate‐Cysteine Ligase Modifier Subunit) (data not shown), In contrast to the in vivo results in the in vitro system, an upregulation in SOD2 could be shown (Figure 3i).
Figure 6.
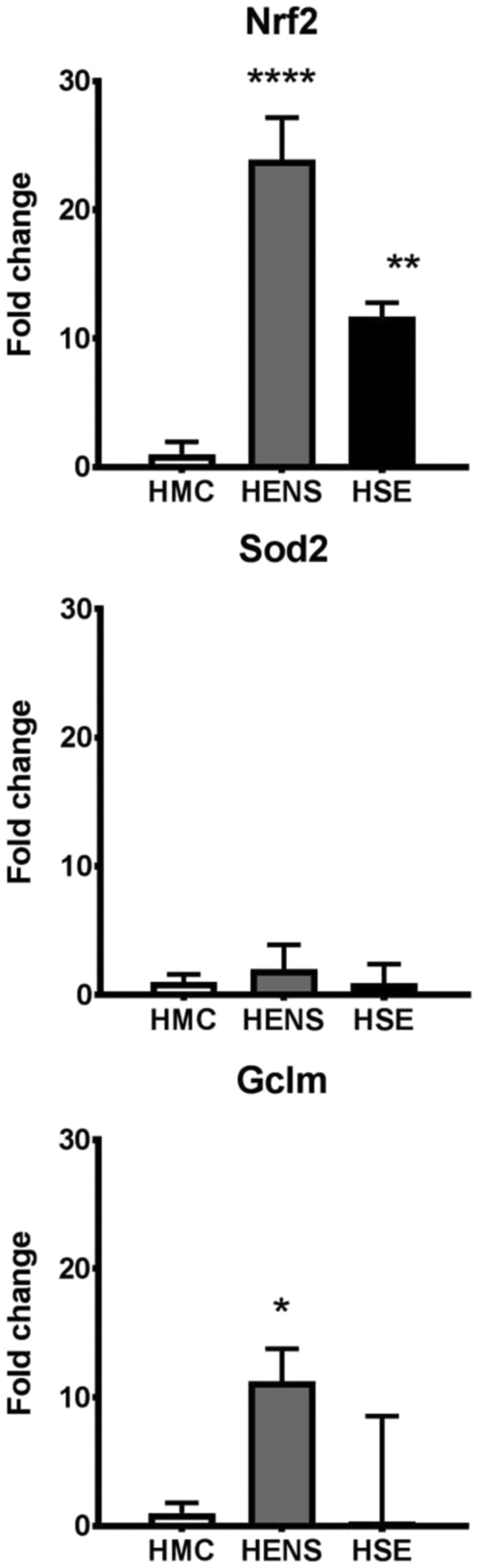
Fold change in mRNA expression (±SD) of oxidative defence genes Sod2, Gclm, and Nrf2 in the quadriceps muscle of Healthy Elderly Sarcopenic (HSE), Healthy Elderly Non‐Sarcopenic (HENS), and healthy middle‐aged control (HMC) groups: HMC (n = 22), HENS (n = 18), HSE (n = 7); fold‐change of mRNA expression is relative to HMC participants. mRNA expression of the oxidative defence genes is increased mainly in HENS (Nrf2 **** P < 0.0001, Gclm*** P < 0.001) but also in HSE (Nrf2 ** P < 0.01) when compared with HMC. Similarly, Sod2 and Gclm had lower expression level in HSE when compared with HMC.
Discussion
We investigated whether myogenesis is altered in healthy elderly adults with or without sarcopenia, diagnosed according to established criteria,21 compared with HMCs using both in vivo and in vitro approaches. The main findings of this study suggest that both pathological and adaptive processes are active in skeletal muscle during healthy ageing, with transcriptomic evidence of increased satellite cell activation. Our data demonstrate that there is activation of countermeasures to oppose increased stress in older muscle cells, and suggest that these countermeasures may also be altered in sarcopenia. Our findings in young and old muscle are consistent with some of the findings described using mouse or rat in vivo models, including increased TNF‐α and myogenic markers.19 This study demonstrates the utility of a combined in vivo and in vitro approach to the investigation of the biology of healthy ageing with or without sarcopenia, and suggests that the process of myogenesis may be altered in sarcopenia.
Using myoblasts obtained from a number of donors across the adult age range, the present study demonstrated a decrease of myoblast fusion in older compared with young donors. Moreover, myogenin and Troponin I transcription were decreased in the myoblast cultures from the older donors indicating reduced or impaired kinetics of myotube formation. The expression of the master regulator of senescence, p16INK4a (Cdkn2), was significantly higher in older donors (Figure 3h) as was the secretion of pro‐inflammatory cytokines. Elevation of inflammatory cytokines has been demonstrated previously in human populations.25 Possibly such upregulation of pro‐inflammatory cytokines with age induces cellular senescence8, 20 and is associated with a reduced rate of differentiation in the myogenic pathway. In addition, TNF‐α is a key up‐regulator of NF‐kB and IL‐6, which increases the inflammatory response. This pathway not only effectively enhances the death of existing muscle fibres but also inhibits the formation of new fibres via MyoD and Mef2 downregulation leading to the loss of skeletal muscle mass and weakness.26, 27 TNF and IL‐6 are also established components of the Senescence‐Associated Secretory Protein response; a set of cytokines secreted by senescent cells that deregulate normal tissue function.
The HENS and HSE groups were all aged > 79 years, thus the effect of older age per se can be inferred by comparison of HENS with the HMC group, whereas changes either resulting from or causing sarcopenia can be assessed by comparison with the HENS and HSE groups. Satellite cell activation transcriptional factors were expressed to a greater degree in the muscles of both HENS and HSE compared with HMC suggesting activation of this pathway in older age. In contrast, significant expression of early myogenesis markers (Myf5 and MyoD), indicative of an orderly pattern of myogenesis, was found only in the HENS group suggesting a possible alteration in the progress of myogenesis associated with sarcopenia. Significantly, 30‐fold increased expression of TNF‐α was detected in the muscles of HENS but not HSE when compared with HMC. These findings might suggest a paradoxical role of TNF‐α in ageing muscle maintenance.28 Alternatively, the active inflammatory phase of muscle loss may occur before clinical sarcopenia is detected (that is, in HENS); by the time clinical sarcopenia is detected using body composition and/or physical function, a more fibrotic process is in play and TNF‐α is no longer elevated. The muscle remodelling factor Mef2 was downregulated in both HENS and HSE, and this may relate to increased pro‐inflammatory cytokine expression1 or other regulatory factors. In contrast with our findings in vitro where terminal differentiation was delayed/ impaired, in vivo the terminal differentiation marker MyoG demonstrated similar increased expression in both HSE and HENS compared with HMC. This result implies that in vivo: (a) the regeneration process is activated and has the potential to progress to completion in old age, (b) the observed alterations in early myogenesis transcription pathways associated with sarcopenia do not necessarily lead to a block in final myotube differentiation. To what extent changes in MyoG mRNA level actually reflect the overall differentiation achieved in vivo cannot be determined from the present data.
Although the expression of the senescence marker p16INK4a (Cdkn2) increased with the donor age in vitro, this finding was not observed in vivo. It is possible that we may have captured only a very small proportion of senescent muscle cells compared with other muscle cell types in the muscle sample. Caution is needed when comparing data from cells in vitro with ageing cells in vivo: The interrelationship and complexity of factors in an in vivo environment that dictate the behaviour and fate of a senescent cell and that influence the recruitment of stem cells into the myogenic lineage still require further investigation.
Cellular senescence is also associated with elevated ROS production, apoptosis, and increased oxidative and glycation damage.29 In the present study, we detected activation of the cellular anti‐oxidant defence pathway Nrf2 in the muscles of both HENS and HSE when compared with HMC. In HSE, the upregulation of Nrf2 was reduced compared with HENS and the Nrf2 downstream gene Gclm was upregulated only in HENS. Thus, although old age seems to be associated with activation of oxidative defence, the presence of sarcopenia is possibly associated with a less vigorous response. It is of interest that HSPA1A (Hsp70) expression was increased in both HENS and HSE compared with HMC, but to a greater extent in HSE, suggesting that misfolded protein stress is increased with advancing age but may be greater in sarcopenia. Reduced cellular defence markers and increased level of misfolded proteins in HSE muscle may contribute to intracellular accumulation of ROS, which may negatively impact on the regeneration process.30 In the present study, significant upregulation of the mitochondrial factors, Bax, and Bcl2 in HENS and HSE, also may indicate increased intracellular stress in elderly adults. For HSPA1A and Sod2, again, our in vitro system yielded different results compared with the in vivo analysis, reflecting not only the complexity of the in vivo environment as explained earlier, but also due to cells being cultured in vitro under optimal conditions within a system representing the earlier stages of regeneration, making it more unlikely that (for HSPA1A) we would see upregulation of the misfolded protein response.
The present study assessed mainly transcriptomic markers of muscle regeneration under basal conditions (at rest, after fasting) and may not reflect the complexity of day to day living. Although the in vitro system has the advantage of a uniform cell population; clearly, it is only a snapshot of a process that in vivo has a much longer timescale. Equally, muscle biopsies are not of single cell origin and some of the changes observed may be ascribed to cells other than those of myocellular lineage. Moreover, data from the biopsies does not discriminate cause from effect. We were unable to compare protein signatures obtained in the elderly groups with the healthy middle age group due to the limited size of samples from HMC. A disadvantage of the in vivo study was the comparison between different ages rather than differences in age matched controls. Moreover, the sample size particularly in the HSE group was small. Future studies in larger cohorts would be valuable to confirm our findings. Furthermore, studies comparing responses of healthy elderly adults with or without sarcopenia to exercise training, which has profound effects on satellite cell function, muscle regeneration markers activation or mitochondrial ROS production in muscle are recommended.
In summary, our study demonstrates that both pathological and adaptive processes are active in skeletal muscle during healthy ageing and are maintained even with sarcopenia, although we suggest that failure of specific adaptive processes may underlie the development of sarcopenia. Muscle regeneration pathways are activated in the healthy ageing elderly without sarcopenia, but may be dysregulated or simply overwhelmed in sarcopenic elderly people. Further understanding of the pathway regulation of activated satellite cell function may be a potentially promising avenue for the identification of strategies to promote healthy ageing and combat sarcopenia.
Author Contributions
J. B., A. M., C. A. G., C. J., K. C. H. F. conceived and designed the experiments. J. B., A. M., A. S. performed the experiments. J. B., A. M., R. M., C. J. analysed and interpreted the data. S. D., R. L., A. S., D. G., R. R., C. A. G., J. A. R., K. C. H. F., C. J. contributed reagents/materials/analysis tools. All authors (led by J. B.) contributed to the writing and review of the final manuscript.
Conflict of interest
None declared.
Supporting information
Table 1. Primer sequences for the custom real‐time PCR (PrimerDesign Ltd) designed to support the MIQE guidelines: minimum information for publication of quantitative real‐time PCR1
Table 2. Donor patient characteristics of the human primary myoblast
Acknowledgements
We are grateful to Dr Nathan Stephens, University of Edinburgh for assistance with participant recruitment and muscle biopsies and to Dr Richard Skipworth, University of Edinburgh for critical evaluation of the manuscript. We thank the staff of the Wellcome Trust Clinical Research Facility, Royal Infirmary Edinburgh, and Department of Medical Physics, Western General Hospital, Edinburgh. Also we thank all our participants. J. B. is grateful to The Daphne Jackson Trust (DJT) and The Leverhulme Trust. We also thank Cook Myosite for provision of human primary skeletal muscle cells. The authors of this manuscript certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia, and Muscle: update 2015.31
Brzeszczyńska, J. , Meyer, A. , McGregor, R. , Schilb, A. , Degen, S. , Tadini, V. , Johns, N. , Langen, R. , Schols, A. , Glass, D. J. , Roubenoff, R. , Ross, J. A. , Fearon, K. C. H. , Greig, C. A. , and Jacobi, C. (2018) Alterations in the in vitro and in vivo regulation of muscle regeneration in healthy ageing and the influence of sarcopenia. Journal of Cachexia, Sarcopenia and Muscle, 9: 93–105. doi: 10.1002/jcsm.12252.
References
- 1. Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One 2011;6: e22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross‐sectional study of muscle strength and mass in 45‐ to 78‐yr‐old men and women. J Appl Physiol 1991;71:644–650. [DOI] [PubMed] [Google Scholar]
- 3. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A 2001;56:B209–B217. [DOI] [PubMed] [Google Scholar]
- 4. Rolland Y, Abellan van Kan G, Gillette‐Guyonnet S, Vellas B. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care 2011;14:15–21. [DOI] [PubMed] [Google Scholar]
- 5. Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, et al. Ageing is associated with diminished muscle re‐growth and myogenic precursor cell expansion early after immobility‐induced atrophy in human skeletal muscle. J Physiol 2013;591:3789–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 2005;4:407–410. [DOI] [PubMed] [Google Scholar]
- 7. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–764. [DOI] [PubMed] [Google Scholar]
- 8. Sasaki M, Ikeda H, Sato Y. Proinflammatory cytokine‐induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study. Free Radic Res 2008;42:625–632. [DOI] [PubMed] [Google Scholar]
- 9. Cannon JG. Cytokines in Aging and Muscle Homeostasis. J Gerontol A 1995;50A:120–123. [DOI] [PubMed] [Google Scholar]
- 10. Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene‐induced senescence relayed by an interleukin‐dependent inflammatory network. Cell 2008;133:1019–1031. [DOI] [PubMed] [Google Scholar]
- 11. Hütter E, Skovbro M, Lener B, Prats C, Rabøl R, Dela F, et al. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell 2007;6:245–256. [DOI] [PubMed] [Google Scholar]
- 12. Masiero E. and M. Sandri M. Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 2010;6:307–309. [DOI] [PubMed] [Google Scholar]
- 13. Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life‐long exercise. Exp Gerontol 2010;45:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 2014. Jan‐Feb;49:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci 2010. Nov;1211:25–36. [DOI] [PubMed] [Google Scholar]
- 16. Farup JL, Madaro L, Puri PL, Mikkelsen UR. Interactions between muscles stem cells, mesenchymal‐derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis 2015;6:e1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, et al. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 2014;28:1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brzeszczyńska J, Johns N, Schilb A, Degen S, Degen M, Langen R, et al. Loss of oxidative defense and potential blockade of satellite cell maturation in the skeletal muscle of patients with cancer but not in the healthy elderly. Aging (Albany NY) 2016. Aug;8;1690–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ibebunjo C, Chick JM, Kendall T, et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol 2013;33:194–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greig CA, Young A, Skelton DA, Pippet E, Butler FM, Mahmud SM. Exercise studies with elderly volunteers. Age Ageing 1994;23:185–189. [DOI] [PubMed] [Google Scholar]
- 21. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–63X. [DOI] [PubMed] [Google Scholar]
- 22. Stephens NA, Gallagher IJ, Rooyackers O, Skipworth RJ, Tan BH, Marstrand T, et al. Using transcriptomics to identify and validate novel biomarkers of human skeletal muscle cancer cachexia. Genome Med 2010;2:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001. Apr;137:231–243. [DOI] [PubMed] [Google Scholar]
- 24. Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, Gillingwater TH, et al. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS One 2013;8: e72457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roubenoff R, Harris TB, Abad LW, Wilson PWF, Dallal JE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol 1998;56A:M20–M26. [DOI] [PubMed] [Google Scholar]
- 26. Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. NF‐kappaB‐induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 2000;289:2363–2366. [DOI] [PubMed] [Google Scholar]
- 27. Calura E, Cagnin S, Raffaello A, Laveder P, Lanfranchi G, Romualdi C. Meta‐analysis of expression signatures of muscle atrophy: gene interaction networks in early and late stages. BMC Genomics 2008;9:630–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moresi V, Adam S, Coletti D. Bimodal effects of TNF‐α on differentiation and hypertrophy of skeletal muscle cell cultures. Basic Appl Myol 2006;16:163–168. [Google Scholar]
- 29. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239–247. [DOI] [PubMed] [Google Scholar]
- 30. Barbieri E, Sestili P. Reactive Oxygen Species in Skeletal Muscle Signaling. J Signal Transduct 2012;2012:982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Primer sequences for the custom real‐time PCR (PrimerDesign Ltd) designed to support the MIQE guidelines: minimum information for publication of quantitative real‐time PCR1
Table 2. Donor patient characteristics of the human primary myoblast


