Abstract
Background
The association of sarcopenia and visceral obesity to treatment outcome is not clear for locally advanced rectal cancer. This study evaluates the influence of skeletal muscle and visceral fat on short‐term and long‐term outcomes in locally advanced rectal cancer patients treated with neoadjuvant chemoradiation therapy followed by curative resection.
Methods
A total of 188 patients with locally advanced cancer were included between January 2009 and December 2013. Neoadjuvant chemoradiotherapy was followed by curative resection. Sarcopenia and visceral obesity were identified in initial staging CT by measuring the muscle and visceral fat area at the third lumbar vertebra level.
Results
Among the 188 included patients, 74 (39.4%) patients were sarcopenic and 97 (51.6%) patients were viscerally obese. Sarcopenia and high levels of preoperative carcinoembryonic antigen were significant prognostic factors for overall survival (P = 0.013, 0.014, respectively) in the Cox regression multivariate analysis. Visceral obesity was not associated with overall survival; however, it did tend to shorten disease‐free survival (P = 0.079).
Conclusions
Sarcopenia is negatively associated with overall survival in locally advanced rectal cancer patients who underwent neoadjuvant chemoradiation therapy and curative resection. Visceral obesity tended to shorten disease‐free survival. Future studies should be directed to optimize patient conditions according to body composition status.
Keywords: Sarcopenia, Visceral obesity, Rectal cancer, Prognosis
Introduction
Reduced muscle mass and increased visceral fat mass are considered negative prognostic factors for colon cancer patients.1, 2, 3, 4 Currently, abdominal CT imaging is gaining interest as a tool to evaluate patients' body composition because it is obtained routinely during the clinical staging process. Previous studies have reported an association between body composition measured on CT and treatment outcomes in colorectal cancer. Sarcopenia and visceral obesity have shown increased incidence of post‐operative complication and delayed recovery.5, 6 Chemotherapy toxicity is frequently observed in patients with sarcopenia. Disease‐free survival (DFS) or overall survival (OS) was poorer in patients with more visceral fat or less muscle than patients with normal body composition.7
Locally advanced rectal cancer is usually treated with preoperative chemoradiation followed by curative resection with total mesorectal excision.8, 9 Adjuvant chemotherapy is generally performed to remove micrometastases.10 Patient baseline physical fitness may affect treatment outcome during the long multimodal treatment process that includes chemotherapy, radiotherapy, and surgery. Body composition of muscle or fat may reflect the patient's function, which may allow the endurance of a challenging treatment process that can influence treatment outcomes. Until now, only few studies have investigated the association between body composition and treatment outcome in non‐metastatic locally advanced rectal cancer. A study showed that visceral obesity was associated with shorter DFS or OS.11 Another study showed that visceral obesity was linked to poor post‐operative outcomes.12 More studies are required to understand the relationship of sarcopenia and visceral obesity with treatment outcomes.
This study evaluates the influence of skeletal muscle and visceral fat for short‐term and long‐term outcomes in locally advanced rectal cancer patients treated with neoadjuvant chemoradiation therapy followed by curative resection.
Materials and methods
Subjects
This retrospective study was approved by the institutional review board (number: KC 16RISI0767), and the informed consent requirement was waived. Patients who underwent neoadjuvant chemoradiotherapy (CRT) were selected from rectal cancer surgery cases between January 2009 and December 2013. Indications of neoadjuvant CRT in our institution are locally advanced rectal cancers with clinical T stage ≥3 or N stage ≥1. We excluded 47 patients from 235 patients initially included for the following reasons: (i) patients who had metastatic lesion at initial diagnosis (n = 22); (ii) patients with recurrent rectal cancer (n = 4); and (iii) patients without available CT examination at initial diagnosis (n = 21). Interval between pre‐CRT CT and initiation of CRT was not considered in excluding patients from this study. Medical records were reviewed to collect patients' basic information that included sex, age, weight, height, and comorbidity. Initial carcinoembryonic antigen (CEA) level, pre‐CRT clinical staging, CRT protocol, and type of surgery were also recorded.
Three radiotherapy protocols were used in our institution for the study period. Standard radiotherapy consisted of 50.4 Gy given as 28 fractions of 1.8 Gy. Two short course radiotherapy protocols included 25 Gy per 5 fractions and 33 Gy per 10 fractions. One of the following concurrent chemotherapy protocols was performed: 5‐fluorouracil (5‐FU) single therapy, FL chemotherapy consisting of 5‐FU and leucovorin, FOLFOX chemotherapy consisting of 5‐FU, leucovorin and oxaliplatin, or Capecitabine (Xeloda®).
Body composition evaluation
Initial CT images before neoadjuvant chemoradiation therapy were retrieved for analysis. One axial portal phase image was selected at the level of the third lumbar vertebral body transverse processes. Total body fat area, visceral fat area, subcutaneous fat area, and abdominal circumference were measured automatically on the selected axial image by a workstation (TeraRecon Aquarius Workstation, TeraRecon, Foster City, California, USA). The measurement of the skeletal muscle area was performed by using a commercially available system (Advantage Windows workstation 4.6, GE Healthcare, Milwaukee, Wisconsin, USA). Skin, visceral organs, and the central spinal canal were excluded manually in the selected axial image to identify specific areas of measurement. The area of the abdominal wall and back muscles (psoas, paraspinal, transversus abdominis, rectus abdominis, internal oblique muscles, and external oblique muscles) were calculated by using the area of pixels with attenuation between −29 and 150 Hounsfield units in demarcated areas.13
Sarcopenia was defined by using sex‐specific cut‐off points for L3 skeletal muscle index (SMI). L3 SMI was calculated as the area of total L3 skeletal muscle (cm2) divided by height square (m2). Cut‐off points of SMI were 52.4 cm2/m2 for men and 38.5 cm2/m2 for women.1 Cut‐off of CT visceral fat area to classify visceral obesity has not been standardized yet. In previous studies, 100 and 130 cm2 were used as cut‐offs.14, 15 In our study, we defined visceral obesity with area of visceral fat >100 cm2.12
Outcome parameters
The primary endpoints of the study were OS and DFS. Overall survival was defined as the time from surgery to death from any cause for expired patients or the last follow‐up for live patients. Disease‐free survival was defined as the time from surgery to the time of recurrence. Secondary endpoints were intraoperative, anastomotic leakage, prolonged admission with more than 30 days, and hospital length of stay.
Statistical analysis
Statistical analysis was performed with SPSS 24.0 (IBM Corporation, Armonk, NY, USA). A P‐value <0.05 was considered statistically significant.
Differences between groups were evaluated by using Student's t‐test and χ 2 test for continuous and categorical variables, respectively. Disease‐free survival or OS in the subgroups was compared by using Kaplan–Meier curves and log‐rank test. Univariate and multivariate Cox regression analyses evaluated factors associated with patient survival period, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Variables with P < 0.15 in univariate analysis were entered into the multivariate analysis.
Results
A total of 188 patients (117 men and 71 women) with median follow‐up of 52 (range 5–91) months were enrolled in this study. Mean interval between initial CT and initiation of treatment was 13.8 ± 9.9 days (range, 0–70 days). Table 1 summarizes the demographics and clinical treatment course. There were 74 patients with sarcopenia and 97 patients with visceral obesity based on initial CT, respectively. Table 2 summarizes differences of variables between patients with and without sarcopenia or visceral obesity. Sarcopenia and visceral obesity were noted more frequently in male patients than in female patients. Mean age was older in sarcopenic and visceral obese patients than in normal patients. BMI and abdominal circumference measured from CT images were smaller in sarcopenic patients and larger in visceral obese patients. There were no significant differences in frequency of complications between patient groups according to sarcopenia and visceral obesity.
Table 1.
General patient characteristics and clinical treatment course
| Characteristics | Mean ± SD (%) [range] |
|---|---|
| Age (years), mean | 61.3 [27–84] |
| Sex (%) | |
| Male | 117 (62.2) |
| Female | 71 (37.8) |
| Weight (kg) | 63.4 ± 10.6 |
| Height (m) | 1.63 ± 0.08 |
| BMI (kg/m2) | 23.8 ± 3.03 |
| Initial CEA (ng/mL) | 2.7 [0–95.9] |
| Pre‐CRT clinical T stage (%) | |
| T2 | 12 (6.4) |
| T3 | 144 (76.6) |
| T4a | 28 (14.9) |
| T4b | 4 (2.1) |
| Pre‐CRT clinical N stage (%) | |
| N0 | 34 (18.1) |
| N1 | 72 (38.3) |
| N2 | 82 (43.6) |
| Clinical TNM stagea (%) | |
| II | 34 (18.1) |
| III | 154 (81.9) |
| Neoadjuvant radiotherapy (%) | |
| Standard | 134 (71.3) |
| Short course, 25 Gy | 25 (13.3) |
| Short course, 33 Gy | 29 (15.4) |
| Neoadjuvant chemotherapy (%) | |
| 5‐FU | 18 (9.6) |
| FL | 140 (74.5) |
| FOLFOX | 2 (1.1) |
| Capecitabine | 28 (14.9) |
| Operation (%) | |
| AR | 1 (0.5) |
| LAR | 129 (68.6) |
| LATA | 32 (17.0) |
| APR | 26 (13.8) |
BMI, body mass index; CEA, carcinoembryonic antigen; CRT, chemoradiotherapy; 5‐FU, 5‐fluorouracil; FL, 5‐FU and leucovorin; FOLFOX, 5‐FU, leucovorin and oxaliplatin; AR, anterior resection; LAR, low anterior resection; LATA, laparoscopic abdominal trans‐anal proctosigmoidectomy with coloanal anastomosis.
Stage according to American Joint Committee on Cancer (AJCC) Cancer Staging Manual 7th edition.
Table 2.
Clinical characteristics according to sarcopenia and visceral obesity
| Sarcopenia | Visceral obesity | |||||
|---|---|---|---|---|---|---|
| No (n = 114) | Yes (n = 74) | P‐value | No (n = 91) | Yes (n = 97) | P‐value | |
| Age (years) | 59.5 ± 10.8 | 64.2 ± 11.1 | 0.005 | 59.9 ± 10.8 | 62.7 ± 11.4 | 0.086 |
| Sex, number of men (%) | 56 (49.1) | 61 (82.4) | <0.001 | 50 (54.9) | 67 (69.1) | 0.051 |
| BMI (kg/m2) | 24.7 ± 3.1 | 22.4 ± 2.4 | <0.001 | 22.2 ± 2.5 | 25.3 ± 2.7 | <0.001 |
| Initial CEA (ng/mL) | 3.11 ± 9.44 | 2.07 ± 1.54 | 0.352 | 2.15 ± 2.94 | 3.22 ± 9.94 | 0.330 |
| Clinical TNM stage III (%)a | 95 (83.3) | 59 (79.7) | 0.564 | 72 (79.1) | 82 (84.5) | 0.350 |
| Abdominal circumference (cm) | 85.2 ± 9.1 | 80.2 ± 7.8 | <0.001 | 77.5 ± 6.8 | 88.6 ± 7.1 | <0.001 |
| Area of skeletal muscle (cm2) | 135.6 ± 31.5 | 123.4 ± 21.1 | 0.002 | 123.9 ± 27.8 | 137.3 ± 27.6 | 0.001 |
| Area of visceral fat (cm2) | 121.2 ± 66.7 | 97.1 ± 62.6 | 0.014 | 58.8 ± 27.7 | 161.3 ± 51.3 | <0.001 |
| Intraoperative complication (%) | 13 (11.4) | 5 (6.8) | 0.324 | 8 (8.8) | 10 (10.3) | 0.807 |
| Anastomotic leak (%) | 11 (9.6) | 5 (6.8) | 0.598 | 11 (12.1) | 5 (5.2) | 0.117 |
| Prolonged admission (%) | 30 (28.9) | 22 (29.7) | 0.620 | 28 (30.7) | 24 (24.7) | 0.416 |
| Length of stay (days) | 10.9 ± 4.0 | 11.6 ± 4.5 | 0.261 | 11.5 ± 4.4 | 10.8 ± 4.0 | 0.690 |
| Recurrence (%) | 29 (25.4) | 18 (24.3) | 0.864 | 18 (19.8) | 29 (29.9) | 0.110 |
| Death (%) | 9 (7.8) | 16 (21.6) | 0.007 | 10 (11.0) | 15 (15.5) | 0.368 |
BMI, body mass index; CEA, carcinoembryonic antigen.
Stage according to American Joint Committee on Cancer (AJCC) Cancer Staging Manual 7th edition.
Overall survival and disease‐free survival
During the follow‐up period, 25 events of death were observed in the patients, 13.3%. The 1, 3, and 5 year OS rates were 99, 89, and 71%, each respectively. Patients with sarcopenia showed significantly shorter OS than patients without sarcopenia (P = 0. 004; Figure 1). Visceral obesity was not associated with OS (P = 0.457; Figure 2). Sarcopenia (P = 0.006) and initial CEA (P = 0.007) were identified as significant predictors of shorter OS in univariate analyses. They remained as independent and significant predictors for poorer OS in multivariate analysis (P = 0.013 and P = 0.014, respectively). Visceral obesity was not a significant prognostic factor for OS (Table 3).
Figure 1.
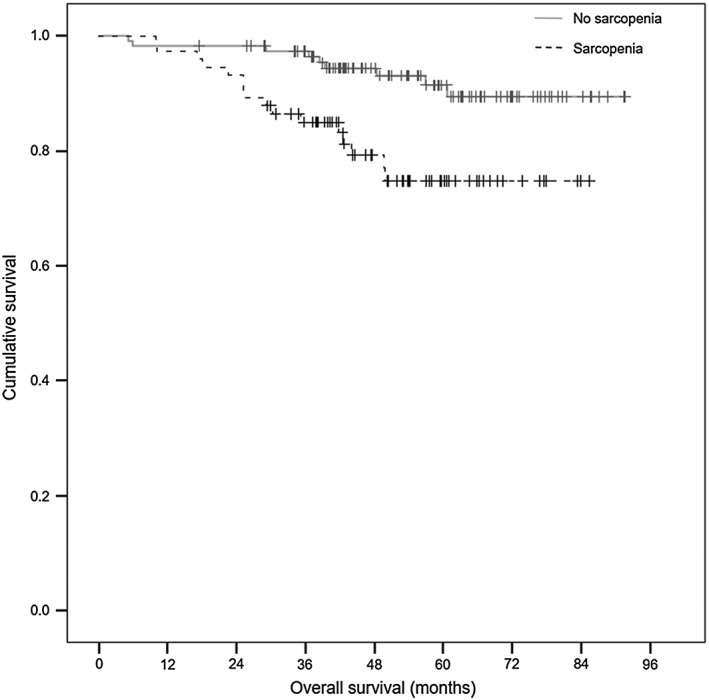
Overall survival (OS) according to sarcopenia.
Figure 2.

Overall survival (OS) according to visceral obesity.
Table 3.
Prognostic factors for overall survival (OS), univariate and multivariate analyses
| Prognostic factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| P‐value | HR (95% CI) | P‐value | HR (95% CI) | |
| Age (years) | 0.834 | 0.996 (0.960–1.033) | ||
| Female gender | 0.132 | 0.494 (0.197–1.238) | 0.483 | 0.691 (0.247–1.937) |
| BMI (kg/m2) | 0.109 | 0.895 (0.781–1.025) | 0.720 | 0.972 (0.832–1.135) |
| Initial CEA (ng/mL) | 0.007 | 1.092 (1.025–1.164) | 0.014 | 1.130 (1.025–1.246) |
| Clinical TNM stage IIIa | 0.799 | 1.149 (0.394–3.349) | ||
| Radiotherapy | ||||
| Standard | Reference | |||
| Short course, 25 Gy | 0.697 | 0.783 (0.229–2.683) | ||
| Short course, 33 Gy | 0.539 | 1.369 (0.503–3.728) | ||
| Sarcopenia | 0.006 | 3.166 (1.396–7.180) | 0.013 | 3.558 (1.311–9.655) |
| Visceral obesity | 0.459 | 1.353 (0.608–3.013) | ||
| Intraoperative complication | 0.357 | 1.654 (0.567–4.822) | ||
| Anastomotic leak | 0.468 | 1.564 (0.468–5.233) | ||
| Prolonged admission | 0.103 | 1.946 (0.873–4.336) | 0.072 | 2.114 (0.935–4.777) |
| Length of stay | 0.822 | 1.010 (0.926–1.102) | ||
CEA, carcinoembryonic antigen.
Stage according to American Joint Committee on Cancer (AJCC) Cancer Staging Manual 7th edition.
Disease recurrence was detected in 47 patients (25.0%) with 1, 3, and 5 year DFS rates of 83, 74, 72%, respectively. Sarcopenia did not shorten DFS (P = 0.900; Figure 3). Viscerally obese patients tended to have shorter DFS than normal patients, but statistical significance was not reached (mean DFS 66.8 months vs 71.4 months, P = 0.121; Figure 4). Univariate analysis found that age was a significant predictor for DFS (HR 0.971, 95% CI 0.947 to 0.996, P = 0.022). Initial CEA was associated with DFS (P = 0.033) as well as OS. After multivariate analysis, visceral obesity showed a tendency to negatively affect DFS (HR 1.701, 95% CI 0.940 to 3.079, P = 0.079; Table 4).
Figure 3.
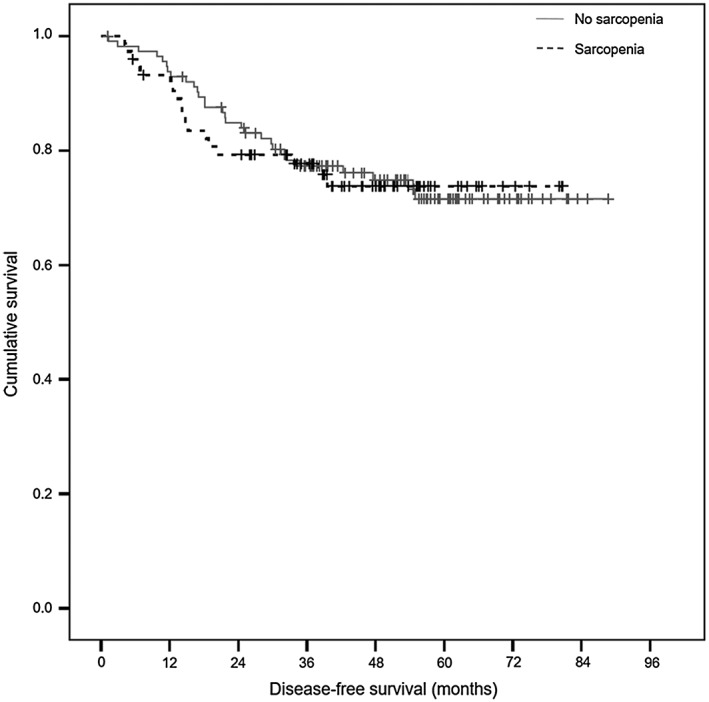
Disease‐free survival (DFS) according to sarcopenia.
Figure 4.
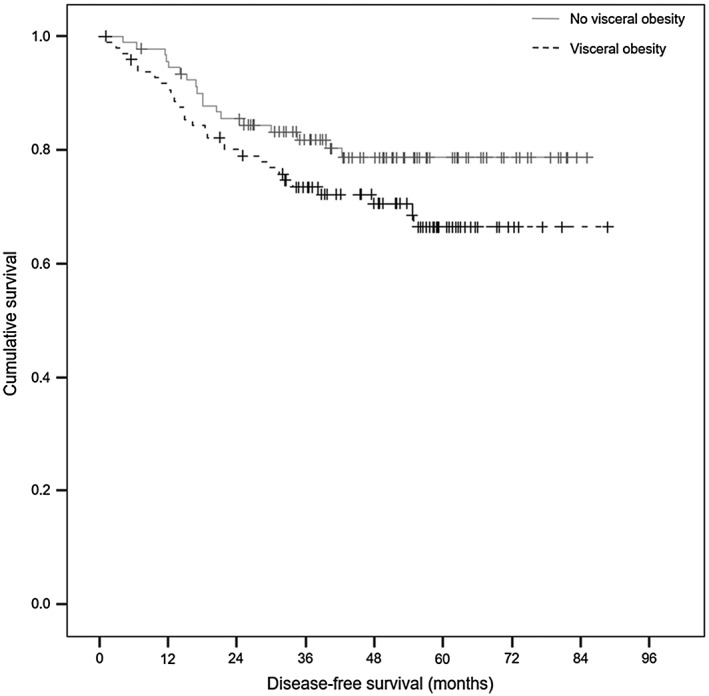
Disease‐free survival (DFS) according to visceral obesity.
Table 4.
Prognostic factors for disease‐free survival (DFS), univariate and multivariate analyses
| Prognostic factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| P‐value | HR (95% CI) | P‐value | HR (95% CI) | |
| Age (years) | 0.024 | 0.972 (0.948–0.996) | 0.022 | 0.971 (0.947–0.996) |
| Female gender | 0.880 | 0.956 (0.531–1.721) | ||
| BMI (kg/m2) | 0.992 | 1.000 (0.911–1.096) | ||
| Initial CEA (ng/mL) | 0.033 | 1.077 (1.006–1.154) | 0.073 | 1.064 (0.994–1.139) |
| Clinical TNM stage IIIa | 0.852 | 0.933 (0.451–1.930) | ||
| Radiotherapy | ||||
| Standard | Reference | |||
| Short course, 25 Gy | 0.190 | 0.501 (0.178–1.409) | ||
| Short course, 33 Gy | 0.735 | 0.869 (0.386–1.956) | ||
| Sarcopenia | 0.900 | 1.038 (0.576–1.871) | ||
| Visceral obesity | 0.124 | 1.586 (0.881–2.856) | 0.079 | 1.701 (0.940–3.079) |
| Intraoperative complication | 0.418 | 1.426 (0.604–3.365) | ||
| Anastomotic leak | 0.441 | 1.441 (0.569–3.647) | ||
| Prolonged admission | 0.569 | 1.199 (0.642–2.242) | ||
| Length of stay | 0.205 | 0.948 (0.872–1.030) | ||
CEA, carcinoembryonic antigen.
Stage according to American Joint Committee on Cancer (AJCC) Cancer Staging Manual 7th edition.
Discussion
Our study showed that sarcopenia was a negative prognostic factor for OS in non‐metastatic locally advanced rectal cancer patients. These patients underwent neoadjuvant CRT followed by surgical resection with total mesorectal excision. This result indicates that patients' muscle mass at initial diagnosis is an important factor in oncologic outcome. Moreover, CT was a useful tool in measuring sarcopenic status of patients.
To the best of our knowledge, this is the first report to describe the prognostic impact of sarcopenia in non‐metastatic locally advanced rectal cancer. Our data add value to the previous literature because they address a more specific group of patients, which include rectal cancer patients undergoing neoadjuvant chemoradiation therapy and curative resection. Only few studies before have described the relationship between sarcopenia and survival in colorectal cancer surgical patients. A few previous studies have included stage IV colorectal cancers with hepatic metastasis.16, 17 One study included rectal cancer surgical patients after neoadjuvant chemoradiation therapy, but it only analysed perioperative outcomes without long‐term oncologic outcomes.12
In our study, sarcopenia had a significant prognostic impact on OS (P = 0.013) but not on DFS (P = 0.900). On the other hand, previous studies regarding colorectal cancer with liver metastasis showed sarcopenia's negative impact on DFS as well as OS.17 As recurrences were more frequent in those stage IV cancers (64.2%), those patients endured more challenging treatment process in the palliative setting. Previous studies observed more frequent post‐operative infection and chemotherapy toxicity in sarcopenic patients, which may result in decreased treatment adherence.18, 19
On the contrary, our study included stages II and III rectal cancer instead of stage IV disease. Multimodal treatment was achieved regardless of sarcopenic status, and most patients adhered to the treatment process. Notably, one previous study noted that neoadjuvant chemoradiation therapy itself did not decrease muscle mass in patients.12 Preoperative chemoradiation therapy might not impose a high burden, which alters the perioperative treatment process or patient function. The quantitative effect of sarcopenia on patient function is still unclear.20 Future study should include tools to assess patient function and seek better relationship among physical function, muscle mass, and clinical progress.
Although BMI and sarcopenia showed no significant association with DFS, visceral obesity tended to shorten DFS with results that agreed with previous studies. Obesity is an important factor in predicting the recurrence of colorectal cancer.2, 18, 19 Especially, viscerally obese rectal cancer patients after neoadjuvant chemotherapy and resection showed shorter DFS than non‐obese patients.11 Other studies also showed that increased visceral fat was a significant predictor of short DFS in resectable colorectal cancer.20, 21
Neither visceral obesity nor sarcopenia showed an association with perioperative and post‐operative surgical outcomes. Intraoperative complications, anastomosis leakage rate, and hospital stay increase were not observed in sarcopenic and viscerally obese patients. The results of our study did not agree with the consistent results of previous studies that showed a negative effect of visceral obesity and sarcopenia on surgical outcome.6, 12, 22, 23, 24, 25, 26 One study showed higher post‐operative complication rates in sarcopenic patients. They included 259 colorectal liver metastasis patients undergoing hepatic resection.16 Yet, their morbidity was mostly related to liver related complications such as bleeding or liver insufficiency. Variable results among studies might be caused by a different stage of rectal cancer, experience of surgeons, and cut‐off value for visceral obesity. Surgeries were performed by experienced surgeons in our institute that provided a lower rate of anastomotic leakage.27
There are several limitations in our study. First, there were relatively small numbers of events regarding patient death and tumour recurrence. As we evaluated only locally advanced rectal cancer patients who underwent neoadjuvant chemoradiation and curative resection, these two conditions might limit the number of eligible patients. However, statistical significance was reached in factors that were consistent with previous studies. Second, initial CT examinations and their protocols were different because some patients performed their initial work up in other hospitals. Differences in CT protocols might cause differences of CT density. However, body composition assessment was performed in the portal phase and area of fat or muscle selected by analysis software was confirmed by a radiologist. Errors from differences of CT protocol may be negligible when considering a wide range of predetermined range of fat and muscle density.
The strength of our study is that we demonstrated the usefulness of CT‐based body composition analysis in locally advanced rectal cancer patients undergoing neoadjuvant CRT. Abdominal CT is usually performed in the clinical staging process; consequently, CT‐based assessment is an efficient way to assess a patient's function and frailty. Moreover, our data add to the building evidence of sarcopenia's prognostic value in the Asian population with relatively large number of rectal cancer cases. The cut‐off value from the Western population was applicable in our data. However, more standardized body composition values and measurements are needed, which consider not only sex but also ethnicity. There are variable cut‐off values for sarcopenia and visceral obesity.12, 17, 22 Area of muscle mass can be measured on abdominal wall or psoas muscle.7 Range of CT density to select muscle area is also variable.28
In conclusion, sarcopenia at initial diagnosis had a negative effect on OS in patients undergoing resection for locally advanced rectal cancer after neoadjuvant CRT. Visceral obesity tendered to shorten DFS. Future studies should be directed to optimize patient conditions according to body composition status.
Funding
This study was funded by Ministry of Education, Republic of Korea(2016R1D1A1B03932876). The funder did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
Moon Hyung Choi, Soon Nam Oh, In Kyu Lee, Seong Taek Oh, and Daeyoun David Won declare that they have no conflict of interest.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.29
Choi, M. H. , Oh, S. N. , Lee, I. K. , Oh, S. T. , and Won, D. D. (2018) Sarcopenia is negatively associated with long‐term outcomes in locally advanced rectal cancer. Journal of Cachexia, Sarcopenia and Muscle, 9: 53–59. doi: 10.1002/jcsm.12234.
References
- 1. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 2. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 2013;62:933–947. [DOI] [PubMed] [Google Scholar]
- 3. Gibson DJ, Burden ST, Strauss BJ, Todd C, Lal S. The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: a systematic review. Eur J Clin Nutr 2015;69:1079–1086. [DOI] [PubMed] [Google Scholar]
- 4. Levolger S, van Vugt JL, de Bruin RW, IJzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg 2015;102:1448–1458. [DOI] [PubMed] [Google Scholar]
- 5. van Vugt JL, Braam HJ, van Oudheusden TR, et al. Skeletal muscle depletion is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2015;22:3625–3631. [DOI] [PubMed] [Google Scholar]
- 6. Lieffers JR, Bathe OF , Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol 2015;41:186–196. [DOI] [PubMed] [Google Scholar]
- 8. Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO‐94 randomized phase III trial after a median follow‐up of 11 years. J Clin Oncol 2012;30:1926–1933. [DOI] [PubMed] [Google Scholar]
- 9. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 10. Murphy CC, Harlan LC, Lund JL, Lynch CF, Geiger AM. Patterns of colorectal cancer care in the United States: 1990–2010. J Natl Cancer Inst 2015;107(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clark W, Siegel EM, Chen YA, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg 2013;216:1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heus C, Cakir H, Lak A, Doodeman HJ, Houdijk AP. Visceral obesity, muscle mass and outcome in rectal cancer surgery after neo‐adjuvant chemo‐radiation. Int J Surg 2016;29:159–164. [DOI] [PubMed] [Google Scholar]
- 13. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. [DOI] [PubMed] [Google Scholar]
- 14. Kang J, Baek SE, Kim T, et al. Impact of fat obesity on laparoscopic total mesorectal excision: more reliable indicator than body mass index. Int J Colorectal Dis 2012;27:497–505. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe J, Tatsumi K, Ota M, et al. The impact of visceral obesity on surgical outcomes of laparoscopic surgery for colon cancer. Int J Colorectal Dis 2014;29:343–351. [DOI] [PubMed] [Google Scholar]
- 16. Peng PD, van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short‐term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550–557. [DOI] [PubMed] [Google Scholar]
- 18. Choi Y, Lee YH, Park SK, Cho H, Ahn KJ. Association between obesity and local control of advanced rectal cancer after combined surgery and radiotherapy. Radiat Oncol J 2016;34:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment‐related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol 2004;22:648–657. [DOI] [PubMed] [Google Scholar]
- 20. Rickles AS, Iannuzzi JC, Mironov O, et al. Visceral obesity and colorectal cancer: are we missing the boat with BMI? J Gastrointest Surg 2013;17:133–143. [DOI] [PubMed] [Google Scholar]
- 21. Moon HG, Ju YT, Jeong CY, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol 2008;15:1918–1922. [DOI] [PubMed] [Google Scholar]
- 22. Cakir H, Heus C, van der Ploeg TJ, Houdijk AP. Visceral obesity determined by CT scan and outcomes after colorectal surgery; a systematic review and meta‐analysis. Int J Colorectal Dis 2015;30:875–882. [DOI] [PubMed] [Google Scholar]
- 23. Ballian N, Lubner MG, Munoz A, et al. Visceral obesity is associated with outcomes of total mesorectal excision for rectal adenocarcinoma. J Surg Oncol 2012;105:365–370. [DOI] [PubMed] [Google Scholar]
- 24. Chen B, Zhang Y, Zhao S, et al. The impact of general/visceral obesity on completion of mesorectum and perioperative outcomes of laparoscopic TME for rectal cancer: a STARD‐compliant article. Medicine (Baltimore) 2016;95:e4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishii Y, Hasegawa H, Nishibori H, Watanabe M, Kitajima M. Impact of visceral obesity on surgical outcome after laparoscopic surgery for rectal cancer. Br J Surg 2005;92:1261–1262. [DOI] [PubMed] [Google Scholar]
- 26. Reisinger KW, Derikx JP, van Vugt JL, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr 2016;35:924–927. [DOI] [PubMed] [Google Scholar]
- 27. Balik E, Asoglu O, Saglam S, et al. Effects of surgical laparoscopic experience on the short‐term postoperative outcome of rectal cancer: results of a high volume single center institution. Surg Laparosc Endosc Percutan Tech 2010;20:93–99. [DOI] [PubMed] [Google Scholar]
- 28. Malietzis G, Currie AC, Athanasiou T, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg 2016;103:572–580. [DOI] [PubMed] [Google Scholar]
- 29. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


