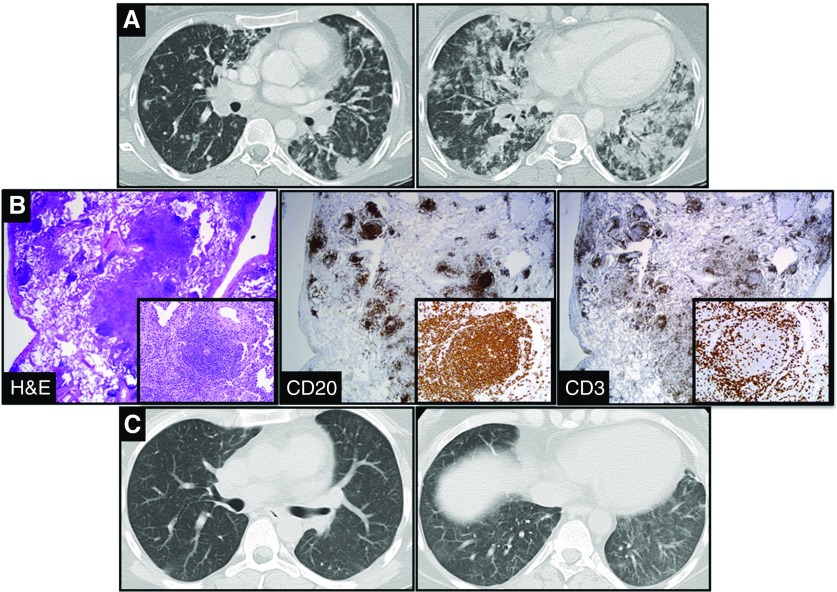
A 28-year-old woman with a medical history significant for Kabuki syndrome with associated hyper-IgM syndrome/common variable immunodeficiency presented with a 3-month history of dyspnea, pleuritic pain, and nonproductive cough. Imaging demonstrated nodular infiltrates increasing toward the lung bases (Figure 1A). Bronchoscopy with lavage and transbronchial biopsies was performed to evaluate for an infectious or inflammatory etiology; however, results were unrevealing, and no infectious etiology was identified. Therefore, she underwent a video-assisted thoracic surgery biopsy that demonstrated nodular lymphoid hyperplasia with follicles centered on small airways and areas of organizing pneumonia (Figure 1B). Immunostains demonstrated follicles composed of CD3+ T cells and CD20+ B cells (Figure 1B) and no morphologic evidence of lymphoma or plasma cell neoplasm. Results were consistent with bronchus-associated lymphoid tissue (BALT). High-dose steroids were administered without resolution of symptoms or radiographic findings. A report of effective responses with rituximab and azathioprine in patients with common variable immunodeficiency who had a similar inflammatory lung process containing tertiary lymphoid structures, granulomas, and organizing pneumonia has been published (1). After four doses of rituximab (weekly), together with the initiation of azathioprine, her symptoms and radiographic findings improved (Figure 1C).
Figure 1.
(A) Coronal chest computed tomography images that demonstrated nodular infiltrates more prominent toward the lung bases in a 28-year-old woman with a 3-month history of dyspnea and a nonproductive cough. (B) Histological sections from video-assisted thoracic surgery biopsy demonstrated nodular lymphoid hyperplasia with follicles centered on small airways and areas of organizing pneumonia. Immunostains demonstrated follicles composed of CD3+ T cells surrounding a center grouping of CD20+ B cells (×20 magnification). Insets represent serial immunostains of a lymphoid follicle in the lower magnification section (×200 magnification). (C) One month after administration of azathioprine and rituximab, findings on coronal computed tomography images were dramatically improved. H&E = hematoxylin and eosin.
BALT is a type of tertiary lymphoid tissue first termed by Bienenstock in 1973 (2) and described as a follicular-like aggregation of lymphoid and structural cells at airway bifurcations (3). Tertiary lymphoid tissues differ from primary (e.g., thymus or bone marrow) and secondary (e.g., lymph node or spleen) lymphoid tissues (3). Tertiary lymphoid tissue develops ectopically and predominately at mucosa sites and can be initiated either by autoimmunity or chronic inflammation (for review, see Reference 3). BALT formation in humans is rarely seen after age 20 years in normal conditions (4). Patients with hyper-IgM syndrome or common variable immunodeficiency have a propensity to develop lymphoid hyperplasia (5). Kabuki syndrome caused by mutations in MLL2, a trithorax group methyltransferase, has not been reported, to our knowledge, to elicit BALT formation (6).
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201511-2305IM on June 8, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chase NM, Verbsky JW, Hintermeyer MK, Waukau JK, Tomita-Mitchell A, Casper JT, Singh S, Shahir KS, Tisol WB, Nugent ML, et al. Use of combination chemotherapy for treatment of granulomatous and lymphocytic interstitial lung disease (GLILD) in patients with common variable immunodeficiency (CVID) J Clin Immunol. 2013;33:30–39. doi: 10.1007/s10875-012-9755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bienenstock J, Johnston N, Perey DY. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973;28:686–692. [PubMed] [Google Scholar]
- 3.Pabst R. Plasticity and heterogeneity of lymphoid organs. What are the criteria to call a lymphoid organ primary, secondary or tertiary? Immunol Lett. 2007;112:1–8. doi: 10.1016/j.imlet.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Hiller AS, Tschernig T, Kleemann WJ, Pabst R. Bronchus-associated lymphoid tissue (BALT) and larynx-associated lymphoid tissue (LALT) are found at different frequencies in children, adolescents and adults. Scand J Immunol. 1998;47:159–162. doi: 10.1046/j.1365-3083.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- 5.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119:1650–1657. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, Huff CD, Shannon PT, Jabs EW, Nickerson DA, et al. Exome sequencing identifies the cause of a Mendelian disorder. Nat Genet. 2010;42:30–35. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]